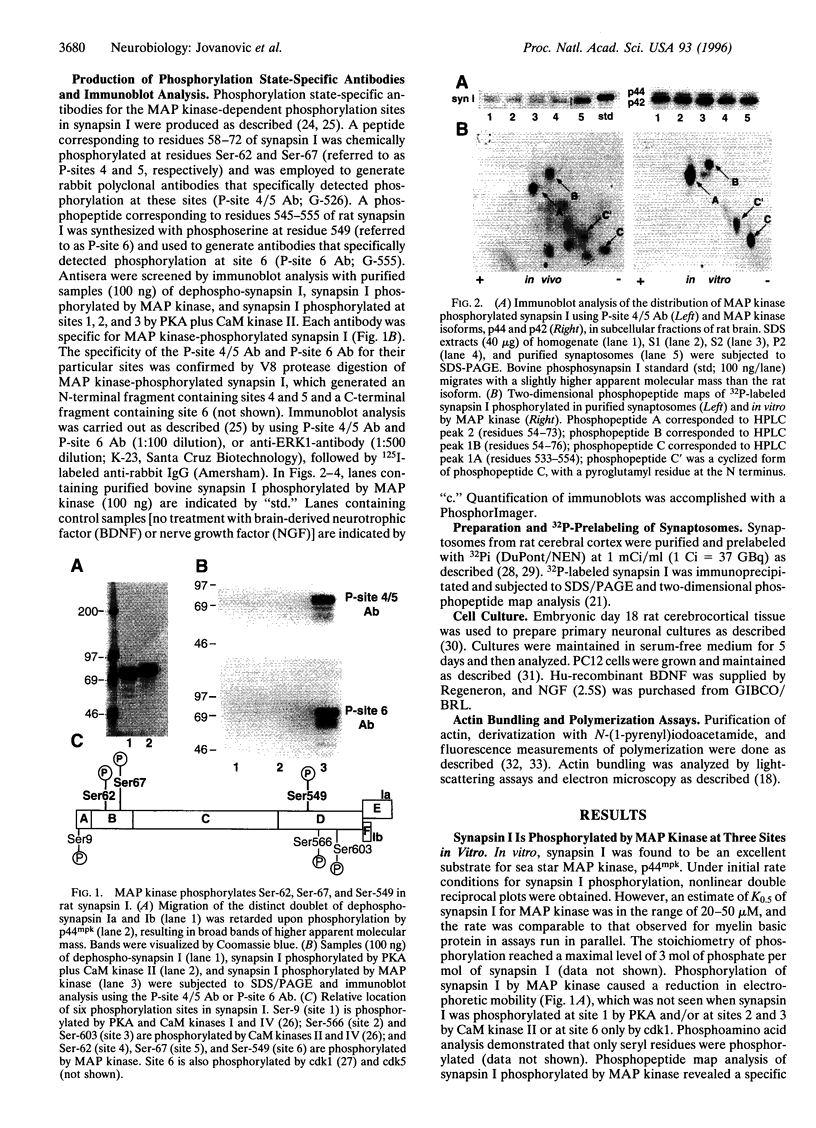
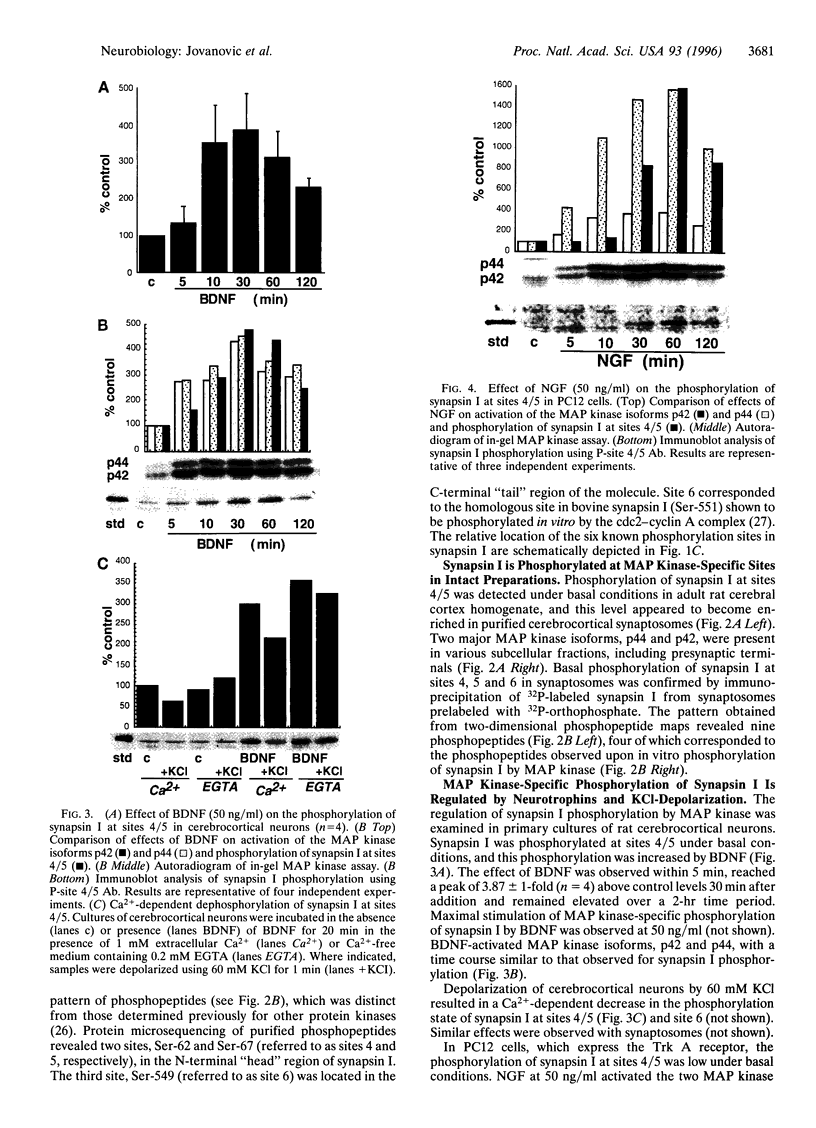
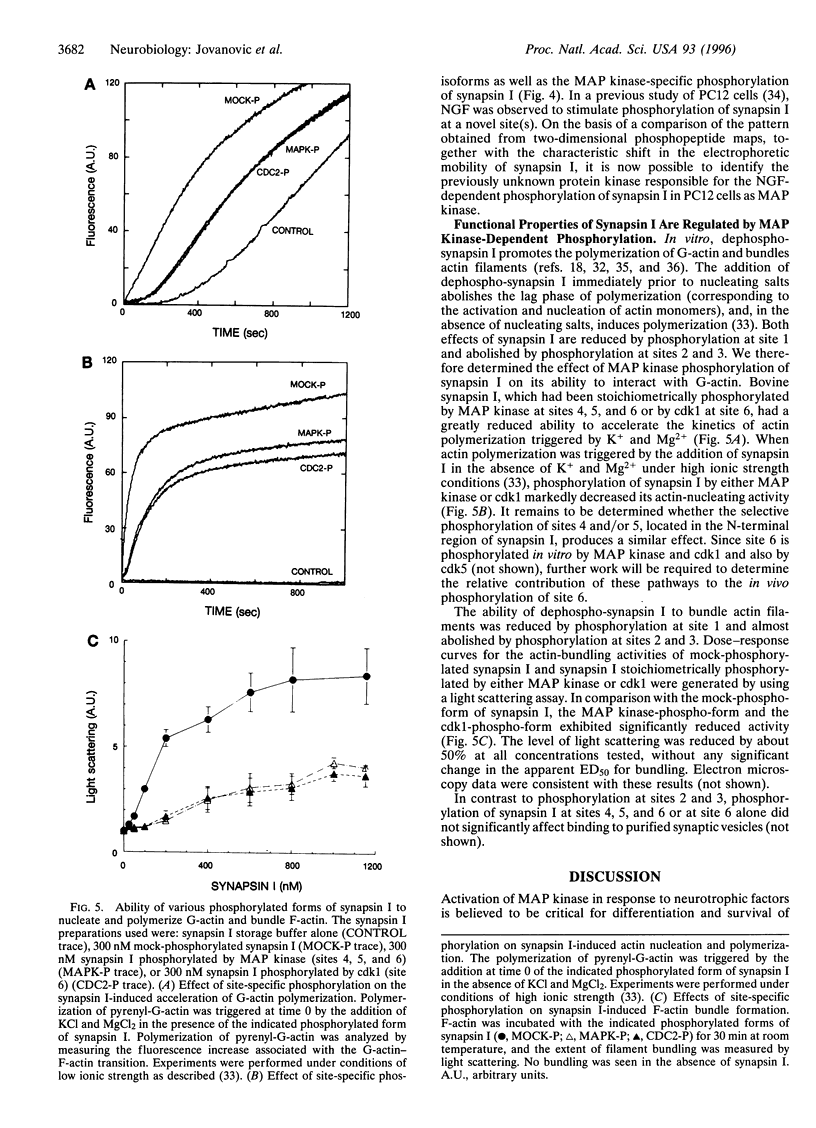
Abstract
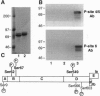
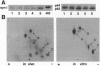
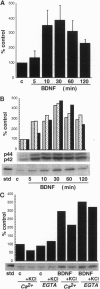
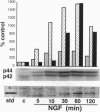
The ability of neurotrophins to modulate the survival and differentiation of neuronal populations involves the Trk/MAP (mitogen-activated protein kinase) kinase signaling pathway. More recently, neurotrophins have also been shown to regulate synaptic transmission. The synapsins are a family of neuron-specific phosphoproteins that play a role in regulation of neurotransmitter release, in axonal elongation, and in formation and maintenance of synaptic contacts. We report here that synapsin I is a downstream effector for the neurotrophin/Trk/MAP kinase cascade. Using purified components, we show that MAP kinase stoichiometrically phosphorylated synapsin I at three sites (Ser-62, Ser-67, and Ser-549). Phosphorylation of these sites was detected in rat brain homogenates, in cultured cerebrocortical neurons, and in isolated presynaptic terminals. Brain-derived neurotrophic factor and nerve growth factor upregulated phosphorylation of synapsin I at MAP kinase-dependent sites in intact cerebrocortical neurons and PC12 cells, respectively, while KCl- induced depolarization of cultured neurons decreased the phosphorylation state at these sites. MAP kinase-dependent phosphorylation of synapsin I significantly reduced its ability to promote G-actin polymerization and to bundle actin filaments. The results suggest that MAP kinase-dependent phosphorylation of synapsin I may contribute to the modulation of synaptic plasticity by neurotrophins and by other signaling pathways that converge at the level of MAP kinase activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bading H., Greenberg M. E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991 Aug 23;253(5022):912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Benfenati F., Valtorta F., Chieregatti E., Greengard P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron. 1992 Feb;8(2):377–386. doi: 10.1016/0896-6273(92)90303-u. [DOI] [PubMed] [Google Scholar]
- Berninger B., García D. E., Inagaki N., Hahnel C., Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. Neuroreport. 1993 Sep 30;4(12):1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- Bähler M., Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987 Apr 16;326(6114):704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Campbell J. S., Seger R., Graves J. D., Graves L. M., Jensen A. M., Krebs E. G. The MAP kinase cascade. Recent Prog Horm Res. 1995;50:131–159. doi: 10.1016/b978-0-12-571150-0.50011-1. [DOI] [PubMed] [Google Scholar]
- Ceccaldi P. E., Grohovaz F., Benfenati F., Chieregatti E., Greengard P., Valtorta F. Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J Cell Biol. 1995 Mar;128(5):905–912. doi: 10.1083/jcb.128.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L. S., Li L., Ferreira A., Kosik K. S., Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernik A. J., Girault J. A., Nairn A. C., Chen J., Snyder G., Kebabian J., Greengard P. Production of phosphorylation state-specific antibodies. Methods Enzymol. 1991;201:264–283. doi: 10.1016/0076-6879(91)01025-w. [DOI] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Harris S. M., Jr, Huttner W. B., Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983 May;96(5):1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley P. R., Jarvie P. E., Heath J. W., Kidd G. J., Rostas J. A. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986 Apr 30;372(1):115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Han H. Q., Greengard P., Kosik K. S. Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9225–9229. doi: 10.1073/pnas.92.20.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Kosik K. S., Greengard P., Han H. Q. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science. 1994 May 13;264(5161):977–979. doi: 10.1126/science.8178158. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Greenberg M. E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995 Apr 14;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hall F. L., Mitchell J. P., Vulliet P. R. Phosphorylation of synapsin I at a novel site by proline-directed protein kinase. J Biol Chem. 1990 Apr 25;265(12):6944–6948. [PubMed] [Google Scholar]
- Han H. Q., Nichols R. A., Rubin M. R., Bähler M., Greengard P. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature. 1991 Feb 21;349(6311):697–700. doi: 10.1038/349697a0. [DOI] [PubMed] [Google Scholar]
- Heumann R. Neurotrophin signalling. Curr Opin Neurobiol. 1994 Oct;4(5):668–679. doi: 10.1016/0959-4388(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Holmes C. F. A new method for the selective isolation of phosphoserine-containing peptides. FEBS Lett. 1987 May 4;215(1):21–24. doi: 10.1016/0014-5793(87)80106-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Schuman E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995 Mar 17;267(5204):1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kim H. G., Wang T., Olafsson P., Lu B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M., Leung L. S., Zhao D., Rylett R. J. Short-term modulation of glutamatergic synapses in adult rat hippocampus by NGF. Neuroreport. 1994 Dec 20;5(18):2433–2436. doi: 10.1097/00001756-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Knipper M., da Penha Berzaghi M., Blöchl A., Breer H., Thoenen H., Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994 Apr 1;6(4):668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Levine E. S., Dreyfus C. F., Black I. B., Plummer M. R. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chin L. S., Shupliakov O., Brodin L., Sihra T. S., Hvalby O., Jensen V., Zheng D., McNamara J. O., Greengard P. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9235–9239. doi: 10.1073/pnas.92.20.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof A. M., Ip N. Y., Poo M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B., Greengard P., Poo M. M. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992 Mar;8(3):521–529. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- Lu B., Yokoyama M., Dreyfus C. F., Black I. B. Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. N., Scholz W. K., Lamballe F., Klein R., Nanduri V., Barbacid M., Palfrey H. C. Signal transduction events mediated by the BDNF receptor gp 145trkB in primary hippocampal pyramidal cell culture. J Neurosci. 1993 Oct;13(10):4281–4292. doi: 10.1523/JNEUROSCI.13-10-04281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Pavlović-Surjancev B., Cahill A. L., Perlman R. L. Nicotinic agonists, phorbol esters, and growth factors activate two extracellular signal-regulated kinases, ERK1 and ERK2, in bovine chromaffin cells. J Neurochem. 1992 Dec;59(6):2134–2140. doi: 10.1111/j.1471-4159.1992.tb10104.x. [DOI] [PubMed] [Google Scholar]
- Pelech S. L. Networking with proline-directed protein kinases implicated in tau phosphorylation. Neurobiol Aging. 1995 May-Jun;16(3):247–261. doi: 10.1016/0197-4580(94)00187-6. [DOI] [PubMed] [Google Scholar]
- Petrucci T. C., Morrow J. S. Synapsin I: an actin-bundling protein under phosphorylation control. J Cell Biol. 1987 Sep;105(3):1355–1363. doi: 10.1083/jcb.105.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone V. A., Shupliakov O., Brodin L., Hilfiker-Rothenfluh S., Czernik A. J., Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995 Jun 8;375(6531):493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- Robbins D. J., Zhen E., Cheng M., Xu S., Ebert D., Cobb M. H. MAP kinases ERK1 and ERK2: pleiotropic enzymes in a ubiquitous signaling network. Adv Cancer Res. 1994;63:93–116. doi: 10.1016/s0065-230x(08)60399-1. [DOI] [PubMed] [Google Scholar]
- Romano C., Nichols R. A., Greengard P. Synapsin I in PC12 cells. II. Evidence for regulation by NGF of phosphorylation at a novel site. J Neurosci. 1987 May;7(5):1300–1306. doi: 10.1523/JNEUROSCI.07-05-01300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Bader S. A., Pelech S. L. Purification and characterization of a maturation-activated myelin basic protein kinase from sea star oocytes. J Biol Chem. 1990 Jan 5;265(1):52–57. [PubMed] [Google Scholar]
- Snider W. D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994 Jun 3;77(5):627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Stoop R., Poo M. M. Potentiation of transmitter release by ciliary neurotrophic factor requires somatic signaling. Science. 1995 Feb 3;267(5198):695–699. doi: 10.1126/science.7839148. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Greengard P., Fesce R., Chieregatti E., Benfenati F. Effects of the neuronal phosphoprotein synapsin I on actin polymerization. I. Evidence for a phosphorylation-dependent nucleating effect. J Biol Chem. 1992 Jun 5;267(16):11281–11288. [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]