Abstract
Chronic alcohol consumption leads to hypertriglyceridemia, which is positively associated with alcoholic liver disease (ALD). However, whether and how it contributes to the development of fatty liver and liver injury are largely unknown. In this study, we demonstrate that chronic alcohol exposure differently regulates the expression of very low-density lipoprotein receptor (VLDLR) in adipose tissue and the liver. Whereas adipose tissue VLDLR is significantly downregulated, its hepatic expression is dramatically increased after chronic alcohol feeding. While HepG2 cells stably overexpressing VLDLR manifests increased intracellular triglyceride accumulation, VLDLR-deficient mice are protective against fatty liver and liver injury after chronic alcohol exposure. Mechanistic investigations using both in vitro and in vivo systems reveal that oxidative stress-induced nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activation plays a critical role in alcohol-induced VLDLR upregulation in hepatocytes, but not in adipocytes. Oxidative stress enhances VLDLR gene expression and protein abundance in primary hepatocytes, concomitant with the Nrf2 activation. Conversely, Nrf2 gene silencing abrogates oxidative stress-induced VLDLR upregulation in the liver, but not in adipose tissue. In mice, alcohol exposure induces hepatic oxidative stress and Nrf2 activation. Supplementation of N-acetylcysteine alleviates fatty liver and liver injury induced by chronic alcohol exposure, which is associated with suppressed Nrf2 activation and attenuated VLDLR increase in the liver. Furthermore, in comparison to wild type counterparts, Nrf2 deficient mice demonstrate attenuated hepatic VLDLR expression increase in response to chronic alcohol exposure.
Conclusion
Chronic alcohol consumption differently alters VLDLR expression in adipose tissue and the liver. Oxidative stress-induced Nrf2 activation is mechanistically involved in VLDLR overexpression in hepatocytes in response to chronic alcohol consumption. Hepatic VLDLR overexpression plays an important role in the pathogenesis of ALD.
Keywords: VLDL, LPL, HL, TG-rich lipoproteins, ARE
INTRODUCTION
Alcohol Liver Disease (ALD) ranks among the major causes of morbidity and mortality in the world, and affects millions of patients worldwide each year.1 Fatty liver is the most common and earliest response of the liver to chronic alcohol consumption. Although can be a completely benign condition, excessive fat accumulation makes hepatocytes vulnerable to the “second hit”, mainly proinflammatory cytokines and oxidative stress, leading to the progression to steatohepatitis.2,3
Alcohol affects hepatic TG synthesis and disposal at multiple steps. Sterol regulatory element binding proteins (SREBP)-1c, a master transcription factor controlling de novo lipogenesis, is upregulated by alcohol,4 and specific knockout of SREBP-1c in the liver protects mouse from alcohol-induced liver damage.5 Moreover, alcohol exposure impaired hepatic fatty acid β-oxidation via suppressing AMP-activated protein kinase and peroxisome proliferator-activated receptor-alpha activation.6,7 Furthermore, enhanced hepatic fatty acids absorption8 and impaired hepatic VLDL secretion are also reported to contribute to fatty liver after chronic alcohol exposure.9
Chronic alcohol consumption induces hypertriglyceridemia.10–12 Interestingly, alcohol-induced hypertriglyceridemia is positively associated with fatty liver and liver injury,13,14 implying that altered hepatocyte lipoproteins absorption may contribute to alcohol-induced hepatic fat accumulation. However, the direct evidence supporting this notion is currently scarce.
Very-low density lipoprotein receptor (VLDLR) is a number of the low density lipoprotein (LDL) receptor superfamily, highly expressed in skeletal muscle, heart, and adipose tissue but only in small amount in the liver.15 In peripheral tissues, VLDLR binds apo-E-triglyceride-rich lipoproteins (TRLs) and mediates their uptake.16 The VLDLR−/− mice are leaner than their wild-type littermates in both genetically and the diet-induced obese models and demonstrate marked hypertriglyceridemia due to inefficient metabolism of TRLs in adipose tissue.17,18 In addition, VLDLR mediates the trans-cytosis of lipoprotein lipase (LPL) across endothelial cells, therefore, LPL activity is significantly reduced in VLDLR−/− mice.19 Alternatively, it was shown that VLDLR is required for the pro-adipogenic effect of the peroxisome proliferator-activated receptors-gamma (PPAR-γ) agonist.20 In cardiomyocytes, hypoxida/ischemia-induced accumulation of lipids is dependent on expression of the VLDLR.21
Due to its low expression in hepatocytes, the role of VLDLR in the development of fatty liver diseases has received little investigative attention. However, indirect evidence exists. Long-term stable overexpression of VLDLR in the liver of LDLR-deficient mice increased liver TG content, although it improved plasma lipid profile and aortic atherosclerosis.22 In hepatoma cells, hypoxia condition leads to intracellular triglyceride accumulation via inducing VLDLR overexpression.23 To our knowledge, the only direct evidence supporting the involvement of hepatic VLDLR in the development of fatty liver diseases was provided by a very recent study showing that hepatic VLDLR overexpression was attributed to ER stress-induced hepatic steatosis in high-fat diet fed mice.24
In this work, we discovered that alcohol increased hepatic VLDLR expression and VLDLR−/− mice are protected from alcohol-induced fatty liver and liver injury. Furthermore, our data reveal that oxidative stress-induced nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway activation plays a critical role in hepatic VLDLR overexpression in response to chronic alcohol exposure.
MATERIALS AND METHODS
Animals and treatments
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago, which is certified by the American Association of Accreditation of Laboratory Animal Care. Male C57BL/6 mice weighing 25 ± 0.5 g (means ± SD) were obtained from the Harlan Laboratory (Indianapolis, IN). Both VLDLR- and Nrf2-deficient mice, as well as their age/sex-matched wild type mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were randomly assigned to two groups and fed for five weeks with liquid diets according to Lieber and De Carli.25
Cells and culture conditions
Primary mouse hepatocytes were purchased from Celsis In Vitro Technologies (M91684). HepG2 cells, a human hepatoma cell line, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Hepatocytes were cultured in DMEM containing 10% (v/v) fetal bovine serum, 2 mM glutamine, 5 U/ml penicillin, and 50 µg/ml streptomycin at 37 °C in a humidified O2/CO2 (19:1) atmosphere. Primary adipocytes were isolated from mouse epididymal fat pad and cultured as previously described.26
Intragastric fat load
Animals were given an intragastric 200 µl olive oil bolus after 5 weeks on Lieber-De Carli liquid diet with/without ethanol and an overnight fast. Blood samples were drawn from tail vein at 0, 1, 2, 4, 6, and 8 hours after bolus administration, and plasma triglyceride concentrations were determined by CardioChek® analyzer using test strips for triglyceride.
Plasma lipid and lipoprotein analyses
Blood was collected from the retro-orbital plexus into tubes containing EDTA. Lipoproteins, VLDL (d <1.006 g/ml), intermediate/low density lipoproteins (IDL/LDL) (d = 1.006 – 1.063 g/ml), and high density lipoproteins (HDL) (d = 1.063 – 1.21 g/ml), were isolated by sequential ultracentrifugation. TG concentrations in lipoprotein fractions (VLDL and IDL/LDL) were determined by commercially available assay kits (Sigma).
Establishment of stable VLDLR-overexpressing HepG2 cells
HepG2 cells grown to 80–90% confluence were transfected with either 0.8 ug/well of the expression vector pcDNA3.1/hVLDLR or empty vector control pcDNA3.1 (+) (a gift from Dr. Kazuhiro Oka at College of Medicine, Baylor University) in 24-well plates using Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY) following the manufacturer's guidelines. For the selection of stable VLDLR overexpression cells, HepG2 cells were passaged at 1/10 dilution after 48 hours. G418 was added (400 ug/mL) for HepG2 cells screening. The culture medium was replaced at 2 to 3-day intervals until G418-resistant clones emerged (3 weeks after plating). Resistant cells were cloned by limiting dilution. The cells were kept under G418 selection. The overexpression of VLDLR was confirmed by western blot analysis and real time PCR.
Intracellular triglyceride determination
Total lipids were extracted and intracellular TG contents were measured as described previously.26
Quantitative real-time RT-PCR
Total RNA, from either frozen liver tissue or cultured cells, was isolated and real-time RT-PCR was performed as described previously.26
Western blotting
Liver tissues were homogenized and hepatocytes were lysed in RIPA buffer and proteins were detected by Western blot using specific antibodies as described previously.26
Gene silencing by siRNA
Transient gene silencing was attained by transfection siRNA into cells using siPORT lipid transfection reagent according to the manufacturer's instructions. Scrambled siRNA was used as a control. Gene silencing was verified by detecting protein with immunoblotting analysis after transient transfection with siRNA.
Immunohistochemistry
HepG2 cells were plated onto sterilized glass coverslips at a density of 1 × 104 cells/cm2 and incubated in complete DMEM medium. After the treatments, hepatocytes were fixed with 4% paraformaldehyde for 20 minutes at room temperature. The fixed cells were incubated overnight at 4°C with primary antibodies. After three PBS washes, the cells were incubated for 1 hour at room temperature with secondary antibodies conjugated to fluorescein isothiocyanate (FITC). Cell nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI). Images were captured using an Olympus fluorescence microscope.
Statistical Analysis
All data are expressed as means ± SD. Statistical analysis was performed using a one-way ANOVA and further analyzed by Newman-Keuls test for statistical difference. Differences between treatments were considered to be statistically significant at P < 0.05.
RESULTS
Early stage alcoholic liver injury is associated with hyperlipidemia
In comparison to pair-fed animals, alcohol-fed mice showed modest elevation of plasma alanine aminotransferase (ALT) (Fig. 1A). Associated with the early-stage alcoholic liver injury was a marked liver triglyceride accumulation in the liver in alcohol-fed mice (Fig. 1B & C). Alcohol feeding resulted in elevated plasma TG, total cholesterol, and free fatty acids (FFAs) concentrations (Fig. 1D). Furthermore, alcohol exposure delayed recovery of plasma TG content after a bolus intragastric fat load (Fig. 1E).
Figure 1. Chronic alcohol exposure causes fatty liver, liver injury, and hyperlipidemia in mice.
Male C57BL/6 mice weighing 25 ± 0.5 g (means ± SD) were pair-fed liquid diets with or without ethanol for 5 weeks. Chronic alcohol exposure increased plasma ALT level (A) and tissue triglyceride (TG) content (B & C) in the liver. Chronic alcohol exposure resulted in hyperlipidemia, manifested by the significant elevations of plasma TG, total cholesterol, and frees fatty acids (FFAs) levels (D), and delayed plasma triglyceride clearance (E). All values are denoted as means ± SD Data (n = 8). * p < 0.05 when compared with corresponding PF. PF, pair-fed; AF, alcohol-fed.
Alcohol exposure differently regulates VLDLR expression in adipose tissue and the liver
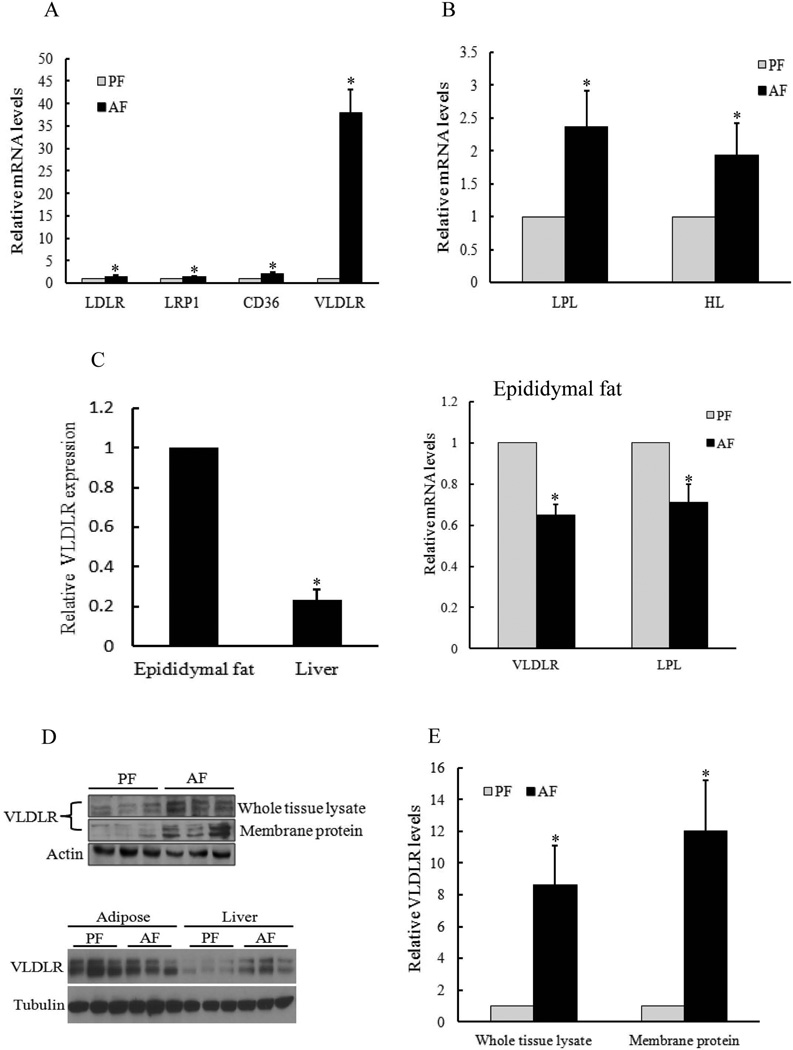
Four proteins critically involved in lipoproteins delivery, including LDLR (low-density lipoprotein receptor), LRP1 (LDLR-related protein 1), CD36, and VLDLR, were examined. In the liver, the gene expressions of all proteins examined were increased by alcohol (Fig. 2A). Among them, the change in VLDLR expression was most dramatic (~ 40 folds). Moreover, alcohol increased gene expressions of both lipoprotein lipase (LPL) and hepatic lipase (HL) in the liver, two critical lipases involved in lipoproteins metabolism (Fig. 2B). In comparison to the liver, adipose tissue demonstrates much higher basal VLDLR expression (Fig. 2C & D) and, in contrast to the liver, both VLDLR and LPL gene expression in adipose tissue (epididymal fat pad) was significantly reduced (Fig. 2C). At protein level, both total and functional (membrane-bound) VLDLR protein abundance in the liver was increased in response to alcohol exposure (Fig. 2D & E).
Figure 2. Chronic alcohol exposure markedly increases hepatic VLDLR expression.
Male C57BL/6 mice weighing 25 ± 0.5 g (means ± SD) were pair-fed liquid diets with or without ethanol for 5 weeks. Total RNAs were isolated from liver and adipose tissues. (A & B) Real time RT-PCR analysis of genes involved in hepatic lipoproteins delivery (A) and metabolism (B). (C) Real time RT-PCR analysis of VLDLR gene expression (relative to epididymal fat) in epididymal fat and the liver, as well as VLDLR and LPL gene expressions (relative to PF) in epididymal fat pad. All values are denoted as means ± SD Data (n = 8). * p < 0.05 when compared with corresponding PF. Both whole liver tissue and plasma membrane proteins were isolated and subjected to Western blot for the detection of VLDLR protein abundance (D & E). All values are denoted as means ± SD Data (n = 8). * p < 0.05 when compared with corresponding PF. PF, pair-fed; AF, alcohol-fed.
Overexpression of VLDLR in hepatocytes increases intracellular lipid contents
A stable VLDLR-overexpressing (OE) hepatocyte cell line and corresponding vector control (VC) cell line were established via transfecting HepG2 cells with a pcDNA3.1/hVLDLR-1 vector or empty vector. The VLDLR overexpression was characterized by RT-PCR (Fig. 3A & B) and Western blot, respectively (Fig. 3C). It is noteworthy here that in comparison to primary hepatocytes, HepG2 cells express a higher level of basal VLDLR, which accounts for the relative less fold increase in VLDLR gene expression in our VLDLR-overexpressing cell line. This observation is in fact in line with previous studies reporting that VLDLR expression plays an important role in cancer cell survival.23 In comparison to VC cells, OE cells exhibited higher basal intracellular TG content, which was further increased when exogenous VLDL was supplemented (Fig. 3D). Interestingly, the presence of exogenous LPL exacerbated intracellular TG accumulation by VLDL, implying that the VLDL remnants, such as intermediate density lipoprotein (IDL), may be a better ligand for VLDLR-regulated lipoproteins absorption in the hepatocytes. Similarly, both intracellular total cholesterol and FFAs levels were increased in VLDLR OE hepatocytes (Supplementary data, Fig. 1), although the effects were less significant. Gene silencing with VLDLR siRNA attenuated the elevation of intracellular lipids contents in VLDLR OE hepatocytes (Fig. 3D; Supplementary data, Fig. 2).
Figure 3. Chronic VLDLR overexpression in hepatocytes increases intracellular triglyceride accumulation.
A stable VLDLR-overexpressing (OE) hepatocyte cell line was established and VLDLR expression was characterized by both RT-PCR (A & B) and Western blot (C). All values are denoted as means ± SD from three or more independent batches of cells. * p < 0.05 when compared with vector control cells. (D) VLDLR overexpression increases intracellular triglyceride accumulation. Both VLDLR overexpressing (OE) and vector control (VC) hepatocytes were exposed to the media containing human VLDL (50 µg/ml) in the presence or absence of human recombinant LPL (0.25 unit/ml) for 16 hours. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different characters differ significantly, p < 0.05. (E) VLDLR siRNA transfection attenuates intracellular triglyceride accumulation in VLDLR OE cells. VLDLR overexpressing (OE) hepatocytes were transfected with VLDLR siRNA for 16 hours before the exposure to the media containing human VLDL (50 µg/ml) in the presence or absence of human recombinant LPL (0.25 unit/ml). Intracellular triglyceride content was determined 16 hours later and expressed as fold of the control cells (OE cells transfected with scramble siRNA, without VLDL and LPL) after being standardized by corresponding protein concentrations. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different characters differ significantly, p < 0.05.
VLDLR-knockout mice are protected from alcohol-induced fatty liver and liver injury
To directly address whether the hepatic VLDLR overexpression contributes to alcoholic liver injury, VLDLR−/− mice and gender-/age-matched wild type (WT) littermates were exposed to control (PF, pair-fed) and ethanol-containing diet (AF) for 5 weeks. VLDLR deficiency alleviated alcoholic fatty liver and liver injury (Fig. 4A–C). In line with previous reports,17,18 VLDLR−/− mice exhibited obvious hyperlipidemia, which were not further affected by alcohol feeding (Supplementary data, Fig. 3). Alcohol exposure was associated with increased TG levels in isolated VLDL, while TG levels in IDL/LDL were decreased. VLDLR−/− mice showed much higher VLDL TG levels than their wild type littermates and alcohol exposure had no effect on TG levels in both VLDL and IDL/LDL in VLDLR−/− mice (Table 1). VLDLR−/− mice exhibited a higher level of nuclear SREBP-1c protein abundance than their wild type littermates under control diet and alcohol-feeding aggravate the difference (Fig. 4D; Supplementary data, Fig. 4). No significant differences were observed in the expressions of LDLR, LRP1, CD36, and HL in the livers between WT and VLDLR−/− mice after chronic alcohol exposure (Fig. 4E & F), although VLDLR−/− mice exhibited higher hepatic CD36 than wild type animals when control diet was fed (Fig. 4E). In contrast, in comparison to WT animals, VLDLR−/− mice exhibited significantly lower LPL expression in both PF and AF group (Fig. 4F).
Figure 4. VLDLR-deficient mice are protective from alcoholic liver injury.
VLDLR−/− and wild type (WT) mice were pair-fed liquid diets with or without ethanol for 5 weeks. In comparison to WT counterparts, VLDLR−/− mice demonstrate lowered plasma ALT levels and decreased hepatic fat accumulation (A–C). All values were denoted as means ± SD (n = 5). Bars with different characters differ significantly, p < 0.05. (D) VLDLR−/− mice demonstrate enhanced hepatic SREBP-1c activation. After 5-week feeding, the nuclear fractions were freshly isolated from liver tissues and subjected to Western blot for the detection of SREBP-1c protein abundance. (E & F) Real time RT-PCR analysis of genes involved in hepatic lipoproteins delivery (A) and metabolism (B). All values were denoted as means ± SD (n = 5). For each gene, bars with different characters differ significantly, p < 0.05. PF, pair-fed; AF, alcohol-fed.
Table 1.
Triglyceride (TG) contents in TG-rich lipoproteins
| TG | ||||
|---|---|---|---|---|
| n | VLDL | IDL/LDL | ||
| mg/dl | ||||
| WT | PF | 6 | 106 ± 19a | 81 ± 12a |
| WT | AF | 6 | 164 ± 25b | 59 ± 18b |
| VLDLR−/− | PF | 5 | 314 ± 54c | 54 ± 14b |
| VLDLR−/− | AF | 5 | 331 ± 47c | 62 ± 17b |
Lipoproteins were isolated by sequential ultracentrifugatio.
Values are expressed as means ± S.D.
Data with different character in the same column differ significantly, p < 0.05.
Oxidative stress-induced Nrf2 activation triggers VLDLR overexpression in hepatocytes
Oxidative stress plays a pathological role in the development of ALD. The effect of oxidative stress on hepatic VLDLR overexpression was subsequently examined via exposing primary mouse hepatocytes to three oxidative stress inducers, including hydrogen peroxide (H2O2), rotenone (a mitochondrial complex I inhibitor), and 4-hydroxynonenal (4-HNE). Oxidative stress inducers increased VLDLR expression in both primary mouse hepatocytes and human HepG2 cells (Fig. 5A). Conversely, N-acetylcysteine (NAC), a widely used antioxidant via serving as glutathione precursor, abrogated VLDLR overexpression induced by oxidative stress-inducers (Fig. 5B). Nrf2 is a master transcriptional regulator activated by oxidative and electrophilic stress.27 To gain insight into the mechanisms underlying oxidative stress-induced VLDLR overexpression in hepatocytes, we examined the role of Nrf2 activation in this process. Exposure of primary mouse hepatocytes to oxidative stress inducers resulted in Nrf2 activation, evidenced by the increased nuclear Nrf2 protein abundance, enhanced Nrf2 DNA binding activity, and elevated gene expression of NAD(P)H:quinone oxidoreductase1 (NQO1), a specific target gene by Nrf2 activation (Fig. 5C). Moreover, chemical inducers for Nrf2 increased VLDLR expression in hepatocytes (Fig. 5D). Conversely, Nrf2 gene silencing via siRNA transfection abrogated VLDLR upregulation induced by either H2O2 or 4-HNE (Fig. 5E).
Figure 5. Oxidative stress-induced Nrf2 activation triggers VLDLR overexpression in hepatocytes.
Primary mouse hepatocytes, or HepG2 cells, were exposed to complete DMEM medium containing hydrogen peroxide (H2O2) (100 µM), rotenone (100 nM), and 4-hydroxynonenal (4-HNE) (20 µM), respectively, for 6 hours. (A) Oxidative stress inducers increases VLDLR expression at both mRNA and protein levels. All values are denoted as means ± SD from three or more independent batches of cells. * p < 0.05. (B) N-acetylcysteine (NAC) prevents oxidative stress-induced VLDLR upregulation. Primary mouse hepatocytes were pretreated with NAC (5mM) for 2 hours before the addition of H2O2 (100 µM) or rotenone (100 nM). (C) Oxidative stress inducers lead to Nrf2 activation. All values were denoted as means ± SD from three or more independent experiments. * p < 0.05. (D) Nrf2 inducers increase VLDLR expression. Primary mouse hepatocytes were exposed to the culture medium containing sulforaphane (2 µM) or tBHQ (10 µM) for 8 hours. VLDLR gene expression and protein abundance were determined. All values were denoted as means ± SD from three or more independent experiments. * p < 0.05. (E) Nrf2 siRNA transfection abolishes VLDLR increases in response to oxidative stress inducers. Primary mouse hepatocytes were transfected with Nrf2 siRNA for 16 hours before the exposure to oxidative stress inducers. Whole cell lysates were collected for the detection of VLDLR protein.
Nrf2 activation plays a minor role in controlling VLDLR expression in adipocytes
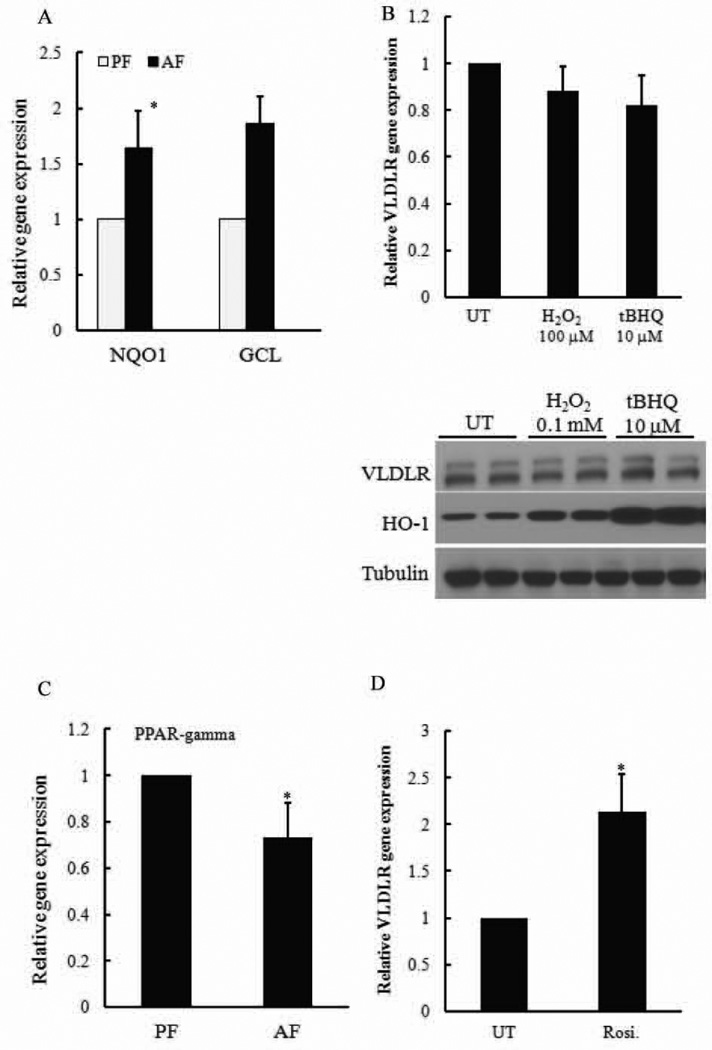
To determine whether Nrf2 activation also regulates VLDLR expression in adipocytes, we first examined adipose tissue Nrf2 activation in response to alcohol feeding. As shown in Fig. 6A, alcohol exposure activated Nrf2 in epididymal fat pads. To directly determine the effect of oxidative stress-induced Nrf2 activation on VLDLR expression in adipocytes, primary adipocytes were treated with either H2O2 or tBHQ for 8 hours. VLDLR expression was determined. Although both chemicals increased expression of HO-1, a target protein of Nrf2, neither of them induced VLDLR expression (Fig. 6B). Conversely, alcohol suppressed adipose tissue PPAR-gamma activity (Fig. 6C) and rosiglitazone, a PPAR-gamma agonist, significantly increased VLDLR expression in adipocytes (Fig. 6D), suggesting the PPAR-gamma is a major player in controlling VLDLR expression in adipocytes.
Figure 6. Nrf2 activation has no effect on VLDLR expression in adipocytes.
(A) Chronic alcohol exposure activates Nrf2 in adipose tissue. Male C57BL/6 mice (10-week old) were exposed to ethanol-containing liquid diet for 5 weeks. Nrf2 target genes in adipose tissue were determined. All values were denoted as means ± SD (n = 6). * p < 0.05 when compared with AF. (B) Nrf2 inducers had no effect on VLDLR expression in adipocytes. Primary adipocytes were treated with H2O2 (100 µM) and tBHQ (10 µM) for 8 hours. VLDLR gene expression and protein abundance were determined. (C & D) Alcohol exposure suppressed PPAR-gamma activation in adipose tissue (C) and PPAR-gamma activation enhanced VLDLR expression in adipocytes. All values were denoted as means ± SD from three or more independent experiments. * p < 0.05 vs. UT.
Nrf2 activation contributes to alcohol-induced hepatic VLDLR overexpression
To verify the in vivo relevance of our in vitro observations, male C57BL/6 mice (10-week old) were exposed to ethanol-containing liquid diet with/without NAC supplementation for 5 weeks. NAC supplementation alleviated alcohol-induced fatty liver, liver injury, and hepatic oxidative stress, evidenced by reduced liver TG contents, plasma ALT levels, and the formation of hepatic 4-HNE-protein adducts, respectively (Fig. 7A). Importantly, NAC supplementation concomitantly prevented hepatic VLDLR overexpression (Fig. 7B). Concordant to previous reports,28 chronic alcohol exposure was associated with strengthened hepatic Nrf2 activation, evidenced by enhanced nuclear Nrf2 DNA binding activity and increased gene expressions of NQO1 and glutamate cysteine ligase (GCL), two target proteins of Nrf2 (Fig. 7C). Importantly, alcohol-induced liver Nrf2 activation was attenuated by NAC supplementation (Fig. 7C). To further confirm the critical role of Nrf2 activation in alcohol-induced liver VLDLR overexpression, Nrf2 gene knockout mice and corresponding wild type littermates were exposed to alcohol-containing diet. VLDLR gene expression in the liver was determined 5 weeks later. As shown in Fig. 7D, Nrf2 knockout mice demonstrated lower hepatic VLDLR expression even under pair-fed condition. In response to alcohol exposure, Nrf2 knockout mice showed attenuated hepatic VLDLR mRNA levels when compared with their wild type counterparts. The knockout of Nrf2 did not impact alcohol-induced VLDLR downregulation in adipose tissue. Interestingly, adipose tissue VLDLR expression was slightly increased in Nrf2 KO mice in response to either control or alcohol diet (Fig. 7D).
Figure 7. Nrf2 activation contributes to alcohol-induced hepatic VLDLR overexpression.
Male C57BL/6 mice (10-week old) were exposed to ethanol-containing liquid diet with/without NAC supplementation (0.16 mg/ml) for 5 weeks. (A) NAC supplementation reduces liver TG contents, plasma ALT levels, and the formation of hepatic 4-HNE-protein adducts, respectively. All values were denoted as means ± SD (n = 6). Bars with different characters differ significantly, p < 0.05. (B & C) NAC supplementation prevents alcohol-induced VLDLR overexpression (B) and Nrf2 activation (C) in the liver. All values were denoted as means ± SD (n = 6). Bars with different characters differ significantly, p < 0.05. (D) Nrf2 deficiency prevents alcohol-induced VLDLR overexpression in the liver, but not in adipose tissue. Nrf2-deficient and wild type mice (10-week old) were exposed to ethanol-containing liquid diet for 5 weeks. All values were denoted as means ± SD (n = 4–5). Bars with different characters differ significantly, p < 0.05. PF, pair-fed; AF, alcohol-fed.
DISCUSSION
In this work, we present evidence that chronic alcohol consumption differently regulates VLDLR expressions in the liver and adipose tissue. We demonstrate for the first time that the upregulation of VLDLR expression in the liver plays a critical role in the initiation and progression of ALD. Our results reveal that the Nrf2 activation triggered by oxidative stress is mechanistically involved in hepatic VLDLR overexpression following chronic alcohol exposure (Supplementary data, Fig. 5).
Adipose tissue dysfunction contributes to alcohol-induced hypertriglyceridemia and the development of alcoholic liver disease.8,29,30 In this work, we demonstrated that chronic alcohol exposure decreased adipose tissue VLDLR and LPL expression, which was associated with delayed plasma triglyceride clearance. Considering its function and expression pattern (mainly in adipose tissue, which represents much larger percentage of body weight than the liver), VLDLR downregulation in adipose tissue, even in a small scale, may play an important role in alcohol-associated hypertriglyceridemia. The data from current study together with others’ report31 suggest that PPAR-gamma suppression contributes to alcohol-induced adipose tissue VLDLR downregulation. Since one of the major phenotypes of VLDLR−/− mice is hypertriglyceridemia, due to weakened TG-rich lipoproteins clearance activity in adipose tissue,17,18 our results implied that the downregulated adipose tissue VLDLR expression represents an important mechanism accounting for alcohol-induced hypertriglyceridemia.
We observed that chronic alcohol feeding upregulated hepatic expressions of the proteins critically involved in lipoproteins metabolism, with the most profound effect on VLDLR. Although VLDLR−/− mice showed more significant hypertriglyceridemia in response to alcohol exposure than their corresponding wild type littermates, they are protected against fatty liver and liver injury, suggesting that VLDLR represents a link between alcohol-induced hypertriglyceridemia and alcoholic liver injury. Noteworthy here is that when chronically fed with alcohol diet, VLDLR−/− mice showed aggravated increase of hepatic expression of SREBP-1c, a master regulator for de novo lipogenic pathway. Although the current study did not directly determine the effect of VLDLR deficiency on hepatic lipogenic process, it is plausible that increased SREBP-1c levels and possible activation of lipogenic pathway may act as a compensatory mechanism due to decreased lipoproteins uptake under the circumstance of VLDLR deficiency.
LPL and HL are key lipases to hydrolyze triglycerides in lipoproteins to produce their corresponding remnants. Whereas HL is highly expressed in the liver, LPL is most widely distributed in adipose, heart, and skeletal muscle tissue. Evidence suggests that the ectopic expression of LPL in the liver of obese subjects may represent a pathological factor contributing to obesity-related fatty liver disease.32 In adipose tissue, VLDLR is a positive regulator of LPL expression and activity.21,22 In the present study, chronic alcohol exposure increased mRNA levels of both LPL and HL in the liver, however, only LPL expression was lowered in the liver of VLDLR−/− mice, suggesting that VLDLR similarly regulates LPL expression in the liver as it does in adipose tissue. Furthermore, our cell culture studies showed that exogenous LPL inclusion in the media further promoted TG accumulation in VLDLR-overexpressing HepG2 cells, indicating that enhanced LPL expression/activity vice versa facilitates VLDLR-regulated lipoprotein uptake and the development of hepatic steatosis.
The transcription factors reported to regulate VLDLR expression include PPAR-γ,30 HIF-1α,21,23 the farnesoid X receptor,33 and PERK-ATF4.24 Although the potential contributions of these transcription factors and pathways to the hepatic VLDLR expression in the setting of chronic exposure cannot be excluded, our present study uncovered that oxidative stress-triggered Nrf2 activation is critically involved in alcohol-induced hepatic VLDLR overexpression. Nrf2 is a ubiquitously expressed transcription factor and highly expressed in the liver.27 Oxidative stress activates Nrf2, thereby regulating gene expression of antioxidants and phase II detoxification enzymes through binding to the antioxidant response element (ARE),34 whose core sequence contains a short cis-acting element with a consensus sequence of 5’-TGAC-nnn-GC-3’.35 Identification of the detailed ARE sequence in the promoter region responsible for VLDLR expression is one of ongoing research subjects in our laboratory, however, it is noteworthy that a putative (albeit not perfect) ARE core sequence exists in the promoter region of mouse VLDLR gene, 502 nucleotides upstream of the first exon (Supplementary Data, Fig. 6).
Although the beneficial effects of Nrf2 activation have been reported by numerous studies in a variety of experimental and clinical settings, chronic Nrf2 activation may be detrimental under certain circumstances. For instant, Nrf2 activation was reported to enhance de novo lipogenic activity and worsened metabolic syndrome in leptin-deficient and high-fat fed mice, leading to hepatic steatosis.36,37 Conversely, Nrf2 knockout mice on an apoE-null background were protective against atherosclerosis.38 Our current study supports that oxidative stress-induced Nrf2 activation contributes to the pathogenesis of ALD via upregulating hepatic VLDLR expression. The further supportive data is our observation that female mice express significantly higher levels of hepatic VLDLR than their age-matched male littermates (Supplementary Data, Fig. 7). It has been well-recognized that females are more susceptible to the toxic effects of alcohol.39 In this context, the gender-specific expression pattern of hepatic VLDLR may account, at least partially, to the gender difference in the susceptibility to alcohol induced liver injury. Further studies using both male and female VLDLR−/− mice are warranted to address these issues.
In summary, our data suggest that hepatic VLDLR overexpression in response to chronic alcohol exposure plays an important role in the pathogenesis of ALD and oxidative stress-induced Nrf2 activation represents a novel mechanism in this process. Although under physiological situation, the VLDLR may not be able to actively participate in the delivery of TRLs in the liver due to its low expression. Increased hepatic VLDLR after chronic alcohol exposure may represent an important mechanism for the development of fatty liver via TRLs absorption. Our results provide evidence that the hepatic VLDLR might be a potential and novel therapeutic target for the treatment of ALD.
Supplementary Material
Acknowledgments
Financial support: This research was supported by a grant from the National Institutes of Health NIAAA grants R01 AA017442 (Z Song)
Abbreviation
- VLDLR
very low-density lipoprotein receptor
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- TRLs
triglyceride rich lipoproteins
- IDL
intermediate density lipoprotein
- DMEM
Dulbecco's Modified Eagle Medium
- LPL
lipoprotein lipase
- HL
hepatic lipase
- LDLR
low-density lipoprotein receptor
- LRP1
LDLR-related protein 1
- ARE
antioxidant response element
- ROS
reactive oxygen species
References
- 1.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 3.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 4.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 5.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–8084. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 8.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, et al. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J Hepatol. 2002;36:157–162. doi: 10.1016/s0168-8278(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 10.Hendriks HF, van Haaren MR, Leenen R, Schaafsma G. Moderate alcohol consumption and postprandial plasma lipids in men with different risks for coronary heart disease. Alcohol Clin Exp Res. 2001;25:563–570. [PubMed] [Google Scholar]
- 11.Van de Wiel A. The effect of alcohol on postprandial and fasting triglycerides. Int J Vasc Med. 2012;862504 doi: 10.1155/2012/862504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessembinders K, Wielders J, van de Wiel A. Severe hypertriglyceridemia influenced by alcohol (SHIBA) Alcohol Alcohol. 2011;46:113–116. doi: 10.1093/alcalc/agq088. [DOI] [PubMed] [Google Scholar]
- 13.Fielding BA, Reid G, Grady M, Humphreys SM, Evans K, Frayn KN. Ethanol with a mixed meal increases postprandial triacylglycerol but decreases postprandial non-esterified fatty acid concentrations. Br J Nutr. 2000;83:597–604. doi: 10.1017/s0007114500000763. [DOI] [PubMed] [Google Scholar]
- 14.Savolainen MJ, Baraona E, Leo MA, Lieber CS. Pathogenesis of the hypertriglyceridemia at early stages of alcoholic liver injury in the baboon. J Lipid Res. 1986;27:1073–1083. [PubMed] [Google Scholar]
- 15.Webb JC, Patel DD, Jones MD, Knight BL, Soutar AK. Characterization and tissue-specific expression of the human ‘very low density lipoprotein (VLDL) receptor’ mRNA. Hum Mol Genet. 1994;3:531–537. doi: 10.1093/hmg/3.4.531. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, et al. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 17.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, et al. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277:10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 18.Goudriaan JR, Tacken PJ, Dahlmans VE, Gijbels MJ, van Dijk KW, Havekes LM, et al. Protection from obesity in mice lacking the VLDL receptor. Arterioscler Thromb Vasc Biol. 2001;21:1488–1493. doi: 10.1161/hq0901.095147. [DOI] [PubMed] [Google Scholar]
- 19.Goudriaan JR, Espirito Santo SM, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BJ, et al. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Tao H, Hajri T. Very low density lipoprotein receptor promotes adipocyte differentiation and mediates the proadipogenic effect of peroxisome proliferator-activated receptor gamma agonists. Biochem Pharmacol. 2011;82:1950–1962. doi: 10.1016/j.bcp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Perman JC, Boström P, Lindbom M, Lidberg U, StÅhlman M, Hägg D, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, et al. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- 23.Shen GM, Zhao YZ, Chen MT, Zhang FL, Liu XL, Wang Y, et al. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem J. 2012;441:675–683. doi: 10.1042/BJ20111377. [DOI] [PubMed] [Google Scholar]
- 24.Jo H, Choe SS, Shin KC, Jang H, Lee JH, Seong JK, et al. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS, DeCarli LM. Effects of mineral and vitamin supplementation on the alcohol-induced fatty liver and microsomal induction. Alcohol Clin Exp Res. 1989;13:142–143. doi: 10.1111/j.1530-0277.1989.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Wang Z, Li J, Gu D, Li S, Shen C, Song Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS One. 2013;8:e70663. doi: 10.1371/journal.pone.0070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 29.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, et al. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- 30.Diehl AM. Obesity and alcoholic liver disease. Alcohol. 2004;34:81–87. doi: 10.1016/j.alcohol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Takazawa T, Yamauchi T, Tsuchida A, Takata M, Hada Y, Iwabu M, et al. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone increases expression of very low density lipoprotein receptor gene in adipocytes. J Biol Chem. 2009;284:30049–30057. doi: 10.1074/jbc.M109.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardina E, Baena-Fustegueras JA, Llamas R, Catalán R, Galard R, Lecube A, et al. Lipoprotein lipase expression in livers of morbidly obese patients could be responsible for liver steatosis. Obes Surg. 2009;19:608–616. doi: 10.1007/s11695-009-9827-5. [DOI] [PubMed] [Google Scholar]
- 33.Sirvent A, Claudel T, Martin G, Brozek J, Kosykh V, Darteil R, et al. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 36.Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211–G1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iimuro Y, Frankenberg MV, Arteel GE, Bradford BU, Wall CA, Thurman RG. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. Am J Physiol. 1997;272:G1186–G1194. doi: 10.1152/ajpgi.1997.272.5.G1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.