Abstract
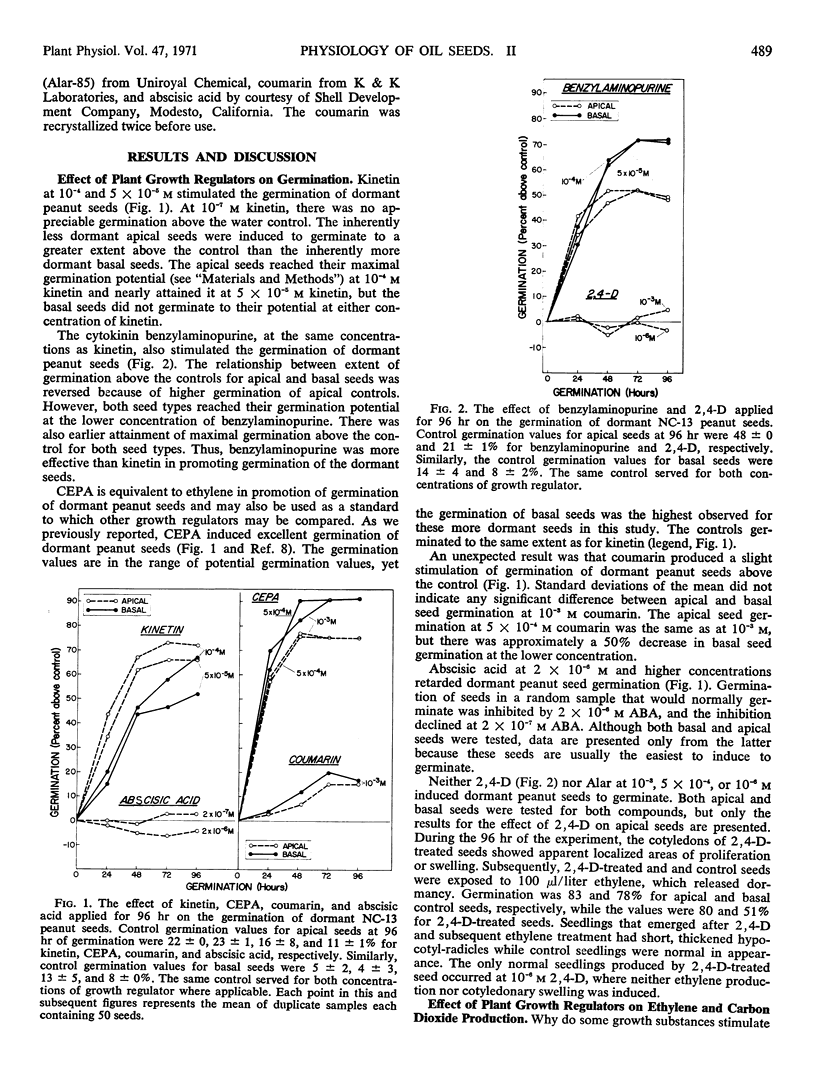
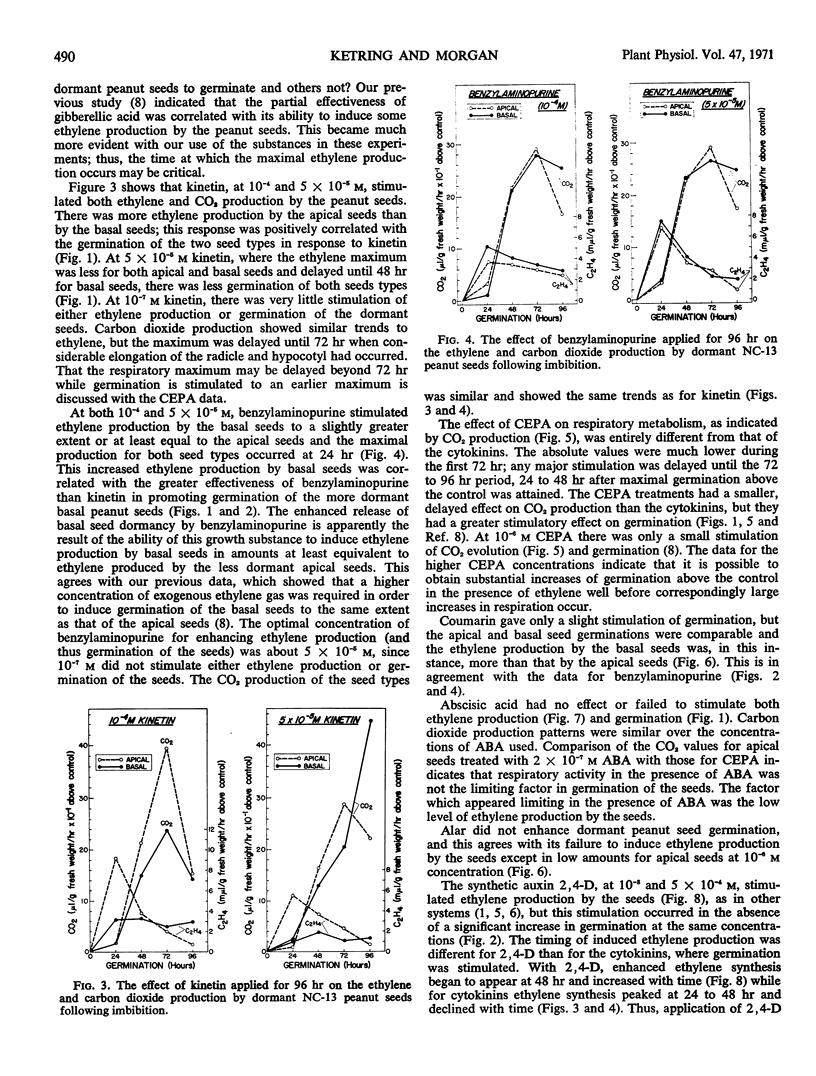
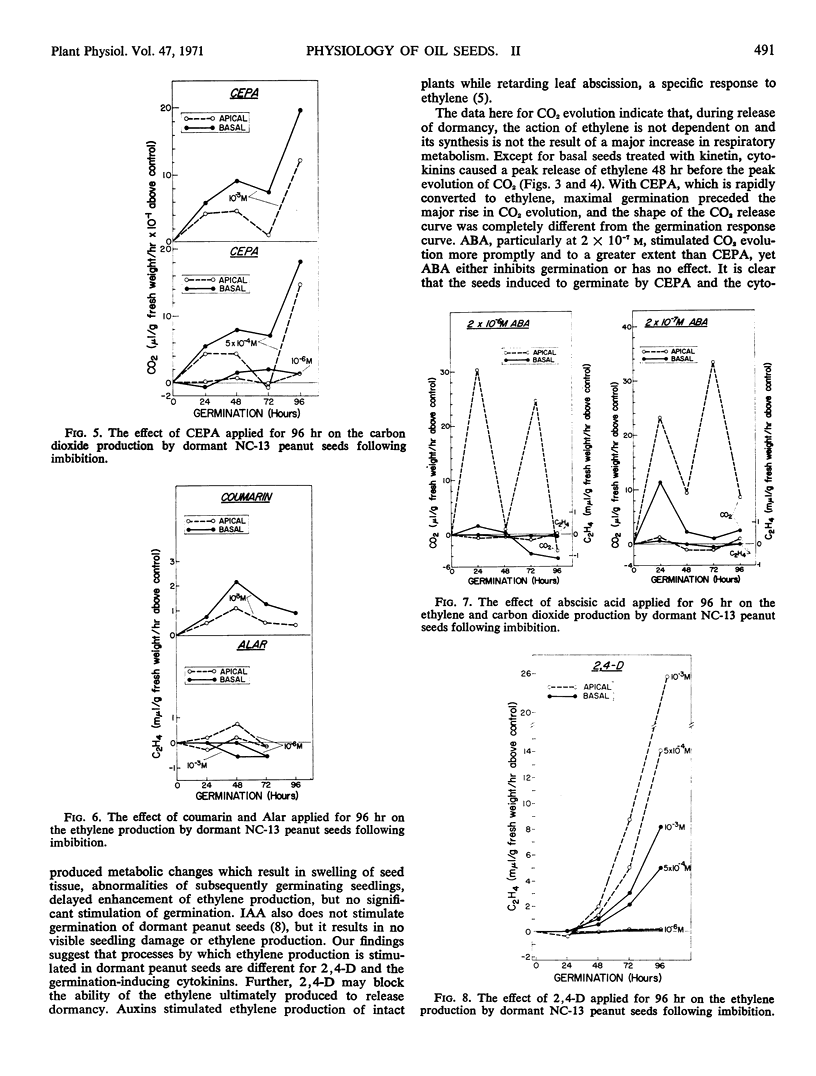
Germination, ethylene production, and carbon dioxide production by dormant Virginia-type peanuts were determined during treatments with plant growth regulators. Kinetin, benzylaminopurine, and 2-chloroethylphosphonic acid induced extensive germination above the water controls. Benzylaminopurine and 2-chloroethylphosphonic acid increased the germination of the more dormant basal seeds to a larger extent above the controls than the less dormant apical seeds. Coumarin induced a slight stimulation of germination while abscisic acid, 2,4-dichlorophenoxyacetic acid, and succinic acid 2,2-dimethylhydrazide did not stimulate germination above the controls. In addition to stimulating germination, the cytokinins also stimulated ethylene production by the seeds. In the case of benzylaminopurine, where the more dormant basal seeds were stimulated to germinate above the control to a larger extent than the less dormant apical seeds, correspondingly more ethylene production was induced in the basal seeds. However, the opposite was true of kinetin for both germination and ethylene production. When germination was extensively stimulated by the cytokinins, maximal ethylene and carbon dioxide evolution occurred at 24 and 72 hours, respectively. Abscisic acid inhibited ethylene production and germinaton of the seeds while carbon dioxide evolution was comparatively high. The crucial physiological event for germination of dormant peanut seeds was enhancement of ethylene production by the seeds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Auxin stimulation of ethylene evolution. Plant Physiol. 1966 Apr;41(4):585–588. doi: 10.1104/pp.41.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esashi Y., Leopold A. C. Dormancy regulation in subterranean clover seeds by ethylene. Plant Physiol. 1969 Oct;44(10):1470–1472. doi: 10.1104/pp.44.10.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring D. L., Morgan P. W. Ethylene as a Component of the Emanations From Germinating Peanut Seeds and Its Effect on Dormant Virginia-type Seeds. Plant Physiol. 1969 Mar;44(3):326–330. doi: 10.1104/pp.44.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring D. L., Morgan P. W. Physiology of oil seeds: I. Regulation of dormancy in virginia-type peanut seeds. Plant Physiol. 1970 Mar;45(3):268–272. doi: 10.1104/pp.45.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney N. E. Control of apple ripening by succinic Acid 2,2-dimethyl hydrazide, 2-chloroethyltrimethylammonium chloride, and ethylene. Plant Physiol. 1969 Aug;44(8):1127–1131. doi: 10.1104/pp.44.8.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. W., Powell R. D. Involvement of ethylene in responses of etiolated bean hypocotyl hook to coumarin. Plant Physiol. 1970 May;45(5):553–557. doi: 10.1104/pp.45.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E. R., Freebairn H. T. Ethylene, seed germination, and epinasty. Plant Physiol. 1969 Jul;44(7):955–958. doi: 10.1104/pp.44.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]


