Summary
Analyses of mutants impaired in assimilate export from chloroplasts revealed that carbohydrates as primary output of photosynthesis control expression of nuclear genes associated with plastidial processes such as acclimation to high light intensities.
Key words: Arabidopsis mutants, carbon partitioning, light acclimation, metabolomics, photosynthesis, retrograde signalling, sugar metabolism, transcriptomics.
Abstract
Retrograde signals from chloroplasts are thought to control the expression of nuclear genes associated with plastidial processes such as acclimation to varying light conditions. Arabidopsis mutants altered in the day and night path of photoassimilate export from the chloroplasts served as tools to study the involvement of carbohydrates in high light (HL) acclimation. A double mutant impaired in the triose phosphate/phosphate translocator (TPT) and ADP-glucose pyrophosphorylase (AGPase) (adg1-1/tpt-2) exhibits a HL-dependent depletion in endogenous carbohydrates combined with a severe growth and photosynthesis phenotype. The acclimation response of mutant and wild-type plants has been assessed in time series after transfer from low light (LL) to HL by analysing photosynthetic performance, carbohydrates, MgProtoIX (a chlorophyll precursor), and the ascorbate/glutathione redox system, combined with microarray-based transcriptomic and GC-MS-based metabolomic approaches. The data indicate that the accumulation of soluble carbohydrates (predominantly glucose) acts as a short-term response to HL exposure in both mutant and wild-type plants. Only if carbohydrates are depleted in the long term (e.g. after 2 d) is the acclimation response impaired, as observed in the adg1-1/tpt-2 double mutant. Furthermore, meta-analyses conducted with in-house and publicly available microarray data suggest that, in the long term, reactive oxygen species such as H2O2 can replace carbohydrates as signals. Moreover, a cross-talk exists between genes associated with the regulation of starch and lipid metabolism. The involvement of genes responding to phytohormones in HL acclimation appears to be less likely. Various candidate genes involved in retrograde control of nuclear gene expression emerged from the analyses of global gene expression.
Introduction
Light is one of the most prominent environmental factors for plants. It not only determines the rate of photosynthesis and biomass production (Sharkey, 1985), but it also controls various aspects of development (Frankhauser and Chory, 1997). Apart from these beneficial effects, excess light can trigger metabolic changes as well as redox imbalances, and thus has the potential to damage thylakoid structures in the chloroplasts. Therefore, the activity and composition of thylakoid proteins, which determine photosynthetic electron transport rates (ETRs), have to be adapted to changing light intensities over both short- and long-term periods. Long-term adaptation can be ascribed as acclimation (Eberhard et al., 2008; Kleine et al., 2009).
The vast majority of chloroplast-localized proteins are nuclear encoded (Leister, 2003). In order to adjust their expression to plastome-encoded photosynthesis genes, a constant flow of information from the chloroplast to the nucleus seems to be required (Beck, 2005; Koussevitzky et al., 2007; Eberhard et al., 2008). It has been proposed that this ‘retrograde signalling’ is based on a variety of components such as tetrapyrroles (Surpin et al., 2002; Beck, 2005; Czarnecki et al., 2012), reactive oxygen species (ROS; Kim et al., 2008; Foyer and Noctor, 2009; Miller et al., 2009; Triantaphylidès and Havaux, 2009; Balazadeh et al., 2012), the redox state of plastoquinone (Pfannschmidt et al., 1999; Pfalz et al., 2012), plastidial gene expression (Ahlert et al., 2003), the metabolic state of the mesophyll including sugars (Smeekens, 1998, 2000; Rolland et al., 2002, 2006; Bräutigam et al., 2009), and plant hormones, such as abscisic acid (ABA), as a response to an enhanced xanthophyll cycle activity (Baier et al., 2004; Baier and Dietz, 2005). More recently phosphoadenosine phosphate (PAP; Estavillo et al., 2012) and 2-C-methyl-d-erythritol-2,4-cyclopyrophosphate (MecPP; Xiao et al., 2012), an intermediate of the plastidial methylerythritol phosphate (MEP) pathway of isoprenoid biosynthesis (Lichtenthaler, 1999), have also been proposed to act as retrograde signals. Despite a long list of compounds and/or conditions involved, detailed information on how these retrograde signals are integrated and modify nuclear gene expression is still missing. It is also conceivable that retrograde signalling is less complex than believed and relies only on a limited number of primary signals, which then use known components, for example those involved in sugar or hormonal signalling (for a review, see Rolland et al., 2006). Mutants defective in the day and night path of photoassimilate export from the chloroplasts can serve as a model system to test this assumption.
The export of triose phosphates (TPs) during the day (day path) is blocked by a knockout of the TP/phosphate translocator (TPT; Schneider et al., 2002). The absence of a functional ADP-glucose pyrophosphorylase (AGPase) in the adg1-1 mutant in turn results in the absence of starch (Lin et al., 1986) and therefore a block in maltose and glucose delivery and export during the night (night path). In contrast, the adg1-1 mutant accumulates high amounts of soluble sugars during the day (Kunz et al., 2010; Schmitz et al., 2012). While a defect in the TPT can be compensated for by an increased starch turnover in the light (Häusler et al., 1998; Schneider et al., 2002; Walters et al., 2004), a simultaneous block in TPT and AGPase in the adg1-1/tpt-2 double mutant results in severe growth retardation, high chlorophyll fluorescence, inhibition of the ETR, and depletion of carbohydrates (Häusler et al., 2009; Schmitz et al., 2012). This harsh phenotype conditionally occurs only under high light (HL) conditions, but is absent in low light (LL)-grown plants (Schmitz et al., 2012). Marked differences in the abundance of plastome- and nuclear-encoded thylakoid proteins in adg1-1/tpt-2 were also HL dependent and could be rescued by feeding sugars to the plants (Heinrichs et al., 2012; Schmitz et al., 2012), suggesting that (i) HL acclimation is impaired in the double mutant and (ii) soluble sugars can restore the acclimation response.
The response of wild-type and mutant plants to continuous growth in HL or LL has been extensively characterized by Schmitz et al. (2012). Here, time series rather than different light intensities (Walters et al., 2004) have been used to study the impact of impaired carbon partitioning and leaf carbohydrate metabolism on the acclimation response of Arabidopsis thaliana towards HL. Photosynthesis parameters, metabolite levels, and global gene expression were measured after LL to HL (LL/HL) transfer in mutant and wild-type plants. The data suggest that endogenous sugars and ROS play a pivotal role in HL acclimation in A. thaliana.
Materials and methods
Plant material, growth conditions, and photosynthesis measurements
Plant material and growth conditions in HL [photon flux density (PFD=300 μmol m–2 s–1) or LL (PFD=30 μmol m–2 s–1) have been described in detail by Schmitz et al. (2012). For LL/HL transfer experiments, plants were grown under long-day conditions (i.e. 16h light/8h dark) for 3 weeks. Photosynthesis parameters at photosystem II (PSII) were determined according to Schreiber et al. (1986) and Genty et al. (1989) with an IMAGING-PAM fluorometer (Walz, Effeltrich, Germany).
Determination of metabolites
Carbohydrates were extracted and measured enzymatically as described by Schmitz et al. (2012). Anthocyanins were determined according to Giraud et al. (2008).
For gas chromatography–time of flight-mass spectrometry (GC-ToF-MS) profiling, primary metabolites were extracted and derivatized according to established procedures (Lisec et al., 2006). Raw data were then normalized following the procedure outlined in Roessner et al. (2001); peaks were identified using the software Tagfinder (Luedemann et al., 2012), with the support of the mass tags deposited in the Golm Metabolome Database (Kopka et al., 2005). Further details regarding the metabolic profiling are reported in Supplementary Table S1 available at JXB online, which is compliant with the recommendations reported in Fernie et al. (2011).
Ascorbic acid (Asc) and dehydroascorbate (DHA) were quantified with ascorbate oxidase according to Horling et al. (2003), and glutathione (GSH) and glutathione disulphide (GSSG) by enzymatic cycling with glutathione reductase and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) (Griffith, 1980) with some modifications, as described by Oelze et al. (2012).
Mg-protoporphrin IX (MgProtoIX) was extracted from 100mg of frozen leaf powder in 2×200 μl and 1×100 μl acetone:methanol:0.1 N NH4OH (10:9:1). After each centrifugation for 10min at 4 °C, the supernatants were collected and aliquots analysed by HPLC-based fluorescence detection to quantify Mg-porphyrins by means of authentic standards (Frontier Scientific) according to Czarnecki et al. (2011).
RNA extraction for transcriptome analyses and quantitative reverse transcription–PCR (qRT–PCR)
Samples for transcriptome analyses were collected from two sets of independently grown plants, with 3–6 plants per sample. Total RNA was isolated according to Logemann et al. (1987), and for transcriptome analyses subsequently purified on Plant RNeasy extraction columns (Qiagen). Processing and hybridization to GeneChip ATH1 Genome Arrays (Affymetrix) was conducted at the ‘Kompetenzzentrum Fluoreszente Bioanalytik’ (KFB, Regensburg). The complete set of microarray data can be obtained from http://www.ebi.ac.uk/miamexpress/ (ArrayExpress accession: E-MEXP-3791). For qRT–PCR with Lhcb1 the following primer combination was used (RL_LhcbI_fwd: TTCCCTGGAGACTACGGATG; RL_LhcbI_rev: CCCACCTGCTGTGGATAACT).
Statistical evaluation of experimental data
Raw fluorescence probe signals of Affymetrix microarrays were evaluated and statistically analysed with the Robin software (Lohse et al., 2010). The data were normalized by the RMA-method (robust multiarray averaging; Wu et al., 2004). Average log2-fold expression changes and adjusted P-values were calculated by Linear Models for Microarray Data (Limma; Smyth, 2004) and false discovery rate (FDR) corrected according to Benjamini and Hochberg (1995). The Tair10 genome release as well as a modified Mapman Binning (Thimm et al., 2004) were used to annotate the probe-IDs. Venn diagrams were constructed with the help of the web-based tool Venny (Oliveros, 2007). The R-tool 2.12.0 (package ‘pcaMethods’; Stacklies et al., 2007) has been implemented for multivariate analyses [principle component analysis (PCA)] as well as for Fisher’s exact tests.
Significant differences between more than two physiological data sets were analysed using single-site analysis of variance (ANOVA) combined with the post-hoc Tukey–Kramer test, which allows the comparison of unequal sample sizes and identifies values which are significantly different (Ludbrook, 1998). For data plotting and fitting, SigmaPlot10.0 for Windows (SPSS Inc.) was used.
Published microarray data were obtained either from NASC (http://affymetrix.arabidopsis.info) or from the supplementary data of the respective publications online. Differentially regulated genes were filtered by log2-fold changes at a threshold of ±1 (for time series at least one time point had to contain the candidate gene at a threshold of ±1) and commonly regulated genes were extracted. The relative abundance of coinciding genes was calculated from , where Ac is the coincidence coefficient, and a0 equals the number of coinciding genes as a fraction of the total number of differentially regulated genes in in-house (XT) or published (YT) experiments. The sum of the Ac values of all foreign arrays was set to 100% and, from the relative contribution, pie charts were constructed. The correlation of log2 ratios between in-house and published arrays was calculated according to Pearson (Galton, 1888).
Results and Discussion
LL/HL transfer leads to rapid photoinhibition followed by a slow recovery
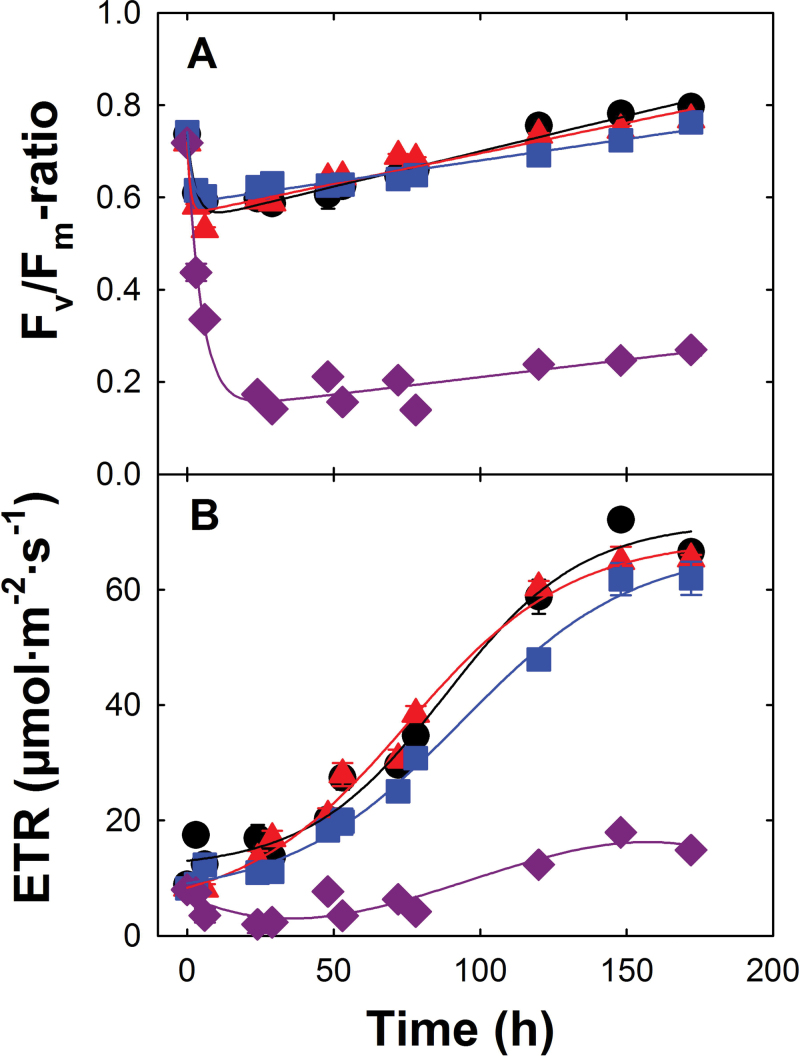
Exposure of LL-grown plants to HL conditions rapidly leads to severe photoinhibition and a re-organization of the photosynthetic apparatus during acclimation (Bailey et al., 2004; Horton et al., 2008). The dynamics of these processes are reflected in changes of the photosynthetic light reaction determined by PAM fluorometry. Modulated chlorophyll a (Chl a) fluorescence in dark-adapted mutant and wild-type plants was monitored over a time period of 1 week (172h) after LL/HL transfer (Fig. 1). HL exposure of LL-grown plants resulted in a drop in the F v/F m ratio within the first 3h based on photoinhibition, followed by a slow recovery (Fig. 1A). In contrast to wild-type and single mutant plants, which exhibit a complete recovery of F v/F m within 1 week, the F v/F m ratio in adg1-1/tpt-2 decreased to a minimum value below 0.2 within the first 2 d upon HL exposure, followed by a small, but incomplete recovery thereafter. The decrease in F v/F m can be attributed to the dissociation of light-harvesting complexes (LHCs) from photosynthetic core complexes (Schmitz et al., 2012). The ETR in all plant lines increased only slowly with time after LL/HL transfer. In Col-0, tpt-2, and adg1-1, the maximum increase in ETR commenced after 48h in HL (Fig. 1B), whereas in the adg1-1/tpt-2 double mutant the ETR remained significantly lower over the whole time course of the experiment. The statistical analysis of the above data is contained in Table 1 of Supplementary Document S1 available at JXB online.
Fig. 1.
Time-dependent response of Chl a fluorescence in the dark-adapted state and photosynthetic ETR upon LL/HL transfer of wild-type and mutant plants. Col-0 (circles), tpt-2 (squares), adg1-1 (triangles), and adg1-1/tpt-2 (diamonds) plants were grown for 3 weeks under LL and long-day conditions. The plants were transferred to HL at t=0 (4h in the light). The maximum quantum yield of ETR through PSII is reflected by the F v/F m ratio (A). The ETR (B) was determined at a PFD of ~300 μmol m–2 s–1. The data represent the mean ±SE of n=3 replicates. In some cases, the error bars are smaller than the symbols. (This figure is available in colour at JXB online.)
LL/HL transfer results in sugar and anthocyanin accumulation
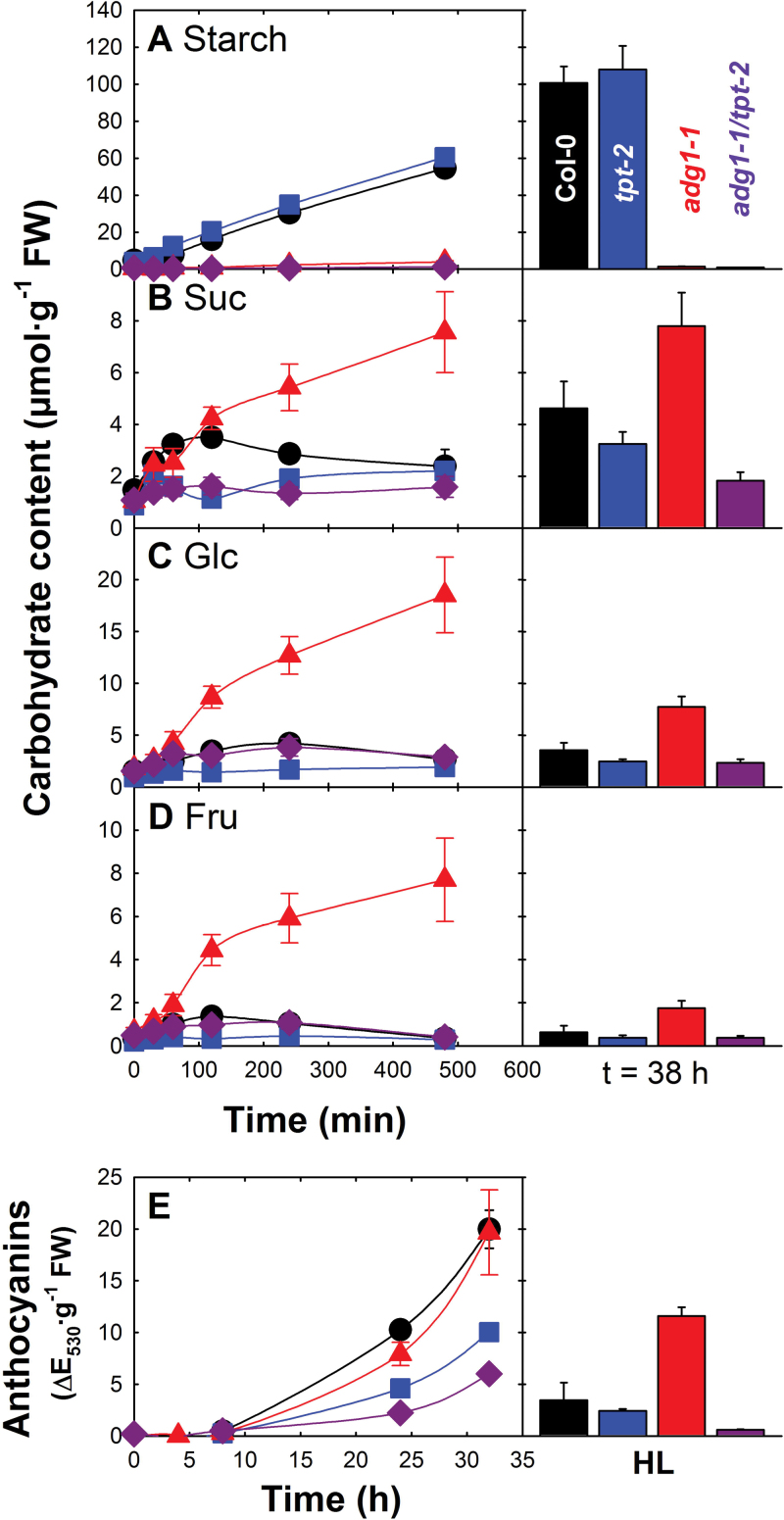
Dynamic changes in carbohydrate contents were assessed in wild-type and mutant plants within the first 8h, and 38h after LL/HL transfer (Fig. 2). Starch contents linearly increased with time only in wild-type and tpt-2 mutant plants and approached levels of ~100 μmol g–1 fresh weight (FW) at the end of day 2 (i.e. after 38h; Fig. 2A). The soluble sugars sucrose (Suc), glucose (Glc), and fructose (Fru) dramatically increased in adg1-1 within the first 8h and 38h after LL/HL transfer, but showed only a transient increase in wild-type, tpt-2, and adg1-1/tpt-2 plants (Fig. 2B–D). Significant differences were observed for most time points only between adg1-1 and the other lines (see Table 2 of Supplementary Document S1 available at JXB online). The transient accumulation of carbohydrates in all lines reflects an increased capacity to assimilate CO2 under HL conditions. However, in adg1-1/tpt-2, a prolonged time in HL led to a depletion of soluble sugars (compare Schmitz et al., 2012).
Fig. 2.
Short-term responses of leaf carbohydrate contents upon LL/HL transfer of wild-type and mutant plants. Col-0 (circles), tpt-2 (squares), adg1-1 (triangles), and adg1-1/tpt-2 (diamonds) plants were grown for 3 weeks in LL under long-day conditions. The plants were transferred to HL at t=0 (4h in the light). The contents of starch (A), Suc (B), Glc (C), and Fru (D) have been monitored within the first 480min (8h; left panel) and after 38h (right panel). In (E), anthocyanin accumulation in wild-type and mutant plants is displayed during the first 32h after transfer from LL to HL and is compared with steady-state levels of anthocyanins in HL-grown plants. The data represent the mean ±SE of n=5 replicates. In some cases, the error bars are smaller than the symbols. (This figure is available in colour at JXB online.)
LL/HL transfer also resulted in anthocyanin accumulation in wild-type and adg1-1 plants (Fig. 2E), which was, however, less pronounced in tpt-2 and adg1-1/tpt-2. Anthocyanin levels in HL-grown plants (most pronounced in adg1-1) appeared to be directly correlated with soluble carbohydrates (Fig. 2B–D, right panel). Suc is the most prominent inducer of anthocyanin production involving the MYB75/PAP1 (Teng et al., 2005) and HY5 transcription factors (Shin et al., 2013) as well as the COP1/SPA ubiquitin ligase (Maier et al., 2013). For further statistical data analyses, see Table 2 of Supplementary Document S1 available at JXB online.
Lhcb1 expression and MgProtoIX levels were not correlated in response to LL/HL transfer
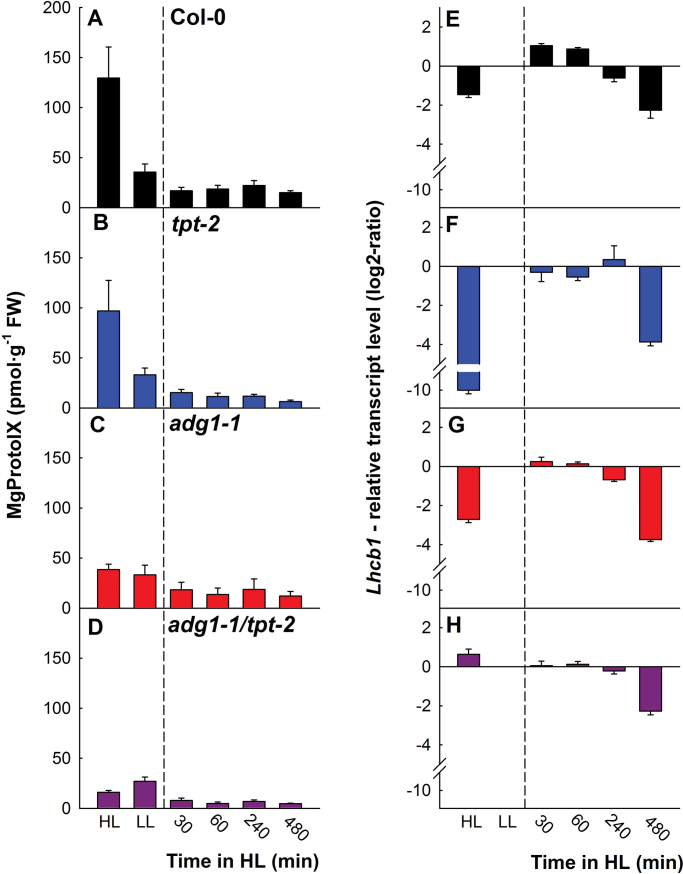
The Chl biosynthesis intermediate, MgProtoIX, was proposed to act as a retrograde signal (Susek et al., 1993; Strand et al., 2003), although this idea has recently been challenged by more refined determinations of tetrapyrroles and the lack of correlation of their content with the abundance of Lhcb1 transcripts (LHCII; Mochizuki et al., 2008; Moulin et al., 2008). Changes in MgProtoIX contents and Lhcb1 expression levels in mutant and wild-type plants in response to short- and long-term HL exposure (Fig. 3) lacked any direct correlation (Supplementary Fig. S1A available at JXB online). In all plants, MgProtoIX initially dropped to lower levels upon HL exposure compared with LL-grown plants (Fig. 3A–D). Long-term HL exposure resulted in higher levels of MgProtoIX in wild-type and tpt-2 plants (Fig. 3A, B), but not in adg1-1/tpt-2 (Fig. 3D), and was less pronounced in adg1-1 (Fig. 3C). Likewise, apart from small differences within the first 60min after LL/HL transfer, the short-term response of Lhcb1 expression was similar between all plant lines (Fig. 3E–H). However, while Lhcb1 expression was suppressed to a different degree in HL-grown wild-type and both single mutant plants (Fig. 3E–G), it was slightly induced in adg1-1/tpt-2 (Fig. 3H). These data further indicate that acclimation to HL is perturbed in the double mutant. A statistical analysis of the data shown in Fig. 3 is given in Table 3 of Supplementary Document S1 available at JXB online.
Fig. 3.
Contents of MgProtoIX (A–D) and transcript levels of Lhcb1 (E–H) in Col-0 wild-type plants, the tpt-2 and adg1-1 single mutants, and the adg1-1/tpt-2 double mutant grown either continuously in HL or LL or at different time intervals after LL/HL transfer. (This figure is available in colour at JXB online.)
Although MgProtoIX did not correlate with Lhcb1 expression, there was a pronounced inverse correlation of Lhcb1 transcript levels with soluble carbohydrate contents in wild-type and mutant plants (Supplementary Fig. S1E available at JXB online). Hence, carbohydrates might play a profound role in the transcriptional acclimation response of A. thaliana. In contrast, MgProtoIX levels were directly correlated with the light reaction of photosynthesis (i.e. ETR; Supplementary Fig. S1F available at JXB online) rather than with products of the dark reaction (Supplementary Fig. S1B, C available at JXB online).
Redox components were similarly affected in mutant and wild-type plants after LL/HL transfer
Light stress leads to the production of ROS, which can cause severe damage to membranes and the photosynthetic apparatus (Apel and Hirt, 2004; Vass, 2012). Besides ROS with a short lifetime such as singlet oxygen (Krieger-Liszkay, 2005), those with a longer lifetime suh as, for instance, H2O2, would have the potential to leave the chloroplast and hence to act as a retrograde signal (Noctor et al., 2000; Karpinski et al., 2013). As H2O2 can also be produced in the peroxisomes during photorespiration and in the apoplast, the mesophyll has to distinguish between different sources of H2O2 signals (Shapiguzov et al., 2013).
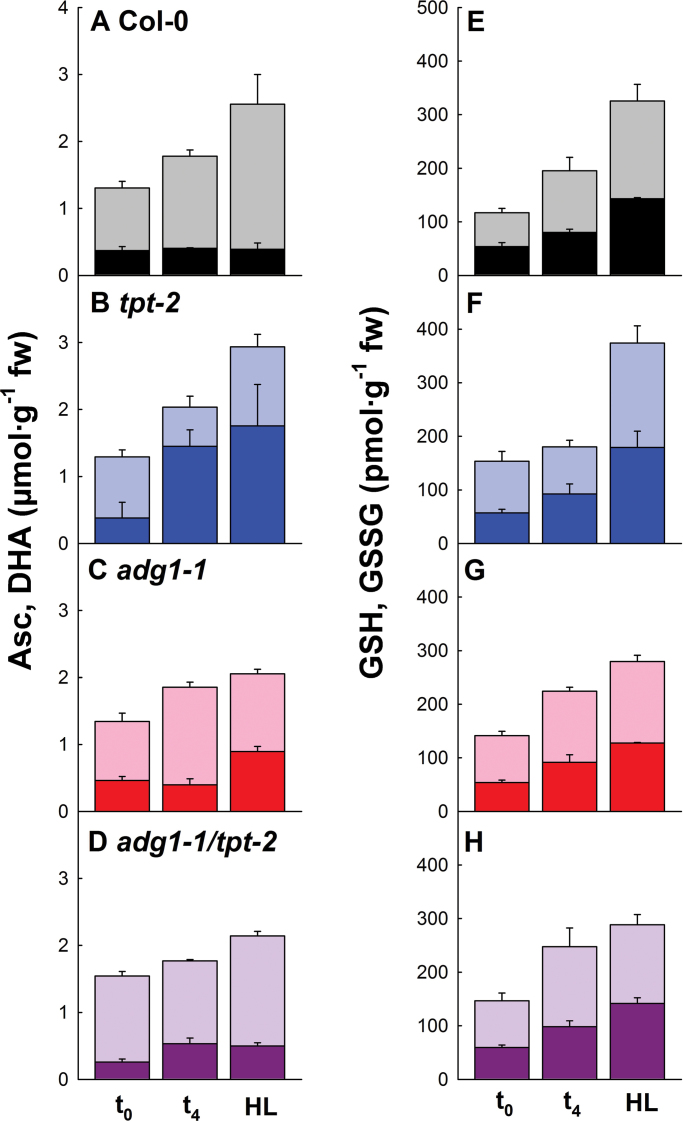
The major H2O2 source in photosynthesis is the Mehler–peroxidase reaction or ‘pseudo cyclic’ electron transport (Badger et al., 2000). H2O2 can be detoxified by either the Asc–GSH cycle (Halliwell–Asada pathway; Noctor and Foyer, 1998) or the Asc-independent thiol peroxidase pathway (Dietz et al., 2006). Asc and DHA as well as GSH and GSSG levels after LL/HL transfer lack large differences in adg1-1/tpt-2 (Fig. 4D, H) compared with wild-type plants (Fig. 4A, E). All plant lines show an increase in the total contents of both redox components depending on the time in HL. Significant changes in Asc and DHA levels were observed in the tpt-2 mutant, which resulted in a drop of the Asc/DHA ratios below one (Fig. 4B). This was, however, not reflected in large changes in GSH/GSSG ratios (Fig. 4F).
Fig. 4.
Contents of redox components in Col-0 wild-type (A, E), tpt-2 (B, F) and adg1-1 (C, G) single mutants, and adg1-1/tpt-2 (D, H) double mutant plants grown in LL (t 0) or HL, or 4h after LL/HL transfer (t 4h). The reduced compounds of the ascorbate (Asc)/dehydroascorbate (DHA) system (D) and the glutathione (GSH)/glutathione disulphide (GSSG) system (E–H) are represented by the lighter portions of the bars, whereas the oxidized compounds are marked by the darker portions. The data represent the mean ±SE of 3–5 replicates. (This figure is available in colour at JXB online.)
Although the exposure of LL-grown adg1-1/tpt-2 plants to HL resulted in severe photoinhibition, the redox components involved in the detoxification of ROS were hardly altered, most probably because photoinhibition also restricts the production of ROS at the thylakoid membrane. Hence, differential redox signalling as a cause of the phenotype of adg1-1/tpt-2 appears to be unlikely. For statistical analyses, see Table 4 of Supplementary Document S1 available at JXB online.
Assessment of transcriptomic changes in response to LL/HL transfer of mutants impaired in carbohydrate metabolism
The role of impaired carbon partitioning in HL acclimation has been assessed in wild-type and mutant plants by microarray-based transcriptomics in time series upon LL/HL transfer. Suitable time points for sampling were obtained from experiments shown in Figs 1 and 2, and were LL (t 0), as well as 4h (t 4h; as short-term response) and 48h (t 48h; as long-term response) after transfer to HL. At the latter time point, the ETR in wild-type and single mutant plants started to increase in response to HL exposure (Fig. 1B).
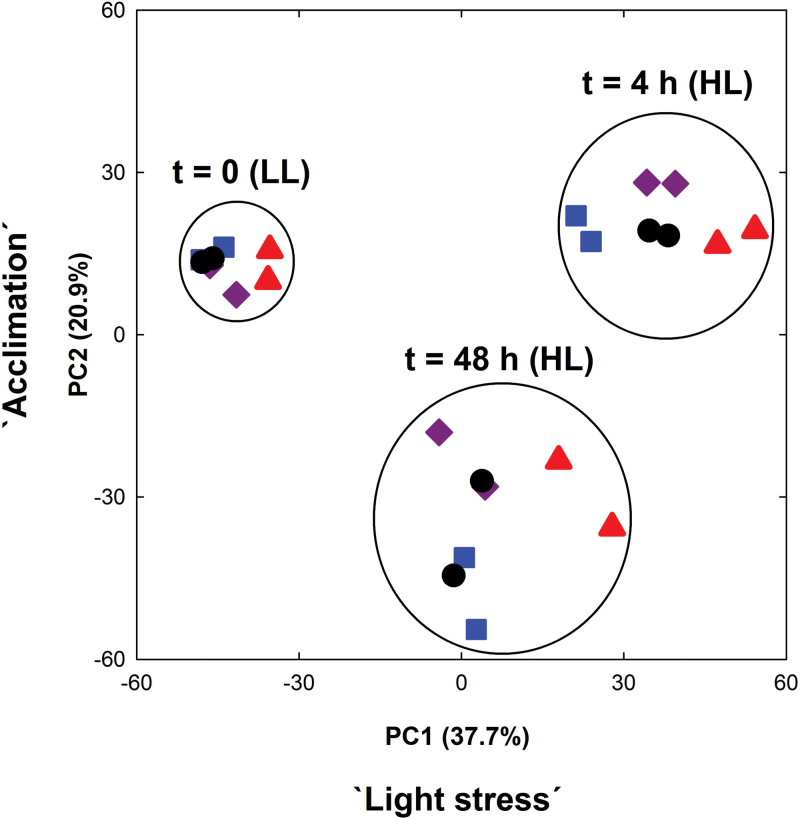
A multivariate analysis of the data sets (PCA; significance level, P>0.01; no threshold) revealed three individual clusters that were governed by the light regime and/or the time in HL rather than by metabolic defects of the respective mutants (Fig. 5). The microarray data were further analysed by ‘static’ and ‘dynamic’ comparisons of gene expression. For the ‘static’ approach (for details, see Supplementary Document 2 available at JXB online), expression levels in the mutants were referred to wild-type plants at each time point. Numbers of significantly altered genes are shown in the Venn diagrams (Fig. 6) and in more detail in Supplementary Tables S2–S4 available at JXB online. For a dynamic assessment of HL-responsive genes in wild-type and mutant plants, their expression level at t 4h and t 48h after LL/HL transfer were referred to t 0 (Fig. 7; Supplementary Tables S5, S6 available at JXB online).
Fig. 5.
Multivariate analysis (principle components) of the microarray data sets of LL/HL transfer experiments with Col-0 wild-type (circles), the tpt-2 (squares) and adg1-1 (triangles) single mutants, and the adg1-1/tpt-2 double mutant (diamonds). (This figure is available in colour at JXB online.)
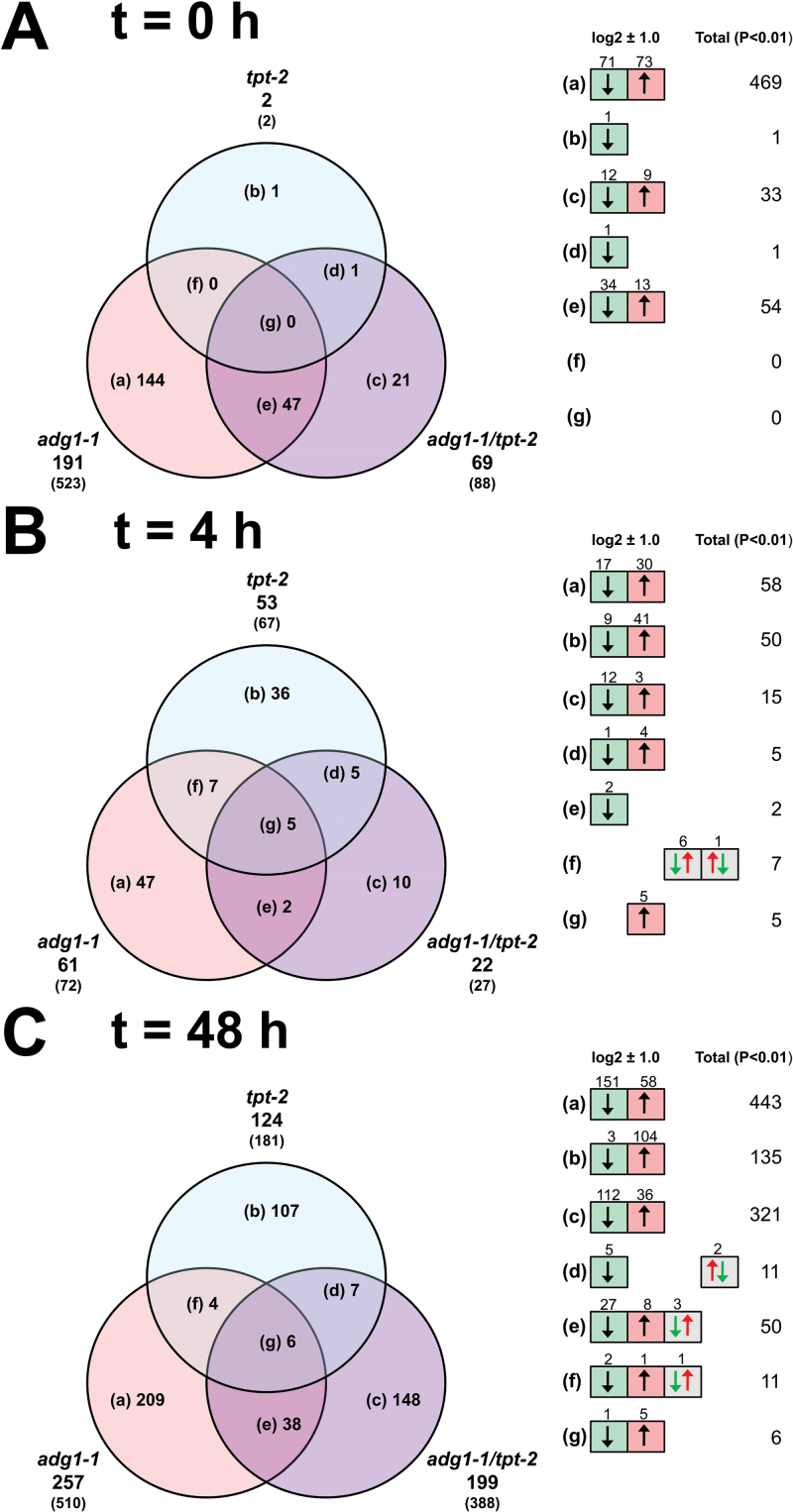
Fig. 6.
Venn diagrams showing ‘static’ comparisons of differentially regulated genes between mutant and wild-type plants at t=0h (A), t=4h (B), and t=48h (C). Genes that were significantly (P<0.01) altered at a threshold of log2 ratios of ±1.0 are displayed. The numbers in parentheses and in the right panel represent total numbers of significantly altered genes without any threshold. (This figure is available in colour at JXB online.)
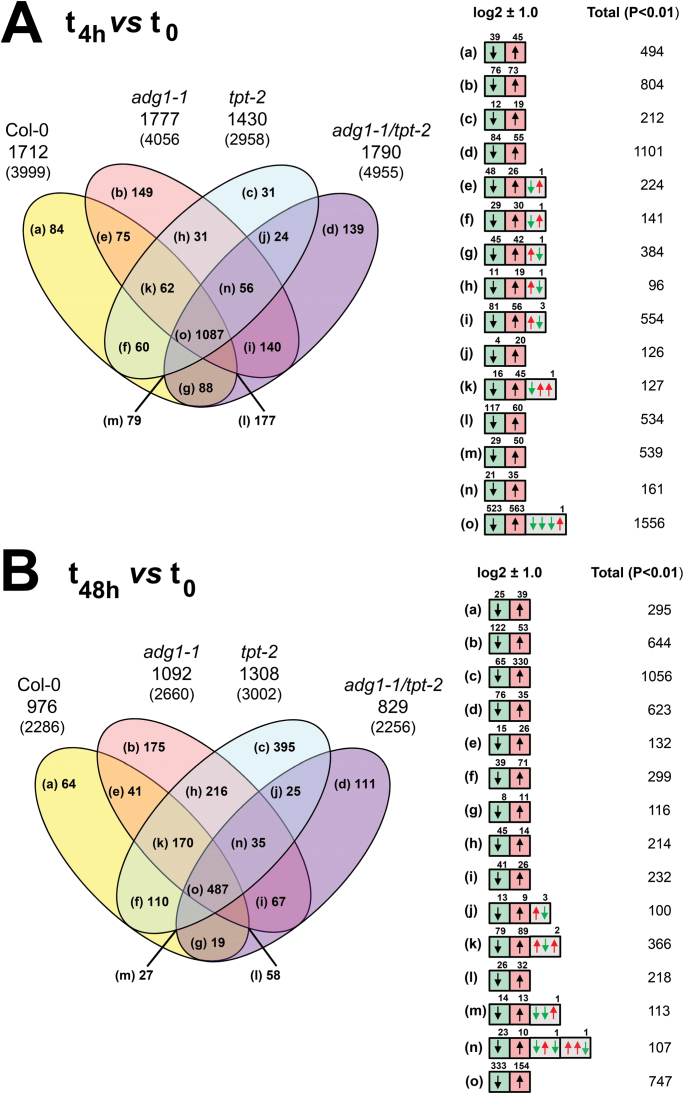
Fig. 7.
Venn diagrams showing ‘dynamic′ comparisons of differentially regulated genes in wild-type and mutant plants at t 4h versus t 0 (A) and t 48h versus t 0 (B). Genes that were significantly (P<0.01) altered at a threshold of log2 ratios of ±1.0 are displayed. The numbers in parentheses and in the right panel represent total numbers of significantly altered genes without any threshold. The number of genes differently regulated at t 4h (A) and t 48h (B) in HL compared with t 0 in LL are displayed. (This figure is available in colour at JXB online.)
Static assessment of global gene expression after LL/HL transfer
Under LL conditions, 523 genes were significantly altered in adg1-1 compared with 88 in adg1-1/tpt-2 and only two in tpt-2 (Fig. 6A). After 4h in HL, the number of differentially regulated genes dropped to lower values in adg1-1 and adg1-1/tpt-2 (Fig. 6B), but increased again after 48h of HL exposure (Fig. 6C). In tpt-2, the number of altered genes increased from 67 to 181 after 4h and 48h, respectively, in HL. However, the highest number was again found in adg1-1 (510) rather than in adg1-1/tpt-2 (388). The number of commonly regulated genes in the central overlapping regions of the Venn diagrams was small, suggesting that the alterations in gene expression compared with wild-type plants observed in the ‘static approach’ were related to the individual lesions in the mutants rather than to changing conditions. Static changes in expression of HL-responsive genes have been analysed and described in more detail in Supplementary Document S2 and in Supplementary Tables S2–S4 available at JXB online. Functional categories containing more than five genes are shown in Table 1. In adg1-1/tpt-2, significantly over-represented genes of the categories ‘amino acid metabolism’, ‘cell wall’, and ‘CHO metabolism’ were down-regulated at t 48h after LL/HL transfer, whereas over-represented plastome-encoded genes belonging to the categories ‘protein – synthesis′ and ‘PS – light reaction’ were up-regulated. Remarkably, in adg1-1/tpt-2, MYC4 (At4g17880), a basic helix–loop–helix (bHLH)-type transcription factor involved in jasmonate responses (Fernández-Calvo et al., 2011) and glucosinolate biosynthesis (Schweizer et al., 2013), was severely down-regulated in both LL and HL. According to the eFP browser (Winter et al., 2007), MYC4 is also inducible by sugars.
Table 1.
Distribution of functional categories following the ‘static’ assessment of expression profiles of adg1-1, tpt-2, and adg1-1/tpt-2 versus wild-type plants at t 0 (LL) and at t 4h and t 48h after LL/HL transferOnly functional categories with five or more altered genes are displayed.
| Functional categories | adg1-1 versus Col-0 | tpt-2 versus Col-0 | adg1-1/tpt-2 versus Col-0 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t 0 | t 4h | t 48h | t 4h | t 48h | t 48h | |||||
| Up | Down | Up | Down | Up | Down | Up | Up | Up | Down | |
| Amino acid metabolism | – | – | – | – | – | – | – | – | – | 5 (4.4)* |
| Cell wall | – | – | – | – | – | – | – | 6 (2.7)* | – | 11 (4.6)* |
| CHO metabolism | – | – | – | – | 5 (8.7)* | – | – | – | – | 6 (5.4)* |
| Development | 5 (2.4) | – | – | – | – | – | – | – | – | – |
| Hormone metabolism | – | – | – | – | – | – | – | 6 (2.8)* | – | – |
| Lipid metabolism | – | – | 5 (10.2)* | – | – | 7 (2.8)* | – | – | – | – |
| Protein – degradation | – | 11 (2.3)* | – | – | – | 14 (1.4) | 6 (2.6)* | 5 (0.7) | – | – |
| Protein – synthesis | – | – | – | – | 7 (5.2)* | – | – | – | – | – |
| Protein – synthesis (plastome) | – | – | – | – | – | – | – | – | 5 (140)* | – |
| PS – light reaction (plastome) | – | – | – | – | – | – | – | – | 16 (129)* | – |
| RNA – regulation of transcription | – | – | – | – | 6 (1.2) | 21 (1.6) | – | 6 (0.7) | – | 9 (0.9) |
| Signalling | – | – | – | – | – | 7 (0.9) | – | 10 (1.7) | – | – |
| Stress | – | – | – | 5 (8.1)* | – | 11 (2.0)* | – | 12 (3.2)* | – | – |
| Transport | – | – | – | – | – | 5 (0.8) | – | 8 (1.9) | – | 5 (1.1) |
The numbers of significantly (P<0.01) altered genes were taken from Supplementary Tables S2–S4 available at JXB online.
The threshold of log2 ratios was kept at ±1.0.
The data in parentheses refer to over- or under-represented functional categories (i.e. numbers above or below 1).
Significantly (P<0.05) over- or under-represented categories according to Fisher’s exact test are marked by an asterisk.
Over-represented categories are shown in bold.
Dynamic changes in gene expression during LL/HL transfer experiments
Compared with the static assessment of global gene expression, which aimed at identifying differentially regulated genes between mutant and wild-type plants at each time point, the dynamic analysis displayed genes particularly responding to the LL/HL transfer and is therefore described in more detail.
Interestingly, 4h after HL exposure, the overlapping areas of all plants in this study comprised the highest number (1087) of significantly altered genes (Fig. 7A), which also showed a relatively high amplitude of differential expression (i.e. log2 ratios of ±3; Supplementary Table S5 available at JXB online). This observation indicates that the short-term response of HL-dependent regulation of a large set of genes was operational in wild-type and mutant plants. After 48h in HL, the number of genes in the central overlapping region of the Venn diagrams dropped by ~50% compared with t 4h (Fig. 7B; Supplementary Table S7B available at JXB online), but increased specifically in tpt-2 from 31 to 395 genes at t 4h and t 48h, respectively. Compared with tpt-2, the number of altered genes was appreciably smaller in adg1-1 (175) and adg1-1/tpt-2 (111).
Strategies for functional analyses of HL-responsive genes
Different strategies were applied to obtain additional information on HL-responsive genes in wild-type and mutant plants. The following observations and assumptions have been addressed. (i) It can be assumed that commonly regulated HL-responsive genes in all lines of this study should be independent from metabolic constraints by individual mutations. (ii) In contrast, specifically regulated or non-regulated genes after LL/HL transfer should be linked to the metabolic constraints in the individual mutant plants. Finally, (iii) comparisons of in-house with publicly available microarray data on hormone and stress treatments might help to identify components associated with signalling pathways involved in HL acclimation.
Wild-type and mutant plants comprise a large set of commonly regulated genes in response to LL/HL transfer
The central regions of the Venn diagrams shown in Fig. 7 comprise genes responding similarly to HL exposure in wild-type and mutant plants and can hence be considered as independent or less dependent than lesions in primary carbohydrate metabolism. These genes are listed in Supplementary Table S7 available at JXB online. The number of genes was narrowed down by adjusting the threshold log2 ratio to ±1.5. Under HL conditions, 562 and 237 genes were differentially regulated 4h and 48h after LL/HL transfer, respectively (Supplementary Table S7A, B available at JXB online). The distribution of functional categories comprising five or more genes per category is shown in Table 2.
Table 2.
Enrichment of functional categories among the commonly regulated genes in wild-type and mutant plants as a response to LL/HL transfer at t 4h versus t 0 and t 48h versus t 0The threshold of log2 ratios was kept at ±1.5. Only functional categories with five or more altered genes are displayed.
| Functional categories | No. of commonly regulated genes | |||
|---|---|---|---|---|
| t 4h versus t 0 | t 48h versus t 0 | |||
| Up-regulated | Down-regulated | Up-regulated | Down-regulated | |
| Cell wall | – | 16 (2.65)* | – | 11 (3.05)* |
| Development | 9 (1.12) | 7 (0.85) | 5 (2.58) | 5 (1.02) |
| DNA synthesis/chromatin structure | 9 (0.96) | – | – | |
| Hormone metabolism | 5 (0.87) | 13 (2.20)* | – | 14 (3.96)* |
| Lipid metabolism | 7 (1.54) | 10 (2.15)* | – | 7 (2.51)* |
| Major CHO metabolism | 11 (8.87)* | – | – | – |
| Misc. – Cytochrome P450 | 5 (2.01) | – | – | – |
| Misc. – (UDP glucosyl- and glucoronyl transferases) | 5 (2.48) | – | – | – |
| Protein – degradation | 11 (0.59)* | 21 (1.10) | – | 10 (0.88) |
| Protein – post-translational modification | – | 10 (1.18) | – | 6 (1.18) |
| Protein – synthesis | 8 (1.24) | – | – | – |
| Protein – targeting | 6 (1.97) | – | – | – |
| RNA – processing | 9 (2.89)* | – | – | – |
| RNA – regulation of transcription (RT) | 30 (1.24) | 44 (1.78)* | – | 22 (1.49) |
| Secondary metabolism – flavonoids | 5 (5.18)* | – | 11 (47.32)* | – |
| Signalling | 8 (0.55)* | 21 (1.42) | – | 15 (1.69) |
| Stress – abiotic | 10 (2.16)* | 9 (1.91) | – | 5 (1.77) |
| Stress – biotic | 6 (1.12) | – | – | – |
| Transport | 14 (1.22) | 18 (1.54) | 7 (2.54)* | 11 (1.57) |
The numbers of significantly (P<0.01) altered genes were taken from Supplementary Table S7 available at JXB online.
The threshold of log2 ratios was kept at ±1.5.
The data in parentheses refer to over- or under-represented functional categories (i.e. numbers above or below 1).
Significantly (P<0.05) over- or under-represented categories according to the Fisher’s exact test are marked by an asterisk.
Over-represented categories are shown in bold letters, whereas under-represented categories are in italics.
Strikingly, the diversity of functional categories was larger at t 4h versus t 0 compared with t 48h versus t 0. In particular, the number of categories containing up-regulated genes was higher at t 4h (i.e. 17 categories) compared with t 48h (i.e. three categories) after LL/HL transfer. In both cases the category ‘unknown’ has been excluded. At t 4h versus t 0, amongst the over-represented categories ‘major CHO metabolism’, ‘secondary metabolism – flavonoids’, ‘RNA processing’, and ‘abiotic stress’ were up-regulated, whereas ‘cell wall’, ‘hormone’, ‘lipid metabolism’, and ‘regulation of transcription (RT)’ were down-regulated (Table 2). With the help of the above approach, it was possible to dissect the data into a large variety of short-term, HL-responsive genes and a limited number of categories that respond in the long term to HL exposure (Table 2).
Genes associated with ‘RT’, ‘signalling’, ‘abiotic stress’, or ‘biotic stress’, as well as ‘hormone metabolism’ can be considered as candidates that might be involved in retrograde signalling. There were 30 genes belonging to the ‘RT’ category up-regulated and 45 down-regulated at t 4h versus t 0 compared with four up- and 22 down-regulated genes at t 48h versus t 0. Strikingly, none of the up-regulated and only 13 of the down-regulated RT genes was found at both t 4h and t 48h upon HL exposure, suggesting that this dynamic response occurred only transiently (Supplementary Table S7 available at JXB online).
Likewise, the expression of genes belonging to the categories ‘signalling’ as well as ‘abiotic stress’ or ‘biotic stress’ was altered to a larger extent at t 4h compared with t 48h, and, again, only a small number of genes belonging to these categories was found at both t 4h and t 48h. The category ‘hormone metabolism’ comprises genes coding for response regulators as well as metabolic enzymes. At t 4h versus t 0, this category contained a broad spectrum of phytohormones, such as ABA, gibberellic acid, brassinosteroids, cytokinins, and jasmonate, whereas at t 48h versus t 0, 13 of the 14 down-regulated genes of the ‘hormone’ category were confined to auxins and only one to ethylene. For further information, see Supplementary Table S7 available at JXB online.
Primary carbon metabolism and transport were commonly affected in the short term after HL exposure in all lines
A number of metabolic genes associated with ‘lipid metabolism’, ‘major CHO metabolism’, and ‘transport’ were differentially regulated as a response to LL/HL transfer, specifically in the short term, namely at t 4h (Table 2). The detailed analysis of these genes, which is contained in Supplementary Document S3 available at JXB online, can be summarized as follows: most of the 11 genes associated with ‘major CHO metabolism’ are involved in starch metabolism (see also Walters et al., 2004). The transcriptional regulation of these genes is obviously independent from the presence or absence of starch, as it also works in the adg1-1 background. An analysis with ATTEDII (Obayashi et al., 2009) revealed that these genes are imbedded in a single regulatory network (Supplementary Fig. S2A, Supplementary Table S7A available at JXB online), which also comprises a chloroplast-localized AMP-activated protein kinase (At5g39790; Supplementary Table S7A available at JXB online). There appears to be a cross-talk between ‘CHO metabolism’ and ‘lipid metabolism’ as three members of this network are also contained in a network of up-regulated genes associated with ‘lipid metabolism’ (Supplementary Fig. S2B available at JXB online). Likewise, down-regulated genes involved in ‘lipid metabolism’ at t 4h versus t 0 (but not at t 48h versus t 0) form a large network (Supplementary Fig. S2C available at JXB online).
Inter- and intracellular transport processes are of particular importance in the adjustment of metabolism to the requirement of the plants. Among 32 differentially regulated genes associated with ‘transport processes’, the expression of two genes belonging to the phosphate translocator family was altered after 4h in HL. The glucose 6-phosphate/phosphate translocator2 (GPT2; At1g61800) and the phosphoenolpyruvate/phosphate translocator2 (PPT2; At3g01550) were up- and down-regulated, respectively. Interestingly, GPT2 strongly responds to elevated soluble sugar levels (Kunz et al., 2010; Schmitz et al., 2012), for example in starch-free mutants or after feeding of exogenous carbohydrates to the plants (Heinrichs et al., 2012). Although GPT2 was highly up-regulated at t 4h versus t 0 in all plants (Supplementary Table S7A available at JXB online), its expression level at t 48h versus t 0 corresponded well to the sugar levels in the individual plant lines; that is, GPT2 expression was further increased in adg1-1, but was not significantly changed in the double mutant (compare Fig. 1). For more information, please refer to Supplementary Document S3 available at JXB online.
Does the mutant-specific regulation or non-regulation of genes provide information on missing signals?
Here the question was asked of whether the analysis of genes specifically regulated or not regulated in the individual mutant plants in response to LL/HL transfer can provide information on the putative retrograde signals involved.
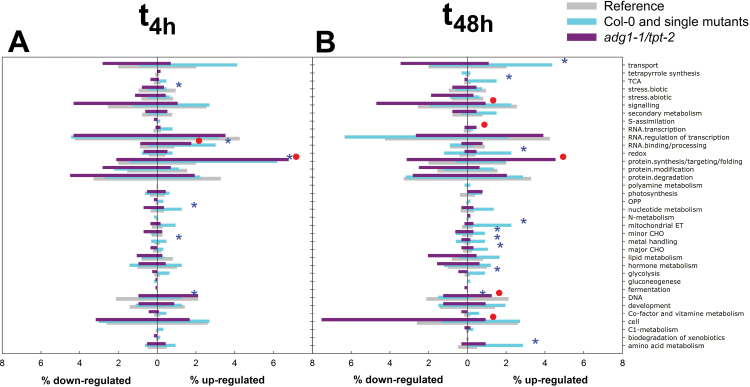
In a first approach, the intention was to identify significantly (Fisher’s exact test; P<0.05) over- or under-represented functional groups or clusters according to MapMan bins (Thimm et al., 2004). Significantly altered (P<0.01) HL-responsive or non-responsive genes in the adg1-1/tpt-2 double mutant (i.e. those genes responding to HL only in wild-type and single mutant plants) were categorized without threshold (compare Fig. 7). The major outcome of this approach is shown in Fig. 8 and can be summarized as follows: the MapMan binning of the expression data resulted in 36 major functional categories. In the short term (i.e. 4h after LL/HL transfer; Fig. 8A), genes belonging to the categories ‘RNA binding/processing’ and ‘protein synthesis/targeting/folding’ were significantly over-represented in both groups, regulated and non-regulated genes, in adg1-1/tpt-2. All other significant alterations can be attributed to genes that were not differentially expressed in adg1-1/tpt-2, and these were the under-represented categories ‘biotic stress’ and ‘DNA’ as well as the over-represented categories ‘nucleotide metabolism’ and ‘metal handling’. In the long term (i.e. 48h after LL/HL transfer; Fig. 8B), the pattern of over- or under-represented functional categories was different compared with t 4h. Only three out of 36 functional categories were specifically altered in the double mutant according to their relative abundance. Interestingly, in the categories ‘signalling’ and ‘cell’, down-regulated genes were over-represented (Fig. 8B), whereas the category ‘RNA – transcription’ contained over-represented up-regulated genes. There were also some interesting changes in the relative abundance of genes that were specifically not regulated in adg1-1/tpt-2. Nine out of the 36 functional categories contained over-represented up-regulated genes in Col-0 and the single mutants related to ‘transport’, ‘tricarboxylic acid cycle’, ‘redox’, ‘mitochondrial electron transport’, ‘minor CHO metabolism’, ‘major CHO metabolism’, ‘metal handling’, ‘glycolysis’, and ‘amino acid metabolism’, suggesting that these processes are less responsive in the double mutant.
Fig. 8.
MapMan binning-based distribution of up- and down-regulated genes in individual functional categories 4h (A) and 48h (B) after LL/HL transfer. The reference value (light grey bars) refers to the number of genes belonging to a certain category expressed as a percentage of all genes on the microarray, whereas intermediate grey and dark grey bars refer to the number of up- or down-regulated genes belonging to a certain category as a percentage of all differentially regulated genes. Intermediate grey bars refer to all genes not regulated in adg1-1/tpt-2 (i.e. genes regulated in Col-0 and the single mutants) and dark grey bars refer to genes specifically regulated in adg1-1/tpt-2. Significant differences (P<0.05) in the relative distribution amongst the individual categories were estimated by Fisher’s exact test and marked by either asterisks or circles for intermediate grey or dark grey bars, respectively. (This figure is available in colour at JXB online.)
In a second approach, genes specifically responding to the constraints in the single and double mutants were separated and are listed in Supplementary Table S8 available at JXB online. For this purpose, the log2-fold change for genes assigned as non-regulated was limited to ±0.5. In particular, adg1-1/tpt-2 exhibited a strong phenotype upon HL exposure and hence further attention was focused on this line (compare MapMan binning, Fig. 8). At t 4h versus t 0 adg1-1/tpt-2 contained five up- and nine down-regulated genes (Supplementary Table S8A available at JXB online). Three of the five up-regulated genes were associated with histone proteins and hence ‘chromatin structure’ and ‘cell organization’. Besides a gene of unknown function (At2g30600), a methylthioalkylmalate synthase gene (At5g23010) involved in methionine-derived glucosinolate biosynthesis (Textor et al., 2004) was up-regulated. Interestingly, of the nine down-regulated genes, four genes were associated with ‘biotic stress’ (three of these genes encode heat shock proteins; At1g53540, At3g46230, and At5g12020, which have also been recognized in the static approach; compare Supplementary Document S2 available at JXB online) and one gene each was linked to ‘calcium signalling’ (At2g41090), ‘RT’ (At5g61010), ‘mitochondrial electron transport’ (At4g05020), ‘minor CHO metabolism’ (At2g37760), and ‘lipid metabolism’ (At2g44300). In particular, heat shock proteins represent the interaction point of various stress response pathways (Swindell et al., 2007) and deserve further attention also with respect to retrograde signalling. In the category ‘RT’ an Exo70 type subunit of an exocytosis complex was down-regulated. Such organelle-like structures are involved in excretion processes by fusion with the plasma membrane (Li et al., 2010; Wang et al., 2010).
At t 48h versus t 0 there were five and six genes up- or down-regulated specifically in adg1-1/tpt-2 (Supplementary Table S8C available at JXB online). Amongst the up-regulated genes, plastome-encoded ribosomal proteins (two genes) and one gene encoding RNA polymerase were found. The further two up-regulated genes were associated with either ‘amino acid degradation’ (At3g16150) or ‘RT’, namely the heat shock transcription factor A9 (At2g26150), which under normal conditions is only weakly expressed in leaves (eFP browser, Winter et al., 2007), again bringing members of heat shock proteins or related transcription factors into focus.
Genes specifically not responding to HL exposure in the double mutant, but responding in the wild type and the single mutants, might provide information on missing signals for induction or repression of these genes. At t 4h versus t 0 only six HL-non-responsive genes were found in adg1-1/tpt-2 (Supplementary Table S8B available at JXB online). In the wild type and both single mutants, the three up-regulated genes are non-characterized transporters (At4g13800 and At5g26220) or are involved in developmental processes (At1g77450). The three down-regulated genes are associated with ‘cell wall degradation’ (At1g02640), ‘redox’ (At3g62950; i.e. a putative glutaredoxin), and a trehalose phosphatase/synthase (TPS) 9 (At1g23870; Leyman et al., 2001). A member of the same gene family (TPS1) is most probably involved in the initiation of flowering (Wahl et al., 2013). There is, however, no direct link to the metabolic lesion in the mutant plant or to the putative signals involved. Likewise, the functional categories of genes not responding to HL in the double mutant are rather heterogeneous.
Further analyses of specifically regulated or non-regulated genes in the single mutant and the wild type are also presented in Supplementary Table S8 available at JXB online. Remarkably there were 87 genes specifically up-regulated in the tpt-2 mutant 48h after LL/HL transfer. Of these genes, at least five genes were associated with Ca2+-dependent signalling and four with glutathione-S-transferases. In wild-type plants, the expression of only a low number of genes was specifically altered (Supplementary Table S8M, N available at JXB online).
Are sugar-, phytohormone-, or oxidative stress-related signals involved in HL acclimation of A. thaliana?
The contribution of putative retrograde signals to global gene expression was assessed by comparing in-house with publicly available microarray data. Genes found to be HL responsive in the experimental set-up were compared with those genes regulated by elevated ‘sugar levels’, ‘oxidative stress’, or ‘hormones’ (for details, see Supplementary Tables S9 and S10 available at JXB online). Elevated sugar levels either were achieved by feeding A. thaliana seedlings with 3% Suc in light series (Michael et al., 2008) or were obtained endogenously in the pho3 mutant defective in SUT2, namely the Suc transporter responsible for phloem loading (Lloyd and Zakhleniuk, 2004). Oxidative stress based on singlet oxygen (1O2) and 1O2-dependent products from lipid or carotenoid oxidation (Shapiguzov et al., 2013) can be observed in the flu mutant (op den Camp et al., 2003). The FLU protein inhibits an early step in tetrapyrrole biosynthesis and thereby prevents the accumulation of the Chl precursor protochlorophyllide (Meskauskiene et al., 2001). The application of the herbicide methyl viologen (MV) to A. thaliana leads to the accumulation of H2O2 (A. Ditzer, D. Bartels, and H.-H. Kirch; NASCARRAYS-143; op den Camp et al., 2003). The effect of the phytohormones ABA, indole acetic acid (IAA), the cytokinin trans-zeatin (tZ), gibberellic acid (GA3), the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), and methyl jasmonate (MJ) on global gene expression has been studied by Goda et al. (2008).
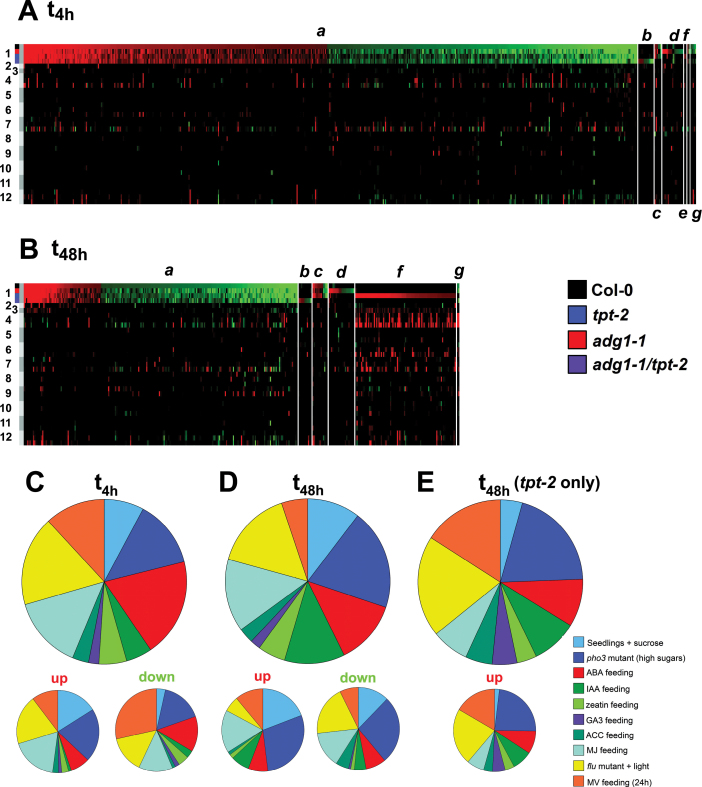
The major outcome of the microarray comparisons is summarized in Table 3, as well as in Fig. 9 and in Supplementary Fig. S3 available at JXB online. Genes responsive to external Suc feeding or enhanced endogenous carbohydrate levels in the pho3 mutant represent a large portion of the commonly de-regulated genes shown in Fig. 9. Remarkably, the overlap of genes responsive to Suc feeding and in the pho3 mutant was rather low (i.e. two out of 81 genes at t 4h and four out of 70 genes at t 48h). Still, the direction of regulation (i.e. up or down) of HL- and sugar-responsive genes coincided to a high degree. More than 50% of the commonly up-regulated genes in the present experiments responded to sugars in the same way (Fig. 9). Furthermore, the log2 ratios of the individual data sets correlated directly with each other and resulted in Pearson correlation coefficients well above 0.7 (Table 3). There was no such coincidence or correlation with genes responding to phytohormones such as ABA (Fig. 9; Table 3). However, genes responding to MV-induced oxidative stress exhibited a positive long-term correlation (i.e. 48h after LL/HL transfer, Table 3), although the portion of ROS-induced genes was lower compared with t 4h versus t 0 (Fig. 9). Interestingly, an entirely different approach undertaken by Oelze et al. (2012) used plants acclimated to extremely LL close to the light compensation point and their transfer to a 10- and 100-fold higher light intensity, respectively. Regulation of selected marker transcripts measured 6h after transfer to HL suggested that metabolite, redox, and lipid-derived signals are particularly important in realizing the acclimation.
Table 3.
Pearson correlation coefficients calculated for comparisons of in-house with publicly available microarray dataIn (A) a list of commonly regulated genes (Supplementary Table S7 available at JXB online) and up-regulated genes in tpt-2, 48h after LL/HL transfer (Supplementary Table S8K* available at JXB online) have been used as basis for comparison. In (B) the gene lists for comparisons were obtained from Supplementary Tables S5A–C and S6A–C (available at JXB online).
| Microarray data sets | A | t 4h all | t 48h all | t 48h tpt-2* | B | t 4h Col-0 | t 48h Col-0 | t 4h adg1-1 | t 48h adg1-1 | t 4h tpt-2 | t 48h tpt-2 | t 4h adg1-1/tpt-2 | t 48h adg1-1/tpt-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation coefficient (0 to ±1) | |||||||||||||
| Seedlings+sucrose | 0.759 | 0.901 | –0.479 | ND | ND | ND | ND | ND | ND | ND | ND | ||
| pho3 mutant | 0.866 | 0.956 | 0.191 | 0.976 | None | 0.357 | 0.601 | 0.627 | 0.322 | –0.933 | None | ||
| ABA feeding | –0.093 | 0.242 | 0.238 | –0.026 | 0.692 | 0.299 | –0.049 | 0.556 | 0.247 | –0.607 | –0.107 | ||
| IAA feeding | –0.278 | 0.061 | 0.176 | 0.282 | 0.735 | –0.063 | –0.514 | None | 0.129 | –0.407 | –0.217 | ||
| Zeatin feeding | 0.110 | –0.089 | 0.251 | –0.369 | –0.991 a | –0.722 | –0.230 | –0.589 | 0.140 | 0.386 | –0.223 | ||
| GA3 feeding | 0.249 | –0.527 | 0.808 | None | None | 0.136 | 0.641 | None | 0.190 | None | None | ||
| ACC feeding | –0.232 | 0.178 | 0.533 | 0.375 | 0.520a | 0.605 | 0.061 | None | 0.026 | 0.650 | None | ||
| MJ feeding | 0.600 | 0.609 | 0.279 | 0.874 | 0.795 | 0.113 | 0.345 | –0.589 | 0.170 | –0.234 | –0.256 | ||
| flu mutant+light | 0.374 | 0.063 | –0.077 | 0.317 | –0.497 | 0.140 | 0.041 | –0.863 | 0.353 | –0.045 | –0.090 | ||
| Wild type+MV (24h) | 0.476 | 0.725 | 0.516 | ND | ND | ND | ND | ND | ND | ND | ND | ||
The colums in (A) refer to threshold log2-fold change of ±1.5, whereas the columns in (B) are based on a threshold log2 ratio of ±1.0. Pearson correlation coefficients above/below 0.7/–0.7 are displayed in bold.
ND, not determined; none, less than three commonly regulated genes.
a Less than four genes.
Fig. 9.
Heat map presentation of up- [red (log2max=3)] and down-regulated [green (log2min= –3)] genes of in-house microarrays at t 4h versus t 0 (A) or t 48h versus t 0 (B) compared with publicly available microarrays based on Supplementary Table S8 available at JXB online. The numbers to the left indicate in-house arrays (1) with Col-0, tpt-2, adg1-1, and adg1-1/tpt-2 labelled with the colours black, blue, red, and dark purple, respectively; (2) seedlings+sucrose; (3) pho3 mutant; (4) flu mutant; (5) flu mutant+MV; and feeding of the wild type with (6) MV, (7) ABA, (8) tZ, (9) IAA, (10) ACC, (11) GA3, or (12) MJ. The small italic letters indicate (a) commonly de-regulated genes in wild-type and all mutant plants of the in-house arrays, (b) specifically altered genes in adg1-1/tpt-2, (c) specifically not regulated genes in adg1-1/tpt-2, (d) specifically altered genes in adg1-1, (e) specifically not regulated genes in adg1-1, (f) specifically altered genes in tpt-2, and (g) specifically not regulated genes in tpt-2. Relative distribution of overlaps between differentially regulated genes in public microarrays compared with altered genes in all biotypes investigated in this study at t=4h (C) and t=48h (D) after LL/HL transfer. In (E), the relative distribution of overlaps between differentially regulated genes in publicly available microarrays is compared with up-regulated genes only found in the tpt-2 mutant. The smaller pie charts refer to the relative distribution of up- or down-regulated genes between the individual experiments.
Furthermore, the positive correlation with MJ is based on the fact that parts of the sugar-responsive genes also respond to MJ (e.g. 30% of the genes differentially regulated in pho3). It appears likely, therefore, that elevated sugar levels and ROS rather than phytohormones such as ABA are the predominant factors involved in the acclimation response in A. thaliana. However, the sugar-dependent response is obviously independent from the constraints in the mutant. Analyses of differentially regulated genes in the individual lines, including wild-type plants, revealed higher contributions of phytohormones or ROS to the HL response (Table 3; Supplementary Fig. S3 available at JXB online). To increase the number of genes for these comparisons, the gene lists from Supplementary Tables S5 and S6 available at JXB online [i.e. Venn areas (a), (b), (c), and (d) depicted in Fig. 7A and B; log2 ratio ±1.0] rather than from Supplementary Table S8 available at JXB online (log2 ratio ±1.5) were used.
Surprisingly, in the tpt-2 single mutant, >25% of the highly up-regulated genes at t 48h and >30% of the moderately down-regulated genes also responded to 1O2 (Fig. 9E; Supplementary Fig. S3C available at JXB online). At t 4h there was a highly negative correlation between ROS- and HL-dependent gene expression in tpt-2 (Table 3). This overall lower responsiveness towards ROS correlates well with aberrant levels of redox components (Asc and DHA) observed in this mutant (compare Fig. 4B). Genes individually up-regulated in tpt-2 at t 48h after LL/HL transfer exhibited a good correlation with genes responsive to GA3. However, the total number of overlapping genes was low (eight genes). In wild-type plants, positive correlations of HL-responsive genes were found for sugars and MJ as well as for the phytohormone IAA, whereas tZ exhibited a negative correlation (Table 3). In the above cases as well as in the case of the negative correlation of HL- and sugar-responsive genes in the adg1-1/tpt-2 double mutant, the portion of total genes available for these analyses was rather low (Supplementary Table S10 available at JXB online).
Soluble sugars have a major impact on HL acclimation in A. thaliana
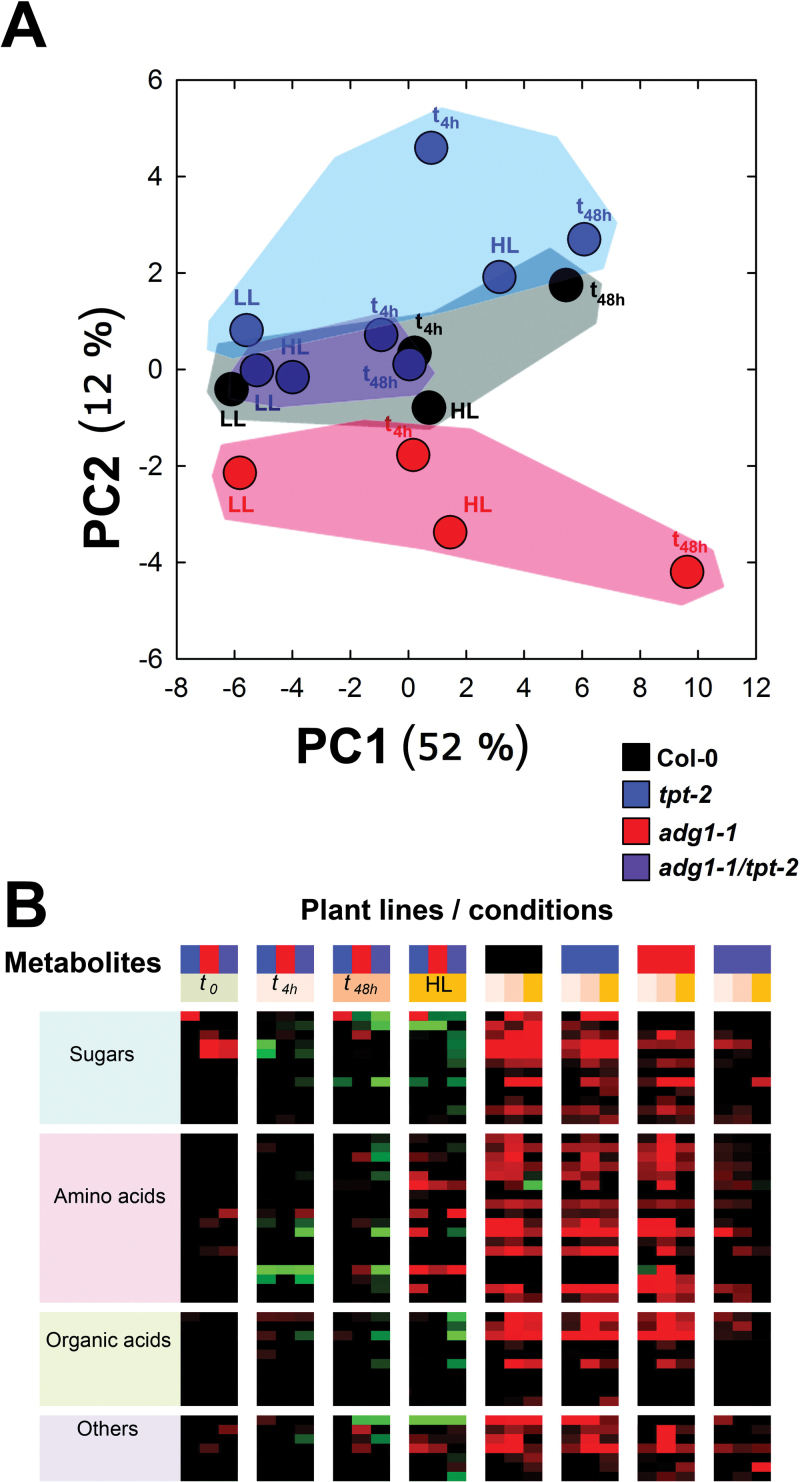
Metabolomics can help to identify retrograde signals (Caldana et al., 2012). Profiles of 47 metabolites distributed amongst three major categories such as sugars (12), amino acids (18), organic acids (10), and others (seven) have been measured for all plants in this study. The plants were either grown continuously in LL or HL or were subjected to a LL/HL transfer and samples taken at t 4h and t 48h. Figure 10 shows an overview of the metabolic profiling. More detailed data are contained in Table 4 and in Supplementary Tables S1, S11, and S12 available at JXB online. PCA (Fig. 10A) indicates that the first component of the metabolic profiles was separated based on the light conditions applied, whereas the second component was separated due to metabolic constraints in the respective mutants. Strikingly, for adg1-1/tpt-2 there was neither a large separation in the first component nor was the second component clearly separated from the wild type, again showing that the double mutant is compromised in HL acclimation. The static and dynamic assessments of metabolite heat maps (Fig. 10B) indicate (i) that at t 0 (i.e. in LL) the most pronounced increase exists for sugars, but not for amino acids or organic acids in adg1-1 versus Col-0 and adg1-1/tpt-2 versus Col-0. (ii) Upon transfer to HL, sugars and organic acids appear to be mostly decreased (most pronounced in adg1-1/tpt-1 versus Col-0), whereas amino acids show a trend of only a transient decrease (at t 4h and t 48h), but an increase at continuous growth in HL. (iii) As expected, most metabolites were elevated in Col-0 and both single mutants, at least transiently, in HL compared with LL. Strikingly, (iv) differences in metabolite contents are less marked in adg1-1/tpt-2 grown in HL compared with LL conditions.
Table 4.
Contents of selected metabolites determined either enzymatically (starch, d-Suc, d-Glc, and d-Fru) or by GC-MS in leaf extracts of (A) Col-0 and tpt-2 or (B) adg1-1 and adg1-1/tpt-2 grown either continuously in LL or HL or after LL/HL transfer at t 4h or t 48h
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | Col-0 t 0 (LL) | Col-0 t 4h | Col-0 t 48h | Col-0 (HL) | tpt-2 t 0 (LL) | tpt-2 t 4h | tpt-2 t 48h | tpt-2 (HL) |
| Starch and sugar content (μmol g–1 FW) | ||||||||
| Starch | 2.86±0.29 | 33.04±1.30 | 70.81±3.66 | 122.50±7.19 | 5.33±0.23 | 36.93±5.60 | 110.17±3.74 | 148.49±5.97 |
| d-Suc | 0.77±0.06 | 1.97±0.04 | 2.66±0.25 | 3.90±0.45 | 0.60±0.03 | 1.06±0.02 | 3.07±0.20 | 1.70±0.23 |
| d-Glc | 1.02±0.12 | 2.05±0.12 | 2.69±0.25 | 8.32±1.06 | 0.95±0.05 | 1.14±0.06 | 3.50±0.22 | 5.95±0.86 |
| d-Fru | 0.10±0.01 | 0.56±0.02 | 0.57±0.06 | 1.69±0.21 | 0.01±0.01 | 0.12±0.02 | 0.53±0.07 | 1.20±0.13 |
| Relative metabolite content, GC-MS (arbitrary units g–1 FW) | ||||||||
| Sugars | ||||||||
| d-Suc | 3.26±0.06 | 6.08±0.10 | 7.33±0.12 | 6.78±0.19 | 3.39±0.06 | 4.18±0.16 | 7.15±0.13 | 8.13±0.25 |
| d-Glc | 0.53±0.04 | 21.35±0.68 | 12.21±1.14 | 13.71±1.25 | 0.67±0.04 | 3.19±0.22 | 22.97±0.69 | 16.01±0.75 |
| d-Fru | 0.74±0.04 | 16.80±0.45 | 9.68±1.85 | 12.78±0.90 | 0.91±0.03 | 3.38±0.24 | 17.42±0.46 | 8.61±0.56 |
| d-Maltose | 0.12±0.01 | 0.25±0.02 | 1.87±0.26 | 0.52±0.03 | 0.89±0.05 | 0.51±0.06 | 10.98±0.81 | 6.71±0.95 |
| α,α′-d-Trehalose | 0.25±0.03 | 0.48±0.03 | 4.10±0.43 | 2.84±0.17 | 0.25±0.02 | 0.31±0.03 | 1.22±0.13 | 0.84±0.08 |
| Amino acids | ||||||||
| dl-Glutamic acid | 32.46±1.86 | 124.73±2.57 | 165.01±2.40 | 39.41±3.02 | 53.29±2.59 | 98.74±6.50 | 169.43±3.91 | 87.26±9.74 |
| Glycine | 3.52±0.23 | 144.51±2.41 | 115.60±1.32 | 14.48±0.33 | 4.41±0.26 | 107.48±1.70 | 109.62±2.31 | 80.02±3.37 |
| dl-Serine | 10.12±0.36 | 28.05±0.75 | 54.53±1.41 | 38.38±0.84 | 15.97±0.71 | 65.01±0.91 | 66.14±1.24 | 63.03±1.45 |
| l-Proline | 7.16±0.83 | 35.89±1.73 | 60.06±3.78 | 14.33±2.1 | 6.84±0.43 | 70.17±4.47 | 42.50±4.54 | 70.52±5.59 |
| Organic acids | ||||||||
| Pyruvic acid | 0.61±0.03 | 0.67±0.06 | 0.97±0.07 | 0.84±0.05 | 0.79±0.06 | 1.64±0.18 | 1.07±0.04 | 1.61±0.19 |
| 2-Methyl-dl-malic acid | 0.09±0.01 | 0.34±0.03 | 1.02±0.03 | 0.51±0.03 | 0.17±0.01 | 0.42±0.02 | 1.21±0.04 | 0.56±0.05 |
| Fumaric acid | 106.36±3.90 | 125.30±1.85 | 111.55±2.94 | 133.31±2.25 | 117.78±3.67 | 101.63±2.49 | 91.51±2.35 | 112.65±3.50 |
| Others | ||||||||
| Glycerol | 1.50±0.07 | 2.97±0.30 | 2.91±0.14 | 3.99±0.47 | 1.42±0.05 | 2. 79±0.24 | 2.41±0.13 | 3.55±0.18 |
| myo-Inositol | 12.82±0.58 | 19.92±0.40 | 23.73±1.37 | 26.92±0.37 | 11.44±0.72 | 18.02±0.44 | 23.18±0.54 | 15.78±0.56 |
| Erythritol | 0.27±0.01 | 0.73±0.09 | 1.06±0.07 | 0.82±0.05 | 0.35±0.03 | 0.78±0.06 | 1.28±0.07 | 1.29±0.16 |
| Putrescine | 1.09±0.07 | 7.53±0.64 | 10.94±0.94 | 3.11±0.49 | 1.14±0.05 | 3.90±0.35 | 16.55±1.00 | 14.76±1.63 |
| B | ||||||||
| Metabolites | adg1-1 t 0 (LL) | adg1-1 t 4h | adg1-1 t 48h | adg1-1 (HL) | adg1-1/ tpt-2 t 0 (LL) | adg1-1/ tpt-2 t 4h | adg1-1/ tpt-2 t 48h | adg1-1/ tpt-2 (HL) |
| Starch and sugar content (μmol g–1 FW) | ||||||||
| Starch | 0.27±0.01 | 0.52±0.03 | 0.67±0.06 | 0.11±0.06 | 0.42±0.03 | 0.85±0.03 | 0.68±0.04 | 1.82±0.79 |
| d-Suc | 1.74±0.14 | 3.90±0.62 | 8.67±0.87 | 3.43±0.74 | 1.34±0.16 | 1.52±0.10 | 1.99±0.25 | 1.02±0.09 |
| d-Glc | 2.40±0.14 | 11.11±0.08 | 7.89±0.56 | 9.65±0.58 | 2.05±0.08 | 3.60±0.04 | 4.00±0.20 | 3.61±0.15 |
| d-Fru | 0.89±0.10 | 6.04±0.16 | 2.68±0.29 | 3.11±0.16 | 0.66±0.01 | 0.98±0.06 | 0.78±0.10 | 0.85±0.06 |
| Relative metabolite content, GC MS (arbitrary units g–1 FW) | ||||||||
| Sugars | ||||||||
| d-Suc | 5.66±0.18 | 6.69±0.06 | 7.08±0.05 | 7.90±0.12 | 3.79±0.15 | 5.20±0.07 | 5.93±0.08 | 4.77±0.19 |
| d-Glc | 6.77±0.53 | 25.97±0.17 | 24.41±0.44 | 26.82±0.37 | 2.92±0.44 | 13.41±1.30 | 10.77±0.74 | 5.73±0.77 |
| d-Fru | 10.91±0.63 | 20.17±0.21 | 19.71±0.37 | 20.95±0.34 | 3.92±0.48 | 5.84±0.47 | 8.44±0.54 | 3.76±0.29 |
| d-Maltose | ND | 0.55±0.03 | 0.52±0.04 | 0.14±0.01 | ND | 0.38±0.04 | 0.21±0.02 | 0.14±0.03 |
| α,α′-d-Trehalose | 0.16±0.01 | 0.44±0.03 | 3.33±0.23 | 1.48±0.07 | 0.15±0.02 | 0.18±0.02 | 0.25±0.03 | 0.76±0.10 |
| Amino acids | ||||||||
| dl-Glutamic acid | 29.83±1.81 | 65.86±5.49 | 176.49±1.89 | 37.77±1.71 | 35.30±1.80 | 73.15±5.63 | 70.01±4.78 | 37.78±4.50 |
| Glycine | 4.78±0.24 | 84.63±3.23 | 116.06±1.69 | 22.94±1.59 | 2.52±0.15 | 20.29±1.63 | 7.62±0.38 | 4.30±0.26 |
| dl-Serine | 6.10±0.22 | 23.16±1.38 | 49.68±1.48 | 25.88±1.76 | 7.70±0.52 | 19.02±1.19 | 16.56±0.49 | 14.93±0.68 |
| l-Proline | 4.01±0.29 | 30.16±2.26 | 68.00±7.02 | 18.81±2.64 | 6.94±1.31 | 26.60±1.85 | 22.41±4.80 | 9.86±1.32 |
| Organic acids | ||||||||
| Pyruvic acid | 0.53±0.04 | 0.53±0.04 | 0.87±0.05 | 1.09±0.06 | 0.62±0.03 | 1.10±0.07 | 1.09±0.12 | 0.62±0.02 |
| 2-Methyl-dl-malic acid | 0.12±0.01 | 0.36±0.03 | 1.13±0.06 | 0.62±0.02 | 0.16±0.02 | 0.38±0.01 | 0.62±0.02 | 0.16±0.02 |
| Fumaric acid | 136.96±4.41 | 115.53±1.70 | 98.82±3.49 | 116.77±2.31 | 96.78±1.61 | 91.04±1.66 | 85.79±1.36 | 82.43±10.17 |
| Others | ||||||||
| Glycerol | 1.24±0.02 | 2.18±0.25 | 2.55±0.05 | 4.42±1.15 | 1.22±0.13 | 2.27±0.16 | 2.33±0.19 | 10.11±3.54 |
| myo-Inositol | 6.35±0.35 | 13.79±0.78 | 18.83±0.74 | 16.17±0.54 | 7.07±0.16 | 13.69±0.51 | 16.35±0.44 | 6.16±0.35 |
| Erythritol | 0.53±0.04 | 1.07±0.13 | 4.69±0.38 | 1.44±0.10 | 0.89±0.02 | 1.57±0.09 | 1.80±0.04 | 1.96±0.21 |
| Putrescine | 2.93±0.10 | 9.60±1.26 | 36.15±2.69 | 11.37±1.12 | 1.92±0.12 | 6.92±0.98 | 5.18±0.39 | 5.95±0.88 |
The samples were harvested in the middle of the light period (i.e. after 8h in the light).
The data represent the mean of five independent samples ±SE; ND, not detected.
Fig. 10.
(A) Principle component analysis of metabolome data (see Supplementary Tables S11 and S12 available at JXB online) obtained with Col-0, adg1-1, tpt-2, and adg1-1/tpt-2 grown in LL or HL and 4h (t 4h) or 48h (t 48h) after LL/HL transfer. (B) Heat map presentation of the same set of metabolome data shown in (A) referred either to Col-0 in a ‘static’ comparison or to t 0 in a ‘dynamic’ comparison. The heatmap colours refer to increased or decreased metabolite contents at log2 ratios of +3 and –3 (bright red or bright green), respectively. Further colours represent Col-0 wild-type (black), tpt-2 (blue), adg1-1 (red), and adg1-1/tpt-2 (dark purple). The different orange colour intensities are defined within the figure.
Table 4 shows that carbohydrate contents determined enzymatically or by GC-MC showed similar relative changes. Remarkably, Glc was the only soluble sugar that showed considerable variations in its content in adg1-1/tpt-2, in particular as a short-term response to LL/HL transfer (i.e. at t 4h). In contrast to adg1-1/tpt-2, Glc contents remained high or further increased in wild-type and single mutant plants 48h after LL/HL transfer and when grown continuously in HL. It is hence tempting to speculate that a diminished Glc content in HL-grown adg1-1/tpt-2 might contribute to the lack of HL acclimation of the double mutant, a notion that is further supported by the rescue of the double mutant’s HL phenotype by feeding of Glc (Heinrichs et al., 2012; Schmitz et al., 2012). Like Glc, Fru and Suc accumulated in wild-type and single mutant plants (at least transiently; Table 4A, 4B), whereas there was no such increase in adg1-1/tpt-2. It recently could be demonstrated that diminished cytosolic Fru contents due to the deficiency of SWEET17, an A. thaliana Fru exporter from the vacuole, resulted in plants with stunted growth (Chardon et al., 2013). Hence, besides Glc, Fru might probably also be considered as a signalling molecule. Moreover, glycine, proline, and myo-inositol showed a similar distribution pattern in adg1-1/tpt-2 to Glc. Proline (Verslues and Sharma, 2010) and myo-inositol (Donahue et al., 2010) are involved in stress responses, and the latter is the structural basis for lipid signalling molecules (Valluru and van den Ende, 2011). Maltose contents were selectively increased in tpt-2 starting 48h after LL/HL transfer, but were close to the limit of detection in the starch-free background. Maltose results from starch degradation via β-amylase and thus supports the idea of daytime starch mobilization in the absence of the TPT. The complete list of metabolites (Supplementary Table S11 available at JXB online) was further evaluated by a static and dynamic assessment, similar to the microarrays, statistically analysed (Table 5 of Supplementary Document S1 available at JXB online) and listed in Supplementary Table S12 available at JXB online.
Conclusions
The genetic approach used here with mutants provides novel insight into the significance of carbohydrates and redox/ROS signalling in retrograde control of nuclear gene expression as a response of A. thaliana to HL exposure. Sugars are the first major products of photosynthesis and their contents are correlated with environmental conditions such as light and temperature. Even with an impaired day and night path of carbon export from the chloroplast in the adg1-1/tpt-2 double mutant, some changes in Glc contents in response to HL were observed in the short term but not in the long term. In the group of commonly regulated genes, which respond to HL in the short term, a cross-talk of genes involved in carbohydrate and lipid metabolism exists. The impaired sugar-based long-term adaptation to HL observed in adg1-1/tpt-2 most probably results in its severe growth and photosynthesis phenotype. To what extent severely down-regulated transcription factors such as MYC4 are involved in the lack of HL acclimation of adg1-1/tpt-2 will be the subject of future experiments. Further elements, probably involved in the fine-tuning of the sugar response, have been identified amongst the genes which were either not or were specifically de-regulated in the single or double mutants. For instance, genes coding for heat shock proteins or genes that are involved in calcium signalling were specifically down-regulated in adg1-1/tpt-2. Detailed analyses of candidate genes would hence be challenging in future research. Meta-analyses of transcriptome data such as, for instance, conducted by van Akelen and Whelan (2012) can also shed more light on the fine-tuning in retrograde signalling. The meta-analyses conducted here support the idea that carbohydrates play a pivotal role in HL acclimation of A. thaliana.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Correlation analyses of Lhcb1 expression levels, ETR, and contents of MgProtoIX, soluble sugars, and total carbohydrates.
Figure S2. Graphviz presentation of co-expression networks obtained with ATTEDII for data shown in Supplementary Table S7A and Supplementary Document S3.
Figure S3. Relative distribution of differentially regulated genes in publicly available microarray experiments compared with in-house expression data for Col-0 (A), tpt-2 (B), adg1-1 (C), and adg1-1/tpt-2 at t 4h and t 48h after LL/HL transfer.
Document S1. Statistical analyses (ANOVA/Tukey–Kramer) of the experimental data containing six tables.
Document S2. Detailed description of the static assessment of global gene expression data after LL/HL transfer between wild-type and mutant plants.
Document S3. Detailed analysis of commonly regulated genes following the dynamic assessment of global gene expression after LL/HL transfer between wild-type and mutant plants.
Table S1. Overview of the metabolite reporter list.
Table S2. Static comparison of global gene expression in adg1-1, tpt-2. and adg1-1/tpt-2 versus Col-0 at t 0 before LL/HL transfer.
Table S3. Static comparison of global gene expression in adg1-1, tpt-2, and adg1-1/tpt-2 versus Col-0 at t 4h after LL/HL transfer.
Table S4. Static comparison of global gene expression in adg1-1, tpt-2, and adg1-1/tpt-2 versus Col-0 at t 48h after LL/HL transfer.
Table S5. Dynamic comparison of global gene expression in Col-0, adg1-1, tpt-2, and adg1-1/tpt-2 at t 4h after LL/HL transfer versus t 0.
Table S6. Dynamic comparison of global gene expression in Col-0, adg1-1, tpt-2, and adg1-1/tpt-2 at t 48h after LL/HL transfer versus t 0.
Table S7. Filtered list of significantly (P<0.01) altered genes in the central overlapping regions of the Venn diagrams shown in Fig. 7A and B.
Table S8. List of genes specifically regulated or not regulated in the individual lines. The gene lists are based on the Venn diagrams shown in Fig. 7.
Table S9. Comparison of HL-responsive genes within the group of commonly regulated genes (Supplementary Table S7) with publicly available microarray data on genes responding to elevated sugars, oxidative stress, or the phytohormones ABA, indole acetic acid (IAA), trans-zeatin (tZ), gibberellic acid (GA3), the ethylene precursur ACC, and methyl jasmonate (MJ).
Table S10. Comparison of HL-responsive genes within the group of individually altered genes (Supplementary Table S8) with publicly available microarray data on genes responding to elevated sugars, oxidative stress, or the phytohormones ABA, indole acetic acid (IAA), trans-zeatin (tZ), gibberellic acid (GA3), the ethylene precursur ACC, and methyl jasmonate (MJ).
Table S11. Contents of metabolites determined by GC-MS in leaves of (A) Col-0, (B) adg1-1, (C) tpt-2, and (D) adg1-1/tpt-2 grown either continuously in LL or HL or after transfer from LL to HL at t 4h or t 48h.
Table S12. Evaluation of metabolite profiles in response to growth in LL or HL or after LL/HL transfer in Col-0 wild-type, adg1-1, and tpt-2 single mutants as well as adg1-1/tpt-2 double mutant plants based on the data in Supplementary Table S10.
Acknowledgements
This study was supported by a grant in frame of the DFG Research unit 804 ‘retrograde signalling’ in plants. FS acknowledges the support of the CRA-Young Investigator programme.
References
- Ahlert D, Ruf S, Bock R. 2003. Plastid protein synthesis is required for plant development in tobacco. Proceedings of the National Academy of Sciences, USA 100, 15730–15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399 [DOI] [PubMed] [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H. 2000. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1433–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Dietz K-J. 2005. Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. Journal of Experimental Botany 56, 1449–1462 [DOI] [PubMed] [Google Scholar]
- Baier M, Ströher E, Dietz K-J. 2004. The acceptor availability at photosystem I and ABA control nuclear expression of 2-Cys peroxiredoxin-A in Arabidopsis thaliana . Plant and Cell Physiology 45, 997–1006 [DOI] [PubMed] [Google Scholar]
- Bailey S, Horton P, Walters RG. 2004. Acclimation of Arabidopsis thaliana to the light environment: the relationship between photosynthetic function and chloroplast composition. Planta 218, 793–802 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Jaspert N, Arif M, Mueller-Roeber B, Maurino VG. 2012. Expression of ROS-responsive genes and transcription factors after metabolic formation of H2O2 in chloroplasts. Frontiers in Plant Science 3, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CF. 2005. Signaling pathways from the chloroplast to the nucleus. Planta 222, 743–756 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B (Methodological) 57, 289–300 [Google Scholar]
- Bräutigam K, Dietzel L, Kleine T, et al. 2009. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. The Plant Cell 21, 2715–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Fernie AR, Willmitzer L, Steinhauser D. 2012. Unraveling retrograde signaling pathways: finding candidate signaling molecules via metabolomics and systems biology driven approaches. Frontiers in Plant Science 3, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon F, Bedu M, Calenge F, et al. 2013. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Current Biology 23, 697–702 [DOI] [PubMed] [Google Scholar]
- Czarnecki O, Gläßer C, Chen JG, Mayer KF, Grimm B. 2012. Evidence for a contribution of ALA synthesis to plastid-to-nucleus signaling. Frontiers in Plant Science 3, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki O, Peter E, Grimm B. 2011. Methods for analysis of photosynthetic pigments and steady-state levels of intermediates of tetrapyrrole biosynthesis. Methods in Molecular Biology 75, 357–385 [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, de Miranda SMN, Baier M, Finkemeier I. 2006. The function of peroxiredoxins in plant organelle redox metabolism. Journal of Experimental Botany 57, 1697–1709 [DOI] [PubMed] [Google Scholar]
- Donahue JL, Alford SR, Torabinejad J, et al. 2010. The Arabidopsis thaliana myo-inositol 1-phosphate synthase1 gene is required for myo-inositol synthesis and suppression of cell death. The Plant Cell 22, 888–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard S, Finazzi G, Wollman F-A. 2008. The dynamics of photosynthesis. Annual Review of Genetics 42, 463–515 [DOI] [PubMed] [Google Scholar]
- Estavillo GM, Crisp PA, Pornsiriwong W, et al. 2011. Evidence for a SAL1–PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. The Plant Cell 23, 3992–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P, Chini A, Fernández-Barbero G, et al. 2011. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. The Plant Cell 23, 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Aharoni A, Willmitzer L, Stitt M, Tohge T, Kopka J, Carroll AJ, Saito K, Fraser PD, DeLuca V. 2011. Recommendations for reporting metabolite data. The Plant Cell 23, 2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2009. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants and Redox Signaling 11, 861–905 [DOI] [PubMed] [Google Scholar]
- Frankhauser C, Chory J. 1997. Light control of plant development. Annual Review of Cell and Developmental Biology 13, 203–229 [DOI] [PubMed] [Google Scholar]
- Galton F. 1888. Co-relations and their measurement, chiefly from anthropometric data. Proceedings of the Royal Society 45, 135–145 [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92 [Google Scholar]
- Giraud E, Ho LH, Clifton R, et al. 2008. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiology 147, 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Sasaki E, Akiyama K, et al. 2008. The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. The Plant Journal 55, 526–542 [DOI] [PubMed] [Google Scholar]
- Griffith OW. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry 106, 207–212 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Geimer S, Kunz HH, Schmitz J, Dörmann P, Bell K, Hetfeld S, Guballa A, Flügge UI. 2009. Chlororespiration and grana hyperstacking: how an Arabidopsis double mutant can survive despite defects in starch biosynthesis and daily carbon export from chloroplasts. Plant Physiology 149, 515–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Schulz B, Flügge UI. 1998. Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204, 366–376 [DOI] [PubMed] [Google Scholar]
- Heinrichs L, Schmitz J, Flügge UI, Häusler RE. 2012. The mysterious rescue of adg1-1/tpt-2—an Arabidopsis thaliana double mutant impaired in acclimation to high light—by exogenously supplied sugars. Frontiers in Plant Sciences 3, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, König J, Finkemeier I, Baier M, Kandlbinder A, Dietz KJ. 2003. Divergent light-, ascorbate- and oxidative stress dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis thaliana . Plant Physiology 131, 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV. 2008. Photosynthetic acclimation: does the dynamic structure and macroorganisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS Journal 275, 1069–1079 [DOI] [PubMed] [Google Scholar]
- Karpiński S, Szechyńska-Hebda M, Wituszyńska W, Burdiak P. 2013. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant, Cell and Environment 36, 736–744 [DOI] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Apel K, Laloi C. 2008. No single way to understand singlet oxygen signaling in plants. EMBO Reports 9, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T, Voigt C, Leister D. 2009. Plastid signaling to the nucleus: messengers still lost in the mists? Trends in Genetics 25, 185–192 [DOI] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, et al. 2005. GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21, 1635–1638 [DOI] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. 2007. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 [PubMed] [Google Scholar]
- Krieger-Liszkay A. 2005. Singlet oxygen production in photosynthesis. Journal of Experimental Botany 56, 337–346 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Häusler RE, Fettke J, Herbst K, Niewiedomski P, Gierth M, Bell M, Steup M, Flügge UI, Schneider A. 2010. The role of plastidial glucose 6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biology 12 Suppl 1, 115–128 [DOI] [PubMed] [Google Scholar]
- Leister D. 2003. Chloroplast research in the genomic age. Trends in Genetics 19, 47–56 [DOI] [PubMed] [Google Scholar]
- Leyman B, Van Dijck P, Thevelein JM. 2001. An unexpected plethora of trehalose biosynthesis genes in Arabidopsis thaliana . Trends in Plant Sciences 6, 510–513 [DOI] [PubMed] [Google Scholar]
- Li S, van Os GM, Ren S, Yu D, Ketelaar T, Emons AM, Liu CM. 2010. Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiology 154, 1819–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. 1999. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology 50, 47–65 [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J. 1988. Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiology 86, 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. 2006. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nature Protocols 1, 387–396 [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Zakhleniuk OV. 2004. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3 . Journal of Experimental Botany 55, 1221–1230 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. 1987. Improved method for the isolation ofRNA from plant tissues. Analytical Biochemistry 163, 16–20 [DOI] [PubMed] [Google Scholar]
- Lohse M, Nunes-Nesi A, Krueger P, et al. 2010. Robin: an intuitive wizard application for R-based expression microarray quality assessment and analysis. Plant Physiology 153, 642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook J. 1998. Multiple comparison procedures updated. Clinical and Experimental Pharmacology and Physiology 25, 1032–1037 [DOI] [PubMed] [Google Scholar]
- Luedemann A, von Malotky L, Erban A, Kopka J. 2012. TagFinder: preprocessing software for the fingerprinting and the profiling of gas chromatography-mass spectrometry based metabolome analyses. Methods in Molecular Biology 860, 255–286 [DOI] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hülskamp M, Hoecker U. 2013. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. The Plant Journal 74, 638–651 [DOI] [PubMed] [Google Scholar]
- Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. 2001. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 98, 12826–12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, et al. 2008. Network discovery pipeline elucidates conserved time of day-specific cis-regulatory modules. PLoS Genetics 4, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2009. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant, Cell and Environment 33, 453–467 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A. 2008. The steady-state level of Mgprotoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis . Proceedings of the National Academy of Sciences, USA 105, 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M, McCormac AC, Terry MJ, Smith AG. 2008. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mgprotoporphyrin IX accumulation. Proceedings of the National Academy of Sciences, USA 105, 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49, 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Foyer CH. 2000. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T, Hayahi S, Saeki M, Ohta H, Kinoshita K. 2009. ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Research 37, 987–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze ML, Vogel MO, Alsharafa K, Kahmann U, Viehhauser A, Maurino VG, Dietz KJ. 2012. Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10- or 100-fold light increment and the possible involvement of retrograde signals. Journal of Experimental Botany 63, 1297–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros JC. 2007. VENNY. An interactive tool for comparing lists with Venn Diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html
- op den Camp RG, Przybyla D, Ochsenbein C, et al. 2003. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. The Plant Cell 15, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Liebers M, Hirth M, Grübler B, Holtzegel U, Schröter Y, Dietzel L, Pfannschmidt T. 2012. Environmental control of plant nuclear gene expression by chloroplast redox signals. Frontiers in Plant Science 3, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF. 1999. Photosynthetic control of chloroplast gene expression. Nature 397, 625–628 [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie A. 2001. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. The Plant Cell 13, 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, BaenGonzalez E, Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Physiology 57, 675–709 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J. 2002. Sugar sensing and signaling in plants. The Plant Cell 14, Suppl., 185–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Schöttler MA, Krueger S, Geimer S, Schneider A, Kleine T, Leister D, Bell K, Flügge UI, Häusler RE. 2012. Defects in leaf carbohydrate metabolism compromise acclimation to high light and lead to a high chlorophyll fluorescence phenotype in Arabidopsis thaliana. BMC Plant Biology 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Häusler RE, Kolukisaoglu U, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge UI. 2002. An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. The Plant Journal 32, 685–699 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research 10, 51–62 [DOI] [PubMed] [Google Scholar]
- Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P. 2013. Arabidopsis basic helix–loop–helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. The Plant Cell 25, 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiguzov A, Vainonen JP, Wrzaczek M, Kangasjärvi J. 2013. R S-talk—how the apoplast, the chloroplast, and the nucleus get the message through. Frontiers in Plant Sciences 3, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Botanical Review 51, 53–105 [Google Scholar]
- Shin DH, Choi M, Kim K, Bang G, Cho M, Choi SB, Choi G, Park YI. 2013. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Letters 587, 1543–1547 [DOI] [PubMed] [Google Scholar]
- Smeekens S. 1998. Sugar regulation of gene expression in plants. Current Opinion in Plant Biology 1, 230–234 [DOI] [PubMed] [Google Scholar]
- Smeekens S. 2000. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology 51, 49–81 [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology 3, Article3. [DOI] [PubMed] [Google Scholar]
- Stacklies W, Redestig H, Scholz M, Walther D, Selbig J. 2007. PCA methods—a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23, 1164–1167 [DOI] [PubMed] [Google Scholar]
- Strand A, Asami T, Alonso J, Ecker JR, Chory J. 2003. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421, 79–83 [DOI] [PubMed] [Google Scholar]
- Surpin M, Larkin R, Chory J. 2002. Signal transduction between the chloroplast and the nucleus. The Plant Cell 14, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J. 1993. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74, 787–799 [DOI] [PubMed] [Google Scholar]
- Swindell WR, Huebner M, Weber AP. 2007. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8, 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. 2005. Sucrose specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiology 139, 1840–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor S, Bartram S, Kroymann J, Falk KL, Hick A, Pickett JA, Gershenzon J. 2004. Biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana: recombinant expression and characterization of methylthioalkylmalate synthase, the condensing enzyme of the chain elongation cycle. Planta 218, 1026–1035 [DOI] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M. 2004. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal 37, 914–939 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Havaux M. 2009. Singlet oxygen in plants: production, detoxification and signaling.Trends in Plant Sciences 14, 219–228 [DOI] [PubMed] [Google Scholar]
- Valluru R, Van den Ende W. 2011. Myo-inositol and beyond—emerging networks under stress. Plant Science 181, 387–400 [DOI] [PubMed] [Google Scholar]
- Van Aken O, Whelan J. 2012. Comparison of transcriptional changes to chloroplast and mitochondrial perturbations reveals common and specific responses in Arabidopsis. Frontiers in Plant Science 3, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I. 2012. Molecular mechanisms of photodamage in the photosystem II complex. Biochimica et Biophysica Acta 1817, 209–217 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Sharma S. 2010. Proline metabolism and its implications for plant–environment interaction. Arabidopsis Book 8, e0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M. 2013. Regulation of flowering by trehalose-6- phosphate signaling in Arabidopsis thaliana . Science 339, 704–707 [DOI] [PubMed] [Google Scholar]
- Walters RG, Ibrahim DG, Horton P, Kruger NJ. 2004. A mutant of Arabidopsis lacking the triose-phosphate/phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiology 135, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, Wang X, Robinson DG, Jiang L. 2010. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. The Plant Cell 22, 4009–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. 2007. An ‘Electronic Fluorescent Pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS One 2, e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Irizarry R, Gentleman R, Murillo FM, Spencer F. 2004. A model-based background adjustment for oligonucleotide expression arrays. Journal of the American Statistics Association 99, 909–917 [Google Scholar]
- Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K. 2012. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149, 1525–1535 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.