Abstract
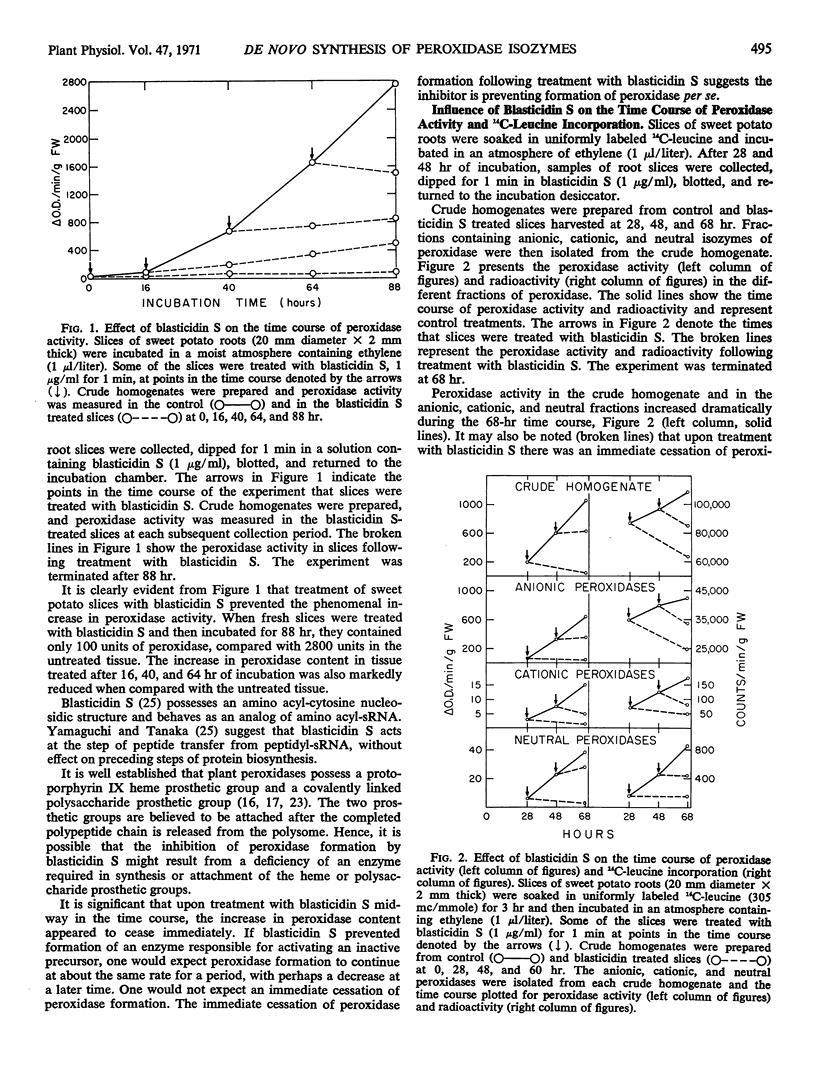
The peroxidase content of sweet potato slices (Ipomoea batatas Lam.) increased nearly 100-fold following 84 hours incubation in an air atmosphere containing ethylene, 1 microliter per liter. The object of experiments reported here is to determine if this increase in peroxidase activity results from synthesis de novo of the enzyme or from activation of a preexisting inactive form of the enzyme.
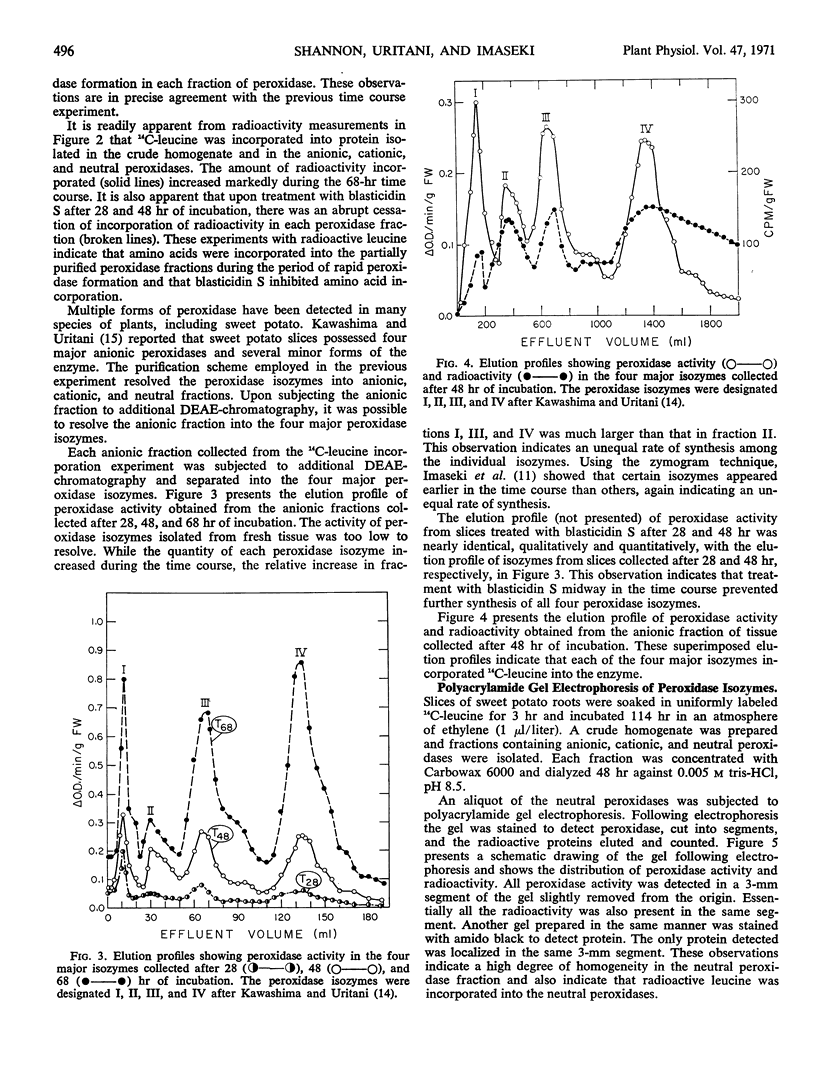
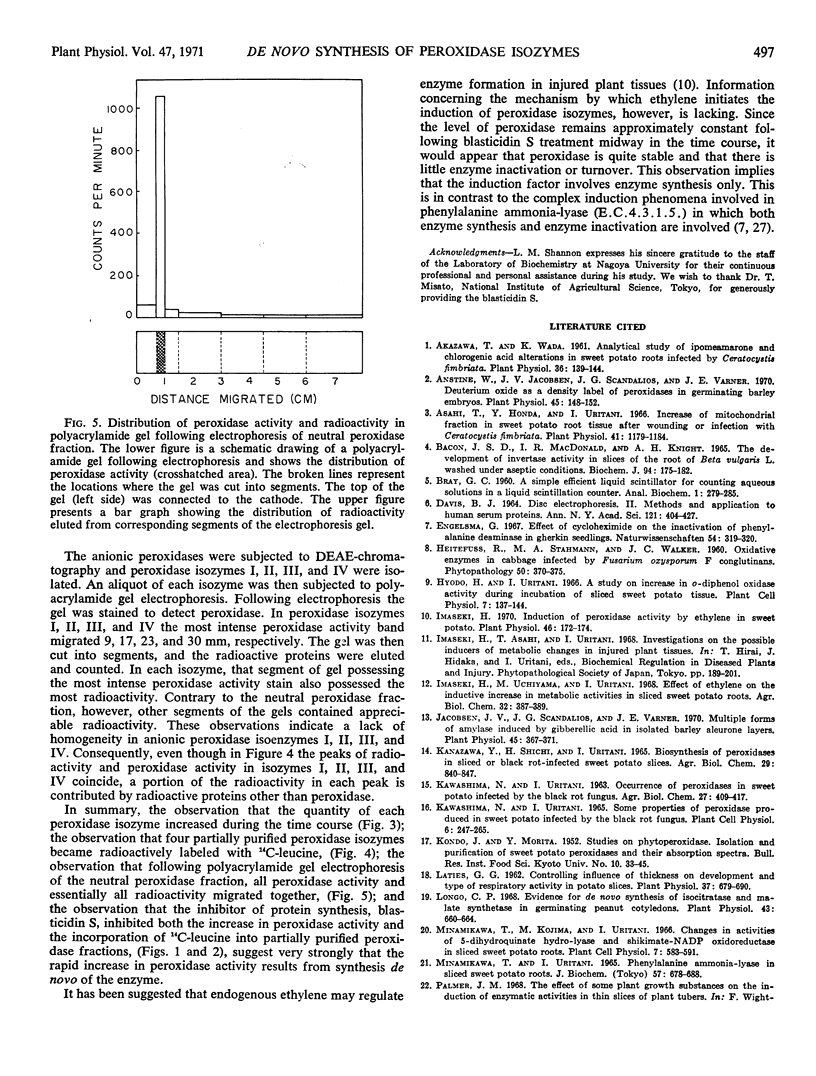
The enzymatic activity of each peroxidase isozyme increased during the incubation period, and each peroxidase isozyme appeared to incorporate 14C-leucine. Polyacrylamide gel electrophoresis of the neutral peroxidase fraction showed that all peroxidase activity and essentially all radioactivity migrated as a single superimposable band. The other peroxidase fractions were less pure. Treatment of fresh slices, or slices collected midway in the time course with the inhibitor of protein synthesis, blasticidin S, (1 microgram per milliliter for one minute) caused an abrupt cessation of peroxidase formation and simultaneously an abrupt cessation of incorporation of 14C-leucine into peroxidase isozymes. These observations indicate that the rapid increase in peroxidase activity in sweet potato slices results from synthesis de novo of the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa T., Wada K. Analytical study of ipomeamarone & chlorogenic acid alterations in sweet potato roots infected by Ceratocystis fimbriata. Plant Physiol. 1961 Mar;36(2):139–144. doi: 10.1104/pp.36.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstine W., Jacobsen J. V., Scandalios J. G., Varner J. E. Deuterium oxide as a density label of peroxidases in germinating barley embryos. Plant Physiol. 1970 Feb;45(2):148–152. doi: 10.1104/pp.45.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi T., Honda Y., Uritani I. Increase of Mitochondrial Fraction in Sweet Potato Root Tissue after Wounding or Infection with Ceratocystis fimbriata. Plant Physiol. 1966 Sep;41(7):1179–1184. doi: 10.1104/pp.41.7.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON J. S., MACDONALD I. R., KNIGHT A. H. THE DEVELOPMENT OF INVERTASE ACTIVITY IN SLICES OF THE ROOT OF BETA VULGARIS L. WASHED UNDER ASEPTIC CONDITIONS. Biochem J. 1965 Jan;94:175–182. doi: 10.1042/bj0940175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Engelsma G. Effect of cycloheximide on the inactivation of phenylalanine deaminase in gherkin seedlings. Naturwissenschaften. 1967 Jun;54(12):319–320. doi: 10.1007/BF00640619. [DOI] [PubMed] [Google Scholar]
- Imaseki H. Induction of peroxidase activity by ethylene in sweet potato. Plant Physiol. 1970 Jul;46(1):172–174. doi: 10.1104/pp.46.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Scandalios J. G., Varner J. E. Multiple forms of amylase induced by gibberellic acid in isolated barley aleurone layers. Plant Physiol. 1970 Apr;45(4):367–371. doi: 10.1104/pp.45.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. Controlling Influence of Thickness on Development & Type of Respiratory Activity in Potato Slices. Plant Physiol. 1962 Sep;37(5):679–690. doi: 10.1104/pp.37.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmann M. A., Clare B. G., Woodbury W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966 Nov;41(9):1505–1512. doi: 10.1104/pp.41.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. E., Pratt H. K., Biale J. B. IDENTIFICATION OF ETHYLENE AS A VOLATILE PRODUCT OF THE FUNGUS PENICILLIUM DIGITATUM. Plant Physiol. 1951 Apr;26(2):304–310. doi: 10.1104/pp.26.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


