Abstract
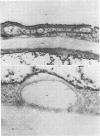
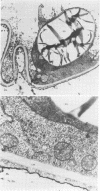
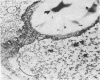
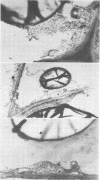
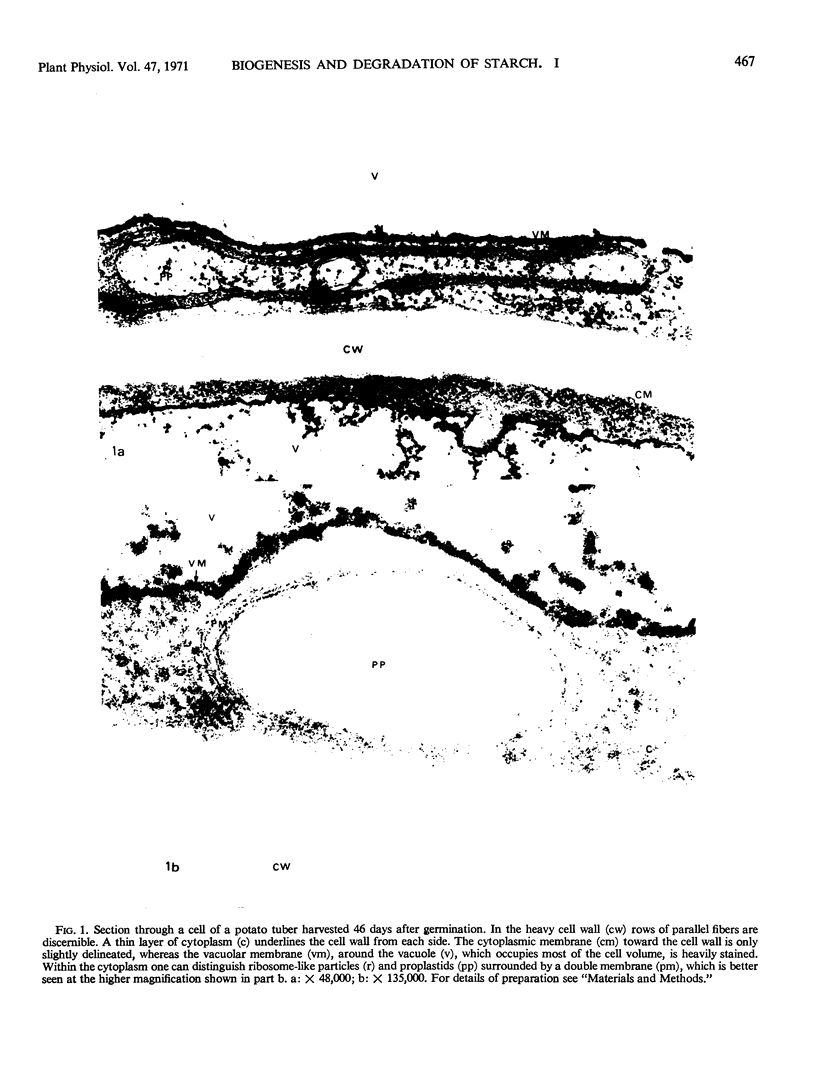
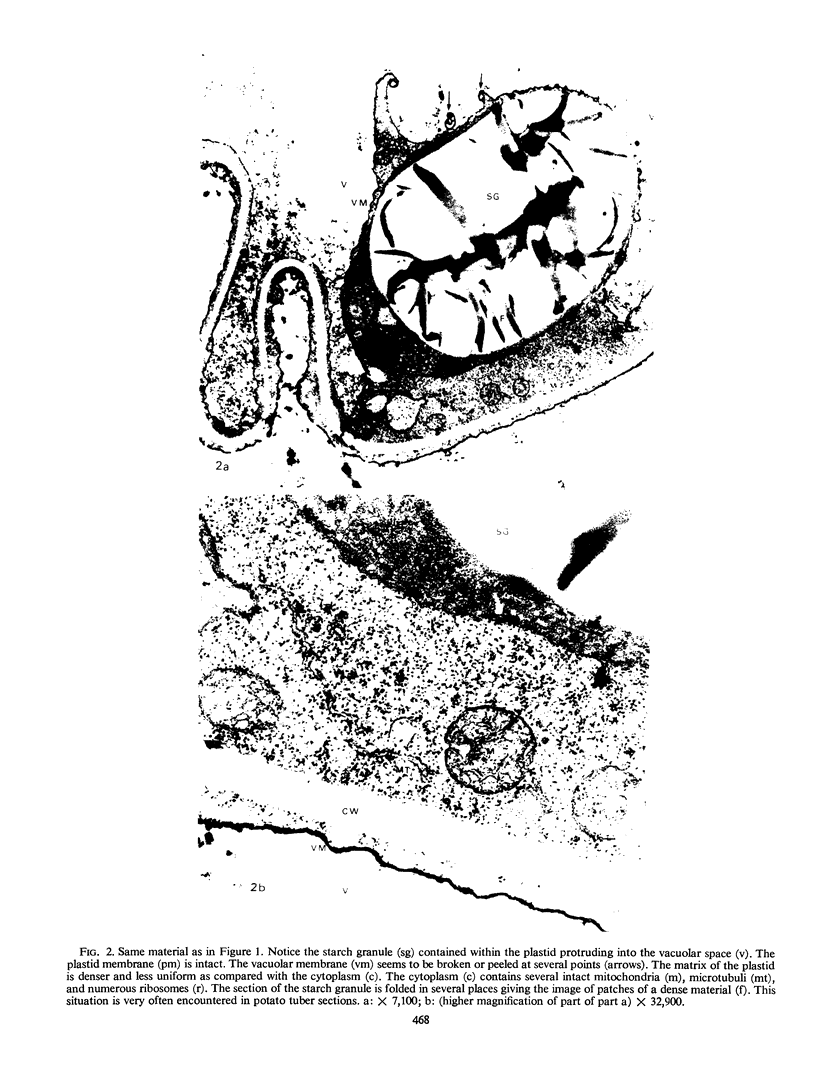
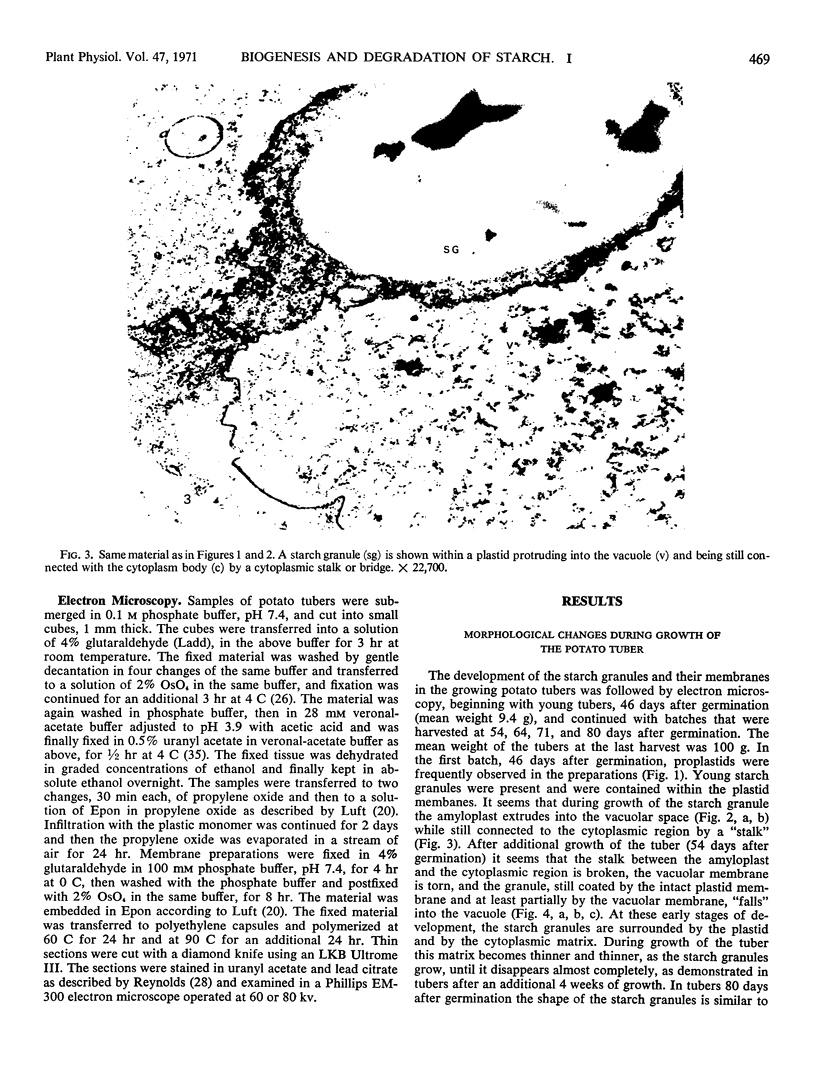
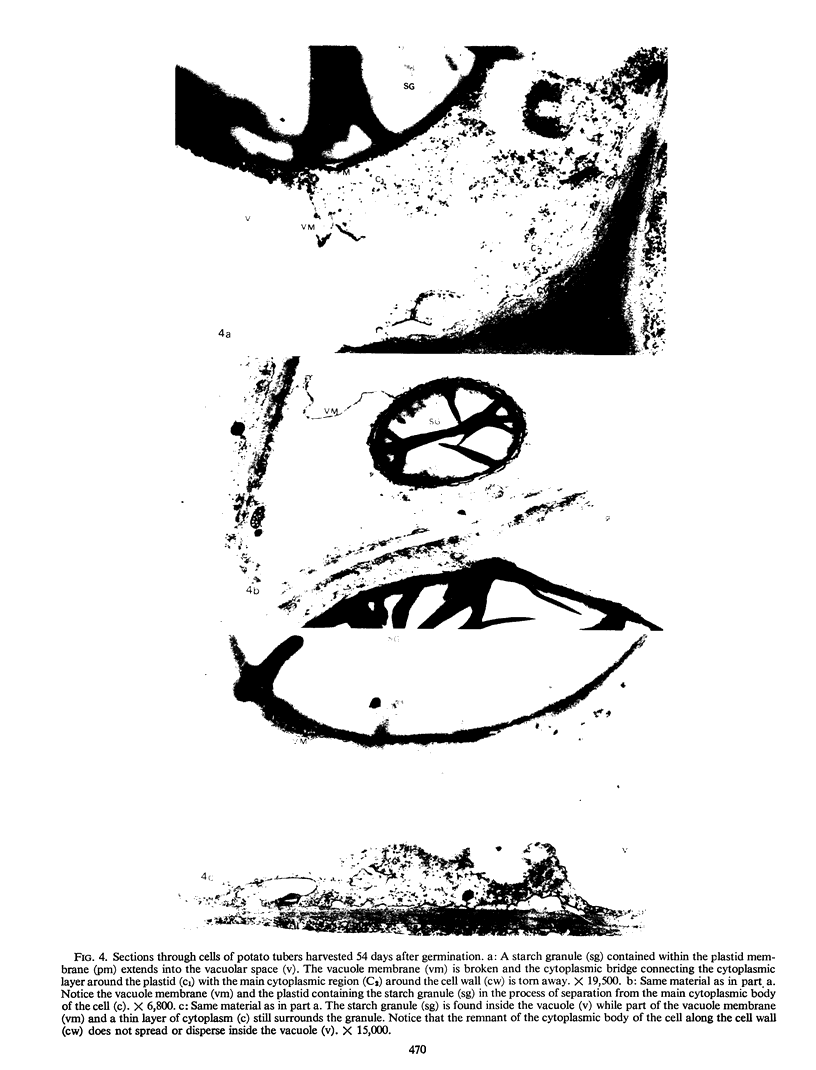
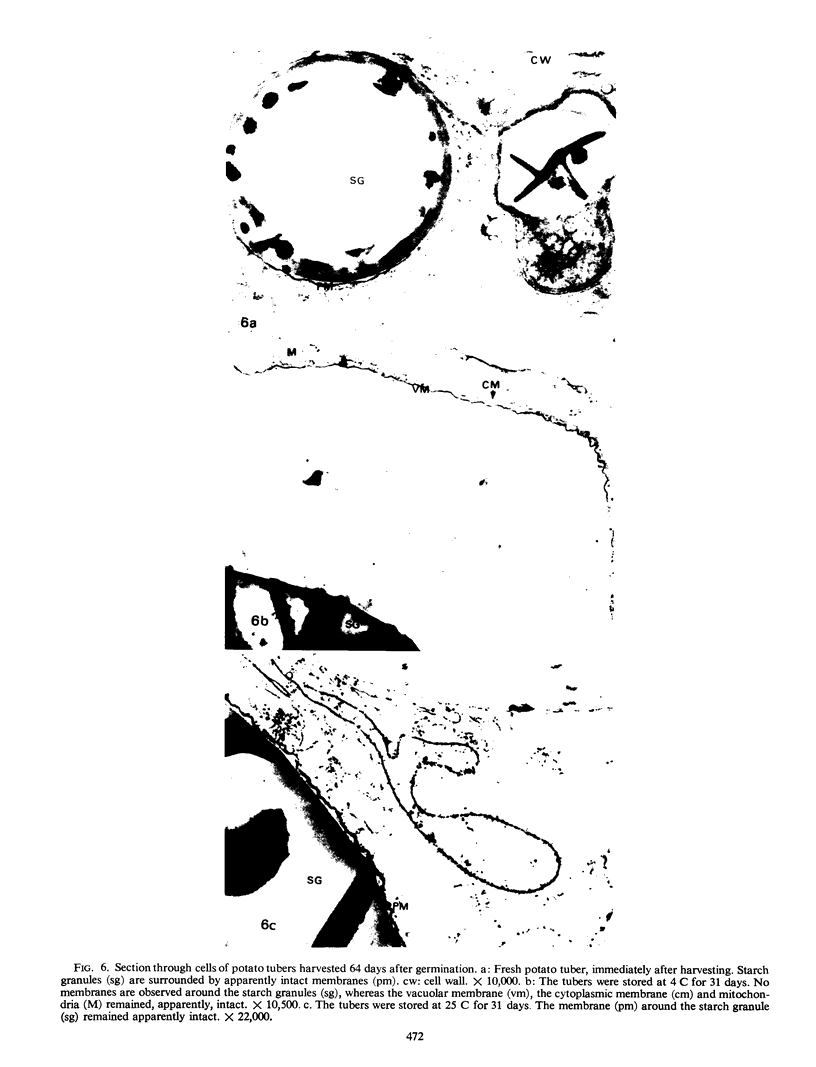
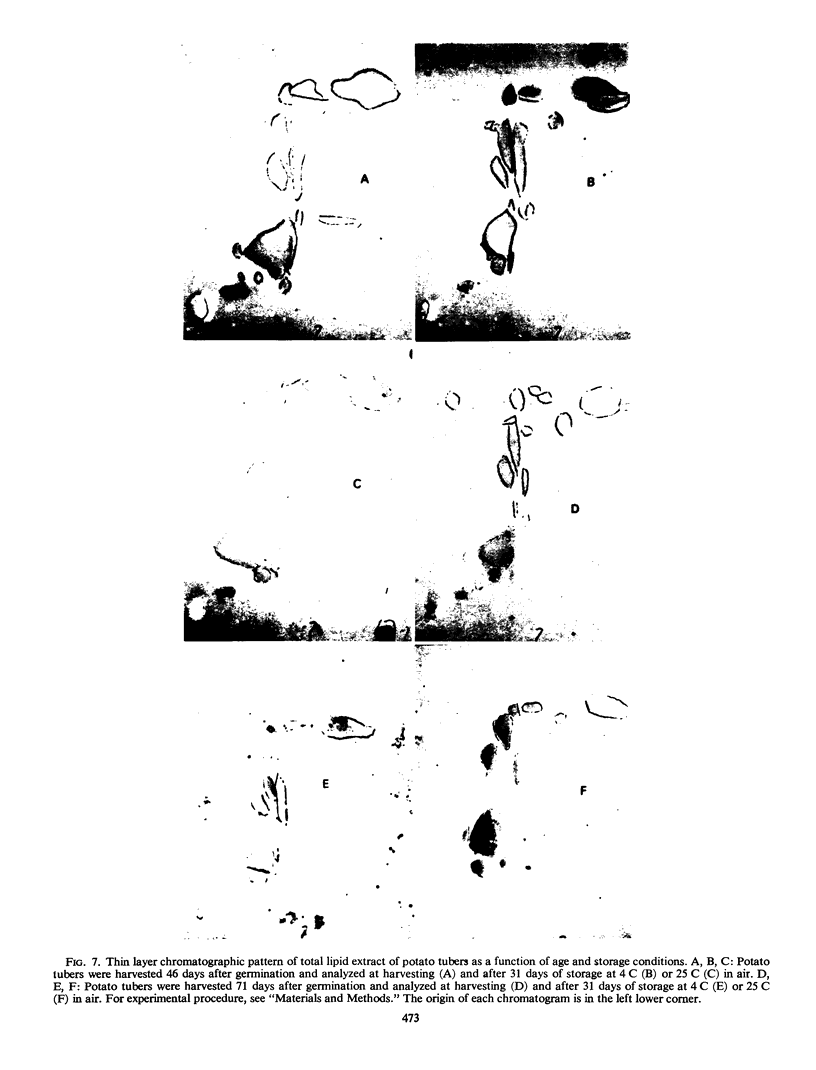
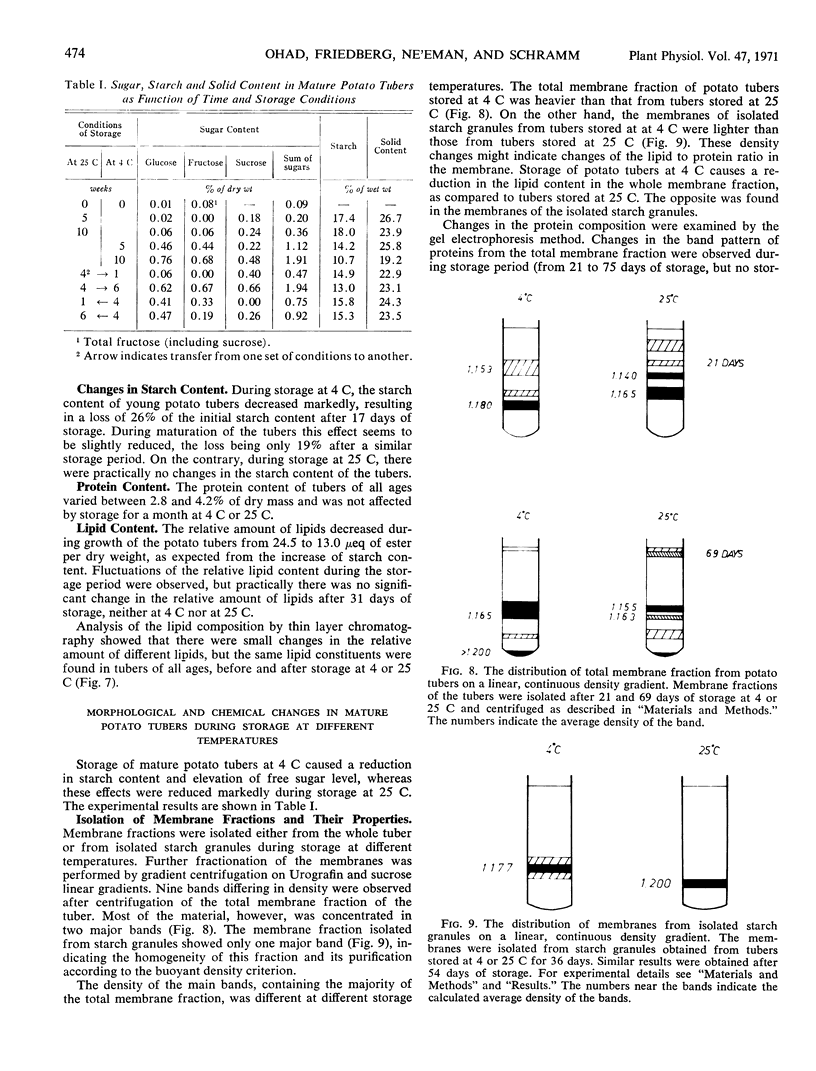
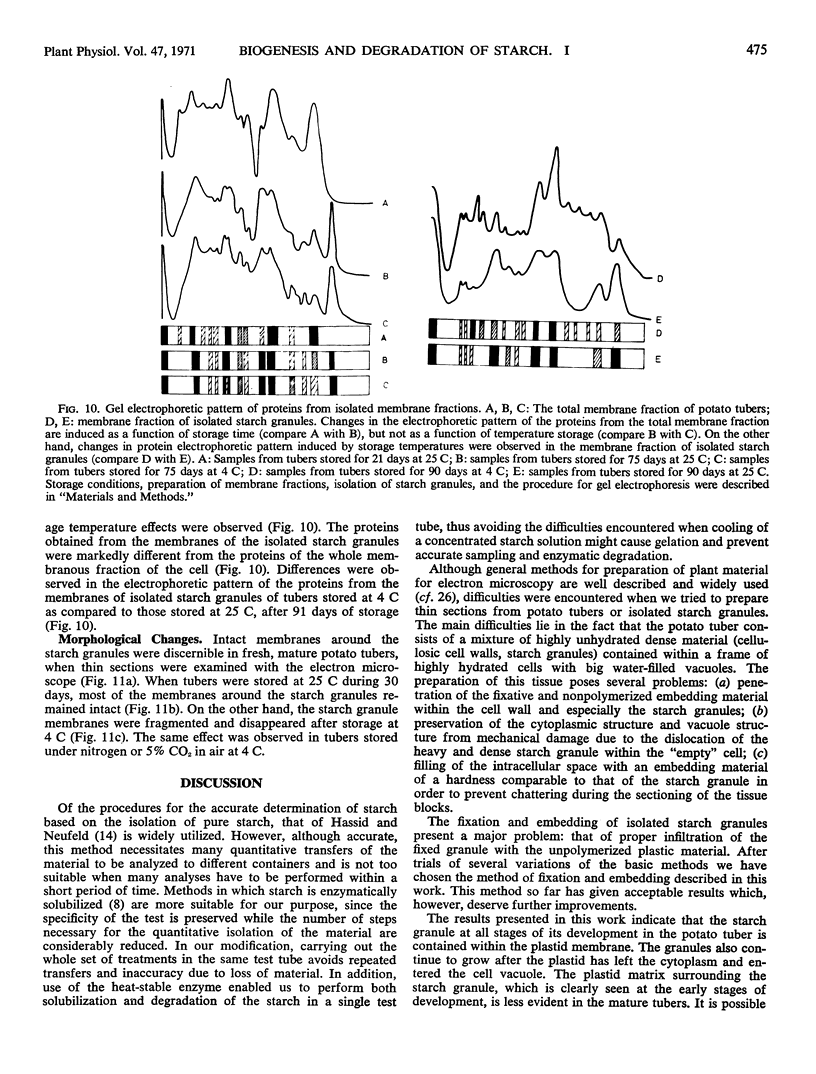
Storage of mature or developing potato tubers (Solanum tuberosum “Up-to-Date” variety) at 4 C causes a reduction in the starch content and the elevation in the level of free sugars. This phenomenon is not observed when the tubers are stored at 25 C. Changes in the morphology of cells from developing or mature tubers after storage at 4 or 25 C have been followed by electron microscopy. During all stages of the tuber development the starch granules are surrounded by a membrane derived from the plastid envelope. Storage in the cold induces disintegration of this membrane. A membrane fraction isolated from starch granules of tubers stored at 4 C has a lower buoyant density, and the electrophoretic pattern of its proteins is different from that of a similar membrane fraction obtained from tubers stored at 25 C. It is suggested that the cold-induced changes in the starch and sugar content during storage of potato tubers might be correlated with damage to the membranes surrounding the starch granules and changes in their permeability to degradative enzymes and substrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREGOFF H. M., ROBERTS E., DELWICHE C. C. Paper chromatography of quaternary ammonium bases and related compounds. J Biol Chem. 1953 Dec;205(2):565–574. [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRYDMAN R. B. STARCH SYNTHETASE OF POTATOES AND WAXY MAIZE. Arch Biochem Biophys. 1963 Aug;102:242–248. doi: 10.1016/0003-9861(63)90177-2. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. II. Some properties of the adenosine diphosphate glucose:starch glucosyltransferase bound to the starch granule. J Biol Chem. 1967 Jan 25;242(2):312–317. [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. The use of glucose oxidase (notatin) for the determination of glucose in biological material and for the study of glucose-producing systems by manometric methods. Biochem J. 1948;42(2):230–238. [PMC free article] [PubMed] [Google Scholar]
- LEPAGE M. THE SEPARATION AND IDENTIFICATION OF PLANT PHOSPHOLIPIDS AND GLYCOLIPIDS BY TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Jan;13:99–103. doi: 10.1016/s0021-9673(01)95078-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Ne'eman Z., Ohad I. Selective reaggregation of solubilized Mycoplasma-membrane proteins and the kinetics of membrane reformation. Biochim Biophys Acta. 1969;193(2):277–293. doi: 10.1016/0005-2736(69)90189-8. [DOI] [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzakis J. A. Uranyl acetate, a stain and a fixative. J Ultrastruct Res. 1968 Jan;22(1):168–184. doi: 10.1016/s0022-5320(68)90055-5. [DOI] [PubMed] [Google Scholar]