Abstract
The ultimate goal of cell division is equal transmission of the duplicated genome to two new daughter cells. Multiple surveillance systems exist that monitor proper execution of the cell division program and as such ensure stability of our genome. One widely studied protein complex essential for proper chromosome segregation and execution of cytoplasmic division (cytokinesis) is the chromosomal passenger complex (CPC). This highly conserved complex consists of Borealin, Survivin, INCENP, and Aurora B kinase, and has a dynamic localization pattern during mitosis and cytokinesis. Not surprisingly, it also performs various functions during these phases of the cell cycle. In this review, we will give an overview of the latest insights into the regulation of CPC localization and discuss if and how specific localization impacts its diverse functions in the dividing cell.
Introduction
During cell division, an exact copy of the genome is transmitted from mother cell to daughter cells. This requires equal segregation of the duplicated chromosomes (sister chromatids) during mitosis followed by cytoplasmic division to form two separate cells. A prerequisite for faithful segregation of the chromosomal content is bi-orientation of the sister chromatids on the mitotic spindle. This is achieved when two sister chromatids bind microtubules emanating from opposite poles of the cell (a state called amphitelic attachment). As long as this attachment state has not been reached for all chromosomes, the mitotic checkpoint is active and prevents progression of the cell cycle into anaphase. If the mitotic checkpoint fails, cells enter anaphase prematurely with unattached or aberrantly attached kinetochores (multi-protein structures that assemble at centromeres and that form the microtubule attachment sites of the chromosomes), resulting in chromosome segregation errors. Generally, when the mitotic checkpoint is completely inactive, the extent of chromosome segregation errors is too severe to be compatible with cell survival (Kops et al. 2005). A weakened checkpoint, on the other hand, is thought to result in infrequent losses and gains of chromosomes (known as chromosomal instability) that can be compatible with life. However, the latter situation gives rise to aneuploidy (a state in which a cell contains a chromosome number deviating from a multiple of a haploid chromosome content) and may predispose to cancer (Kops et al. 2005; Ricke and van Deursen 2013; Thompson et al. 2010).
A major regulator of mitosis and cytokinesis is the evolutionarily conserved chromosomal passenger complex (CPC) consisting of the enzymatic core Aurora B kinase (AURKB), the scaffold protein inner centromere protein (INCENP), and two other non-enzymatic subunits Survivin (BIRC5) and Borealin (CDCA8). The Baculovirus Inhibitor of Apoptosis Protein repeat (BIR) protein Survivin, together with Borealin, binds the N-terminal part of INCENP, while Aurora B interacts with the C-terminal IN-box of INCENP. The N- and C-terminal regions of INCENP are separated by a large unstructured region that harbors a Heterochromatin Protein 1 (HP1) binding motif, multiple (potential) Cdk1 phosphorylation sites, and a predicted coiled-coil domain (Fig. 1) (Ainsztein et al. 1998; Carmena et al. 2012b; Dephoure et al. 2008; Hegemann et al. 2011; Honda et al. 2003; Jeyaprakash et al. 2007; Kaitna et al. 2000; Mackay et al. 1993, 1998; Malik et al. 2009; Nousiainen et al. 2006; Olsen et al. 2010). Because protein interactions within the CPC support protein stability of the individual CPC subunits, knockdown or depletion of any CPC member as well as (chemical) inhibition of Aurora B in either fungi, fly, worm, frog, or mammalian cells gives rise to very similar phenotypes (Honda et al. 2003; Klein et al. 2006; Vader et al. 2006a). Disturbance of CPC function results in chromosome congression and segregation defects due to stabilization of incorrect kinetochore–microtubule attachments, an impaired function of the mitotic checkpoint and improper spindle formation. Moreover, cytokinesis is also impaired and cells that exit mitosis without a functional CPC are tetraploid and eventually die or senesce. The severity of these defects seems to depend on the level of knockdown or kinase inhibition; certain functions of the CPC are already disturbed when the complex is only partially inhibited (e.g., correction of merotelic attachments—see below), while others (e.g., its function in the mitotic checkpoint) may require complete inhibition (Adams et al. 2001; Biggins and Murray 2001; Carvalho et al. 2003; Cimini et al. 2006; Ditchfield et al. 2003; Gassmann et al. 2004; Giet and Glover 2001; Girdler et al. 2006; Hauf et al. 2003; Honda et al. 2003; Kallio et al. 2002; Lens et al. 2003; Santaguida et al. 2011; Schumacher et al. 1998; Speliotes et al. 2000; Tanaka et al. 2002; Xu et al. 2010). Importantly, Aurora B heterozygous knockout mice have an increased cancer incidence underscoring the essential role of the CPC in maintaining chromosomal stability (Fernandez-Miranda et al. 2011).
Fig. 1.
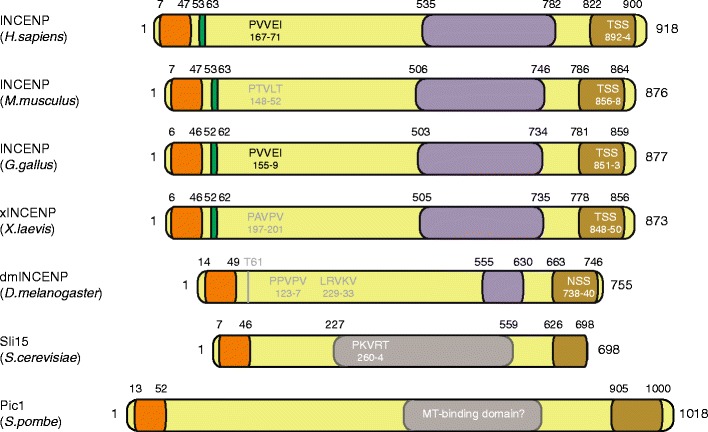
Schematic depiction of INCENP in humans and the most commonly used model organisms to study the function of the CPC. The CEN-box is shown in orange (Jeyaprakash et al. 2007; Mackay et al. 1998). The green box is the β-tubulin binding region (which includes the Cdk1-phosphorylated Threonine-59; amino acid number in Homo sapiens) (Wheatley et al. 2001). Note that Drosophila INCENP contains a potential N-terminal Cdk1 site (TP) but not a recognizable β-tubulin binding region. The amino acids shown to be responsible for binding to HP1 (PxVxL/I) are depicted in black (Kang et al. 2011; Nozawa et al. 2010). Gray and white motifs are potential HP1 binding sites. Note that although Sli15 has a potential HP1 binding site, budding yeast lacks an HP1 homolog (Hickman et al. 2011). The domain responsible for central spindle localization of human INCENP is shown in purple (Vader et al. 2007). This domain has a predicted coiled-coil structure conserved from Xenopus to humans (Mackay et al. 1993). Therefore, these coiled-coil domains are also depicted in purple. Drosophila INCENP has a short coiled-coil domain (shown in purple as well), the function of which has not been studied yet. Moreover, Sli15 contains a microtubule-binding domain that is not a coiled-coil (shown in gray) (Kang et al. 2001). For Pic1, no data are available on microtubule binding. The brown region is the IN-box including the TSS motif (if indicated), which binds and activates Aurora B and is itself phosphorylated by Aurora B (Adams et al. 2000; Kaitna et al. 2000; Xu et al. 2009)
Mammalian Aurora B is closely related to members of the AGC (cAMP-dependent, cGMP-dependent, protein kinase C) family of Serine/Threonine kinases, and the Aurora family also comprises Aurora A and Aurora C (Gold et al. 2006; Vader and Lens 2008). These kinases share a common consensus phosphorylation motif ([R/K]x[S/T]Φ, in which x can be any amino acid and Φ is a hydrophobic residue) and substrate specificity is mainly achieved by specific kinase distribution patterns within cells or between cells: Aurora A localizes to the mitotic spindle, centrosomes, and midbody, while Aurora B is present at chromosomal arms, inner centromeres, the central spindle, and the midbody (Alexander et al. 2011; Cheeseman et al. 2002). Aurora C behaves very similar to Aurora B with respect to localization and function, but is mainly expressed in germ cells and in the morula (Avo Santos et al. 2011; Fernandez-Miranda et al. 2011; Sasai et al. 2004; Schindler et al. 2012; Slattery et al. 2009; Yanai et al. 1997). The differential mitotic localization of Aurora A and Aurora B is dictated by a distinct repertoire of interaction partners. Aurora A localizes to centrosomes through its interaction with Ajuba, while its association with the mitotic spindle depends on binding to TPX2 (Hirota et al. 2003; Kufer et al. 2002). Aurora B localization is mediated by its interaction with INCENP that in turn binds to HP1, implicated in chromosomal arm localization, and to Survivin and Borealin required for centromere localization (see below) (Ainsztein et al. 1998; Nozawa et al. 2010). Interestingly, just a single point mutation (G198N) in Aurora A changes its binding preference for TPX2 into a preference for INCENP and rescues the loss of Aurora B in chromosome alignment and mitotic checkpoint function (Fu et al. 2009; Hans et al. 2009). This underscores that substrate specificity of the Aurora A and Aurora B kinases is predominantly dictated by specific localization of these kinases. Indeed, the prevailing view is that the non-enzymatic subunits of the CPC activate Aurora B and guide it to its different locations in the dividing cell to encounter and phosphorylate substrates and hence to execute its local functions (Carmena et al. 2012b; Vader et al. 2006b).
Aurora B and INCENP are first seen in late S phase nuclei at pericentromeric regions and near nucleoli, concomitant with an increase in their protein levels. Aurora B remains pericentromeric until late G2 when the kinase becomes more diffusely localized on chromatin (Fig. 2) (Andersen et al. 2005; Bischoff et al. 1998; Cooke et al. 1987; Hayashi-Takanaka et al. 2009; Monier et al. 2007; Terada 2006; Zeitlin et al. 2001b). Co-localization of Aurora B and Survivin at pericentromeric regions in G2 has been observed, yet the precise localization of Borealin in interphase cells has not been studied carefully (Monier et al. 2007; Zeitlin et al. 2001b). The presence of Aurora B at pericentromeres coincides with phosphorylation of Serine-10 of Histone H3 (H3-S10) at these foci indicating that the kinase is already active in late S phase. Although its function in interphase is unknown, transient inhibition of Aurora B kinase activity in interphase gives rise to segregation defects in anaphase, suggesting that already before mitosis Aurora B may regulate proper kinetochore formation or function (Emanuele et al. 2008; Hayashi-Takanaka et al. 2009).
Fig. 2.
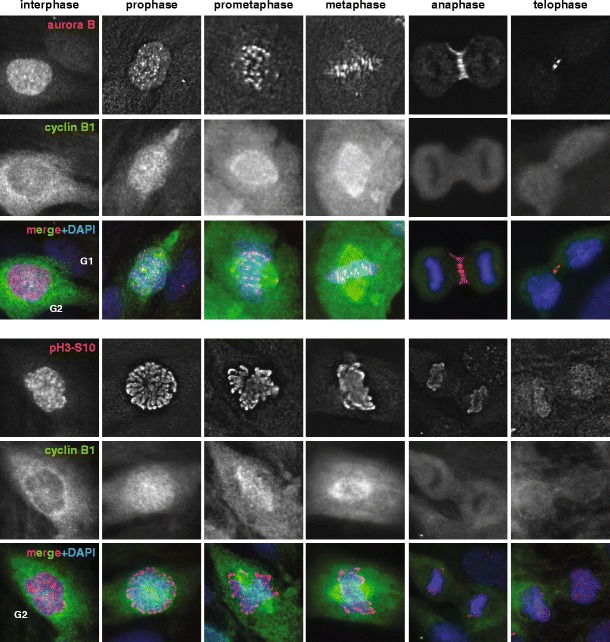
Localization of Aurora B and Histone H3 phosphorylated on Serine-10 in different phases of the cell cycle. Asynchronously growing RPE1 cells were stained using antibodies against Aurora B (A300-431A, Bethyl Laboratories) or phospho-Histone H3S10 (06–570, Merck-Millipore) and Cyclin B1 (sc-245, Santa Cruz Biotechnology). Cyclin B1 staining was used to determine cell cycle stage. Aurora B levels are higher in G2 cells than in G1 cells. In late G2 (when cytoplasmic levels of Cyclin B1 are high), Aurora B localizes diffusely in the nucleus and only in prophase Aurora B starts to accumulate clearly on centromeres, where it stays during prometaphase and metaphase. When cells enter anaphase, Aurora B moves to the midzone and subsequently, in telophase, it localizes to the midbody. Phosphorylation of Histone H3-S10 increases dramatically when cells enter mitosis and stays high until cells enter anaphase. All images were acquired on a deconvolution system (DeltaVision RT; Applied Precision) with a 100 x/1.40 NA U Plan S Apochromat objective (Olympus) using softWoRx software (Applied Precision). Images are average intensity projections of stacks except for the DAPI images (maximum intensity)
In early prophase, Aurora B and the other passenger proteins are briefly present on chromosomal arms (Fig. 2). Here, the CPC appears to contribute to sister chromatid resolution (Dai et al. 2006; Gimenez-Abian et al. 2004; Losada et al. 2002; Nishiyama et al. 2013). The CPC acts in concert with Cdk1 to phosphorylate the cohesin-stabilizing protein Sororin (Dreier et al. 2011; Nishiyama et al. 2013). These phosphorylation events cause dissociation of Sororin from the cohesin subunit precocious dissociation of sisters protein 5 (Pds5) leading to Wapl-mediated release of acetylated cohesin from chromosome arms and loss of cohesion (Dreier et al. 2011; Kueng et al. 2006; Nishiyama et al. 2013). In addition, the CPC may enhance chromosome compaction by loading of Condensin I onto chromosomal arms (Lipp et al. 2007; Takemoto et al. 2007). However, unlike fission yeast Bir1p mutants that display impaired chromosome condensation, inhibition of Aurora B or depletion of CPC subunits does not have a pronounced effect on chromosome condensation at the onset of mitosis in mammalian cells (Carvalho et al. 2003; Ditchfield et al. 2003; Hauf et al. 2003; Lens et al. 2003; Morishita et al. 2001; Rajagopalan and Balasubramanian 2002).
During (pro)metaphase, when the CPC becomes concentrated at the inner centromere (Fig. 2), the CPC ensures chromosome bi-orientation by detaching erroneous kinetochore–microtubule attachments (a process dubbed “error correction”) (Gassmann et al. 2004; Zeitlin et al. 2001a; Hauf et al. 2003; Tanaka et al. 2002). These erroneous attachments can be monotelic (a condition in which only one sister kinetochore in a pair of sister kinetochores is bound by microtubules), syntelic (attachment in which two sister kinetochores are bound by microtubules from the same spindle pole), and merotelic (attachment in which a single kinetochore is bound to microtubules emanating from both spindle poles). Furthermore, at this stage, the CPC supports proper functioning of the mitotic checkpoint through kinetochore recruitment and activation of the mitotic checkpoint kinase Mps1 (Biggins and Murray 2001; Kallio et al. 2002; Santaguida et al. 2011; Saurin et al. 2011). At anaphase onset, the CPC transfers to the cleavage furrow and to the overlapping ends of the midzone microtubules (Fig. 2). Here, it contributes to furrow ingression, central spindle formation, and to axial shortening of chromosomal arms (Douglas et al. 2010; Miyauchi et al. 2007; Mora-Bermudez et al. 2007; Neurohr et al. 2011; Petronczki et al. 2007; Xu et al. 2009). Finally, when present at the midbody, the CPC supports timely abscission (Carlton et al. 2012; Norden et al. 2006; Steigemann et al. 2009).
This review focuses on the regulation of CPC localization in mitosis and during cytokinesis, and discusses recent findings that both support and challenge the idea that precise localization of the CPC is key to its proper function before and after anaphase onset.
Centromere recruitment of the CPC
Inner centromere localization of the CPC depends on Survivin and Borealin interacting with the N-terminus of INCENP in a three-helical bundle arrangement (Jeyaprakash et al. 2007; Klein et al. 2006; Vader et al. 2006a). Survivin interacts with histone H3 phosphorylated on Threonine-3 (H3-T3) by the kinase Haspin, while Borealin binds to the Bub1-dependent histone H2A Threonine-120 (H2A-T120) phosphorylation site via the Shugoshin proteins (Shugoshin-1 and Shugoshin-2 in human cells, Shugoshin-2 in fission yeast; Fig. 3) (Kelly et al. 2010; Wang et al. 2010; Yamagishi et al. 2010; Kawashima et al. 2010; Tsukahara et al. 2010). The inner centromere is the site where these two histone phosphorylation marks seem to overlap providing an explanation as to why the CPC concentrates at this site (Yamagishi et al. 2010). Moreover, the observation that the interaction between Borealin and Shugoshin requires the phosphorylation of Borealin by Cdk1 may explain why centromere accumulation of the CPC starts in late prophase (Fig. 2) (Tsukahara et al. 2010).
Fig. 3.
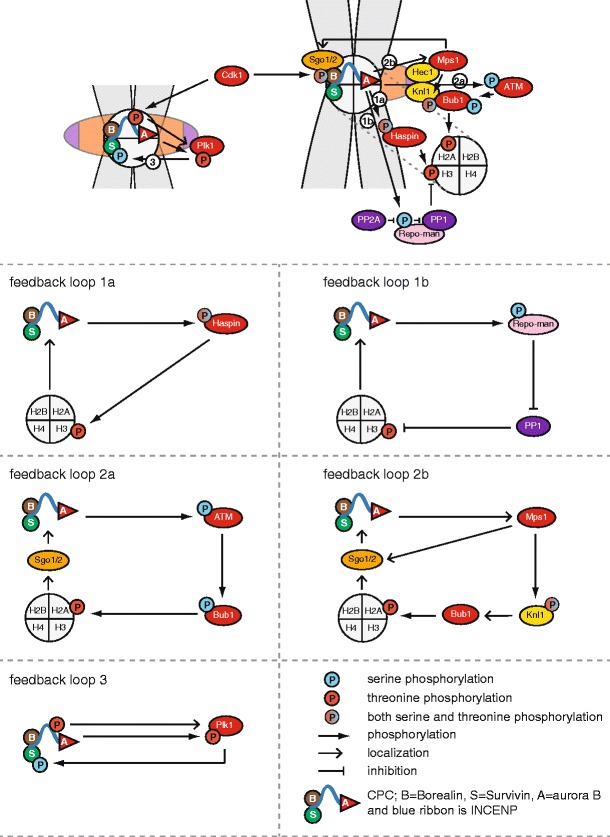
Feedback loops that regulate accumulation of the CPC on the inner centromere. The numbers in white circles in the summarizing image at the top indicate the different feedback loops. These loops are clarified in the lower part of the figure. Loop 1a covers the Aurora B-Haspin circuit and loop 1b the Aurora B-Repo-Man-PP1 loop. Loop 2a involves Aurora B-ATM-Bub1 and loop 2b Aurora B-Mps1-Bub1. Loop 3 involves INCENP/Aurora B-Plk1. See main text for details. Kinases are shown in red and phosphatases in purple
Interestingly, in fission yeast, the Survivin homolog Bir1p mediates both the interaction with phosphorylated H3-T3 and the interaction with Shugoshin-2 required for CPC centromere recruitment (Tsukahara et al. 2010; Yamagishi et al. 2010). Recently, mammalian Survivin was also found to directly interact with Shugoshin-1 via a stretch of amino acids (AKER) in Shugoshin-1 that resembles the phosphorylated N-terminus of histone H3 (ARTphK) (Jeyaprakash et al. 2011). Since these interactions depend on the same domain in Survivin, they are thought to be mutually exclusive, and it was proposed that Survivin binds to Shugoshin-1 before H3-T3 is phosphorylated. Upon H3-T3 phosphorylation, Survivin interacts with the phosphorylated histone, while Borealin binds Shugoshin (Jeyaprakash et al. 2011).
Feedback loops regulating centromeric localization of the CPC
Since Haspin and Bub1 generate the CPC centromere docking sites, upstream regulators of Haspin and Bub1 localization and activity also control CPC localization and activity. These signaling cascades are in turn controlled by Aurora B kinase activity suggesting the existence of extensive feedback mechanisms (Fig. 3).
Feedback between Haspin and Aurora B
The crystal structure of Haspin suggests that the activation loop in the kinase domain naturally adopts an active conformation and, unlike other kinases, it does not contain any phosphorylatable residues in its activation loop (Villa et al. 2009). This most likely explains why Haspin isolated from interphase or mitotic cells is equally active in vitro (Villa et al. 2009; Wang et al. 2011). Still, phosphorylation of H3-T3 occurs only in mitosis indicating that the cellular activity of Haspin is regulated (Dai and Higgins 2005; Wang et al. 2011). Indeed, Haspin is phosphorylated on multiple serine and threonine residues by Aurora B in mitosis, and mutation of these sites affects the cellular activity of Haspin towards H3-T3 (Wang et al. 2011). In line with the phenotype induced by a non-phosphorylatable mutant of Haspin, Aurora B inhibition in mitotic cells strongly reduces H3-T3 phosphorylation, suggesting that Aurora B enhances its own recruitment to centromeres by regulating Haspin. The mechanism by which Aurora B phosphorylation affects Haspin function is currently not known, but it could be mediated through release of an inhibitory factor or supporting substrate binding (Wang et al. 2011).
Additionally, posttranslational modification of Haspin substrates or the activity of phosphatases could also affect Haspin-dependent substrate phosphorylation. In line with this, methylation of H3-K4, which neighbors H3-T3, inhibits phosphorylation on H3-T3 by Haspin, at least in vitro (Eswaran et al. 2009). Moreover, H3-T3 is dephosphorylated by the PP1γ-Repo-Man phosphatase and this was shown to restrict H3-T3 phosphorylation to centromeres in prometaphase and metaphase (Qian et al. 2011). However, the activity of PP1γ-Repo-Man needs to be restrained in mitosis to allow H3-T3 phosphorylation in the first place. Recent work suggests a role for Aurora B in this regulation. Aurora B was found to phosphorylate Serine-893 in Repo-Man preventing its chromosomal targeting and thereby supporting phosphorylation of H3-T3. Repo-Man not only binds PP1γ but it also interacts with PP2A and the latter phosphatase in turn counteracts the phosphorylation of Serine-893 by Aurora B (Qian et al. 2013). This thus implies that the positive feedback between Haspin and Aurora B and feedback between Aurora B and its counteracting phosphatases determine the level of phosphorylated H3-T3 at the centromere and thereby the centromeric accumulation of the CPC (Fig. 3, feedback loop 1a and 1b).
Finally, the localization of Haspin is also expected to affect the level of centromeric H3-T3 phosphorylation. GFP-tagged Haspin is found on chromatin, but due to the lack of specific antibodies, the exact localization pattern of the endogenous mammalian kinase and the factors that regulate its localization are not known (Dai et al. 2005). In Schizosaccharomyces pombe, the Haspin homolog Hrk1 is recruited to chromatin in mitosis in a Pds5-dependent manner. Pds5 is a cohesin-associated protein implicated in sister chromatid cohesion (Hartman et al. 2000; Panizza et al. 2000; Sumara et al. 2000). Currently, there is no clear evidence that human Pds5A or Pds5B is enriched in centromeric regions, but chromosome spreads derived from Pds5B-depleted cells show an increased frequency of cells with reduced centromere localization of Aurora B (Losada et al. 2005). Moreover, Pds5B knockout mouse embryonic fibroblasts display centromere cohesion defects, reduced H3-T3 phosphorylation, and impaired centromeric accumulation of Aurora B (A. Losada, personal communication). This suggests that in mammalian cells Pds5B may be responsible for Haspin recruitment.
Recruitment of Haspin to cohesin-rich areas could be an interesting way to regulate centromere-specific CPC recruitment, since centromeres are the regions where the two sister chromatids are held together by cohesin until anaphase. Cohesin is removed from the chromosomal arms during prophase by the so-called prophase pathway (Hauf et al. 2005; Sumara et al. 2000). Centromeric cohesin is protected from the prophase pathway by Bub1-dependent recruitment of Shugoshin to centromeres. (McGuinness et al. 2005; Salic et al. 2004; Tang et al. 2004). Since the Shugoshin proteins in turn recruit the CPC via Borealin, this may create the ideal setting for feedback loops between Bub1, Aurora B, and Haspin to ensure centromeric accumulation of the CPC at the end of prophase.
Feedback between Bub1 and Aurora B
Bub1 kinase activity is required for H2A-T120 phosphorylation, but little is known about direct activation of Bub1 kinase by upstream kinases and whether these inputs affect H2A-T210 phosphorylation (Kawashima et al. 2010; Ricke et al. 2012). The DNA damage kinase ATM, which appears to have a DNA damage-independent role during mitosis, was shown to phosphorylate Bub1 and support Bub1-dependent H2A phosphorylation. Interestingly, the mitotic activity of ATM requires phosphorylation of Serine-1403 by Aurora B implying feedback between Bub1 and Aurora B via ATM (Fig. 3, feedback loop 2a) (Matsuoka et al. 2007; Yang et al. 2011).
Besides direct regulation of kinase activity, kinetochore localization of Bub1 is also essential for H2A phosphorylation (Kawashima et al. 2010; Ricke et al. 2012; Yamagishi et al. 2010). Kinetochore localization of Bub1 depends on Mps1 activity both in yeast and human cells (London et al. 2012; Maciejowski et al. 2010; Shepperd et al. 2012; Sliedrecht et al. 2010; van der Waal et al. 2012; Yamagishi et al. 2012). In yeast, this recruitment is mediated via phosphorylation of the kinetochore protein Spc105/Knl1 by Mps1, which enhances binding of Bub1 to Knl1 (London et al. 2012; Shepperd et al. 2012; Yamagishi et al. 2012). In mammalian cells, however, also Bub3 may contribute to Bub1 kinetochore localization (Krenn et al. 2012; Taylor et al. 1998; Meraldi et al. 2004).
Given its role in Bub1 kinetochore recruitment, it is not surprising that Mps1 inhibition delays the centromeric accumulation of the CPC. Yet, Mps1 not only supports CPC recruitment via kinetochore localization of Bub1 but also via a process that appears to act more directly on the localization of Shugoshin-1, downstream of Bub1 (van der Waal et al. 2012). Since active Aurora B in turn is required for kinetochore localization of both Mps1 and Bub1, and phosphorylation of H2A-T120, positive feedback appears to regulate this CPC recruitment pathway as well (Fig. 3, feedback loop 2b) (Hauf et al. 2003; Santaguida et al. 2011; Saurin et al. 2011; Vazquez-Novelle and Petronczki 2010; van der Waal et al. 2012). The effect of Aurora B inhibition on Bub1 kinetochore localization and phosphorylation of H2A might in part be due to impaired Ndc80/Hec1-dependent kinetochore recruitment and activation of Mps1 (Nijenhuis et al. 2013; Santaguida et al. 2011; Saurin et al. 2011).
Feedback between Plk1 and the CPC
Another feedback loop involved in CPC centromere recruitment, in particular to misaligned chromosomes, involves Plk1 (Fig. 3, feedback loop 3). In nontransformed diploid RPE1 cells and primary fetal fibroblasts, Aurora B is enriched on misaligned chromosomes in a Plk1- and Aurora B-dependent manner (Salimian et al. 2011). Although levels of phosphorylated H2A-T120 are clearly increased on these misaligned chromosomes, inhibition of Plk1 does not affect phosphorylation of either of the two centromere recruitment marks (H3-T3 and H2A-T120). Survivin, however, may be more directly involved because Plk1 can phosphorylate Serine-20 in Survivin, which appears to be required for Aurora B activation (Chu et al. 2011). INCENP in turn, while phosphorylated on Threonine-388 by Cdk1, mediates kinetochore localization of Plk1 (Goto et al. 2006). Moreover, in Drosophila cells, Aurora B was shown to directly phosphorylate Polo in its activation loop at centromeres in early mitosis (Carmena et al. 2012a). Also in human cells, phosphorylation of the T-loop residue Threonine-210 in Plk1 is reduced in cells from which Aurora B or INCENP has been depleted, suggesting that this is a conserved mechanism (Carmena et al. 2012a).
Overall, these data indicate extensive feedback between the CPC, Haspin, Bub1, Mps1, and Plk1 to ensure rapid accumulation of the CPC at centromeres when cells enter mitosis and kinetochore-microtubule attachments are being established. In addition, these feedback loops may warrant high CPC levels on aberrantly attached chromosomes where efficient error correction is particularly needed.
Is centromere localization of the CPC required for its function in (pro)metaphase?
In all commonly studied model organisms, Aurora B localization is confined to (inner) centromeres during prometaphase and metaphase. Moreover, the evolutionarily conserved meticulous mode of regulation of CPC recruitment to the inner centromere implies that centromeric accumulation of the CPC is crucial to Aurora B function. In fact, it is central to the prevailing model explaining how chromosome bi-orientation is achieved (Andrews et al. 2004; Liu et al. 2009; Tanaka et al. 2002). In this model, erroneous kinetochore–microtubule attachments that fail to generate (sufficient) tension across sister kinetochores are detached because kinetochore-localized microtubule binding proteins of the KMN network (consisting of the Knl1 complex, the Mis12 complex, and the Ndc80/Hec1 complex) are in close proximity of the centromere-localized kinase and are phosphorylated by Aurora B (Cheeseman et al. 2006; Welburn et al. 2010). Phosphorylation of Ndc80/Hec1 and Knl1 reduces their microtubule-binding affinity resulting in the detachment of kinetochore microtubules (Cheeseman et al. 2002, 2006; De Luca et al. 2006). Correct, amphitelic attachments that generate tension across sister kinetochores are stabilized because these microtubule-binding outer kinetochore substrates are pulled out of reach of Aurora B. This spatial restriction model thus relies on the strict localization of the CPC to inner centromeres.
Interestingly, recent work in budding yeast challenges this spatial restriction model. Yeast cells expressing an N-terminal deletion mutant of the INCENP homolog Sli15 (Sli15ΔNT) that does not interact with Bir1 (budding yeast homolog of Survivin) and as a consequence fails to localize Ipl1 (budding yeast homolog of Aurora B) to centromeres are viable and capable of supporting proper chromosome segregation (Campbell and Desai 2013). Strikingly, even loss of viability and segregation defects caused by Bir1 deletion mutants or mutants of the centromere recruitment proteins Bub1 and Shugoshin-1 (the latter is not involved in cohesion protection in budding yeast mitosis) are suppressed by Sli15ΔNT, strongly suggesting that centromere localization of the CPC is not required for cell viability or chromosome bi-orientation (Campbell and Desai 2013; Indjeian et al. 2005). Unlike wild-type Sli15, which localizes to centromeres, the Sli15 truncation mutant was found at the pre-anaphase spindle and weakly at kinetochores (Campbell and Desai 2013). Spindle localization of Sli15 before anaphase is prevented by both Cdk1- and Ipl1-dependent phosphorylation of the microtubule-binding domain in Sli15 (Nakajima et al. 2011; Pereira and Schiebel 2003). Mutation of these Cdk1 or Ipl1 phosphorylation sites into alanines causes both Sli15 and Ipl1 to prematurely localize to the pre-anaphase spindle, quite similar to Sli15ΔNT (Campbell and Desai 2013; Nakajima et al. 2011). Interestingly, expression of such a non-phosphorylatable Sli15 mutant (Sli15-6A) partially rescued viability of Bir1 deletion mutants. This argues that mere clustering of Sli15/Ipl1 on the spindle might be sufficient to activate Ipl1 and to phosphorylate kinetochore substrates when inter-kinetochore tension is low. Furthermore, it implies that tension sensing cannot be explained by a change in distance between centromere-localized kinase and kinetochore-localized substrates (see below).
While centromeric localization of the CPC may not be important for proper chromosome segregation and viability in budding yeast, it remains to be determined if this also applies to higher organisms. Expression of a Survivin BIR domain mutant (SurvivinD72A/D73A) that fails to localize the CPC to centromeres but does localize the complex to the central spindle in chicken DT40 cells rescues cytokinesis failure and survival of cells lacking endogenous Survivin (Yue et al. 2008). However, this mutant does not rescue a taxol-induced mitotic checkpoint response, a phenotype that is strongly correlated with an impaired chromosome segregation function of the CPC (Lens et al. 2006; Lens et al. 2003), and expression of a similar mutant (SurvivinD70A/D71A) in HeLa cells fails to rescue chromosome alignment defects caused by depletion of endogenous Survivin (Wang et al. 2010). Similarly, depletion or inhibition of Haspin, the kinase responsible for phosphorylation of H3-T3 required for centromeric CPC recruitment, also causes chromosome alignment defects, but not cytokinesis failure (Dai et al. 2005; De Antoni et al. 2012; Huertas et al. 2012; Wang et al. 2012). This suggests that centromeric localization of the CPC is needed for proper chromosome segregation in mammalian cells and that rescue of cytokinesis failure by Survivin BIR domain mutants might be the reason why cells expressing these mutants are viable.
Similar to Sli15ΔNT, deletion of the first ~40–60 N-terminal amino acids in human or chicken INCENP (INCENP-ΔCEN) disturbs the interaction with Survivin and Borealin and disrupts CPC centromere localization (Ainsztein et al. 1998; Klein et al. 2006; Mackay et al. 1993; Vader et al. 2006a). However, in marked contrast to Sli15ΔNT, these INCENP deletion mutants do not rescue Aurora B activity or mitotic checkpoint function in response to taxol in INCENP or Survivin-depleted cells (Vader et al. 2006a). Instead of localizing to the mitotic spindle, human and chicken INCENP-ΔCEN localize weakly and diffusely to chromatin (Mackay et al. 1998; Vader et al. 2006a). Similarly, expression of the Survivin-BIR domain mutants or inhibition of Haspin also results in diffuse localization of the CPC over chromosomal arms. Because antibody-mediated cross-linking of a Xenopus INCENP-ΔCEN mutant does rescue Aurora B activity in Xenopus egg extracts, one could argue that this weak chromatin association may cluster INCENP/Aurora B insufficiently (Kelly et al. 2007). Hence, it would be interesting to tether mammalian INCENP-ΔCEN to the mitotic spindle and test if this type of localization clusters the bimolecular complex sufficiently to support functional activity of Aurora B in human cells.
Bi-orientation without centromere-localized CPC
If error-free chromosome segregation does not require centromere localization of the CPC, how can the CPC discriminate between correctly and incorrectly attached kinetochore microtubules? In other words: How is the lack of bi-orientation and inter-kinetochore tension translated into Aurora B-dependent error correction, and how is this error correction activity silenced to allow the stabilization of amphitelically attached kinetochore microtubules?
One explanation is that tension sensing is an intrinsic property of the kinetochore (Campbell and Desai 2013). Indeed, kinetochores themselves can be stretched when attached to microtubules (intra-kinetochore stretching) (Akiyoshi et al. 2010; Maresca and Salmon 2009; Uchida et al. 2009). However, tension is thought to be the result of pulling forces exerted by amphitelically attached microtubules that are resisted by centromeric cohesin, a ring-shaped protein complex embracing the sister chromatids and holding them together until anaphase onset. Based on this definition, tension can only build up once chromosomes have bi-oriented, and this is usually visualized in cells by an increase in inter-kinetochore distance (Tanaka 2005). Because intra-kinetochore stretching is already observed in mono-oriented chromosomes, it is not correlated with bi-orientation or tension (Akiyoshi et al. 2010; Maresca and Salmon 2009; Uchida et al. 2009).
Although intra-kinetochore stretching may not be related to inter-kinetochore tension, in vitro force studies on purified budding yeast kinetochores demonstrated that the mere application of tension by a laser trap is sufficient to stabilize kinetochore–microtubule attachments similar to what was already proposed by Nicklas and colleagues based on micro-needle pulling experiments in grasshopper spermatocytes (Akiyoshi et al. 2010; Ault and Nicklas 1989; Nicklas and Koch 1969; Nicklas and Ward 1994). Although binding of the isolated budding yeast kinetochores to purified microtubules depended on Ndc80/Hec1 and Spc105/Knl1, the force-induced stabilization of attachments were unlikely due to reduced microtubule destabilizing activity of Ipl1, Mph1 (the budding yeast homolog of Mps1), or possibly other kinases because the in vitro laser trap experiments were performed in the absence of ATP (Akiyoshi et al. 2010; Maure et al. 2007). Instead, stabilization of kinetochore–microtubule attachments induced by mechanical tension was proposed to occur as a result of a change in the net balance between microtubule tip assembly (associated with a low kinetochore–microtubule detachment rate) and disassembly (associated with a high kinetochore–microtubule detachment rate) (Akiyoshi et al. 2010). In line with this, by using optical tweezers to pull on beads coated with the microtubule polymerizing protein XMAP215, Trushko and coworkers showed that the growth speed of microtubules can be increased by tension, most likely because tensile force enhances the microtubule polymerization activity of XMAP215 (Trushko et al. 2013). Interestingly, when purifiying kinetochores Akiyoshi et al. copurified Stu2p, which is the budding yeast homolog of XMAP215, the microtubule polymerization activity of which does not depend on ATP (Brouhard et al. 2008; Akiyoshi et al. 2010).
When translated back into a cellular setting in which Aurora B is present, this could imply that even when Aurora B is constitutively active (either at the centromere or at another site near the kinetochore), once chromosomes become bipolarly attached, the microtubule stabilizing effect of the mechanical force may simply outweigh the microtubule detaching activity of Aurora B kinase. If this model was true, then stable positioning of Aurora B at kinetochores using a Mis12-INCENP fusion protein should not affect the stability of amphitelically attached kinetochore microtubules. Mis12-INCENP expressing cells are capable of chromosome alignment and increasing inter-kinetochore distances, suggesting chromosome bi-orientation can occur. However, (bipolar) attachments are not stabilized and cells are delayed in mitosis with chromosomes frequently falling out of the metaphase plate (Liu et al. 2009). Although the possibility that the artificially kinetochore-localized Aurora B prevents the establishment of proper attachments cannot be excluded, it may also suggest that the attachment status of the kinetochore (unattached or syntelically attached versus amphitelically attached) is somehow translated into more or less Aurora B kinase activity or into more or less phosphorylation of Aurora B kinetochore substrates, via for instance recruitment and/or exchange of PP2A and PP1 phosphatases (Foley et al. 2011; Liu et al. 2010; Trinkle-Mulcahy et al. 2003). The question then remaining is how this is accomplished when the CPC is not localized at inner centromeres and the spatial restriction model cannot be applied? Work from De Luca et al. suggests that a very small, but active pool of Aurora B might reside at unattached kinetochores and diminishes upon microtubule attachment and establishment of tension (DeLuca et al. 2011). How this putative kinetochore-localized pool of active Aurora B is regulated remains to be determined, but it might, at least to some extent, explain why Aurora B detaches incorrectly attached kinetochore microtubules and not amphitelic attachments. In case of the latter, while the pool of active Aurora B at kinetochores becomes smaller, the levels of PP1γ at kinetochores increase. These changes likely ensure a switch-like transition from an incorrectly attached kinetochore with phosphorylated substrates into an amphitelically attached kinetochore with dephosphorylated substrates (Liu et al. 2010; Trinkle-Mulcahy et al. 2003). Interestingly, also in the Sli15ΔNT budding yeast strain, a pool of Ipl1 was found at kinetochores. Since a Sli15 mutant that prematurely localizes to the pre-anaphase spindle can only partially rescue the segregation defects of a Bir1 mutant, this kinetochore-bound pool may explain the superior rescue activity of Sli15ΔNT.
What is the function of centromere-localized Aurora B?
If centromere localization of Aurora B is not required for tension sensing and chromosome bi-orientation, the question remains what the role of centromeric-localized Aurora B is. An important difference between budding yeast chromosomes and fission yeast or mammalian chromosomes is the presence of multiple microtubule attachment sites per kinetochore in the latter versus only one attachment site per kinetochore in budding yeast. Mammalian cells (and fission yeast cells alike) could simply more heavily depend on high and local centromeric Aurora B activity because these cells have to deal with multiple kinetochore-bound microtubules that need to be destabilized in case of an aberrant attachment. In addition, the centromeric pool might support the active pool of Aurora B at the kinetochore (DeLuca et al. 2011). Alternatively, an explanation why Sli15ΔNT is effective in suppressing chromosome segregation defects in Bir1 mutant budding yeast might be because syntelic error correction does not require centromere localization of CPC/Aurora B while correction of merotelic attachments, which cannot occur in budding yeast, does (Campbell and Desai 2013). When left unresolved, merotelic attachments are particularly hazardous as these attachments are not sensed by the mitotic checkpoint and are likely to missegregate sister chromatids in anaphase (Cimini et al. 2001). Since fission yeast does have multiple microtubule attachment sites per kinetochore, it would be interesting to generate a deletion mutant of Pic1 (the fission yeast homolog of INCENP and Sli15), which cannot localize to centromeres and study its effect on fidelity of chromosome segregation and cell viability. The reason why merotelic kinetochore–microtubule attachments would specifically require centromeric Aurora B for correction could be because the merotelically attached microtubules may be in the vicinity of the centromeres or distort the kinetochore in such a way that the kinetochore attachment site of the incorrectly attached microtubule is positioned towards the centromere (Andrews et al. 2004; Cimini et al. 2006; Gregan et al. 2011; Knowlton et al. 2006). Interestingly, active Aurora B is enriched on merotelic attachment sites (Knowlton et al. 2006). Since microtubules can be co-activators of Aurora B kinase in vitro, this could mean that the merotelically attached microtubules might in turn contribute to the activity of the centromeric pool of Aurora B (Rosasco-Nitcher et al. 2008; Tseng et al. 2010).
Alternatively, Aurora B could be required at centromeres to prevent merotelic attachments. In fission yeast, Ark1 (Aurora B homolog) mediates clamping of the different microtubule binding sites in one kinetochore via the monopolin and condensin complexes to reduce the chance of acquiring merotelic attachments (Gregan et al. 2007; Tada et al. 2011). Obvious homologs of members of the monopolin complex in mammalian cells have not been found, although the evolutionarily conserved Spc24/25 heterodimer, which is part of the Ndc80/Hec1 complex, shows structural resemblance to the fission yeast Csm1 homodimer, a component of the monopolin complex (Wei et al. 2006). Moreover, in mammalian cells, localization of Condensin I depends on Aurora B activity and depletion of Condensin I gives rise to merotelic attachments (Gerlich et al. 2006; Samoshkin et al. 2009). These data suggest that Aurora B may not only be able to correct but could also prevent merotelic attachments in mammalian cells, and this latter activity might require centromere localization of the CPC.
Translocation of the CPC from centromeres to the central spindle in anaphase
Even if centromeric localization of the CPC is not required for certain kinetochore regulatory functions of the CPC, the fact that the complex is predominantly found at this site in mitosis and the evolutionary conservation of the centromeric recruitment pathways imply that in most cells the centromere is the preferred spot for CPC clustering and hence activation of Aurora B before anaphase (Kelly et al. 2010; Wang et al. 2010; Yamagishi et al. 2010). As mentioned earlier, perturbations in centromeric localization of the CPC in mammalian cells result in chromosome segregation defects, indicating that the complex is not readily clustered on an alternative spot in the mitotic cell. In anaphase, the CPC leaves the centromeres and is found at the central spindle and equatorial cortex. This change in localization is thought to promote the loss of Aurora B kinase activity from a place where it is no longer needed and gain of activity at a location where its function is now required.
Although the pool of Aurora B found at the equatorial cortex in anaphase may not be necessarily derived from centromeres, the pool localizing to the spindle midzone is (Murata-Hori and Wang 2002). The translocation from centromeres to the central spindle changes the CPC from a Borealin/Survivin-directed, histone-bound state into an INCENP/Aurora B-directed, microtubule-bound state and the drop in Cdk1 activity due to the degradation of Cyclin B, is a major contributing factor in yeast and mammalian cells (Fig. 4) (Murata-Hori and Wang 2002; Pereira and Schiebel 2003). In Saccharomyces cerevisiae, it depends on the Cdc14 phosphatase, which dephosphorylates multiple Cdc28 (Cdk1 homolog in budding yeast) and Ipl1 sites that reside mainly in the microtubule-binding domain of Sli15. Phosphorylation of these sites prevents microtubule-binding of Sli15 before mitotic exit (Mirchenko and Uhlmann 2010; Nakajima et al. 2011; Pereira and Schiebel 2003). In addition, Cdc28-dependent phosphorylation of Ipl1 represses its interaction with the microtubule plus-end tracking protein Bim1 (Zimniak et al. 2012). Notably, the putative microtubule-binding domain in mammalian INCENP (the coiled-coil domain, Fig. 1) does not contain any Cdk1 or Aurora B consensus sites suggesting that this mode of regulation may not be applicable to mammalian INCENP. However, mammalian INCENP is heavily phosphorylated in mitosis on Cdk1 consensus sites residing in the unstructured region of the protein. Except for Threonine-59 (T59; see below), the function of these phosphorylation events and their contribution to CPC translocation in anaphase is unknown (Dephoure et al. 2008; Hegemann et al. 2011; Malik et al. 2009; Nousiainen et al. 2006; Olsen et al. 2010; Hummer and Mayer 2009).
Fig. 4.
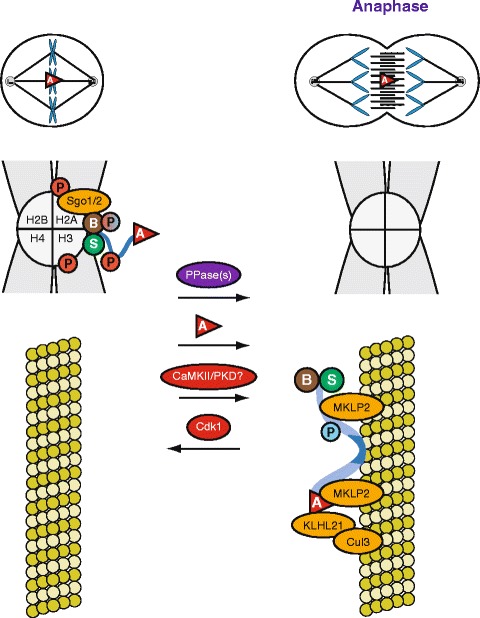
Scheme illustrating the regulation of translocation of the CPC from centromeres (in metaphase) to the central spindle (in anaphase). The mechanism behind the requirement for Aurora B activity is unknown. The phosphatase(s) (PPase) required for dephosphorylation of Threonine-59, important for binding of MKLP2 to INCENP, has not been identified yet (Hummer and Mayer 2009). Phosphorylation of Serine-197 has been suggested to be involved in central spindle/midbody localization (Yang et al. 2007). The motif surrounding this serine fits the consensus motifs for Calmodulin-Dependent Protein Kinase II (CaMKII) and protein kinase D (PKD). The part in sapphire that binds to microtubules is INCENP's coiled-coil domain
Translocation of the mammalian CPC seems to depend on a reduction in the centromere binding affinity and an increase in the microtubule binding affinity of the CPC. In line with this idea, upon Cdk1 inhibition, the H3-T3 and H2A-T120 phosphorylation marks are lost, most likely due to the activity of PP1γ phosphatase (van der Horst and Lens, unpublished data) (Qian et al. 2011; Vagnarelli et al. 2011). In addition, a Borealin-7A mutant (in which seven putative Cdk1 sites were mutated into alanine) no longer localizes to centromeres due to reduced affinity for Shugoshin suggesting that Borealin dephosphorylation at anaphase onset could also play a role (Tsukahara et al. 2010). The increase in microtubule binding affinity is in part mediated by dephosphorylation of the T59 Cdk1 site in INCENP. Dephosphorylation of T59 in anaphase is required for INCENP to interact with the kinesin MKLP2, to bind microtubules and translocate to the central spindle midzone (Goto et al. 2006; Gruneberg et al. 2004; Hummer and Mayer 2009). Like most kinesins, MKLP2 can multimerize and this multimerization could be responsible for the characteristic localization of MKLP2 and the CPC at the site where the anti-parallel microtubules of the central spindle overlap (Lee et al. 2010; McDonald et al. 1979). Knockdown of MKLP2 or expression of an INCENP mutant in which T59 is changed into a phospho-mimicking glutamic acid precludes the CPC from translocating to the central spindle resulting in cytokinesis failure (Gruneberg et al. 2004; Hummer and Mayer 2009). While the phosphatase that dephosphorylates INCENP in anaphase in yeast is known (Cdc14), in mammalian cells the T59 phosphatase remains to be identified. In addition to INCENP's interaction with MKLP2, the coiled-coil domain of INCENP itself is required for central spindle localization, potentially by increasing the microtubule affinity of the CPC-MKLP2 complex (van der Horst et al., manuscript in preparation) (Vader et al. 2007). Apart from INCENP, also Aurora B can directly interact with MKLP2 as well as with the Cul3-KLHL21 complex that localizes to the central spindle in anaphase (Gruneberg et al. 2004; Maerki et al. 2009). Interestingly, in KLHL21-depleted cells, MKLP2 localization to the central spindle is reduced, which probably reflects the reduced localization of the CPC to this region. Whether localization of KLHL21 also depends on the presence of MKLP2 or the CPC, similar to the mutual dependency of MKLP2 and INCENP, is currently unknown.
Finally, in mammalian cells Aurora B kinase activity is required for translocation of the CPC to the central spindle as the CPC remains on chromosomes in anaphase when Aurora B activity is inhibited (Xu et al. 2009). While in budding yeast Aurora B-dependent phosphorylation sites in the microtubule-binding domain of INCENP need to be dephosphorylated for efficient translocation of the CPC to the central spindle (Mirchenko and Uhlmann 2010; Nakajima et al. 2011), in mammalian cells the Aurora B substrates that need to be phosphorylated and the ones that potentially need to be dephosphorylated to support CPC translocation remain to be determined.
Interestingly, INCENP may have to be phosphorylated on Serine-197 to localize to the midbody in telophase as mutation of this residue to alanine prevents midbody localization (Yang et al. 2007). The amino acid sequence surrounding Serine-197 (LPRTLS197PT) does not conform to the optimal Aurora B consensus motif ([R/K]x[S/T]Φ), but does fit the Cdk1, protein kinase D (LxRxx[S/T]), and CaMKII consensus motifs (Φx[K/R]xx[S/T]Φ[D/E]) (Doppler et al. 2005; Ubersax and Ferrell 2007). A role for Cdk1 is unlikely as its activity drops significantly during mitotic exit, although low or localized residual Cdk1 activity at this stage of the cell cycle cannot be formally excluded. It remains to be explored whether protein kinase D or CaMKII can function as the INCENP-Serine-197 kinase and how CPC localization depends on phosphorylation of Serine-197.
Is CPC (re-)localization required for anaphase progression and cytokinesis?
As mentioned earlier, relocalization of the CPC and thus Aurora B activity in anaphase most likely serves a dual purpose: elimination of activity from a site where it is no longer needed or wanted and increase in activity at a site where its function is now required. In both Xenopus egg extracts containing sperm chromatin and Caenorhabditis elegans embryos, nuclear envelope reformation is impaired when Aurora B cannot be extracted from chromatin upon knockdown of Cdc48/p97, suggesting that the removal of the CPC from centromeres or chromatin in anaphase might be important for reformation of the nucleus in telophase (Ramadan et al. 2007). In addition, it might prevent microtubule destabilization and reactivation of the mitotic checkpoint during anaphase due to relieve of inter-kinetochore tension after cohesin cleavage. Indeed, a mutant of the mitotic exit phosphatase Cdc14 in budding yeast caused Bub1 kinetochore localization and Mad1 phosphorylation in anaphase indicative of mitotic checkpoint reactivation (Mirchenko and Uhlmann 2010). This phenotype was rescued by expression of a mutant of Sli15 that could no longer be phosphorylated by Cdk1 and allowed the CPC to leave centromeres in the absence of Cdc14. Also in mammalian cells, preventing CPC translocation by depleting the mitotic kinesin MKLP2 resulted in the recruitment of the mitotic checkpoint proteins BubR1 and Bub1 to anaphase kinetochores (Vazquez-Novelle and Petronczki 2010). However, sister chromatid separation and Cyclin B degradation still took place indicating that the anaphase-promoting complex was active and the mitotic checkpoint had been silenced. Moreover, chromosome segregation in MKLP2-depleted cells was not impaired indicating that retention of Aurora B at anaphase centromeres does not affect the stability of kinetochore–microtubule attachments (Vazquez-Novelle and Petronczki 2010). Recent data provided an explanation for these findings in MKLP2-depleted cells, since Aurora B activity towards the kinetochore protein Dsn1 in anaphase is counteracted by PP1 activity and the two PP1-targeting subunits Sds22 and Repo-Man are responsible for this effect (Wurzenberger et al. 2012). Taken together, removal of Aurora B from centromeres may only be essential for some aspects of mitotic exit, such as nuclear envelope reformation (Ramadan et al. 2007).
CPC translocation from centromeres to the central spindle might be more important for gain of function since the presence of the complex at the central spindle appears to be required for proper execution of cytokinesis. Alternatively, the central spindle may merely be a site where clustering and thus activation of the CPC occurs in anaphase, and precise localization in anaphase may not be required for CPC function during mitotic exit. However, the following pieces of evidence do to some extent suggest central spindle localization of the CPC to be important. Chromatid arm shortening that occurs in early anaphase to prevent chromatin from being trapped in the cleavage furrow, is most evident in chromosomal arms that are close to the spindle midzone, and appears to depend on the pool of Aurora B present on the midzone microtubules (Mora-Bermudez et al. 2007; Neurohr et al. 2011). Moreover, suppression of Aurora B activity in anaphase or telophase or inhibition of translocation of the CPC from centromeres to the central spindle by expression of an INCENP T59 phosphomimicking mutant causes binucleation possibly due to a failure to generate and/or maintain a stable midbody. Midbody instability may in turn be a consequence of misregulation of the kinesins Kif2a and Kif4a, both of which are substrates of Aurora B and regulate central spindle size (Gruneberg et al. 2004; Guse et al. 2005; Hummer and Mayer 2009; Nunes Bastos et al. 2013; Steigemann et al. 2009; Uehara et al. 2013). Further details on how the CPC controls mitotic exit and cytokinesis have recently been reviewed (Carmena et al. 2012b).
Notably, inhibition of Aurora B activity before the onset of furrow ingression prevents ingression resulting in binucleation (Ahonen et al. 2009). However, if one inhibits Aurora B when ingression has just started, ingression continues for some time before the furrow regresses (Ahonen et al. 2009; Guse et al. 2005). In contrast, inhibition of Aurora B at the stage that a cell is ready for abscission forces fission of the intercellular bridge, indicating that Aurora B activity has to drop below a certain threshold to allow abscission (Steigemann et al. 2009). These results also provide a rationale as to why completion of cytokinesis is such a slow process because it seems to depend on a gradual decrease in Aurora B activity in the midbody, which may in part be degradation-mediated and in part phosphatase-regulated (Floyd et al. 2008; Mallampalli et al. 2013; Steigemann et al. 2009). In line with this, defective nuclear pore assembly or a chromosome bridge traversing the midbody delays abscission and this is associated with prolonged Aurora B activity in the midbody (Mackay et al. 2010; Steigemann et al. 2009). The induction of an abscission delay by potentially hazardous situations has been dubbed abscission checkpoint and appears to act via Aurora B-mediated phosphorylation of the abscission inhibiting protein CHMP4C on Serine-210 (Capalbo et al. 2012; Carlton et al. 2012; Steigemann et al. 2009). This serine sits in a region that is not present in the other CHMP proteins that are required for abscission and is involved in CHMP4C's recruitment to the site of abscission (Carlton et al. 2012; Guizetti et al. 2011). One important question that remains is how seemingly divergent defects that can cause an abscission delay are communicated to Aurora B. Finally, recent findings suggest that the assembly of CHMP proteins into filaments that support abscission can only take place when daughter cells have attached to a substrate (Lafaurie-Janvore et al. 2013). This adhesion would release tension in the intercellular bridge and stimulate filament assembly. Whether this type of tension release is somehow related to the very recently described role for Aurora B in the regulation of cell spreading via the formin FHOD1 is presently unclear (Floyd et al. 2013).
Concluding remarks
One of the most striking features of the CPC is its dynamic localization in the dividing cell. Tight regulation of its localization has for long been thought essential for execution of its various functions ranging from kinetochore–microtubule error correction, mitotic checkpoint control, and cytokinesis. Especially its localization at the inner centromere is key to a spatial restriction model explaining how Aurora B can discriminate between correctly and incorrectly attached kinetochore microtubules (leaving the former intact, but destabilizing the latter). Recent data in budding yeast challenge this model and they suggest that mere clustering of the CPC near kinetochores may be sufficient to promote proper chromosome segregation. Future research will have to tell whether this also applies to higher organisms in which one kinetochore binds more than one microtubule. However, if this turns out to be the case then we have to re-evaluate how the CPC discriminates between amphitelic, syntelic, and merotelic attachments. In addition, if centromeric localization is not essential for error correction, why is its centromeric localization under such tight control throughout evolution? Could it be that certain specific functions of the CPC, such as for instance prevention of merotely or activation of the mitotic checkpoint, do require its specific recruitment to this site? Alternatively, centromere recruitment of the CPC might guarantee that in anaphase the central spindle pool is strictly dependent on the centromere-localized pool, which may prevent premature execution of anaphase events. Finally, in early anaphase, the CPC is not only present on the central spindle but also on the equatorial cortex. The exact contribution of these two CPC pools to the early and late events of cytokinesis is a topic for future research.
Acknowledgments
We thank Geert Kops and Gerben Vader for critically reading the manuscript and for helpful suggestions. We acknowledge Ana Losada and Arshad Desai for sharing data before publication. This work was supported by grants from the Netherlands Organisation for Scientific Research (NWO-VENI 91610036 to A.v.d.H,; NWO-VICI 91812610 to S.M.A.L.), and the Dutch Cancer Society (KWF UU2009-4311) to S.M.A.L.).
References
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153(4):865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10(17):1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Ahonen LJ, Kukkonen AM, Pouwels J, Bolton MA, Jingle CD, Stukenberg PT, Kallio MJ. Perturbation of Incenp function impedes anaphase chromatid movements and chromosomal passenger protein flux at centromeres. Chromosoma. 2009;118(1):71–84. doi: 10.1007/s00412-008-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsztein AM, Kandels-Lewis SE, Mackay AM, Earnshaw WC. INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol. 1998;143(7):1763–1774. doi: 10.1083/jcb.143.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468(7323):576–579. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, Fry AM, Musacchio A, Stukenberg PT, Mechtler K, Peters JM, Smerdon SJ, Yaffe MB. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Science signaling. 2011;4(179):ra42. doi: 10.1126/scisignal.2001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M. Nucleolar proteome dynamics. Nature. 2005;433(7021):77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Developmental cell. 2004;6(2):253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 1989;98(1):33–39. doi: 10.1007/BF00293332. [DOI] [PubMed] [Google Scholar]
- Avo Santos M, van de Werken C, de Vries M, Jahr H, Vromans MJ, Laven JS, Fauser BC, Kops GJ, Lens SM, Baart EB. A role for Aurora C in the chromosomal passenger complex during human preimplantation embryo development. Hum Reprod. 2011;26(7):1868–1881. doi: 10.1093/humrep/der111. [DOI] [PubMed] [Google Scholar]
- Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15(23):3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. The EMBO journal. 1998;17(11):3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132(1):79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497(7447):118–121. doi: 10.1038/nature12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo L, Montembault E, Takeda T, Bassi ZI, Glover DM, D'Avino PP. The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open biology. 2012;2(5):120070. doi: 10.1098/rsob.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 2012;336(6078):220–225. doi: 10.1126/science.1217180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Pinson X, Platani M, Salloum Z, Xu Z, Clark A, Macisaac F, Ogawa H, Eggert U, Glover DM, Archambault V, Earnshaw WC. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS biology. 2012;10(1):e1001250. doi: 10.1371/journal.pbio.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nature reviews Molecular cell biology. 2012;13(12):789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116(Pt 14):2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111(2):163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127(5):983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Chu Y, Yao PY, Wang W, Wang D, Wang Z, Zhang L, Huang Y, Ke Y, Ding X, Yao X. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. Journal of molecular cell biology. 2011;3(4):260–267. doi: 10.1093/jmcb/mjq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153(3):517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16(17):1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105(5):2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Higgins JM. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle. 2005;4(5):665–668. doi: 10.4161/cc.4.5.1683. [DOI] [PubMed] [Google Scholar]
- Dai J, Sullivan BA, Higgins JM. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Developmental cell. 2006;11(5):741–750. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Dai J, Sultan S, Taylor SS, Higgins JM. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005;19(4):472–488. doi: 10.1101/gad.1267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Antoni A, Maffini S, Knapp S, Musacchio A, Santaguida S. A small-molecule inhibitor of Haspin alters the kinetochore functions of Aurora B. J Cell Biol. 2012;199(2):269–284. doi: 10.1083/jcb.201205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006;5(3):296–303. doi: 10.4161/cc.5.3.2392. [DOI] [PubMed] [Google Scholar]
- DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore–microtubule attachment stability during mitosis. J Cell Sci. 2011;124(Pt 4):622–634. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105(31):10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161(2):267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem. 2005;280(15):15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- Douglas ME, Davies T, Joseph N, Mishima M. Aurora B and 14-3-3 coordinately regulate clustering of centralspindlin during cytokinesis. Curr Biol. 2010;20(10):927–933. doi: 10.1016/j.cub.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier MR, Bekier ME, 2nd, Taylor WR. Regulation of sororin by Cdk1-mediated phosphorylation. J Cell Sci. 2011;124(Pt 17):2976–2987. doi: 10.1242/jcs.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CS, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181(2):241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran J, Patnaik D, Filippakopoulos P, Wang F, Stein RL, Murray JW, Higgins JM, Knapp S. Structure and functional characterization of the atypical human kinase haspin. Proc Natl Acad Sci U S A. 2009;106(48):20198–20203. doi: 10.1073/pnas.0901989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miranda G, Trakala M, Martin J, Escobar B, Gonzalez A, Ghyselinck NB, Ortega S, Canamero M, Perez de Castro I, Malumbres M. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development. 2011;138(13):2661–2672. doi: 10.1242/dev.066381. [DOI] [PubMed] [Google Scholar]
- Floyd S, Pines J, Lindon C. APC/C Cdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase. Curr Biol. 2008;18(21):1649–1658. doi: 10.1016/j.cub.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Floyd S, Whiffin N, Gavilan MP, Kutscheidt S, De Luca M, Marcozzi C, Min M, Watkins J, Chung K, Fackler OT, Lindon C. Spatiotemporal organization of Aurora-B by APC/CCdh1 after mitosis coordinates cell spreading via FHOD1. J Cell Sci. 2013 doi: 10.1242/jcs.123232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EA, Maldonado M, Kapoor TM. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nature cell biology. 2011;13(10):1265–1271. doi: 10.1038/ncb2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bian M, Liu J, Jiang Q, Zhang C. A single amino acid change converts Aurora-A into Aurora-B-like kinase in terms of partner specificity and cellular function. Proc Natl Acad Sci U S A. 2009;106(17):6939–6944. doi: 10.1073/pnas.0900833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich D, Hirota T, Koch B, Peters JM, Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr Biol. 2006;16(4):333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152(4):669–682. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Abian JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters JM. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14(13):1187–1193. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119(Pt 17):3664–3675. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- Gold MG, Barford D, Komander D. Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol. 2006;16(6):693–701. doi: 10.1016/j.sbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Goto H, Kiyono T, Tomono Y, Kawajiri A, Urano T, Furukawa K, Nigg EA, Inagaki M. Complex formation of Plk1 and INCENP required for metaphase–anaphase transition. Nature cell biology. 2006;8(2):180–187. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- Gregan J, Polakova S, Zhang L, Tolic-Norrelykke IM, Cimini D. Merotelic kinetochore attachment: causes and effects. Trends in cell biology. 2011;21(6):374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan J, Riedel CG, Pidoux AL, Katou Y, Rumpf C, Schleiffer A, Kearsey SE, Shirahige K, Allshire RC, Nasmyth K. The kinetochore proteins pcs1 and mde4 and heterochromatin are required to prevent merotelic orientation. Curr Biol. 2007;17(14):1190–1200. doi: 10.1016/j.cub.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166(2):167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, Leonhardt H, Muller-Reichert T, Gerlich DW. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331(6024):1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15(8):778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Hans F, Skoufias DA, Dimitrov S, Margolis RL. Molecular distinctions between Aurora A and B: a single residue change transforms Aurora A into correctly localized and functional Aurora B. Mol Biol Cell. 2009;20(15):3491–3502. doi: 10.1091/mbc.E09-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman T, Stead K, Koshland D, Guacci V. Pds5p is an essential chromosomal protein required for both sister chromatid cohesion and condensation in Saccharomyces cerevisiae. J Cell Biol. 2000;151(3):613–626. doi: 10.1083/jcb.151.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS biology. 2005;3(3):e69. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takanaka Y, Yamagata K, Nozaki N, Kimura H. Visualizing histone modifications in living cells: spatiotemporal dynamics of H3 phosphorylation during interphase. J Cell Biol. 2009;187(6):781–790. doi: 10.1083/jcb.200904137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann B, Hutchins JR, Hudecz O, Novatchkova M, Rameseder J, Sykora MM, Liu S, Mazanek M, Lenart P, Heriche JK, Poser I, Kraut N, Hyman AA, Yaffe MB, Mechtler K, Peters JM. Systematic phosphorylation analysis of human mitotic protein complexes. Sci Signal. 2011;4(198):rs12. doi: 10.1126/scisignal.2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MA, Froyd CA, Rusche LN. Reinventing heterochromatin in budding yeasts: Sir2 and the origin recognition complex take center stage. Eukaryotic cell. 2011;10(9):1183–1192. doi: 10.1128/EC.05123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114(5):585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14(8):3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas D, Soler M, Moreto J, Villanueva A, Martinez A, Vidal A, Charlton M, Moffat D, Patel S, McDermott J, Owen J, Brotherton D, Krige D, Cuthill S, Esteller M. Antitumor activity of a small-molecule inhibitor of the histone kinase Haspin. Oncogene. 2012;31(11):1408–1418. doi: 10.1038/onc.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer S, Mayer TU. Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr Biol. 2009;19(7):607–612. doi: 10.1016/j.cub.2009.02.046. [DOI] [PubMed] [Google Scholar]
- Indjeian VB, Stern BM, Murray AW. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science. 2005;307(5706):130–133. doi: 10.1126/science.1101366. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural basis for the recognition of phosphorylated histone h3 by the survivin subunit of the chromosomal passenger complex. Structure. 2011;19(11):1625–1634. doi: 10.1016/j.str.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin–Borealin–INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131(2):271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10(19):1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002;12(11):900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- Kang J, Chaudhary J, Dong H, Kim S, Brautigam CA, Yu H. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol Biol Cell. 2011;22(8):1181–1190. doi: 10.1091/mbc.E11-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, Chan CS. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J Cell Biol. 2001;155(5):763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327(5962):172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated Histone H3 Threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330(6001):235–239. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Developmental cell. 2007;12(1):31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17(6):2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16(17):1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5(10):773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Krenn V, Wehenkel A, Li X, Santaguida S, Musacchio A. Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. J Cell Biol. 2012;196(4):451–467. doi: 10.1083/jcb.201110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127(5):955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158(4):617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaurie-Janvore J, Maiuri P, Wang I, Pinot M, Manneville JB, Betz T, Balland M, Piel M. ESCRT-III assembly and cytokinetic abscission are induced by tension release in the intercellular bridge. Science. 2013;339(6127):1625–1629. doi: 10.1126/science.1233866. [DOI] [PubMed] [Google Scholar]
- Lee SH, McCormick F, Saya H. Mad2 inhibits the mitotic kinesin MKlp2. J Cell Biol. 2010;191(6):1069–1077. doi: 10.1083/jcb.201003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Rodriguez JA, Vader G, Span SW, Giaccone G, Medema RH. Uncoupling the central spindle-associated function of the chromosomal passenger complex from its role at centromeres. Mol Biol Cell. 2006;17(4):1897–1909. doi: 10.1091/mbc.E05-08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. The EMBO journal. 2003;22(12):2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp JJ, Hirota T, Poser I, Peters JM. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci. 2007;120(Pt 7):1245–1255. doi: 10.1242/jcs.03425. [DOI] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323(5919):1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188(6):809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 2012;22(10):900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16(23):3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Yokochi T, Hirano T. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci. 2005;118(Pt 10):2133–2141. doi: 10.1242/jcs.02355. [DOI] [PubMed] [Google Scholar]
- Maciejowski J, George KA, Terret ME, Zhang C, Shokat KM, Jallepalli PV. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190(1):89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Ainsztein AM, Eckley DM, Earnshaw WC. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J Cell Biol. 1998;140(5):991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Eckley DM, Chue C, Earnshaw WC. Molecular analysis of the INCENPs (inner centromere proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. J Cell Biol. 1993;123(2):373–385. doi: 10.1083/jcb.123.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DR, Makise M, Ullman KS. Defects in nuclear pore assembly lead to activation of an Aurora B-mediated abscission checkpoint. J Cell Biol. 2010;191(5):923–931. doi: 10.1083/jcb.201007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerki S, Olma MH, Staubli T, Steigemann P, Gerlich DW, Quadroni M, Sumara I, Peter M. The Cul3-KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J Cell Biol. 2009;187(6):791–800. doi: 10.1083/jcb.200906117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Lenobel R, Santamaria A, Ries A, Nigg EA, Korner R. Quantitative analysis of the human spindle phosphoproteome at distinct mitotic stages. J Proteome Res. 2009;8(10):4553–4563. doi: 10.1021/pr9003773. [DOI] [PubMed] [Google Scholar]
- Mallampalli RK, Glasser JR, Coon TA, Chen BB. Calmodulin protects Aurora B on the midbody to regulate the fidelity of cytokinesis. Cell Cycle. 2013;12(4):663–673. doi: 10.4161/cc.23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184(3):373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Maure JF, Kitamura E, Tanaka TU. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol. 2007;17(24):2175–2182. doi: 10.1016/j.cub.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KL, Edwards MK, McIntosh JR. Cross-sectional structure of the central mitotic spindle of Diatoma vulgare. Evidence for specific interactions between antiparallel microtubules. J Cell Biol. 1979;83(2 Pt 1):443–461. doi: 10.1083/jcb.83.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS biology. 2005;3(3):e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Developmental cell. 2004;7(1):45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Mirchenko L, Uhlmann F. Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr Biol. 2010;20(15):1396–1401. doi: 10.1016/j.cub.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi K, Zhu X, Foong C, Hosoya H, Murata-Hori M. Aurora B kinase activity is required to prevent polar cortical ingression during cytokinesis. Cell Cycle. 2007;6(20):2549–2553. doi: 10.4161/cc.6.20.4817. [DOI] [PubMed] [Google Scholar]
- Monier K, Mouradian S, Sullivan KF. DNA methylation promotes Aurora-B-driven phosphorylation of histone H3 in chromosomal subdomains. J Cell Sci. 2007;120(Pt 1):101–114. doi: 10.1242/jcs.03326. [DOI] [PubMed] [Google Scholar]
- Mora-Bermudez F, Gerlich D, Ellenberg J. Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nature cell biology. 2007;9(7):822–831. doi: 10.1038/ncb1606. [DOI] [PubMed] [Google Scholar]
- Morishita J, Matsusaka T, Goshima G, Nakamura T, Tatebe H, Yanagida M. Bir1/Cut17 moving from chromosome to spindle upon the loss of cohesion is required for condensation, spindle elongation and repair. Genes Cells. 2001;6(9):743–763. doi: 10.1046/j.1365-2443.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- Murata-Hori M, Wang YL. Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol. 2002;159(1):45–53. doi: 10.1083/jcb.200207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Cormier A, Tyers RG, Pigula A, Peng Y, Drubin DG, Barnes G. Ipl1/Aurora-dependent phosphorylation of Sli15/INCENP regulates CPC-spindle interaction to ensure proper microtubule dynamics. J Cell Biol. 2011;194(1):137–153. doi: 10.1083/jcb.201009137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr G, Naegeli A, Titos I, Theler D, Greber B, Diez J, Gabaldon T, Mendoza M, Barral Y. A midzone-based ruler adjusts chromosome compaction to anaphase spindle length. Science. 2011;332(6028):465–468. doi: 10.1126/science.1201578. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Koch CA. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol. 1969;43(1):40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC. Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol. 1994;126(5):1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis W, von Castelmur E, Littler D, De Marco V, Tromer E, Vleugel M, van Osch MH, Snel B, Perrakis A, Kops GJ. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol. 2013;201(2):217–231. doi: 10.1083/jcb.201210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Sykora MM, Veld PJ HI't, Mechtler K, Peters JM. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci U S A. 2013;110(33):13404–13409. doi: 10.1073/pnas.1305020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125(1):85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci U S A. 2006;103(14):5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa RS, Nagao K, Masuda HT, Iwasaki O, Hirota T, Nozaki N, Kimura H, Obuse C. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nature cell biology. 2010;12(7):719–727. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- Nunes Bastos R, Gandhi SR, Baron RD, Gruneberg U, Nigg EA, Barr FA. Aurora B suppresses microtubule dynamics and limits central spindle size by locally activating KIF4A. J Cell Biol. 2013;202(4):605–621. doi: 10.1083/jcb.201301094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3(104):ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Panizza S, Tanaka T, Hochwagen A, Eisenhaber F, Nasmyth K. Pds5 cooperates with cohesin in maintaining sister chromatid cohesion. Curr Biol. 2000;10(24):1557–1564. doi: 10.1016/s0960-9822(00)00854-x. [DOI] [PubMed] [Google Scholar]
- Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302(5653):2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Developmental cell. 2007;12(5):713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Qian J, Beullens M, Lesage B, Bollen M. Aurora B defines its own chromosomal targeting by opposing the recruitment of the phosphatase scaffold repo-man. Curr Biol. 2013;23(12):1136–1143. doi: 10.1016/j.cub.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr Biol. 2011;21(9):766–773. doi: 10.1016/j.cub.2011.03.047. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Balasubramanian MK. Schizosaccharomyces pombe Bir1p, a nuclear protein that localizes to kinetochores and the spindle midzone, is essential for chromosome condensation and spindle elongation during mitosis. Genetics. 2002;160(2):445–456. doi: 10.1093/genetics/160.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450(7173):1258–1262. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol. 2012;199(6):931–949. doi: 10.1083/jcb.201205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke RM, van Deursen JM. Aneuploidy in health, disease, and aging. J Cell Biol. 2013;201(1):11–21. doi: 10.1083/jcb.201301061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319(5862):469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118(5):567–578. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Salimian KJ, Ballister ER, Smoak EM, Wood S, Panchenko T, Lampson MA, Black BE. Feedback control in sensing chromosome biorientation by the Aurora B kinase. Curr Biol. 2011;21(13):1158–1165. doi: 10.1016/j.cub.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoshkin A, Arnaoutov A, Jansen LE, Ouspenski I, Dye L, Karpova T, McNally J, Dasso M, Cleveland DW, Strunnikov A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PloS one. 2009;4(8):e6831. doi: 10.1371/journal.pone.0006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Vernieri C, Villa F, Ciliberto A, Musacchio A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. The EMBO journal. 2011;30(8):1508–1519. doi: 10.1038/emboj.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, Earnshaw WC, Brinkley WR, Sen S. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59(4):249–263. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- Saurin AT, van der Waal MS, Medema RH, Lens SM, Kops GJ. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun. 2011;2:316. doi: 10.1038/ncomms1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Davydenko O, Fram B, Lampson MA, Schultz RM. Maternally recruited Aurora C kinase is more stable than Aurora B to support mouse oocyte maturation and early development. Proc Natl Acad Sci U S A. 2012;109(33):E2215–E2222. doi: 10.1073/pnas.1120517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JM, Golden A, Donovan PJ. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J Cell Biol. 1998;143(6):1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol. 2012;22(10):891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of Aurora-B. Cell Cycle. 2009;8(18):2984–2994. [PubMed] [Google Scholar]
- Sliedrecht T, Zhang C, Shokat KM, Kops GJ. Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PloS one. 2010;5(4):e10251. doi: 10.1371/journal.pone.0010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes EK, Uren A, Vaux D, Horvitz HR. The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol Cell. 2000;6(2):211–223. doi: 10.1016/s1097-2765(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136(3):473–484. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol. 2000;151(4):749–762. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K, Susumu H, Sakuno T, Watanabe Y. Condensin association with histone H2A shapes mitotic chromosomes. Nature. 2011;474(7352):477–483. doi: 10.1038/nature10179. [DOI] [PubMed] [Google Scholar]
- Takemoto A, Murayama A, Katano M, Urano T, Furukawa K, Yokoyama S, Yanagisawa J, Hanaoka F, Kimura K. Analysis of the role of Aurora B on the chromosomal targeting of condensin I. Nucleic Acids Res. 2007;35(7):2403–2412. doi: 10.1093/nar/gkm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU. Chromosome bi-orientation on the mitotic spindle. Philos Trans R Soc Lond B Biol Sci. 2005;360(1455):581–589. doi: 10.1098/rstb.2004.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108(3):317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci U S A. 2004;101(52):18012–18017. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Ha E, McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J Cell Biol. 1998;142(1):1–11. doi: 10.1083/jcb.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y. Aurora-B/AIM-1 regulates the dynamic behavior of HP1alpha at the G2-M transition. Mol Biol Cell. 2006;17(7):3232–3241. doi: 10.1091/mbc.E05-09-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20(6):R285–R295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Andrews PD, Wickramasinghe S, Sleeman J, Prescott A, Lam YW, Lyon C, Swedlow JR, Lamond AI. Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol Biol Cell. 2003;14(1):107–117. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushko A, Schaffer E, Howard J. The growth speed of microtubules with XMAP215-coated beads coupled to their ends is increased by tensile force. Proc Natl Acad Sci U S A. 2013;110(36):14670–14675. doi: 10.1073/pnas.1218053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BS, Tan L, Kapoor TM, Funabiki H. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Developmental cell. 2010;18(6):903–912. doi: 10.1016/j.devcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nature reviews Molecular cell biology. 2007;8(7):530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184(3):383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R, Tsukada Y, Kamasaki T, Poser I, Yoda K, Gerlich DW, Goshima G. Aurora B and Kif2A control microtubule length for assembly of a functional central spindle during anaphase. J Cell Biol. 2013;202(4):623–636. doi: 10.1083/jcb.201302123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Cruijsen CW, van Harn T, Vromans MJ, Medema RH, Lens SM. The chromosomal passenger complex controls spindle checkpoint function independent from its role in correcting microtubule kinetochore interactions. Mol Biol Cell. 2007;18(11):4553–4564. doi: 10.1091/mbc.E07-04-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Kauw JJ, Medema RH, Lens SM. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO reports. 2006;7(1):85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochimica et biophysica acta. 2008;1786(1):60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Vader G, Medema RH, Lens SM. The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173(6):833–837. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P, Ribeiro S, Sennels L, Sanchez-Pulido L, de Lima AF, Verheyen T, Kelly DA, Ponting CP, Rappsilber J, Earnshaw WC. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Developmental cell. 2011;21(2):328–342. doi: 10.1016/j.devcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waal MS, Saurin AT, Vromans MJ, Vleugel M, Wurzenberger C, Gerlich DW, Medema RH, Kops GJ, Lens SM. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO reports. 2012;13(9):847–854. doi: 10.1038/embor.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Novelle MD, Petronczki M. Relocation of the chromosomal passenger complex prevents mitotic checkpoint engagement at anaphase. Curr Biol. 2010;20(15):1402–1407. doi: 10.1016/j.cub.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Villa F, Capasso P, Tortorici M, Forneris F, de Marco A, Mattevi A, Musacchio A. Crystal structure of the catalytic domain of Haspin, an atypical kinase implicated in chromatin organization. Proc Natl Acad Sci U S A. 2009;106(48):20204–20209. doi: 10.1073/pnas.0908485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;303(6001):231–235. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ, Higgins JM. Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J Cell Biol. 2012;199(2):251–268. doi: 10.1083/jcb.201205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Ulyanova NP, van der Waal MS, Patnaik D, Lens SM, Higgins JM. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol. 2011;21(12):1061–1069. doi: 10.1016/j.cub.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei RR, Schnell JR, Larsen NA, Sorger PK, Chou JJ, Harrison SC. Structure of a central component of the yeast kinetochore: the Spc24p/Spc25p globular domain. Structure. 2006;14(6):1003–1009. doi: 10.1016/j.str.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore–microtubule interface. Mol Cell. 2010;38(3):383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Experimental cell research. 2001;262(2):122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- Wurzenberger C, Held M, Lampson MA, Poser I, Hyman AA, Gerlich DW. Sds22 and Repo-Man stabilize chromosome segregation by counteracting Aurora B on anaphase kinetochores. J Cell Biol. 2012;198(2):173–183. doi: 10.1083/jcb.201112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ogawa H, Vagnarelli P, Bergmann JH, Hudson DF, Ruchaud S, Fukagawa T, Earnshaw WC, Samejima K. INCENP-aurora B interactions modulate kinase activity and chromosome passenger complex localization. J Cell Biol. 2009;187(5):637–653. doi: 10.1083/jcb.200906053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Vagnarelli P, Ogawa H, Samejima K, Earnshaw WC. Gradient of increasing Aurora B kinase activity is required for cells to execute mitosis. J Biol Chem. 2010;285(51):40163–40170. doi: 10.1074/jbc.M110.181545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330(6001):239–243. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nature cell biology. 2012;14(7):746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- Yanai A, Arama E, Kilfin G, Motro B. ayk1, a novel mammalian gene related to Drosophila aurora centrosome separation kinase, is specifically expressed during meiosis. Oncogene. 1997;14(24):2943–2950. doi: 10.1038/sj.onc.1201144. [DOI] [PubMed] [Google Scholar]
- Yang C, Tang X, Guo X, Niikura Y, Kitagawa K, Cui K, Wong ST, Fu L, Xu B. Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Molecular cell. 2011;44(4):597–608. doi: 10.1016/j.molcel.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Camp DG, 2nd, Gritsenko MA, Luo Q, Kelly RT, Clauss TR, Brinkley WR, Smith RD, Stenoien DL. Identification of a novel mitotic phosphorylation motif associated with protein localization to the mitotic apparatus. J Cell Sci. 2007;120(Pt 22):4060–4070. doi: 10.1242/jcs.014795. [DOI] [PubMed] [Google Scholar]
- Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Ogawa H, Gudmundsdottir E, Gassmann R, Morrison CG, Ruchaud S, Earnshaw WC. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183(2):279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin SG, Barber CM, Allis CD, Sullivan KF. Differential regulation of CENP-A and histone H3 phosphorylation in G2/M. J Cell Sci. 2001;114(Pt 4):653–661. doi: 10.1242/jcs.114.4.653. [DOI] [PubMed] [Google Scholar]
- Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155(7):1147–1157. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak T, Fitz V, Zhou H, Lampert F, Opravil S, Mechtler K, Stolt-Bergner P, Westermann S. Spatiotemporal regulation of Ipl1/Aurora activity by direct Cdk1 phosphorylation. Curr Biol. 2012;22(9):787–793. doi: 10.1016/j.cub.2012.03.007. [DOI] [PubMed] [Google Scholar]


