Abstract
Objective
Preclinical and clinical studies have shown beneficial effects of infusions of apolipoprotein A-I (ApoA-I) on atherosclerosis. ApoA-I is also a target for myeloperoxidase (MPO)-mediated oxidation, leading in vitro to a loss of its ability to promote ABCA1-dependent macrophage cholesterol efflux. Therefore, we hypothesized that MPO-mediated ApoA-I oxidation would impair its promotion of reverse cholesterol transport (RCT) in vivo and the beneficial effects on atherosclerotic plaques.
Approach and Results
ApoA-I−/− or ApoE−/− mice were subcutaneously injected with native human ApoA-I, oxidized human ApoA-I (oxApoAI; MPO/hydrogen peroxide/chloride treated) or carrier. While early post injection (8 hours) levels of total ApoA-I in plasma were similar for native versus oxApoA-I, native ApoA-I primarily resided within the HDL fraction, whereas the majority of oxApoA-I was highly cross-linked and not HDL particle associated, consistent with impaired ABCA1 interaction. In ApoA-I−/− mice, ApoA-I oxidation significantly impaired RCT in vivo. In advanced aortic root atherosclerotic plaques of ApoE−/− mice, native ApoA-I injections led to significant decreases in lipid content, macrophage number, and an increase in collagen content; in contrast, oxApoA-I failed to mediate these changes. The decrease in plaque macrophages with native ApoA-I was accompanied by significant induction of their chemokine receptor CCR7. Furthermore, only native ApoA-I injections led to a significant reduction of inflammatory M1 and increase in anti-inflammatory M2 macrophage markers in the plaques.
Conclusions
MPO-mediated oxidation renders ApoA-I dysfunctional and unable to: (i) promote RCT; (ii) mediate beneficial changes in the composition of atherosclerotic plaques; and (iii) pacify the inflammatory status of plaque macrophages.
Keywords: Atherosclerosis, ApoA-I, Myeloperoxidase, Dysfunctional HDL, Reverse Cholesterol Transport
Introduction
Epidemiological studies have clearly shown an inverse relationship of HDL cholesterol (HDL-C) levels and the risk for coronary artery disease.1 The atheroprotective activities of HDL particles are attributed to their central role in reverse cholesterol transport (RCT), anti-inflammatory, antithrombotic and antioxidant effects and improvement of endothelial function.2 Pharmacological raising of HDL-C or apolipoprotein A-I (ApoA-I, the major protein of HDL) is being pursued as a potential therapeutic strategy to provide additional benefit to statins in lowering cardiovascular risk. Preclinical animal studies have shown that infusion or genetic overexpression of ApoA-I leads to delayed atherosclerotic lesion progression and even accelerated lesion regression accompanied by beneficial effects on the inflammatory status of the plaque.3-11 Infusion of ApoA-I has also shown promise in small clinical studies including patients with acute coronary syndrome and with peripheral vascular disease.12-15 However, recent large outcome studies with CETP inhibitors and niacin, as well as a Mendelian randomization study, failed to show a positive correlation of elevated HDL-C levels with cardiovascular outcome.16-19 These studies challenge the “HDL hypothesis” and raise the issue that HDL-C levels do not reflect HDL function.
HDL and ApoA-I are prone to posttranslational modifications, which can directly lead to HDL dysfunction.6 We have previously shown that ApoA-I is a preferred and selective target for myeloperoxidase- (MPO) mediated modification, which leads in vitro to a loss of its ability to promote cholesterol efflux from macrophages – a crucial step in RCT.20 Furthermore, increased levels of MPO-modified ApoA-I have been found in the circulation of patients with coronary artery disease (CAD) and in human atherosclerotic plaques.21-24 We therefore hypothesized that MPO-mediated oxidation of ApoA-I would impair its function to promote RCT in vivo and to mediate beneficial effects on atherosclerotic plaques. Here we test these hypotheses in mouse models after injections of native and MPO-treated ApoA-I.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Levels and distribution of human native and oxidized ApoA-I in the plasma after subcutaneous injection
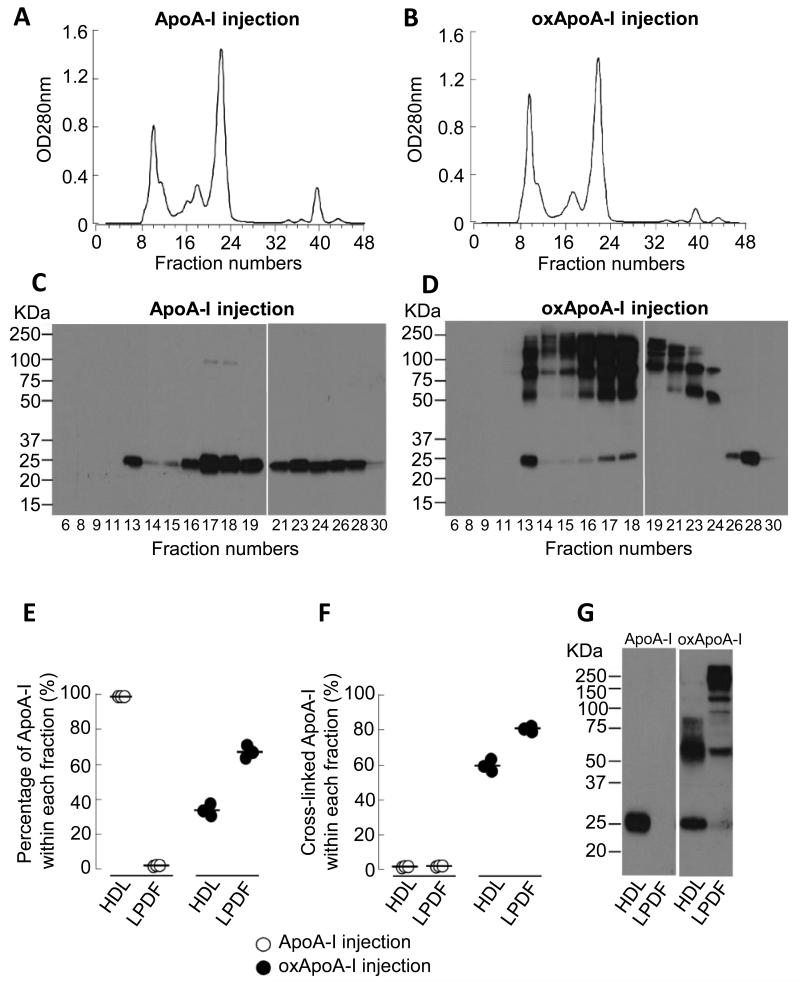
First, we determined the levels of human native and oxidized ApoA-I in the plasma of ApoE−/− mice at 8 and 24 hours after subcutaneous (s.c.) injection of both forms (15 mg each). At 8 hours after the injection, there were comparable plasma levels of native and oxidized ApoA-I, with the latter declining by 24 hours (Supplemental Figure I). Next, we sought to evaluate the distribution of native and oxidized ApoA-I in the plasma of mice 8 hours after the injection by fast performance liquid chromatography (FPLC), with subsequent Western blot analyses of the individual FPLC fractions using anti-total human ApoA-I monoclonal antibody 10G1.5 (mAb 10G1.5)24 (Figure 1A-D). After the injection of native ApoA-I we found the expected immunoreactive band migrating at ~27 kDa, corresponding to the ApoA-I monomer protein (Figure 1C). After the injection of oxidized ApoA-I, high amounts of slower migrating immunoreactive ApoA-I-containing protein bands with molecular weights around ~50, ~75 and ~100 kDa appeared in addition to the ApoA-I monomer band at ~27 kDa (Figure 1D). These bands represent oxidatively cross-linked dimeric and multimeric forms of ApoA-I, as we have previously reported from human aortas.24 For technical reasons, the large amount of cross-linked protein in oxidized ApoA-I renders it confusing to examine its distribution by FPLC as it is simultaneously associated with a wide range of molecular weights. Thus, we evaluated the plasma distribution of native and oxidized ApoA-I by Western blot analysis after buoyant density gradient centrifugation (Figure 1E-G). In contrast to native ApoA-I, which was almost completely recovered within the HDL fraction, only ~35% of oxidized ApoA-I was associated with HDL particles, while ~65% resided within the lipoprotein-deficient fraction (LPDF) (Figure 1E and G). While there was almost no cross-linked ApoA-I detectable after the injection of native ApoA-I in the HDL or LPDF fraction, high levels of cross-linked ApoA-I were present in both fractions after the injection of the oxidized form (Figure 1F and G).
Figure 1. Distribution of injected human native and oxidized ApoA-I in the plasma of ApoE−/− mice.
ApoE−/− mice (16 weeks on Western diet) were injected s.c. with 15 mg of either native or oxidized (oxApoA-I) human ApoA-I. Blood was collected at 8 hours after the injections. A-B) Fast performance liquid chromatography (FPLC) on a Superdex 200 column was performed on pooled plasma (100 μl) from 3 mice. C-D) Western blot analysis of human ApoA-I in numbered FPLC fractions probed with anti-total human ApoA-I monoclonal antibody (mAb 10G1.5). E-G) HDL-containing lipoprotein fraction and lipoprotein deficient fraction (LPDF) were isolated from 40 μl of plasma after sequential buoyant density ultracentrifugation. Human ApoA-I levels were quantified by Western blot analysis using mAb 10G1.5. E) Percentage of injected ApoA-I within HDL versus LPDF, F) percentage of cross-linked ApoA-I within HDL versus LPDF and G) illustrative Western blot analyses probed with mAb 10G1.5 of the distribution of injected human native and oxidized ApoA-I in the plasma compartments.
Impact of oxidation of ApoA-I on plasma HDL-C levels and reverse cholesterol transport (RCT)
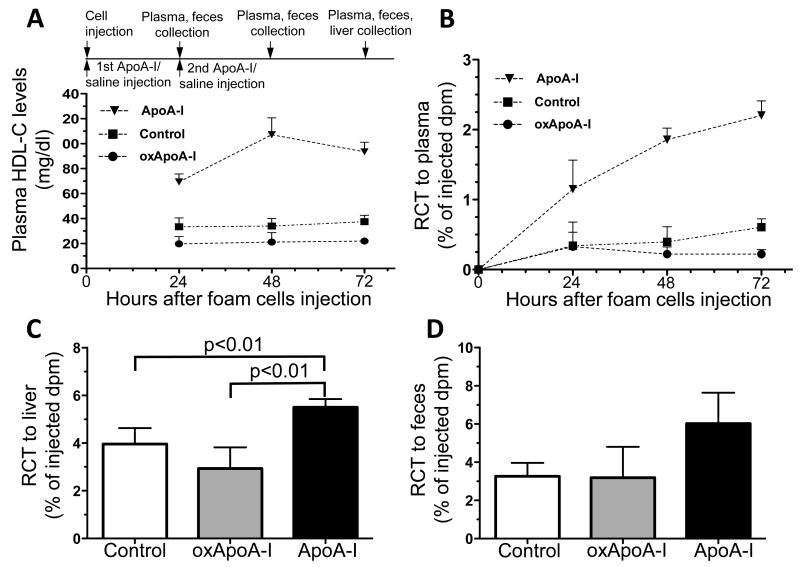
In vitro studies have shown that MPO-oxidized ApoA-I loses the ability to facilitate cholesterol efflux from cells via the ABCA1-dependent pathway.20 To test the hypothesis whether MPO-mediated oxidation of human ApoA-I impairs its RCT activity in vivo, we quantified plasma HDL-C levels and RCT (to plasma, liver and feces) in ApoA-I−/− mice injected subcutaneously at time 0 and 24 hours with 0.4 mg/g of body weight of native ApoA-I, oxidized ApoA-I or the saline carrier (control). We observed that plasma HDL-C levels were significantly increased in mice injected with native ApoA-I compared to the control group at 24, 48 and 72 hours (2.1-, 3.2- and 2.5-fold respectively, p<0.001, Figure 2A). Meanwhile, plasma HDL-C levels were significantly lower in mice injected with oxidized ApoA-I compared to the control group at 24 and 72 hours (by about 40% and 41.5% respectively, p<0.01).
Figure 2. Plasma HDL-C levels and reverse cholesterol transport (RCT) after treatment with human native or oxidized ApoA-I.
A) Experimental timeline and plasma HDL-cholesterol (HDL-C) levels, B) RCT to the plasma, C) liver and D) feces over 3 days after s.c. injection of [3H]cholesterol labeled foam cells into ApoA-I−/− mouse hosts. Significance values for (A) and (B) are given in the text. RCT to plasma, liver and feces is shown as the % of the injected radioactivity. Data are shown as mean ± SD, n = 5-6 mice for controls and n = 4 mice each for native ApoA-I and oxidized ApoA-I (oxApoA-I).
RCT was measured by determining the [3H]cholesterol transported from subcutaneously injected foam cells to plasma, liver and feces over three days. RCT to the plasma was significantly increased in mice injected with native ApoA-I at all three time points by 3.4-, 4.7-, and 3.6-fold, respectively (p<0.01 for 24 hours plasma RCT and p<0.001 for 48 and 72 hours, Figure 2B). RCT to the plasma was lower in mice injected with oxidized ApoA-I compared to the control group, reaching statistical significance at the 72 hour time point (64% lower, p<0.01). RCT to the liver was significantly higher in mice injected with native ApoA-I when compared to the control group (39% increase, p<0.01, Figure 2C). Meanwhile, RCT to the liver was 26% lower in mice injected with oxidized ApoA-I compared to the control group. The same pattern was observed for fecal RCT; and, although statistically not significant, fecal RCT was increased by 84% in mice injected with native ApoA-I compared to the control group, while it remained almost unchanged in mice injected with oxidized ApoA-I (−2% compared to the control group, Figure 2D). Thus, native ApoA-I increased RCT and plasma HDL-C, while oxidized ApoA-I failed to promote RCT or raise HDL-C.
In order to determine whether in addition to its impaired intrinsic efflux activity, oxidized ApoA-I inhibits the cholesterol efflux activity of native ApoA-I, we performed a mixing experiment in vitro. As previously shown20, oxidized ApoA-I has very limited cholesterol efflux activity, however, adding an equal concentration to native ApoA-I did not inhibit the cholesterol efflux activity of the native protein (Supplemental Figure II).
Effects of native and oxidized ApoA-I on plaque composition
Preclinical and clinical studies have shown that raising ApoA-I by infusion or overexpression leads to beneficial changes in the plaque composition, including fewer macrophages, reduced lipid content, and more collagen.6 To test the hypothesis whether MPO-mediated oxidation of human ApoA-I impairs these effects of ApoA-I, hyperlipidemic ApoE−/− mice were injected s.c. with 15 mg (per injection) of human native ApoA-I, oxidized ApoA-I or the carrier (control) four times (every other day); the baseline plasma total cholesterol levels of the three injection groups were 1220 ± 66, 1059 ± 50 and 1044 ± 86 mg/dl respectively, which did not differ significantly from each other (p=0.2). The injections were well tolerated by all mice. Enrichment of either native or oxidized human ApoA-I was detectable in aortic root plaques of the injected mice (Supplemental Figure III), indicating that both forms of ApoA-I were able to leave the circulation and gain access to the intimal areas.
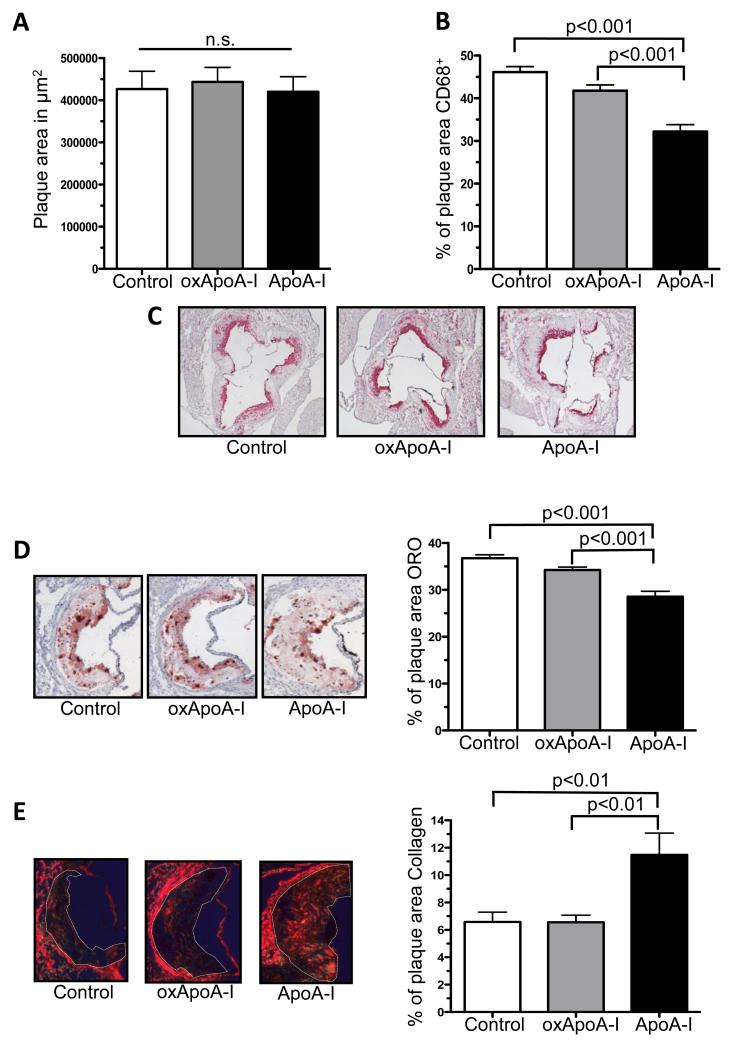
We did not observe a significant difference (p=0.9) in the absolute plaque size between the three groups within this short-term treatment period (Figure 3A). There was, however, a significant decrease in plaque (CD68+) macrophage content in aortic root plaques only in the native ApoA-I treated group (32.2 ± 1.6% of plaque area compared to the control group- 46.1 ± 1.3% of plaque area, p<0.001; Figure 3B and C). Similarly, lipid content (i.e., Oil Red O staining) was found to be significantly decreased in the native ApoA-I group (28.6 ± 1.5% of plaque area) compared to the control group (36.8 ± 0.7% of plaque area; P<0.001) and the oxidized ApoA-I group (34.2 ± 0.6% of plaque area, P<0.001) (Figure 3D). In contrast, there were no significant differences in the plaque macrophage and lipid contents between the oxidized ApoA-I and the control group.
Figure 3. Plaque size, macrophages, lipid and collagen content after treatment with human native or oxidized ApoA-I.
Aortic root sections: A) plaque size, B-C) macrophage (CD68+) plaque cells (magnification 4×, n = 10 in each group), D) lipid content (Oil Red O, magnification 10×, n = 10 in each group), E) collagen content (Sirius Red, magnification 5×, n = 5 in each group) after s.c. injection (every other day) of ApoE−/− mice with native ApoA-I, oxidized ApoA-I (oxApoA-I) or carrier (control) over 1 week. Data are shown as mean ± SEM; n.s. = not significant.
Next, we evaluated the effect of native and oxidized ApoA-I on plaque collagen content, a marker associated with increased stability in human plaques. Only the native ApoA-I treated group had significantly increased plaque collagen content (11.2 ± 1.3%) compared to the oxidized ApoA-I (6.6 ± 0.5%; p<0.01) and control (6.6 ± 0.7%; p<0.01) groups, while the latter two groups did not differ significantly (Figure 3E). This may have reflected the combined trends in the gene expression of a stimulator of collagen synthesis, TGF-β, in laser-captured CD68+ macrophages (Supplemental Figure IVA), and in the in situ activity of collagen degrading matrix metalloproteinases (Supplemental Figure IVB).
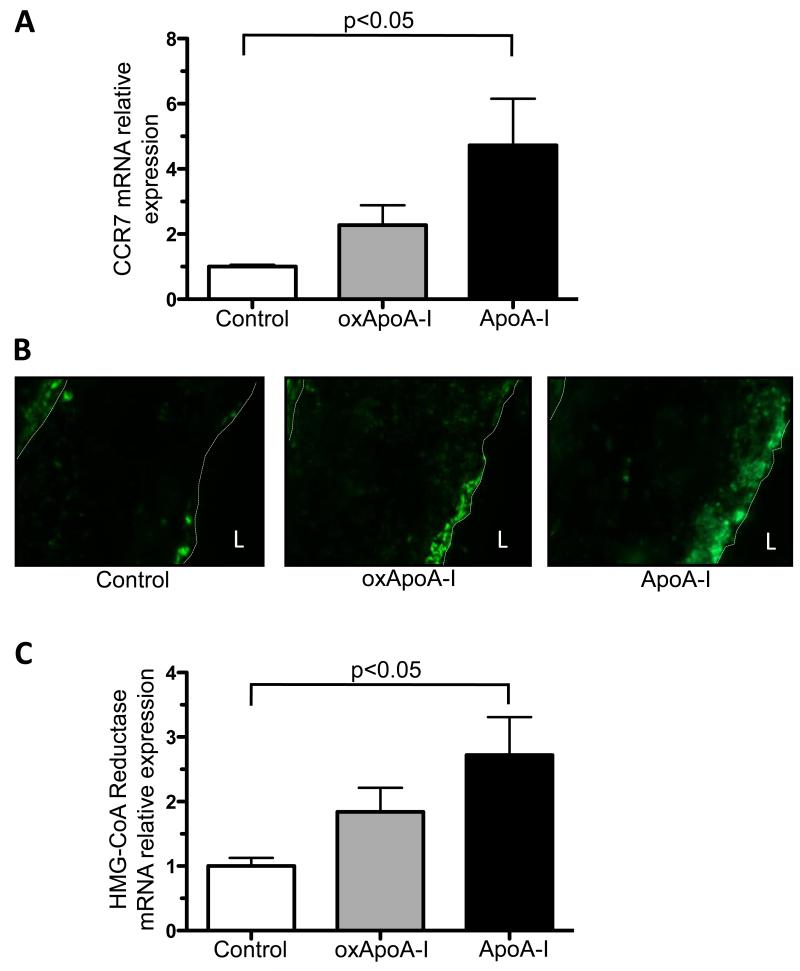
Attenuated induction of CCR7 and HMG-CoA reductase expression in plaque macrophages by oxidized ApoA-I
We have previously demonstrated in multiple mouse models that during the regression phase, CD68+ cells emigrate from the plaques in a process in which the chemokine receptor CCR7 is up-regulated.9, 25 Notably, CCR7 mRNA expression was significantly increased in laser-captured CD68+ plaque cells of mice injected with native ApoA-I (4.7 ± 1.4 fold, p<0.05), but not oxidized ApoA-I (2.3 ± 0.6 fold) compared to the control group (Figure 4A). The changes at the CCR7 mRNA levels were consistent with those observed at the protein level (Figure 4B). The human and mouse CCR7 promoter contain Sterol Response Elements (SRE), hence the cellular sterol content is an important regulator of CCR7 gene expression.26 The reduction in plaque cholesteryl ester content (as judged by Oil red O staining) in the plaques of native ApoA-I injected mice and the significant increase in the expression of the sterol-regulated gene, HMG-CoA reductase, in laser-captured CD68+ plaque cells from these mice (2.7 ± 0.6 fold compared to the control group; p<0.05) support this finding. There was a more modest and non significant increase in HMG-CoA reductase expression after the injection of oxidized ApoA-I (1.8 ± 0.4 fold compared to the control group) (Figure 4C). Consistent with these results is an experiment in which cells of the murine macrophage line RAW 264.7 were incubated with native or oxidized ApoA-I. In accordance with the cholesterol efflux activity of each form of ApoA-I reported above, significant induction of CCR7 gene expression, presumably through the promoter SRE, was observed after treatment with native ApoA-I, but not with oxidized ApoA-I. As the addition of oxidized ApoA-I to native ApoA-I did not impair cholesterol acceptor activity of native ApoA-I (Supplemental Figure II), induction of CCR7 gene expression was also observed in a mixing experiment (Supplemental Figure V).
Figure 4. Attenuated induction CCR7 expression and HMG-CoA reductase in plaque macrophages by oxidation of ApoA-I.
A) Chemokine receptor CCR7 mRNA from laser-captured CD68+ aortic root plaque cells measured by qRT-PCR, B) representative immunohistochemistry for CCR7 in aortic root plaques (magnification 20×), and C) HMG-CoA reductase mRNA expression from laser-captured CD68+ aortic root plaque cells measured by qRT-PCR from ApoE−/− mice (16 weeks on Western diet) after s.c. injection (every other day) of native ApoA-I, oxidized ApoA-I (oxApoA-I) or carrier (control) over 1 week; minimum of 7 mice in each group. Data are shown as mean ± SEM; L = lumen.
Effect of oxidized ApoA-I on the inflammatory state of plaque macrophages
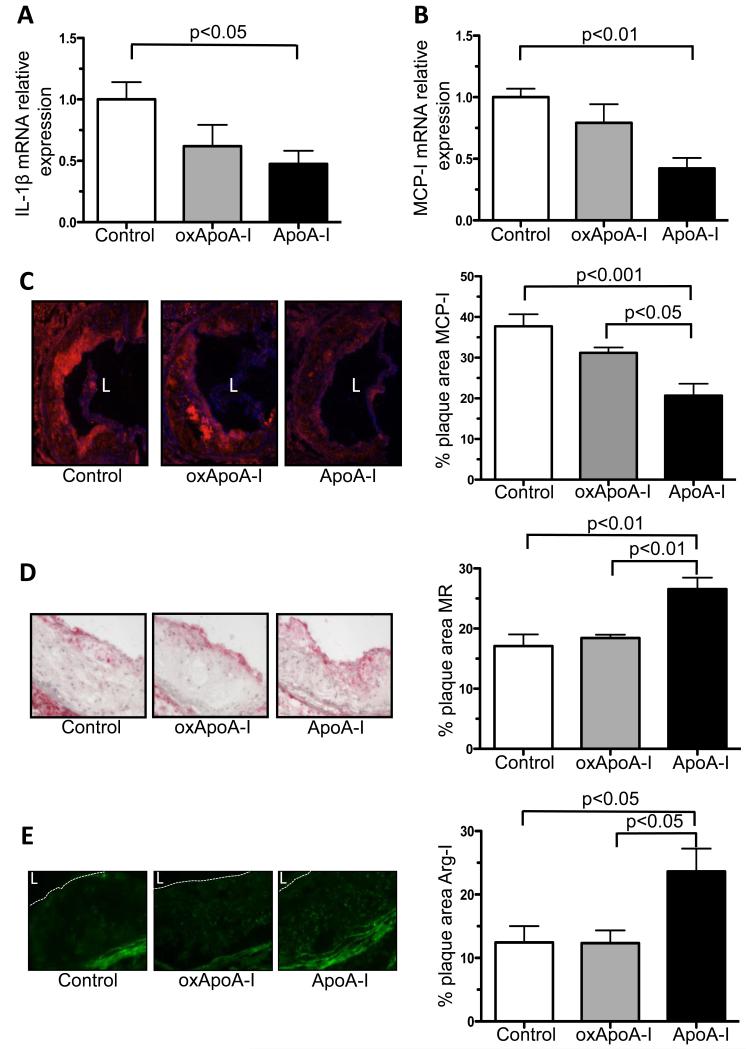
In addition to the changes in plaque macrophage content, we evaluated their inflammatory state. The mRNA expression of the pro-inflammatory M1 macrophage marker interleukin-1 beta (IL1-β) in laser-captured plaque CD68+ cells was significantly reduced after the injection of native ApoA-I (to 0.5 ± 0.1, p<0.05). In marked contrast, no reduction was noted in IL1-β in plaque-derived CD68+ cells after the injection of oxidized ApoA-I compared to the control group (Figure 5A). Consistent with this was the finding that the mRNA expression of monocyte chemoattractant protein-I (MCP-I), a M1 macrophage marker and a target of nuclear factor kappa B (NF-kB) that is involved in the recruitment of monocytes into atherosclerotic plaques, was significantly reduced only in laser-captured CD68+ plaque cells in the native ApoA-I group (to 0.4 ± 0.1, p<0.01) compared to the control group (Figure 5B). The changes in the MCP-I mRNA level were consistent with those at the protein level (Figure 5C). In contrast, there was a significant increase in anti-inflammatory M2 macrophage markers mannose receptor 1 and arginase-I protein in the plaques of the native ApoA-I injected group (26.6 ± 1.9% of plaque area, p<0.01 and 23.6 ± 3.6% of plaque area, respectively) compared to the control group (17.1 ± 1.9% of plaque area p<0.01 and 12.4 ± 2.6% of plaque area, p<0.05 respectively) and the oxidized ApoA-I group (18.4 ± 0.6% of plaque area, p<0.01 and 12.3 ± 2.0% of plaque area, p<0.05, respectively). Again, in marked contrast to the results for native ApoA-I, the results in the oxidized ApoA-I and control groups did not differ significantly (Figure 5D and E).
Figure 5. Inflammatory state of the plaque: changes in M1 and M2 macrophage markers.
A) IL1-β and B) MCP-I mRNA expression of laser-captured CD68+ aortic root plaque cells measured by qRT-PCR; minimum of 8 mice in each group, and immunohistochemistry for C) MCP-I, D) mannose receptor 1 (MR) and E) arginase-I (Arg-I) in aortic root plaques from ApoE −/− mice (16 weeks on Western diet) after s.c. injection (every other day) of native ApoA-I, oxidized ApoA-I (oxApoA-I) or carrier (control) over 1 week; magnification 10×, arginase-I 20×; minimum of 5 mice in each group; Data are shown as mean ± SEM; L = lumen.
Discussion
Epidemiological studies have clearly shown an inverse relationship between levels of HDL-C and cardiovascular risk.1 HDL particles play a central role in RCT, and exhibit antioxidant, anti-inflammatory, antithrombotic activities and improve endothelial function.6 Preclinical studies and small clinical studies have shown that raising HDL-C by providing functional particles or ApoA-I can halt the progression, and even induce the regression, of atherosclerosis.3, 5, 7-9, 11, 13, 15 However, recent reports from the large clinical outcome studies aimed at raising HDL-C levels with either CETP inhibitors or niacin (as add-on therapy to statins) have thus far failed to demonstrate clinical benefit.17, 18, 27 Similarly, results from a recent Mendelian randomization study failed to show an association between genetic variants controlling HDL-C levels and cardiovascular disease (CVD) risks, raising doubts about HDL-C targeted therapeutic strategies for the treatment or prevention of CVD.17, 19, 27-30 Importantly, most of these clinical intervention or Mendelian genetics studies have thus far focused on HDL-C levels, and not ApoA-I. Further, neither the functional properties of the HDL particles were examined with the above pharmacologic interventions (or genetic studies) nor was the HDL particle composition or oxidation state examined.
HDL is a complex macromolecular assembly of heterogeneous particles. Similar to LDL, it is prone to modifications, such as oxidation. We have previously shown that HDL and ApoA-I are selective targets of MPO-mediated oxidation, and increased levels of MPO-modified ApoA-I have been found in the circulation and atherosclerotic plaques of subjects with cardiovascular disease.20-22 Furthermore, we have previously shown in vitro that MPO-modified HDL or ApoA-I loses its ability to promote cholesterol efflux from macrophages - a crucial first step in RCT.21
In the present study we tested the effect of human native ApoA-I and oxidized (MPO-modified) ApoA-I on RCT and atherosclerosis in mice. The degree of oxidation of ApoA-I in all our experiments was comparable to the degree found in ApoA-I isolated from human plaques based upon protein bound 3-chlorotyrosine levels.22 After initial injection, circulating levels of native ApoA-I or oxidized ApoA-I at early time points (within 8 hours) were similar (Supplemental Figure I). While the injected native ApoA-I was almost completely associated with HDL particles in the plasma, the highly cross-linked oxidized ApoA-I was primarily found in the lipoprotein deficient (~65%) versus the HDL fraction (~35%), similar to the state of ApoA-I recovered from human aortas.24 Perhaps not surprisingly, then, only the native ApoA-I led to a significant increase of HDL-C levels and of RCT in ApoA-I deficient mice, whereas this effect was impaired by MPO-catalyzed oxidation of ApoA-I.
In addition to its intrinsic deficiency in promoting cholesterol efflux, a negative effect of oxidized ApoA-I on RCT may be indirect, for example, by competing with native ApoA-I. As noted in Results and Supplemental Figure II, experiments in vitro failed to disclose such a competition. Nonetheless, definitive evidence that these results reflect what occurs in vivo would require the administration of various ratios of native and oxidized ApoA-I, to determine how much of the latter is needed to significantly impair the function of the former.
Assuming that a major atheroprotective effect of ApoA-I is based on its involvement in RCT, we then went on to test whether oxidized ApoA-I injection also was able to mediate changes in the composition and cellular phenotype of atherosclerotic plaques. In the face of severe hyperlipidemia in ApoE−/− mice, we did not observe significant changes in the plaque composition (reduced macrophage and lipid content, increased collagen content) within the short-term treatment with oxidized ApoA-I whereas treatment with native ApoA-I was successful in this regard. In the present study, the analysis of atherosclerosis was performed only in aortic root plaques, the most commonly examined site in mouse studies. Whether the same results would be found throughout the arterial tree is an open question.31 However, we have previously reported similar beneficial effects of ApoA-I on the plaque composition in aortic arches using a model in which an atherosclerotic aortic arch from an ApoE−/− donor mouse is transplanted into a hyperlipidemic ApoE−/− recipient mouse transgenic for human ApoA-I (i.e., a mouse in the naturally low HDL-C levels of the ApoE−/− mouse are restored to wild-type levels).9
The homeostasis of plaque macrophages is regulated by the recruitment of circulating monocytes, their fate (apoptosis or secondary necrosis) within the plaque, their emigration from the plaques, and their local proliferation32. In an aortic transplant model (including the aforementioned one in which ApoA-I and HDL-C levels were raised in ApoE−/− mice) and other models of plaque regression, we have previously shown that macrophage emigration was associated with the up-regulation of the chemokine receptor CCR7.9, 25, 33 Similarly, we found in the present study that after the injection of native ApoA-I the reduction of plaque macrophage (CD68+) cells was accompanied by a significant increase in their CCR7 expression, while this induction was dampened in the oxidized ApoA-I group in vivo and in vitro. The human and mouse CCR7 promoter contains Sterol Response Elements (SRE), thus the cellular sterol content represents an important regulator of CCR7 gene expression.26 In vivo the reduction of cellular sterol content is indicated by the decrease in plaque cholesteryl ester content (as judged by Oil red O staining), which was significant solely in the native ApoA-I treatment group and was consistent with a significant increase in the expression of another sterol-regulated gene, HMG-CoA reductase, in the plaque macrophages. We note, however, that the macrophages remaining in the native ApoA-I group still contained significant lipid, which might explain their persistence and we speculate that additional treatment would have eventually cleared these cells from the plaques. In any case, the present study is the first report on CCR7 induction in plaque macrophages after the injection of exogenous ApoA-I, which also further emphasizes that this induction occurs in multiple models of the regression of atherosclerosis.
In our previous studies, we have also shown that during regression of atherosclerosis the remaining macrophages in the plaques resemble more the anti-inflammatory M2 state.9, 33-35 In the present study, the injection of native ApoA-I led to a decrease in the expression of inflammatory M1 markers (IL-1β, MCP-1) and increases in the expression of anti-inflammatory M2 markers (arginase-I, mannose receptor 1); these effects were attenuated by the oxidation of ApoA-I. MCP-1 is a potent recruitment factor of monocytes to atherosclerotic plaques and is expressed in macrophages and endothelial cells in response to inflammatory cytokines, such as IL-1. Consistent with our in vivo data, ApoA-I has previously been shown to inhibit IL-1β mRNA and protein expression of monocytes in vitro36, and a reduction of circulating MCP-I has been described in ApoE−/− mice after injection of ApoA-I.37 Furthermore, Kirii et al. have reported that the knock-out of IL-1β in ApoE−/− mice attenuates atherosclerotic lesion progression, which is accompanied by a decrease of MCP-I expression in the aortas of these mice.38 Taken together, our data suggests that even short-term treatment with native ApoA-I may reduce plaque inflammation through effects on multiple chemokine and cytokine-mediated pathways.
Despite the reduction in plaque lipid and macrophage content, we did not observe a significant reduction in plaque size in the native ApoA-I injected mice within the short-term treatment period. The loss of plaque area from fewer macrophages could be compensated by an increase in extracellular matrix (ECM), as indicated by an increase in plaque collagen content (a marker associated with increased plaque stability in humans). In turn, the increase of collagen content in ApoA-I injected mice likely resulted from increased synthesis, as indicated by an increased TGF-β and arginase-I expression (which is thought to promote collagen synthesis by M2 macrophages) in plaque CD68+ cells, and a concomitant reduction of ECM degradation, as indicated by the trend of decreased matrix metalloproteinases activity (Supplemental Figure IV). Based on our findings, it is tempting to speculate that the injection of native ApoA-I during the acute phase of a coronary event might efficiently and rapidly dampen the inflammatory processes and promote plaque stability.
The underlying mechanism of the differential effects of native and oxidized ApoA-I is presumably based in part on the impairment of oxidized ApoA-I to promote ABCA1 - dependent cholesterol efflux in vitro and to reduce Oil red O staining in vivo, as well as on the pro-inflammatory activity reported for MPO-modified HDL.22 Anti-inflammatory effects of ApoA-I on macrophages have been attributed to the interaction between ApoA-I and the ABC-transporters39, thus providing a link between the impaired cholesterol efflux activity of oxidized ApoA-I and its lack of suppression of inflammation. There could also be differential effects of the ApoA-I forms on different underlying signal cascades, including the activation of the Janus Kinase 2 (JAK 2)/STAT3 pathway40, the STAT6 pathway (required for polarization to the M2 macrophage state41), or the reduction of toll-like receptor signaling for example via MydD88-NFkB42, 43, all pathways suggested to mediate anti-inflammatory effects in macrophages.
Also as a likely result of its impaired ABCA1-mediated cholesterol acceptor activity, oxidized ApoA-I was cleared more rapidly from the plasma compared to the native form, presumably because it remained lipid poor (as suggested by its high abundance in the lipoprotein deficient fraction of the plasma). Though it cannot be excluded that this also had an impact on the attenuated effects of oxidized ApoA-I on many parameters, including a reduction in the level of endogenous ApoA-I as a result of the more rapid clearance of the oxidized protein, at the time of tissue harvesting, ApoA-I of either form (native or oxidized) was equally and abundantly detected in the aortic root plaques of the mice (Supplemental Figure III).
It is interesting to consider our results in the context of the small clinical studies that have shown promising results on atherosclerosis by the infusion of reconstituted HDL or lipid poor ApoA-I.12-15 Regression of coronary atheroma volume as assessed by intravascular ultrasound (IVUS) was found after the infusion of the Milano variant of ApoA-I and a reconstituted HDL (containing wild-type ApoA-I) in patients with acute coronary syndrome.13, 15 In patients with peripheral artery disease undergoing femoral atherectomy, a single infusion of HDL led to a significant reduction in lipid content and markers of macrophage inflammatory activity, as shown by a lower percentage of cells expressing vascular cell adhesion molecule-1 and a smaller macrophage cell size (attributable to the diminished lipid content) in the excised plaque samples.12 However, it still remains to be shown to which degree the observed beneficial effects on atherosclerosis translate into changes of cardiovascular outcome. More conclusive answers on this can be expected from on-going larger clinical ApoA-I infusion studies and other HDL-raising approaches, such as by induction of endogenous ApoA-I44 or by infusion of autologous delipidated HDL particles.45
In conclusion, we have shown that injection of native (functional) ApoA-I into mice associates with plasma HDL particles, increases HDL-C levels, promotes RCT, and exerts beneficial effects on the plaque composition and the inflammatory state of plaque macrophages within a short-term treatment period. Our data complement the results from previous ApoA-I injection studies3-5, 8, 11-13, 15, 46 and support the use of functional ApoA-I as a potential therapeutic to stabilize vulnerable plaques. In patients with high levels of MPO-activity, already established as a risk factor for CAD,47, 48 these benefits may be lost by modifications to ApoA-I that render it dysfunctional. Thus, our results not only emphasize the importance of the functionality of HDL to reduce cardiovascular risk, but also suggest that the prevention of oxidation of ApoA-I by MPO remains an interesting and potential therapeutic target for CVD.
Supplementary Material
Significance.
In the present study, we have shown that subcutaneous injection of native human ApoA-I (the major protein in HDL) into mice rapidly promotes both reverse cholesterol transport in vivo and exerts beneficial effects on atherosclerotic plaque composition and the inflammatory state of plaque macrophages. These effects are nearly completely abolished by myeoloperoxidase (MPO)-mediated oxidation of ApoA-I. Therefore, our findings not only emphasize the ability of native ApoA-I to rapidly produce changes thought to be atheroprotective, but also highlight that its function can be impaired by MPO, whose activity has been implicated in clinical studies to be associated with increased cardiovascular risk.
Acknowledgments
We are grateful for the excellent technical assistance of Ms. Camille McNally and Dr. Anna Rokhlina.
Sources of Funding
This work was supported by the NIH grants HL084312, P01 HL098055 and P01HL076491. Partial support was also provided by a grant from the Leducq Foundation. Dr. Hewing was supported by a fellowship from the German Research Foundation (DFG: HE 6092/1-1). Dr. Hazen also reports being partially supported by the Leonard Krieger Endowed chair fund. Mass spectrometry studies were performed in the Cleveland Clinic Mass Spectrometry core facility, which is partially supported by a Center of Innovation Award from AB SCIEX.
Footnotes
Disclosures
Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens. Dr. Smith reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Smith reports having been paid as a consultant for Esperion, and receiving research funds from Esperion. Dr. Smith reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab. and Esperion. Dr. Fisher is a member of the Merck Global Advisory Board for Atherosclerosis, is a member of the Merck Speakers Bureau, and has a Merck investigator-initiated medical school grant on the effects of niacin on VLDL metabolism.
References
- 1.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr Opin Lipidol. 2012;23:372–376. doi: 10.1097/MOL.0b013e328353ef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. Dose-related effects of repeated etc-216 (recombinant apolipoprotein a-i milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: In vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51:1098–1103. doi: 10.1016/j.jacc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG, Zafar MU, Santos-Gallego CG, Krause B, Badimon L, Fuster V, Badimon JJ. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein a-i milano (etc-216) administration: Magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51:1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 5.Giannarelli C, Cimmino G, Ibanez B, Chiesa G, Garcia-Prieto J, Santos-Gallego CG, Alique-Aguilar M, Fuster V, Sirtori C, Badimon JJ. Acute apoa-i milano administration induces plaque regression and stabilisation in the long term. Thrombosis and haemostasis. 2012;108:1246–1248. doi: 10.1160/TH12-08-0556. [DOI] [PubMed] [Google Scholar]
- 6.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury RP, Rong JX, Trogan E, Elmalem VI, Dansky HM, Breslow JL, Witztum JL, Fallon JT, Fisher EA. High-density lipoproteins retard the progression of atherosclerosis and favorably remodel lesions without suppressing indices of inflammation or oxidation. Arterioscler Thromb Vasc Biol. 2004;24:1904–1909. doi: 10.1161/01.ATV.0000142808.34602.25. [DOI] [PubMed] [Google Scholar]
- 8.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein a-i(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 9.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B. Regression and stabilization of advanced murine atherosclerotic lesions: A comparison of ldl lowering and hdl raising gene transfer strategies. J Mol Med (Berl) 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiesa G, Monteggia E, Marchesi M, Lorenzon P, Laucello M, Lorusso V, Di Mario C, Karvouni E, Newton RS, Bisgaier CL, Franceschini G, Sirtori CR. Recombinant apolipoprotein a-i(milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res. 2002;90:974–980. doi: 10.1161/01.res.0000018422.31717.ee. [DOI] [PubMed] [Google Scholar]
- 12.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 13.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant apoa-i milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. Jama. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein a-i milano. J Am Coll Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. Jama. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 16.Hewing B, Moore KJ, Fisher EA. Hdl and cardiovascular risk: Time to call the plumber? Circ Res. 2012;111:1117–1120. doi: 10.1161/CIRCRESAHA.112.280958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 18.Michos ED, Sibley CT, Baer JT, Blaha MJ, Blumenthal RS. Niacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: Reconciling the aim-high (atherothrombosis intervention in metabolic syndrome with low hdl/high triglycerides: Impact on global health outcomes) trial with previous surrogate endpoint trials. J Am Coll Cardiol. 2012;59:2058–2064. doi: 10.1016/j.jacc.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma hdl cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein a-i is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein a-i catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in abca1-dependent cholesterol efflux from macrophages. The Journal of biological chemistry. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 22.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. The Journal of biological chemistry. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent hdl reveals a key functional domain for particle maturation and dysfunction. Nature structural & molecular biology. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 24.Didonato JA, Huang Y, Aulak KS, Even-Or O, Gerstenecker G, Gogonea V, Wu Y, Fox PL, Tang WH, Plow EF, Smith JD, Fisher EA, Hazen SL. Function and distribution of apolipoprotein a1 in the artery wall are markedly distinct from those in plasma. Circulation. 2013;128:1644–1655. doi: 10.1161/CIRCULATIONAHA.113.002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the ccr7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 28.Hps2-thrive randomized placebo-controlled trial in 25 673 high-risk patients of er niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. European heart journal. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholls SJ. The aim-high (atherothrombosis intervention in metabolic syndrome with low hdl/high triglycerides: Impact on global health outcomes) trial: To believe or not to believe? J Am Coll Cardiol. 2012;59:2065–2067. doi: 10.1016/j.jacc.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Sorci-Thomas MG, Thomas MJ. Why targeting hdl should work as a therapeutic tool, but has not. Journal of cardiovascular pharmacology. 2013;62:239–246. doi: 10.1097/FJC.0b013e31829d48a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewing B, Fisher EA. Preclinical mouse models and methods for the discovery of the causes and treatments of atherosclerosis. Expert Opin Drug Discov. 2012;7:207–216. doi: 10.1517/17460441.2012.660143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewing B, Parathath S, Mai CK, Fiel MI, Guo L, Fisher EA. Rapid regression of atherosclerosis with mtp inhibitor treatment. Atherosclerosis. 2013;227:125–129. doi: 10.1016/j.atherosclerosis.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, Burger D. Apolipoprotein a-i inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by t lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 37.Bursill CA, Castro ML, Beattie DT, Nakhla S, van der Vorst E, Heather AK, Barter PJ, Rye KA. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1773–1778. doi: 10.1161/ATVBAHA.110.211342. [DOI] [PubMed] [Google Scholar]
- 38.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 39.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30:1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter abca1 functions as an anti-inflammatory receptor. The Journal of biological chemistry. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanson M, Distel E, Fisher EA. Hdl induces the expression of the m2 macrophage markers arginase 1 and fizz-1 in a stat6-dependent process. PLoS One. 2013;8:e74676. doi: 10.1371/journal.pone.0074676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific abca1 knock-out mice enhances pro-inflammatory response of macrophages. The Journal of biological chemistry. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholls SJ, Gordon A, Johannson J, Ballantyne CM, Barter PJ, Brewer HB, Kastelein JJ, Wong NC, Borgman MR, Nissen SE. Apoa-i induction as a potential cardioprotective strategy: Rationale for the sustain and assure studies. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2012;26:181–187. doi: 10.1007/s10557-012-6373-5. [DOI] [PubMed] [Google Scholar]
- 45.Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB., Jr. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 46.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein a-i(milano) on aortic atherosclerosis in apolipoprotein e-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 47.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 48.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The epic-norfolk prospective population study. J Am Coll Cardiol. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.