Summary
BPC factors are important regulators of developmental processes. Here we assign a role to BPCs in the control of meristem size, characterizing them as direct regulators of several HOMEOBOX genes
Key words: Arabidopsis, BPC, cytokinin, HOMEOBOX, meristem.
Abstract
The BASIC PENTACYSTEINE (BCP) family is a poorly characterized plant transcription factor family of GAGA BINDING PROTEINS. In Arabidopsis, there are seven members (BPC1–7) that are broadly expressed, and they can potentially bind more than 3000 Arabidopsis GAGA-repeat-containing genes. To date, BPCs are known to be direct regulators of the INNER NO OUTER (INO), SEEDSTICK (STK), and LEAFY COTYLEDON 2 (LEC2) genes. Because of the high functional redundancy, neither single knockout nor double bpc mutant combinations cause aberrant phenotypes. The bpc1-2 bpc2 bpc3 triple mutant shows several pleiotropic developmental defects, including enlargement of the inflorescence meristem and flowers with supernumerary floral organs. Here, we demonstrated through expression analysis and chromatin immunoprecipitation assays that this phenotype is probably due to deregulation of the expression of the SHOOTMERISTEMLESS (STM) and BREVIPEDICELLUS/KNAT1 (BP) genes, which are both direct targets of BPCs. Moreover, we assigned a role to BPCs in the fine regulation of the cytokinin content in the meristem, as both ISOPENTENYLTRANSFERASE 7 (IPT7) and ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) genes were shown to be overexpressed in the bpc1-2 bpc2 bpc3 triple mutant.
Introduction
The Arabidopsis genome contains more than 1900 transcription factor-encoding genes. Based on sequence homology, function, and activity, these factors are subdivided into 64 transcription factor families (Guo et al., 2005). The BASIC PENTACYSTEINE/BARLEY B RECOMBINANT (BPC/BBR) family is a poorly characterized plant-specific transcription factor family. BPC factors might share functional similarity with the Trithorax-like protein named GAGA-associated factor (GAF) of Drosophila melanogaster, which transcriptionally regulates expression of the homeotic HOX genes and is involved in nucleosome spacing processes (Botas, 1993; Orphanides et al., 1998; Lehmann, 2004; Berger and Dubreucq, 2012). BPC-encoding genes have been identified in different plant species, such as Glycine max (soybean), Hordeum vulgare (barley), Oryza sativa (rice) and Arabidopsis thaliana (Sangwan and O’Brian, 2002; Santi et al., 2003; Meister et al., 2004; Kooiker et al., 2005). BPC family members are characterized by the ability to bind the DNA at GA-rich sequences: the GAGA BINDING PROTEIN (GBP) of soybean specifically binds a (GA)9 repeat sequence located in the Glutamate 1-Semialdehyde Aminotransferase (Gsa1) gene promoter (Sangwan and O’Brian, 2002), the BARLEY B RECOMBINANT (BBR) factor binds (GA)8 sequences in vitro (Santi et al., 2003), and the Arabidopsis BPC proteins specifically recognize (GA)6 and (GA)9 repeats in vitro and in vivo (Meister et al., 2004; Kooiker et al., 2005; Simonini et al., 2012).
The seven BPCs encoded by the Arabidopsis genome sequence are divided into three classes, namely class I (BPC1–3), class II (BPC4–6), and class III (BPC7). Except for BPC5, which is thought to be a pseudogene, they are all ubiquitously expressed transcriptional activators and repressors (Meister et al., 2004; Monfared et al., 2011).
More than 3000 Arabidopsis genes contain at least one GA-rich stretch in their regulatory region, and combining multiple bpc mutant alleles together results in a broad range of developmental defects (Meister et al., 2004; Monfared et al., 2011), suggesting that the function of BPCs are not specific for one developmental process and/or tissue. For instance, BPCs are known to be regulators of YABBI transcription factors, such as INNER NO OUTER (INO), a gene involved in ovule development (Meister et al., 2004). BPCs are also involved in seed development, being regulators of the B3-domain LEAFY COTYLEDON 2 gene (LEC2; Berger et al., 2011). Moreover BPCs regulate the expression of the ovule identity MADS-domain transcription factor encoding gene SEEDSTICK (STK; Pinyopich et al., 2003; Favaro et al., 2003) by looping its regulatory region and through an interaction with a MADS-domain transcription factor containing repressor complex (Kooiker et al., 2005; Simonini et al., 2012).
In 2003, Santi and colleagues demonstrated that, in barley, the BBR factor directly regulates transcription of the HOMEOBOX transcription factor BKN3. The orthologue of BNK3 in Arabidopsis is named SHOOTMERISTEMLESS (STM), and is strongly expressed in meristematic tissues where it is necessary for setting up and maintaining the meristem (Endrizzi et al., 1996; Long et al., 1996). STM promotes cytokinin (CK) synthesis, a class of plant hormones involved in the maintenance of meristem identity, size, and activity (Jasinski et al., 2005; Leibfried et al., 2005; Yanai et al., 2005; Bartrina et al., 2011). Plants with hyperproduction or slow degradation of CK display compact inflorescences, extra floral organs, and altered phyllotaxis caused by an enlarged and overproductive inflorescence meristem (IM) (Venglat and Sawhney, 1996; Bartrina et al., 2011; Bencivenga et al., 2012).
Here, we unravelled the role of class I BPCs in the control of meristem size, characterizing them as direct regulators of several HOMEOBOX genes, such as STM and KNOTTED-LIKE FROM ARABIDOPSIS THALIANA (KNAT) genes like BREVIPEDICELLUS/KNAT1 (BP). Moreover, we linked the bpc1-2 bpc2 bpc3 triple mutant IM phenotype to increased CK synthesis in the IM.
Materials and methods
Plant material and growth conditions
The A. thaliana ecotype used in this work was Col-0; plants were grown under short-day conditions for 2 weeks (22 °C, 8h light/16h dark) and then moved to long-day conditions (22 °C, 16h light/8h dark). The bpc1-2 bpc2 bpc3 triple mutant was kindly provided by Professor C. Gasser. The pBP::GUS and the pCLV3::GUS lines were obtained from the Nottingham Arabidopsis Stock Centre.
In situ hybridization and β-glucuronidase (GUS) staining
In situ hybridization experiments were performed as described previously by Dreni et al. (2011). The STM antisense probe was prepared according to the method of Long et al. (1996) and the ARR7 probe according to the method of Buechel et al. (2010). GUS staining was performed as described by Simonini et al. (2012).
Plasmid construction and ethanol induction experiments
The EAR motif was added at the C terminus of the BPC1-coding sequence (see primer sequences in Supplementary Table S2 available at JXB online). The fragment was cloned into the pB2GW7 plasmid (35S) and the binary pFLUAR (pAlc) vector carrying DsRed as visual selection markers (Battaglia et al., 2006) passing through the pENTRY-D-TOPO vector (Life technologies). Arabidopsis plants were transformed by the floral-dip method (Clough and Bent, 1998).
The 35S::BPC1-EAR lines were selected by BASTA treatment whereas the seeds of the pAlc-BPC1-EAR motif were selected under a Leica MZ FLIII stereomicroscope and immediately transferred on soil. The pALC::BPC1-EAR plants were inducted for 4–6 d for 8h per day using ethanol vapour, which was applied at bolting. Inflorescences were collected at 4 and 6 d of induction.
RNA isolation, reverse transcription-PCR and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from young inflorescences (meristem, floral buds, and young flowers) using the LiCl method (Verwoerd et al., 1989) for all expression analyses (STM, BP, IPT7, and WUS). Total RNA was treated with an Ambion TURBO DNA-free DNase kit and then reverse transcribed using an ImProm-II™ Reverse Transcription System (Promega). The cDNAs were standardized relative to UBIQUITIN10 (UBI10) and PROTEIN PHOSPHATASE 2A SUBUNIT A3 (PP2A; At1g13320) transcripts, and gene expression analyses were performed using an iQ5 Multi Colour Real-Time PCR detection system (Bio-Rad) with a SYBR Green PCR Master Mix (Bio-Rad). Baseline and threshold levels were set according to the manufacturer’s instructions.
For reverse transcription-PCR and qRT-PCR primers, see Supplementary Table S2 available at JXB online.
Chomatin immunoprecipitation (ChIP) assays
ChIP experiments were performed as reported previously using a polyclonal antibody raised against the entire BPC1 protein (Simonini et al., 2012). Chromatin was extracted from wild-type plant (Col-0) inflorescences and from the bpc1-2 bpc2 bpc3 triple mutant, which was used as negative control. The DNA fragments obtained from the immunoprecipitated chromatin were amplified by qRT-PCR using specific primers (see Supplementary Table S2 available at JXB online). Three real-time PCR amplifications were performed for three independent chromatin extractions. For the complete primer sets see Supplementary Table S2 available at JXB online. Enrichment of the target region was determined using an iQ5 Multi Colour Real-Time PCR detection system (Bio-Rad) with a SYBR Green PCR Master Mix (Bio-Rad). The qRT-PCR assays and the fold enrichment calculations were performed as described by Gregis et al. (2008).
Optical, confocal, and scanning electron microscopy.
Samples for GUS and in situ hybridization analyses were imaged using a Zeiss Axiophot D1 microscope (http://www.zeiss.com/) equipped with differential interface contrast optics. Images were captured on an Axiocam MRc5 camera (Zeiss) using the AXIOVISION program (version 4.4).
Propidium iodide staining was performed as described by Truernit et al. (2008). Samples were imaged with an SP5 Leica confocal microscope. Images were subsequently analysed with Fiji software (Schindelin et al., 2012).
Scanning electron microscopy (SEM) analysis was performed according to the method of Grandi et al. (2012).
Accession numbers
Details of accession numbers are as follows: BPC1, AT2G01930; BPC2, AT1G14685; BPC3, AT1G68120; STM, AT1G62360; BP, AT4G08150; KNAT4, AT5G11060; KNAT5, AT4G32040; KNAT6, AT1G23380; KNAT7, AT1G62990; WUS, AT2G17950; WOX3, AT2G28610; WOX9, AT2G33880; RPL, AT5G02030; BLH1, AT2G35940; CRN, AT1G52150; CLV3, AT2G27250; ARR7, AT1G19050; IPT7, AT3G23630.
Results
class I BPCs regulate inflorescence and flower development
Phenotypic analysis of the Arabidopsis bpc1-2, bpc2, and bpc3 single mutants or double mutant combinations did not show any obvious developmental defect, probably due to the functional redundancy among the different BPC genes (Monfared et al., 2011). However, when the three mutants were combined in the bpc1-2 bpc2 bpc3 triple mutant, there were not only defects in reproductive organs (which also affect plant fertility; Monfared et al., 2011) but also evident developmental aberrations in the structure and organization of the inflorescence and flowers.
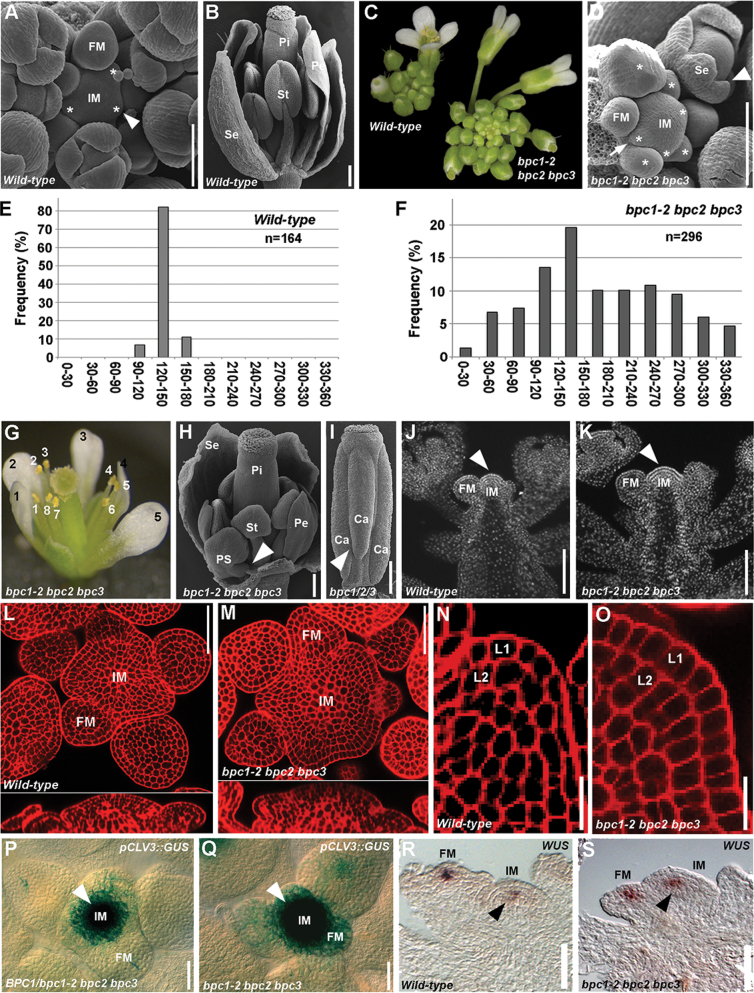
A wild-type Arabidopsis IM is a symmetrical dome-shaped structure that produces floral meristems (FMs) in a spiral phyllotaxy at distances of 137.5°. Typically, three FMs that have not yet developed floral organs can be observed (Fig. 1A). From the FM, four types of floral organs develop in concentric whorls. From the outer to the inner whorl the following develop: four sepals, four petals, six stamens, and a pistil composed of two fused carpels (Fig. 1B). Careful phenotypic analysis of the bpc1-2 bpc2 bpc3 triple mutant showed that the inflorescence developed more flowers than the wild-type plant and that they seemed to be randomly positioned (Fig. 1C). SEM analysis showed that more FMs developed: four or more FMs could be detected at the same time, and these were randomly positioned on the IM surface (Fig. 1D).
Fig. 1.
The bpc1-2 bpc2 bpc3 mutant has enlarged IMs. (A) SEM of a wild-type inflorescence apex. Asterisks indicate developing FMs. (B) SEM of wild-type flower. One sepal has been removed. (C) A bpc1-2 bpc2 bpc3 inflorescence (right) compared a wild-type inflorescence (left). (D) SEM of a bpc1-2 bpc2 bpc3 inflorescence apex showing the IM with several developing floral primordia (arrow). Young floral buds presented fused extra sepals (arrowhead). Asterisks indicate developing FMs. (E) Frequency of divergence angle of siliques in wild-type plants: the majority of the angles fell in the 120–150° class. (F) Frequency of divergence angle of siliques in bpc1-2 bpc2 bpc3 mutant plants: the siliques were randomly distributed along the stem. (G) A bpc1-2 bpc2 bpc3 mutant flower displaying eight stamens (white numbers) and five petals (black numbers). (H) SEM of a bpc1-2 bpc2 bpc3 mutant flower. One sepal has been removed to reveal a petaloid extra stamen developing from the second whorl (arrowhead). (I) SEM of a bpc1-2 bpc2 bpc3 pistil with a third fused carpel (arrowhead). (J) DAPI staining of a longitudinal section of a wild-type inflorescence. The arrowhead indicates the IM. (K) DAPI staining of a longitudinal section of a bpc1-2 bpc2 bpc3 inflorescence. Compare the IM (arrowhead) size with the wild-type one in (J). (L, M) mPS-PI staining of a wild-type (L) and bpc1-2 bpc2 bpc3 (M) inflorescence apex in transversal (upper panel) and longitudinal (lower panel) sections. Note the increased meristem dimension in the bpc1-2 bpc2 bpc3 triple mutant. (N, O) Magnification of mPS-PI staining of the L1 and L2 layers of an inflorescence apex of a wild-type (N) and bpc1-2 bpc2 bpc3 triple mutant (O): the cells of the bpc1-2 bpc2 bpc3 mutant were slightly bigger than those of the wild type. (P) Expression of pCLV3::GUS in the BPC1/bpc1-2 bpc2 bpc3 background (plant does not present the bpc1-2 bpc2 bpc3 phenotype). The arrowhead indicates the IM. (Q) Expression of pCLV3::GUS in the bpc1-2 bpc2 bpc3 background. Note the increase of GUS expression at the IM (arrowhead) compared with that in (L). (R) In situ hybridization with a WUS-specific antisense (as) probe in wild-type IMs (arrowhead) and FMs. (S) In situ hybridization with WUS specific antisense (as) probe in the bpc1-2 bpc2 bpc3 IM (arrowhead) and FMs. Ca, carpel; Pe, petal; Pi, pistil; PS, petaloid stamen; Se, sepal; St, stamen. Bars, 100 µm. (This figure is available in colour at JXB online.)
The characterization of the phyllotactic pattern of the flowers by measuring the divergence angle between successive siliques along the main inflorescence stem (Peaucelle et al., 2007; Pinon et al., 2013) further indicated the random positioning of flowers. In wild-type plants, the majority of the angles fell into the 120–150° class (which contain the theoretical angle 137.5°; Fig. 1E), whereas in the bpc1-2 bpc2 bpc3 mutant, the siliques were randomly distributed along the stem (Fig. 1F and Supplementary Fig. S1 available at JXB online) and extreme distributions were frequently observed (i.e. angles in the 30–60° and 300–330° classes).
Moreover, more than 90% of the bpc1-2 bpc2 bpc3 mutant flowers were composed of five or more sepals, which were often fused along their margins, five or more petals, eight or more stamens (which sometimes arose from the second whorl and presented petaloid features), and up to three carpels (Fig. 1G–I).
As a similar phenotype was observed in plants with hyperproliferative IM tissue (Laufs et al., 1998), the size of the bpc1-2 bpc2 bpc3 IM was analysed by 4′,6-diamidino-2-phenylindole (DAPI) staining and compared with that of the wild-type (Fig. 1J, K). This analysis revealed that the bpc1-2 bpc2 bpc3 triple mutant IM was significantly enlarged when compared with that of the wild-type. Detailed morphological analyses of the inflorescence apex by modified pseudo-Schiff propidium iodide (mPS-PI) staining (Truernit et al., 2008) confirmed that the bpc1-2 bpc2 bpc3 IM was larger than that in wild-type plants, being more expanded and rounded (Fig. 1L, M). The enlargement of the meristem could be a consequence of an increase in cell proliferation and/or cell size. The cells of the L1 and L2 layers of the bpc1-2 bpc2 bpc3 IM were clearly increased in size and had a more rectangular shape with respect to that of the wild-type (Fig. 1N, O). Although the phenotype suggested that cell numbers were increased, a more detailed analysis is needed to confirm this. To obtain further support for an increase in size and activity of the meristem, we investigated the expression of CLAVATA3 (CLV3) and WUSCHEL (WUS) in the bpc1-2 bpc2 bpc3 triple mutant, as an increase in their expression domain has shown to be indicative of a larger meristem (Clark et al., 1995; Schoof et al., 2000). The pCLV3::GUS reporter construct (Gross-Hardt et al., 2002) was introduced in the bpc1-2 bpc2 bpc3 triple mutant background. GUS assays showed that, in triple mutant plants, CLV3 expression was stronger and more expanded when compared with that in the heterozygous mutant plants belonging to the same segregating population (Fig. 1P, Q). To investigate WUS expression in the inflorescence, we performed in situ hybridization analysis using a WUS-specific probe (Brambilla et al., 2007). This analysis showed that, in the triple mutant, the expression domain of WUS was both in the IMs and FMs similar to that observed in the wild type (Fig. 1R, S, and Supplementary Fig. S2 available at JXB online).
Taken together, these data suggested that BPC proteins of class I are involved in regulating IM and FM size by the negative control of meristem activity.
BPC1 is involved in many aspects of plant development
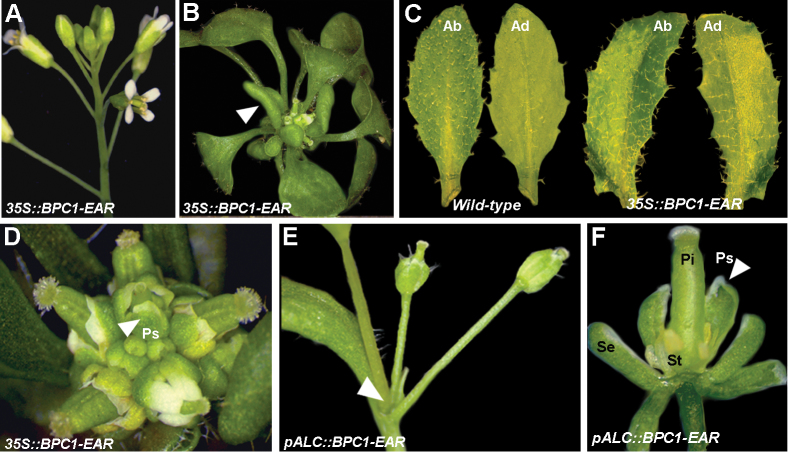
The BPCs are transcriptional regulators that are thought to function both as activators and repressors of gene expression (Meister et al., 2004; Kooiker et al., 2005; Berger and Dubreucq, 2012; Simonini et al., 2012). To investigate the regulatory potential of these factors in more detail, we fused BPC1 to the strong EAR repressor domain (Hiratsu et al., 2003). The BPC1–EAR chimeric open reading frame was placed under the control of the 35S cauliflower mosaic virus promoter and introduced into wild-type Arabidopsis plants. Of the 270 transformants, 90% (n=239) did not show any phenotype, being completely indistinguishable from the wild type (Fig. 2A), whereas the remaining 10% of plants (n=31), which showed the highest expression of the transgene (Supplementary Fig. S3 available at JXB online), exhibited severe defects during vegetative and reproductive development. These 35S::BPC1–EAR plants had few small curved leaves, which never reached the wild-type size (Fig. 2B).
Fig. 2.
Expression of the BPC1–EAR chimeric protein causes strong developmental defects. (A) A 35S::BPC1–EAR plant with wild-type phenotype. (B) A 35S::BPC1–EAR plant with a severe phenotype. Leaves are small and curled (arrowhead). (C) Leaf of a 35S::BPC1–EAR plants with both abaxial and adaxial sides covered by trichomes. (D) Inflorescence of a 35S::BPC1–EAR plant with a severe phenotype. Flowers displayed petaloid sepals (arrowhead). (E) A pALC::BPC1–EAR plant after 6 d of ethanol induction in which the IM arrested prematurely (arrowhead). (F) A flower of a pALC::BPC1–EAR plant after 4 d of ethanol induction with petaloid sepals (arrowhead), no petals, and short and aberrant stamens. Pi, pistil; Se, sepal; St, stamen; Ps, petaloid sepals; Up, upper side; Lo, lower side. (This figure is available in colour at JXB online.)
Analysis of the number of trichomes on the adaxial side of the leaf is a useful criterion for understanding whether a leaf has (partially) lost its adaxial/abaxial symmetry identity. During Arabidopsis leaf development, the first two to three leaves lack trichomes on their adaxial (lower) surface. Later during development, trichomes can be observed on both sides of the leaf but are most abundant on the abaxial (upper) surface (Larkin et al., 1996). Analysis of the fifth developing leaf suggested that the 35S::BPC1–EAR leaves partially lacked adaxial/abaxial identity, as a conspicuous number of trichomes was present on the adaxial side of the leaf (Fig. 2B, C). This features was also present in most of the curved leaves of the 35S::BPC1–EAR rosette.
Furthermore, the plants developed a compact and disorganized inflorescence, bearing aberrant flowers, which remained attached to the rosette due to the inability to develop a stem (Fig. 2D). To avoid the strong pleiotropic phenotypes observed in the 35S::BPC1–EAR lines during vegetative growth and to be able to investigate better the role of BPC1 during flower development, the chimeric BPC1–EAR fusion gene was placed under the control of the AlcR/AlcA ethanol-inducible promoter system (Roslan et al., 2001). Ten wild-type plants and 18 pAlcA::BPC1–EAR plants were treated with ethanol vapour for 8h d–1 for 4 and 6 d, consecutively. The treatment was applied when the plants had switched from the vegetative to the reproductive phase, and a small cluster of floral buds was visible at the centre of the basal rosette. Whereas the wild-type plants treated with ethanol vapour showed no altered phenotype (data not shown), all 18 pAlcA::BPC1–EAR plants showed a strong phenotype when treated with ethanol. In these plants, the inflorescence was composed of only a few flowers, probably due to a premature arrest of IM activity (Fig. 2E). Moreover, the flowers were aberrant and sterile, and the perianth organs were composed only of sepals of which some had petaloid features (Fig. 2F).
The severe pleiotropic phenotypes observed in these BPC1–EAR plants during vegetative and reproductive growth suggested that this factor is involved in many different developmental processes, including meristem activity.
The KNOX genes STM and BP are direct targets of BPCs of class I
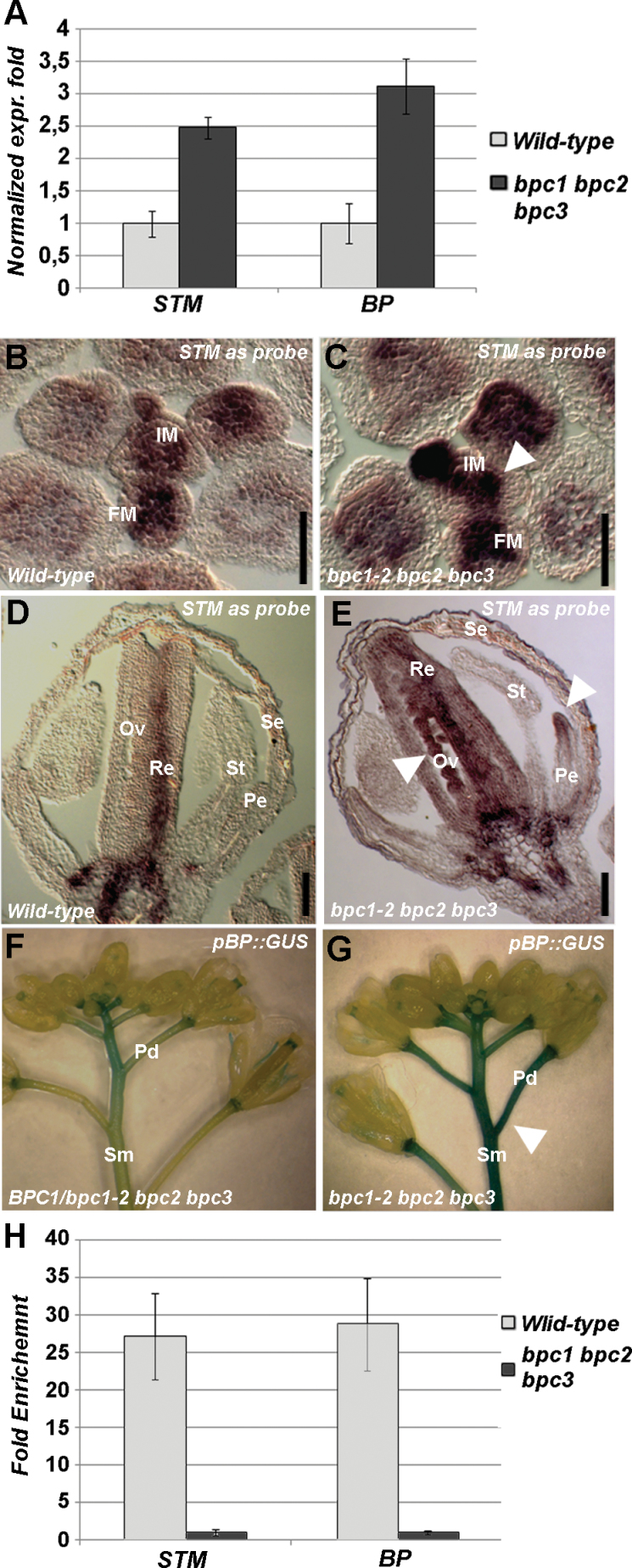
The HOMEOBOX gene family is large in Arabidopsis and can be subdivided in different subfamilies (Chan et al., 1998; Ariel et al., 2007). The KNOTTED1-like homeobox (KNOX) family includes several genes essential for meristem maintenance and floral organ development such as STM, BP and KNAT2–7. As STM and BP are important for meristem maintenance and inflorescence architecture (Long et al., 1996; Venglat et al., 2002), the expression levels of STM and BP were investigated by qRT-PCR of wild-type and bpc1-2 bpc2 bpc3 triple mutant inflorescences. Both STM and BP expression levels were higher (2.5-fold more for STM and 3.2-fold more for BP) in the bpc1-2 bpc2 bpc3 triple mutant than in the wild-type (Fig. 3A), suggesting that BPCs of class I act as repressors of STM and BP.
Fig. 3.
STM and BP are direct targets of class I BPCs. (A) qRT-PCR of the wild type and bpc1-2 bpc2 bpc3 triple mutant to determine STM and BP expression levels. (B–E) In situ hybridization using an STM-specific antisense probe of transversal sections of (B, D) and wild-type (C, E) bpc1-2 bpc2 bpc3 inflorescences. The signal was more intense and expanded in the meristems of the mutant (arrowhead in B). In the wild-type flower, the signal was localized in the replum and at the base of the flower, whereas in the triple mutant it was also in the petal and ovules (arrowheads in E). (F, G) GUS staining of BPC1/bpc1-2 bpc2 bpc3 (F) and bpc1-2 bpc2 bpc3 (G) inflorescences of plants containing the pBP::GUS construct. In the homozygous triple mutant, the signal was expanded and more persistent in both the pedicels and stems (arrowhead in G). (H) ChIP analysis revealing that BPCs of class I directly bind the STM and BP promoter; the bpc1-2 bpc2 bpc3 was used as a negative control. Each bar shows the average of three independent ChIP experiments (±standard deviation). Pe, petals; Se, sepals; St, stamen; Re, replum; Ov, ovules; Sm, stem; Pd, pedicel. Bars, 50 µm. (This figure is available in colour at JXB online.)
In situ hybridization using an STM-specific antisense probe (Long et al., 1996) revealed that its expression domain seemed to be enlarged in the bpc1-2 bpc2 bpc3 triple mutant IM when compared with that of the wild-type, which is probably due to enlargement of the meristem as observed in the triple mutant (Fig. 3B, C). Later during flower development, STM expression was detected ectopically in bpc1-2 bpc2 bpc3 triple mutant petals and ovules (Fig. 3D, E), further supporting the hypothesis that BPCs are repressors of STM.
To analyse temporal and spatial BP expression in the bpc1-2 bpc2 bpc3 triple mutant background, we introduced in this mutant the BP::GUS reporter construct (Ori et al., 2000). GUS assays showed that BP expression was stronger and more persistent, and was expanded throughout the stem and pedicels of the bpc1-2 bpc2 bpc3 triple mutant plants when compared with the segregating genotypes belonging to the same population (Fig. 3F, G). This was in agreement with the upregulation of BP expression detected by RT-PCR and strengthened the hypothesis that BPCs of class I act as repressors of BP transcription.
Analysis of the promoter regions of STM and BP revealed that they contained GA-rich sequences that differed from the GA-rich consensus sequences found in the promoter of the MADS-box gene STK (Kooiker et al., 2005). The latter are relatively short in sequence (9–15bp) and distributed along the 2900bp of the STK regulatory region, whereas the GA repeats located in the STM and BP promoters were extremely long (up to 50bp), unique, and located within 500bp of the transcription start site (Supplementary Fig. S4 available at JXB online). The ability of class I BPCs to bind these GA-rich sequences was tested in three independent ChIP assays (Fig. 3H) using chromatin extracted from wild-type inflorescences and a polyclonal antibody that recognized BPCs of class I (Simonini et al., 2012). Chromatin extracted from inflorescences of the bpc1-2 bpc2 bpc3 triple mutant was used as a negative control, and as a positive control the STK promoter was used (results not shown; Simonini et al., 2012). In all three biological ChIP replicates, the GA-rich stretches in both the STM and BP promoters were strongly enriched (Fig. 3H), confirming that, in Arabidopsis inflorescences, the class I BPCs directly bind and regulate the expression of STM and BP.
HOMEOBOX genes are direct target of BPCs
Analysis of the putative promoter regions of other HOMEOBOX transcription factor-encoding genes belonging to different families showed that 53 out of 88 genes that we analysed contained one or more GA-rich repeats that were similar to those observed in the STM promoter (Supplementary Table S1 available at JXB online). We selected a few genes that were expressed in the Arabidopsis inflorescence, belonging to the KNOX, BELL, WUS, and HD-ZIP families, and that contained GA-rich sequences 500bp upstream of their transcription start site. Using ChIP assays, we verified whether class I BPCs directly bound them. The genes that we selected were KNAT4, KNAT5, KNAT6, and KNAT7 from the KNOX family; WUS, WUSCHEL RELATED HOMEOBOX 3 (WOX3), and WOX9 belonging to the WUS family; REPLUMLESS (RPL) and BELL-LIKE HOMEOBOX1 (BLH1) belonging to the BELL family; and CORONA (CRN) from the HD-ZIP family. Except for CRN, the GA-rich sequences located in the putative promoter regions of all these genes was shown to be highly enriched in three independent ChIP experiments (Fig. 4A), suggesting that they are all direct targets of class I BPC factors.
Fig. 4.
HOMEOBOX and cytokinin pathway genes are regulated by class I BPCs. (A) ChIP analysis revealing that class I BPCs directly bind the GA-rich sites in the promoter of different HOMEOBOX transcription factors; the bpc1-2 bpc2 bpc3 triple mutant was used as a negative control. Each bar shows the average of three independent ChIP experiments (±standard deviation). (B) Expression analyses of IPT7 in wild-type and bpc1-2 bpc2 bpc3 young inflorescences. (C, D) In situ hybridization with ARR7-specific antisense probe using wild-type (C) and bpc1-2 bpc2 bpc3 (D) inflorescences. A stronger and more diffuse signal was detectable in the mutant IM (arrowhead), FM, pedicels, and stem (arrow). (E) ChIP analysis revealing that class I BPCs directly bind the GA-rich site in the ARR7 promoter; the bpc1-2 bpc2 bpc3 triple mutant was used as a negative control. Each bar shows the average of three independent ChIP experiments (±standard deviation). Pd, pedicel; Sm, stem. Bars, 50 µm. (This figure is available in colour at JXB online.)
The CK pathway is upregulated in the bpc1-2 bpc2 bpc3 triple mutant
CKs form a class of plant hormones involved in many aspects of plant development, such as shoot and root meristem formation and activity (Werner et al., 2003), vascular tissue formation (Mähönen et al., 2000), apical dominance, leaf senescence, cell differentiation (Dello Ioio et al., 2007), and cell division (Dewitte et al., 2007). Mutants with overproduction or slow degradation of CKs display enlarged IMs and extrafloral organs (Venglat and Sawhney, 1996; Bartrina et al., 2011; Bencivenga et al., 2012). On the other hand, in mutants with impaired CK biosynthesis or CK perception, the meristem differentiates and terminates prematurely (Bartrina et al., 2011).
A few KNOX genes such as STM and BP are known to be involved in the CK pathway (Venglat et al., 2002; Yanai et al., 2005; Jasinski et al., 2005; Leibfried et al., 2005; Bartrina et al., 2011; Scofield et al., 2013) by directly activating transcription of the ISOPENTENYLTRANSFERASE 7 (IPT7) gene, which encodes a key enzyme involved in the CK synthesis pathway (Kakimoto, 2001). Subsequently, CK signalling is propagated through a set of more than 20 response regulators (ARRs; Heyl et al., 2008) and one of the final goals is the activation of WUS in the meristem to promote the maintenance of meristematic tissue (Jasinski et al., 2005; Leibfried et al., 2005).
The enlarged IM and the increase in STM and BP expression levels as observed in the bpc1-2 bpc2 bpc3 triple mutant were in agreement with a possible increase in the CK content at the IM. To support this hypothesis, we investigated by qRT-PCR the IPT7 expression levels in wild-type and bpc1-2 bpc2 bpc3 inflorescences (meristem and young floral buds; Fig. 4B). This revealed that the expression level of IPT7 was significantly higher in the bpc1-2 bpc2 bpc3 mutant, which was in accordance with the previously detected overexpression of STM. To further support the hypothesis of an increase in CK concentration in the triple bpc mutant meristem, the expression pattern of the ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) gene was investigated by in situ hybridization using a specific ARR7 probe (Buechel et al., 2010). ARR7 is a primary CK response gene and is rapidly upregulated by exogenous CK application (Buechel et al., 2010; Zhao et al., 2010). This revealed that, in the wild-type IM, ARR7 mRNA levels were rather low (Fig. 4C; Buechel et al., 2010; Zhao et al., 2010), whereas in the bpc1-2 bpc2 bpc3 triple mutant, the ARR7 hybridization signal was stronger (Fig. 4D), suggesting that CK-mediated signalling was more active in the IM. Interestingly, a palindromic GAGA box localized in the ARR7 promoter at –178bp from the transcription start site (Supplementary Fig. S4 available at JXB online) was highly enriched when tested in three independent ChIP experiments using antibodies against class I BPCs (Fig. 4E). This strongly supports an involvement of class I BPCs at multiple levels in regulation of the CK pathway in the meristem.
Discussion
The functional characterization of BPC genes has only recently been initiated (Meister et al., 2004; Monfared et al., 2011: Simonini et al., 2012). Interestingly, all these genes are widely expressed throughout the plant, and higher-order bpc mutant combinations have shown developmental defects in both vegetative and reproductive tissues (Monfared et al., 2011). These studies clearly revealed that they act redundantly during plant development. Previously, it was shown that the bpc1-1 bpc2 double mutant had a more severe phenotype than the bpc1-1 bpc2 bpc3 triple mutant (Monfared et al., 2011), suggesting that the loss of BPC3 activity compensates for the loss of BPC1 and BPC2. Under our greenhouse conditions, this observation was not observed with respect to the meristem defects that we described here. These defects were only observed in the bpc1-2 bpc2 bpc3 triple mutant and not in the bpc1-2 bpc2 double mutant. However, in our study, we used for all our analyses the bpc1-2 complete knockout allele (Simonini et al., 2012), and, indeed, in the bpc1-1 bpc2 bpc3 triple mutant, such meristem defects were more rare and milder, suggesting that complete loss of BPC1 activity is needed to observe the meristem phenotypes that we described here.
The class I BPC factors seem to directly regulate HOMEOBOX genes of different classes. The fact that many HOMEOBOX genes controlling meristem functions are directly bound by class I BPC proteins underlines their potential importance in the control of plant development. Considering that the antibodies specifically recognize the class I BPC proteins (Simonini et al., 2012), we can, of course, not exclude that also BPCs of other classes are involved in the regulation of these genes. However, as the bpc1-2 bpc2 bpc3 mutant has larger IMs and FMs (whereas for instance the bpc4 bpc6 double mutant does not have this phenotype), it is clear that class I BPCs at least are important for the regulation of genes controlling meristem size.
STM is a key gene for meristem tissue maintenance and is strongly expressed in both vegetative and reproductive meristems. Loss-of-function alleles of STM display precocious deprivation of meristem tissue in the IM. Therefore, these plants produce only a few flowers with fewer floral organs (Durbak and Tax, 2011). In contrast, upregulation of STM expression leads to an IM enlargement connected to an increase in meristem activity (Yanai et al., 2005). STM is involved in the CK pathway, a class of hormones tightly linked to meristem activity; indeed, loss of meristem function in the stm mutant can be rescued by exogenous CK application or by the expression of a CK biosynthetic gene driven by the STM promoter (Yanai et al. 2005).
STM is upregulated in the bpc1-2 bpc2 bpc3 background, and this is consistent with enlargement of the IM detected in this mutant. Moreover, this regulation seems to be direct, as BPCs of class I strongly bind the STM promoter.
As STM is responsible for CK synthesis in the meristem (Yanai et al., 2005), we investigated whether CK levels were altered in the bpc1-2 bpc2 bpc3 triple mutant by checking the expression profiles of pCLV3:GUS (Gross-Hardt et al., 2002), WUS (Mayer et al., 1998), and ARR7 (Buechel et al., 2010; Zhao et al., 2010).
The pCLV3::GUS expression domain, which is not only a marker for meristem size but is also indicative of CK signalling (Gordon et al., 2009), is expanded in the bpc1-2 bpc2 bpc3 triple mutant IM. This expansion of the pCLV3::GUS domain could be a consequence of the meristem enlargement but could also be a response to the increment in CK content, as exogenous CK treatment stimulates the expansion of pCLV3::GFP–EAR reporter gene expression within IMs and FMs (Gordon et al., 2009).
WUS, which promotes meristem proliferation (Laux et al., 1996) and which is positively regulated by CK (Gordon et al., 2009), did not significantly expand its expression domain in the bpc1-2 bpc2 bpc3 background. This seems to be in contrast with the enlarged meristem and expanded CLV3 domain. However, expansion of the CLV3 expression domain is not always correlated with an expansion in WUS expression, suggesting that the feedback loop that regulates WUS expression in the meristem could occur through both CLAVATA-dependent and -independent pathways (Gordon et al., 2009; Yoshida et al., 2011). This could be the case for the bpc1-2 bpc2 bpc3 mutant, in which the expansion of the CLV3 domain did not seem to be accompanied by a WUS domain expansion. Thus, expansion of the CLV3 expression domain might be more a consequence of the increased meristem size rather than being caused directly by the loss of BPC protein activities. The fact that WUS seems to be a direct target of BPCs (whereas CLV3 does not have BPC-binding sites in its genomic region and therefore is probably not a direct target of BPCs) might place CLV3 and WUS in two different pathways.
The expression levels of both IPT7 and ARR7, which are a CK biosynthetic and a CK responsive gene, respectively (Kakimoto, 2001; Buechel et al., 2010; Zhao et al., 2010), were upregulated in the bpc1-2 bpc2 bpc3 triple mutant, suggesting that, in this mutant, CK levels are increased. Moreover, ARR7 is a direct target of BPCs, strengthening their direct role in regulation of the CK pathway at multiple levels in the meristem.
These data therefore support the hypothesis that, in the bpc1-2 bpc2 bpc3 triple mutant IM, the activity is higher due to increased production of CK, which is probably caused by the upregulation of KNOXI genes like STM and BP (Jasinski et al., 2005; Yanai et al., 2005: Sakamoto et al., 2006). Indeed, in the 35S::STM::GR inducible line, the levels of several CKs increased within 24h of induction (Yanai et al., 2005); moreover, an expansion of the ARR5 expression domain is observed in 35S::BP lines, where BP is constitutively misexpressed in leaves (Yanai et al., 2005).
BP and RPL are two other HOMEOBOX transcription factors to which BPCs of class I directly bind. Both genes are involved in stem elongation and in inflorescence architecture (Ori et al., 2000; Venglat et al., 2002; Smith and Hake, 2003; Kanrar et al., 2008), and their repression could be responsible for the inability of the 35S::BPC1–EAR motif plants to produce a stem and a well-organized inflorescence. The loss of the spiral pattern in the bpc1-2 bpc2 bpc3 inflorescence is reminiscent of rpl mutant plants, and it will be interesting to investigate whether this gene is regulated by BPCs.
BPC factors form a plant-specific transcription factor family. However, despite the fact that their amino acid sequence seems to be unrelated to animal GAGA-binding proteins, they have been suggested to play similar roles in plants (Berger and Dubreucq, 2012; Simonini et al., 2012). The analysis that we have described here points again to an evolutionary relationship between the animal and plant GAGA-binding proteins. In Drosophila, the GAGA factor (dGAF) has been shown to be important in particular for the regulation of HOMEOBOX genes and in this way controlling a wide range of developmental events (Botas, 1993; Graba et al., 1997). The fact that GAGA-binding proteins of animals and plants are important in controlling the activity of HOMEOBOX genes might, of course, be a coincidence, but it remains an interesting parallel between these ‘unrelated’ factors.
The molecular mechanisms by which BPC proteins regulate their target genes are not yet clear. However, recently we showed that they loop the promoter region of the ovule identity gene STK and that they interact with a MADS-domain protein containing repressor complex to silence STK expression in the FM (Kooiker et al., 2005; Gregis et al., 2006; Simonini et al., 2012). It is likely that BPC proteins interact with transcription factor complexes to facilitate their binding to the DNA. Therefore, also in the case of genes like STM and BP, it might well be that BPCs interact with the upstream regulators to recruit them to the promoters. ASYMMETRIC LEAVES1 (AS1) and AS2 are known to directly repress STM expression in leaves (Uchida et al., 2007). It will be interesting to verify whether BPCs interact with AS1 and AS2. In the bpc1-2 bpc2 bpc3 triple mutant, we observed mainly an upregulation of STM in the meristem and flowers, and no ectopic expression in leaves. It seems, therefore, that for the regulation of STM and BP in these tissues, the class I BPC proteins have more of a role in fine-tuning the expression of these genes, rather than acting as the main regulators.
A similar observation was shown for the STK gene (Simonini et al., 2012). When the BPC binding sites were mutated in the STK promoter, its expression, which is normally active only in developing ovules, was completely deregulated, and promoter activity was observed throughout the flower. However, also in this case, no expression was observed in the vegetative parts of the plant. It might be that the repression mechanisms facilitated by BPCs are different between reproductive and vegetative tissues. Further investigations will be needed to obtain a better understanding of these molecular mechanisms.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Angle divergence in the bpc1-2 bpc2 bpc3 mutant.
Supplementary Fig. S2. Expression level of WUS in bpc1-2 bpc2 bpc3 mutant.
Supplementary Fig. S3. Expression level of the BPC1–EAR chimeric gene in plants with a strong phenotype.
Supplementary Fig. S4. Localization of GAGA boxes in STM, BP, and ARR7 promoters.
Supplementary Table S1. HOMEOBOX genes with a GAGA stretch in their promoter sequence (500bp upstream of the transcription start site).
Supplementary Table S2. Primers used in this study.
Acknowledgements
We thank Professor C. Gasser for providing the bpc1-2 bpc2 bpc3 triple mutant, Professor E. Caporali for assistance with the SEM analysis, and Dr S. Masiero and Dr S. Bencivenga for critical comments on the manuscript. SS was supported by the Università degli Studi di Milano. This work was supported by the FLOWER POWER project (ID AGRO-11 and ref. no. 16976) of the Lombardy region, Italy.
Glossary
Abbreviations:
- ChIP
chomatin immunoprecipitation
- CK
cytokinin
- DAPI
4′,6-diamidino-2-phenylindole
- FM
floral meristem
- GUS
β-glucuronidase
- IM
inflorescence meristem
- mPS-PI
modified pseudo-Schiff propidium iodide
- qRT-PCR
quantitative real-time PCR
- SEM
scanning electron microscopy.
References
- Ariel FD, Manavella PA, Dezar CA, Chan RL. 2007. The true story of the HD-Zip family Trends in Plant Science 124, 19–26 [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana . Plant Cell 23, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia R, Brambilla V, Colombo L, Stuitje AR, Kater MM. 2006. Functional analysis of MADS-box genes controlling ovule development in Arabidopsis using the ethanol-inducible alc gene-expression system. Mechanisms of Development 123, 267–726 [DOI] [PubMed] [Google Scholar]
- Bencivenga S, Simonini S, Benkova E, Colombo L. 2012. The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis . Plant Cell 24, 2886–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N, Dubreucq B. 2012. Evolution goes GAGA: GAGA binding proteins across kingdoms. Biochimica et Biophysica Acta 1819, 863–868 [DOI] [PubMed] [Google Scholar]
- Berger N, Dubreucq B, Roudier F, Dubos C, Lepiniec L. 2011. Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell 23, 4065–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botas J. 1993. Control of morphogenesis and differentiation by HOM/Hox genes. Current Opinion in Cell Biology 5, 1015–1022 [DOI] [PubMed] [Google Scholar]
- Brambilla V, Battaglia R, Colombo M, Masiero S, Bencivenga S, Kater MM, Colombo L. 2007. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis . Plant Cell 19, 2544–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechel S, Leibfried A, To JP, Zhao Z, Andersen SU, Kieber JJ, Lohmann JU. 2010. Role of A-type ARABIDOPSIS RESPONSE REGULATORS in meristem maintenance and regeneration. European Journal of Cell Biology 89, 279–284 [DOI] [PubMed] [Google Scholar]
- Chan RL, Gago GM, Palena CM, Gonzalez DH. 1998. Homeoboxes in plant development. Biochimica et Biophysica Acta 1442, 1–19 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067 [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. 2007. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biology 17, 678–682 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, et al. 2007. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proceedings of the National Academy of Sciences USA 104, 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreni L, Pilatone A, Yun D, Erreni S, Pajoro A, Caporali E, Zhang D, Kater MM. 2011. Functional analysis of all AGAMOUS subfamily members in rice reveals their roles in reproductive organ identity determination and meristem determinacy. Plant Cell 23, 2850–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbak AR, Tax FE. 2011. CLAVATA signaling pathway receptors of Arabidopsis regulate cell proliferation in fruit organ formation as well as in meristems. Genetics 189, 177–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. 1996. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE . The Plant Journal 10, 967–979 [DOI] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. 2003. MADS-box protein complexes control carpel and ovule development in Arabidopsis . Plant Cell 15, 2603–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM. 2009. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proceedings of the National Academy of Sciences, USA 106, 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graba Y, Aragnol D, Pradel J. 1997. Drosophila Hox complex downstream targets and the function of homeotic genes. Bioessays 19, 379–388 [DOI] [PubMed] [Google Scholar]
- Grandi V, Gregis V, Kater MM. 2012. Uncovering genetic and molecular interactions among floral meristem identity genes in Arabidopsis thaliana . The Plant Journal 69, 881–893 [DOI] [PubMed] [Google Scholar]
- Gregis V, Andrés F, Sessa A, et al. 2013. Identification of pathways directly regulated by SHORT VEGETATIVE PHASE during vegetative and reproductive development in Arabidopsis . Genome Biology 14, R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. 2006. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis . Plant Cell 18, 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. 2008. AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis . The Plant Journal 56, 891–902 [DOI] [PubMed] [Google Scholar]
- Gross-Hardt R, Lenhard M, Laux T. 2002. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes & Development 16, 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, He K, Liu D, Bai S, Gu X, Wei L, Luo J. 2005. DATF: a database of Arabidopsis transcription factors. Bioinformatics 21, 2568–2569 [DOI] [PubMed] [Google Scholar]
- Heyl A, Ramireddy E, Brenner WG, Riefler M, Allemeersch J, Schmulling T. 2008. The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis . Plant Physiology 147, 1380–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M. 2003. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis . The Plant Journal. 34, 733–739 [DOI] [PubMed] [Google Scholar]
- Jasinski S., Piazza P., Craft J., Hay A., Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M. 2005. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Current Biology 15, 1560–1565 [DOI] [PubMed] [Google Scholar]
- Kakimoto T. 2001. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant and Cell Physiology 42, 677–685 [DOI] [PubMed] [Google Scholar]
- Kanrar S, Bhattacharya M, Arthur B, Courtier J, Smith HM. 2008. Regulatory networks that function to specify flower meristems require the function of homeobox genes PENNYWISE and POUND-FOOLISH in Arabidopsis. The Plant Journal 54, 924–937 [DOI] [PubMed] [Google Scholar]
- Kooiker M, Airoldi CA, Losa A, Finzi L, Kater MM, Colombo L. 2005. BASIC PENTACYSTEINE1 a GA-binding protein that induces conformational changes in the regulatory region of the homeotic Arabidopsis gene SEEDSTICK . Plant Cell 17, 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks MD. 1996. The control of trichome spacing and number in Arabidopsis. Development 122, 997–1005 [DOI] [PubMed] [Google Scholar]
- Laufs P, Grandjean O, Jonak C, Kiêu K, Traas J. 1998. Cellular parameters of the shoot apical meristem in Arabidopsis . Plant Cell 10, 1375–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jürgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis . Development 122, 87–96 [DOI] [PubMed] [Google Scholar]
- Lehmann M. 2004. Anything else but GAGA: a nonhistone protein complex reshapes chromatin structure. Trends in Genetics 2, 15–22 [DOI] [PubMed] [Google Scholar]
- Leibfried A., To J.P., Busch W., Stehling S., Kehle A., Demar M., Kieber JJ, Lohmann JU. 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438, 1172–1175 [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK. 1996. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. 2000. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes & Development 14, 2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815 [DOI] [PubMed] [Google Scholar]
- Meister RJ, Williams LA, Monfared MM, Gallagher TL, Kraft EA, Nelson CG, Gasser CS. 2004. Definition and interaction of a positive regulatory element of the Arabidopsis INNER NO OUTER promoter. The Plant Journal 37, 426–438 [DOI] [PubMed] [Google Scholar]
- Monfared MM, Simon MK, Meister RJ, Roig-Villanova I, Kooiker M, Colombo L, Fletcher JC, Gasser CS. 2011. Overlapping and antagonistic activities of BASIC PENTACYSTEINE genes affect a range of developmental processes in Arabidopsis . The Plant Journal 66, 1020–1031 [DOI] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Chuck G, Bowman JL, Hake S. 2000. Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127, 5523–5532 [DOI] [PubMed] [Google Scholar]
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. 1998. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92, 105–116 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Morin H, Traas J, Laufs P. 2007. Plants expressing a miR164-resistant CUC2 gene reveal the importance of post-meristematic maintenance of phyllotaxy in Arabidopsis . Development 134, 1045–1050 [DOI] [PubMed] [Google Scholar]
- Pinon V, Prasad K, Grigg SP, Sanchez-Perez GF, Scheres B. 2013. Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis . Proceedings of the National Academy of Sciences, USA 110, 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. 2003. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 42, 85–88 [DOI] [PubMed] [Google Scholar]
- Roslan HA, Salter MG, Wood CD, et al. 2001. Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana . The Plant Journal 28, 225–235 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, Sato Y, Matsuoka M. 2006. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiology 142, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I, O’Brian MR. 2002. Identification of a soybean protein that interacts with GAGA element dinucleotide repeat DNA. Plant Physiology 129, 1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi L, Wang Y, Stile MR, et al. 2003. The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene Bkn3 . The Plant Journal 34, 813–826 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644 [DOI] [PubMed] [Google Scholar]
- Scofield S, Dewitte W, Nieuwland J, Murray JA. 2013. The Arabidopsis homeobox gene SHOOT MERISTEMLESS has cellular and meristem-organisational roles with differential requirements for cytokinin and CYCD3 activity. The Plant Journal 75, 53–66 [DOI] [PubMed] [Google Scholar]
- Simonini S, Roig-Villanova I, Gregis V, Colombo B, Colombo L, Kater MM. 2012. BASIC PENTACYSTEINE proteins mediate MADS domain complex binding to the DNA for tissue-specific expression of target genes in Arabidopsis . Plant Cell 24, 4163–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HM, Hake S. 2003. The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15, 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano S, Niihama M, Smith HM, Tasaka M, Aida M. 2010. gorgon, a novel missense mutation in the SHOOT MERISTEMLESS gene, impairs shoot meristem homeostasis in Arabidopsis. Plant and Cell Physiology 51, 621–634 [DOI] [PubMed] [Google Scholar]
- Truernit E, Bauby H, Dubreucq B, Grandjean O, Runions J, Barthélémy J, Palauqui JC. 2008. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis . Plant Cell 20, 1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Townsley B, Chung KH, Sinha N. 2007. Regulation of SHOOT MERISTEMLESS genes via an upstream-conserved noncoding sequence coordinates leaf development. Proceedings of the National Academy of Sciences USA 104, 15953–15958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, Martienssen R, Selvaraj G, Datla R. 2002. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis . Proceedings of the National Academy of Sciences, USA 99, 4730–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat SP, Sawhney VK. 1996. Benzylaminopurine induces phenocopies of floral meristem and organ identity mutants in wild-type Arabidopsis plants. Planta 198, 480–487 [DOI] [PubMed] [Google Scholar]
- Verwoerd T.C., Dekker BM, Hoekema A. 1989. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Research 17, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van, Onckelen H, Schmulling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N. 2005. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Current Biology 15, 1566–1571 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Mandel T, Kuhlemeier C. 2011. Stem cell activation by light guides plant organogenesis. Genes & Development 25, 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU. 2010. Hormonal control of the shoot stem-cell niche. Nature 465, 1089–1092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.