Abstract
Wnt regulates bone formation through β-catenin-dependent canonical and -independent noncanonical signaling pathways. However, the cooperation that exists between the two signaling pathways during osteoblastogenesis remains to be elucidated. Here, we showed that the lack of Wnt5a in osteoblast-lineage cells impaired Wnt/β-catenin signaling due to the reduced expression of Lrp5 and Lrp6. Pretreatment of ST2 cells, a stromal cell line, with Wnt5a enhanced canonical Wnt ligand-induced Tcf/Lef transcription activity. Short hairpin RNA-mediated knockdown of Wnt5a, but not treatment with Dkk1, an antagonist of Wnt/β-catenin signaling, reduced the expression of Lrp5 and Lrp6 in osteoblast-lineage cells under osteogenic culture conditions. Osteoblast-lineage cells from Wnt5a-deficient mice exhibited reduced Wnt/β-catenin signaling, which impaired osteoblast differentiation and enhanced adipocyte differentiation. Adenovirus-mediated gene transfer of Lrp5 into Wnt5a-deficient osteoblast-lineage cells rescued their phenotypic features. Therefore, Wnt5a-induced noncanonical signaling cooperates with Wnt/β-catenin signaling to achieve proper bone formation.
Wnt signaling plays critical roles in the development, growth, and homeostasis of various organs including the skeletal system1,2,3,4. The binding of Wnt to receptor complexes activates β-catenin-dependent canonical and β-catenin-independent noncanonical signaling pathways5.
In the absence of Wnt, a complex of APC, axin, and glycogen synthase kinase-3β (GSK-3β) phosphorylates β-catenin. Phosphorylated β-catenin subsequently undergoes ubiquitination and degradation. Canonical Wnt such as Wnt3a binds to the receptor complex of Frizzled (Fzd) and low density lipoprotein receptor-related protein 5 (Lrp5) or Lrp6. This complex inhibits the kinase activity of GSK-3β, which in turn induces the accumulation of β-catenin in the target cells. The accumulation of β-catenin leads to its translocation into the nucleus, where it interacts with T-cell factor/lymphoid enhancer factor (Tcf/Lef) family members to initiate the transcription of target genes. TAZ, a transcription factor for the hippo pathway, has also recently been shown to function as an inducer for osteoblastogenesis and a suppressor for adipogenesis during canonical Wnt signaling6. On the other hand, Wnt5a binds to the receptor complex of Fzd, Ror1/2 or Ryk, and activates β-catenin-independent noncanonical signaling including Wnt/Ca2+ and Wnt/planar cell polarity pathways7.
The importance of Lrp5 in bone formation was exemplified by identification of mutations within the LRP5 gene of patients with osteoporosis-pseudoglioma syndrome (OPPG)8. The number of osteoblasts and bone mass in Lrp5−/− mice was reduced9. Lrp5 signaling in the duodenum was shown to regulate bone formation by inhibiting serotonin synthesis10. The findings of the study indicated that Lrp5 may function in the gut to regulate bone mass. However, the following studies highlighted us of the importance of Lrp5 in osteoblast-lineage cells. Mice with the osteocyte-specific, but not gut-specific expression of a gain-of-function mutant of Lrp5 (G171V or A214V) exhibited a high bone mass associated with an increase in bone formation11. Lrp5 signaling is recently reported to promote bone formation through direct reprogramming of glucose metabolism in osteoblasts12. These findings suggest that Lrp5 signaling is important for the regulation of bone formation. However, the regulation of Lrp5 and Lrp6 expression in osteoblasts has not been fully elucidated.
Wnt5a-induced noncanonical Wnt signaling has been shown to suppress adipogenesis, which, in turn, promotes the differentiation of mesenchymal stem cells into osteoblast lineage cells13. Wnt5a+/− mice exhibited a low bone mass with increased adipogenesis and decreased osteoblastogenesis. Wnt5a suppressed Ppar-γ transactivation by a co-repressor complex through calcium-calmodulin-dependent protein kinase II-TGF-β activated kinase 1-Nemo-like kinase signaling and induced the expression of Runx2, leading to promotion of osteoblastogenesis13. Moreover, osteoblast-lineage cell-specific Wnt5a-deficient mice (Wnt5a cKO) exhibited a low bone mass with decreased bone formation14. Thus, noncanonical Wnt signals also promote osteoblastogenesis. These previous studies have indicated that both canonical and noncanonical Wnt signalings are required for proper bone formation. However, there is little information about how these two signaling pathways might cooperate with each other during osteoblastogenesis.
Here we showed that Wnt5a-induced noncanonical signaling promoted osteoblast differentiation through the up-regulation of Lrp5 and Lrp6. Osteoblast-lineage cells from the calvariae of Wnt5a−/− mice showed impaired mineralization due to the decreased expression of Lrp5 and Lrp6. The overexpression of Lrp5 in Wnt5a−/− calvarial cells rescued their phenotypes. Thus, Wnt5a induced the up-regulation of Lrp5 and Lrp6, which promoted osteoblast differentiation by canonical Wnt protein-mediated signaling.
Results
Pretreatment with Wnt5a enhances Wnt/β-catenin signaling
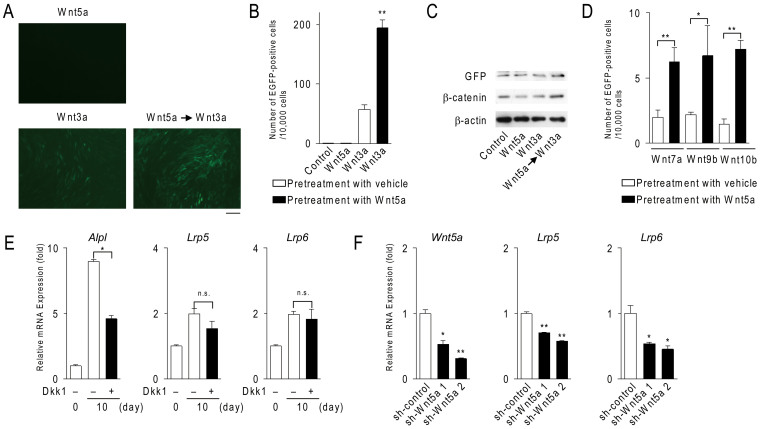
Using bone marrow stromal ST2 cells, in which the Tcf/Lef-EGFP reporter was stably expressed, we found that pretreatment of ST2 cells with Wnt5a enhanced Wnt3a-induced EGFP expression while Wnt5a failed to induce EGFP expression (Fig. 1, A and B). Western blot analysis confirmed that the pretreatment with Wnt5a enhanced Wnt3a-induced EGFP expression and also the Wnt3a-induced accumulation of cytosolic β-catenin in ST2 cells (Fig. 1C). This pretreatment also enhanced Wnt/β-catenin signaling induced by canonical Wnt ligands such as Wnt7a, Wnt9b, and Wnt10b in ST2 cells (Fig. 1D).
Figure 1. Wnt5a-induced noncanonical signals regulate the expression of Lrp5 and Lrp6.
(A–D) The effects of Wnt5a on canonical Wnt ligand-induced Tcf/Lef activity in ST2 cells. The Tcf/Lef-EGFP reporter was stably expressed in ST2 cells. After culturing with 200 ng/ml of Wnt5a or vehicle for 24 hours, ST2 cells were washed with α-MEM and further cultured for 48 hours in the presence or absence of 100 ng/ml Wnt ligands. EGFP-positive cells were counted. (A) Micrographs of ST2 cell cultures. Scale bar, 30 μm. (B, D) Number of EGFP-positive cells per 10,000 cells. (C) Western blot analysis of ST2 cell cultures. ST2 cells were cultured using the same method as described above. (E) Effects of Dkk1 on Lrp5/6 expression in calvarial cells. Calvarial cells were cultured for the indicated time in the presence or absence of 1 μg/ml Dkk1. The expression of Alpl, Lrp5/6 mRNA was detected using real-time PCR. (F) Effects of the shRNA-mediated knockdown of Wnt5a on Lrp5/6 expression in calvarial cells. shRNAs were transfected into calvarial cells using retrovirus. After the transfection, calvarial cells were cultured for 10 days in osteogenic medium. The expression of Lrp5/6 mRNA was detected. In (B, D–F), data are expressed as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, n.s.; not significant. In (C), the full length blots were presented in Supplementary Fig. S5.
Expression of Wnt, Wnt co-receptors, and Fzd during osteoblast differentiation
The above findings prompted us to clarify the roles of Wnt5a in the enhancement of Wnt/β-catenin signaling during osteoblastogenesis. We examined the expression of Wnt ligands such as Wnt5a, Wnt7b, and Wnt10b in calvarial cells under osteogenic culture conditions (Supplementary Fig. S1A online). The expression of Wnt5a and Wnt10b mRNA, but not Wnt7b mRNA was increased in these cultures. Consistently, Wnt5a and Wnt10b, but not Wnt7b, protein levels were increased in these cultures in a time-dependent manner (Supplementary Fig. S1B).
We next examined the expression of Wnt receptors in those cells. The expression of Lrp5 and Lrp6, but not Ror2 or Ryk, was increased in calvarial cells during osteoblast differentiation (Supplementary Fig. S1C). Western blot analysis revealed that the expression of Lrp5 and Lrp6 was increased in these calvarial cell cultures (Supplementary Fig. S1D). These results suggest that the expression of Lrp5 and Lrp6 is increased in calvarial cells during osteoblast differentiation.
The expression of Lrp5 and Lrp6 in calvarial cells was increased with increasing expression of Wnt ligands such as Wnt10b and Wnt5a. To clarify the roles of Wnt/β-catenin signaling in the up-regulation of Lrp5 and Lrp6 in calvarial cells, Dickkopf1 (Dkk1), which disrupts Wnt/Lrp5 or Lrp6 interactions to inhibit Wnt/β-catenin15 and Wnt/TAZ signals6, was added to calvarial cell cultures, and the expression of Lrp5 and Lrp6 was examined (Fig. 1E). The expression of Alpl (encoding alkaline phosphatase), a marker of osteoblasts, was increased during osteoblastogenesis. The treatment of calvarial cells with Dkk1 suppressed the expression of Alpl, but not that of Lrp5 or Lrp6. These results indicate that canonical Wnt signals do not promote the expression of Lrp5/6 in calvarial cells.
Therefore, we examined whether noncanonical Wnt5a regulates Lrp5 and Lrp6 expression in calvarial cells using short hairpin-RNA-mediated knockdown of Wnt5a in calvarial cells. The suppression of Wnt5a expression led to the reduced expression of Lrp5 and Lrp6 (Fig. 1F).
Wnt5a regulates Lrp5/6 expression
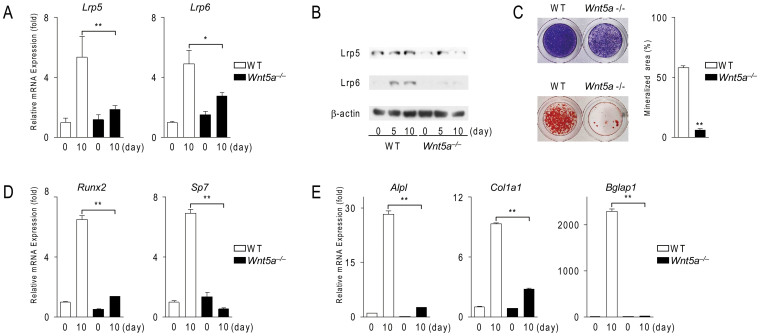
To confirm the up-regulation of Lrp5/6 expression by Wnt5a in calvarial cells, we examined their expressions in Wnt5a−/− calvarial cells compared with wild-type cells. The expression of Lrp5/6 was significantly lower in Wnt5a−/− cells (n = 3, P = 0.00003) (Fig. 2A). The expression levels of the Lrp5 and Lrp6 proteins in Wnt5a−/− cells was lower than those in wild-type cells (Fig. 2B). These results suggest that Wnt5a positively regulates the expression of Lrp5/6 in calvarial cells during their differentiation into osteoblasts.
Figure 2. Wnt5a-deficient calvarial cells exhibit impaired osteoblastogenesis associated with a reduction in Lrp5 and Lrp6 expression.
(A) The expression of Lrp5 and Lrp6 in wild-type (WT) and Wnt5a−/− calvarial cells. Calvarial cells were cultured in osteogenic medium and subjected to real-time PCR analysis. (B) Western blot analysis of Lrp5/6 expression in WT and Wnt5a−/− calvarial cells. Cells were cultured using the same method as that described in (A). The full length blots were presented in Supplementary Fig. S5. (C) Cytochemical staining for alkaline phosphatase activity (top. blue) and alizarin red S staining (bottom, red) in WT and Wnt5a−/− calvarial cell cultures in osteogenic medium in the presence of BMP2 (200 ng/ml). (D, E) The expression of osteoblastic transcription factors (D) and marker genes (E) in WT and Wnt5a−/− calvarial cell cultures. Cells were cultured using the same method as that described in (A). In (A, C–E), data are expressed as the mean ± SD (n = 3). *p < 0.05, **p < 0.01.
Wnt5a−/− calvarial cells exhibited impaired mineralization, a criterion for osteoblast differentiation. Consistent with a previous study14, alkaline phosphatase activity and mineralization were significantly lower in Wnt5a−/− cells (n = 3, P = 0.000000006) (Fig. 2C). The expression of Runx2 and Sp7 (encoding Osterix), transcription factors necessary for osteoblast differentiation16,17,18, was increased in wild-type calvarial cells under osteogenic culture conditions at day 10. However, the expression of these genes was lower in Wnt5a−/− cells (Fig. 2D). As expected, the expression of Alpl, Col1a1 (encoding type I collagen α1), and Bglap1 (encoding osteocalcin), markers for osteoblasts, was also lower in Wnt5a−/− calvarial cells (Fig. 2E). These results suggest that osteoblast differentiation is impaired in Wnt5a−/− calvarial cells.
Wnt5a−/− calvarial cells exhibited impaired Wnt/β-catenin signaling
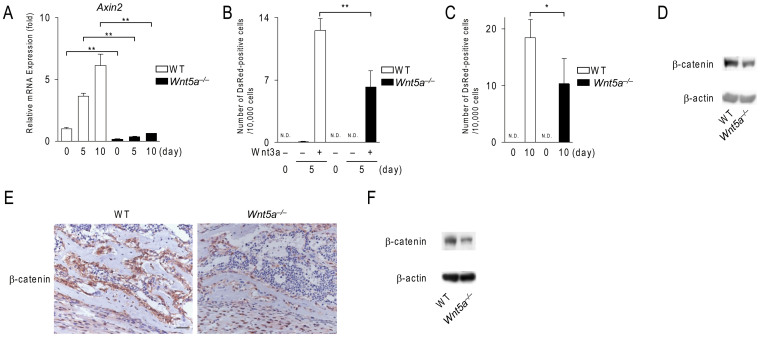
Experiments using Wnt5a−/− calvarial cells indicated that endogenous Wnt5a up-regulated the expression of Lrp5/6 in calvarial cells during osteoblastogenesis. Therefore, we examined whether Wnt/β-catenin signaling was impaired in Wnt5a−/− calvarial cells. The expression of Axin2, a target gene of Wnt/β-catenin signaling, was decreased in Wnt5a−/− calvarial cells (Fig. 3A). We further confirmed impaired Wnt/β-catenin signaling in Wnt5a−/− calvarial cells using adenoviral-mediated gene transfers of the Super-TOP-DsRed reporter (Supplementary Fig. S2). The number of DsRed-positive cells was significantly lower in Wnt5a−/− calvarial cells treated with Wnt3a (n = 4, P = 0.002) (Fig. 3B) and under osteogenic conditions without any exogenous Wnt proteins (Fig. 3C). Western blot analysis revealed that the expression level of β-catenin was lower in Wnt5a−/− calvarial cells than in wild-type cells at day 10 (Fig. 3D). Immunohistochemical analysis revealed that the protein level of β-catenin in bone-lining osteoblasts was lower in the scapulae from Wnt5a−/− mice (Fig. 3E). Western blot analysis also confirmed that the expression level of β-catenin in the scapula of Wnt5a−/− mice was lower than that in the scapula of wild-type mice (Fig. 3F). These results indicate that Wnt/β-catenin signaling is impaired in Wnt5a−/− osteoblast-lineage cells.
Figure 3. Wnt5a-deficient calvarial cells exhibit impaired Wnt/β-catenin signals.
(A) The expression of Axin2 in wild-type (WT) and Wnt5a−/− calvarial cell cultures. Cells were cultured in osteogenic medium for the indicated time and subjected to real-time PCR analysis. (B, C) The number of DsRed-positive cells in WT and Wnt5a−/− calvarial cell cultures transfected with the Tcf/Lef DsRed reporter adenovirus. In (B), cells were treated with Wnt3a (100 ng/ml) under growth culture conditions. In (C), cells were cultured under osteogenic conditions. (D) Western blot analysis of β-catenin expression in WT and Wnt5a−/− calvarial cells. Cells were cultured in osteogenic medium for 10 days. (E) Immunohistochemical staining (brown) of β-catenin in the scapulae of WT and Wnt5a−/− mice at E18.5. (F) Western blot analysis of β-catenin in the scapulae from WT and Wnt5a−/− mice at E18.5. Scale bar, 30 μm. In (A–C), data are expressed as the mean ± SD (n = 3–4). *p < 0.05, **p < 0.01, N.D.; not detected. In (D, F), the full length blots were presented in Supplementary Fig. S5.
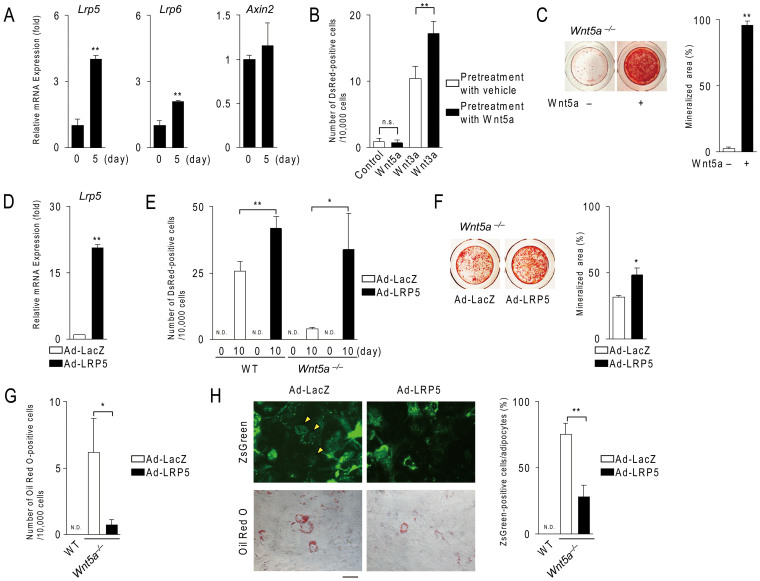
We next examined the effects of exogenous Wnt5a on osteoblastogenesis in Wnt5a−/− calvarial cells. The treatment of these cells with Wnt5a enhanced the expression of Lrp5 and Lrp6 (Fig. 4A), but failed to increase the expression of Axin2 mRNA at day 5, and this may have been due to the insufficient expression of endogenous canonical Wnt ligands at this time point. We, therefore, examined the effects of pretreatment with Wnt5a on Wnt3a-induced Wnt/β-catenin signaling in Wnt5a−/− calvarial cells. Similar to ST2 cell cultures (Fig. 1A), the pretreatment with Wnt5a enhanced Wnt3a-induced the expression of DsRed in those cultures (Fig. 4B). Western blot analysis confirmed that the pretreatment with Wnt5a increased the protein levels of Lrp5/6 in Wnt5a−/−, but not wild-type, calvarial cell cultures (Supplementary Fig. S3). These findings suggest that Wnt5a is abundantly secreted from wild-type calvarial cells in osteogenic medium, and that Wnt5a enhances expression of Lrp5/6 although Wnt5a does not directly activate Wnt/β-catenin signaling. Furthermore, treatments of Wnt5a−/− calvarial cells with Wnt5a rescued mineralization within 14 days (Fig. 4C).
Figure 4. Treatment with Wnt5a or the expression of Lrp5 rescues the phenotypic features of Wnt5a−/− calvarial cells.
(A) The effect of Wnt5a on the expression of Lrp5, Lrp6 and Axin2 in Wnt5a−/− calvarial cells. Cells were cultured in the presence of Wnt5a (500 ng/ml) under osteogenic conditions and subjected to real-time PCR analysis. (B) Effects of the pretreatment with Wnt5a on Wnt3a-induced Wnt/β-catenin signaling in Wnt5a−/− calvarial cells. Cells transfected with the Tcf/Lef DsRed reporters were treated with Wnt5a (500 ng/ml) or vehicle for 5days and further cultured for 48 hours with or without Wnt3a (100 ng/ml). (C) The effect of Wnt5a on mineralization in Wnt5a−/− calvarial cells. Cells were cultured in the presence of Wnt5a (500 ng/ml) plus BMP2 (200 ng/ml) under osteogenic conditions for 14 days. (D) The expression of Lrp5 in Wnt5a−/− calvarial cells infected with LacZ- and Lrp5-adenovirus. After the adenovirus-mediated gene transfer of Lrp5, cells were further cultured for 1 day. (E) The number of DsRed-positive cells in wild-type (WT) and Wnt5a−/− calvarial cells infected with LacZ- and Lrp5-adenovirus together with the Tcf/Lef DsRed reporter adenovirus. After adenovirus-mediated gene transfer, cells were cultured in osteogenic medium for 10 days. (F) The effect of the adenoviral-gene transfer of Lrp5 or LacZ on mineralization in Wnt5a−/− calvarial cell cultures. After adenovirus-mediated gene transfer, cells were cultured using the same method as that described in (C). (G) The number of adipocytes in Wnt5a−/− calvarial cell cultures infected with LacZ- and Lrp5-adenovirus. Cells having oil droplets stained with oil Red O were counted as adipocytes. (H) The percent of ZsGreen-positive cells in total adipocytes. The transfection of Lrp5 and LacZ cDNA, and cell cultures were performed according to the described method in (G). Upper and lower photographs show the expression of ZsGreen and cytochemical staining with oil Red O, respectively. Scale bar, 15 μm. In (B), (E), and (G), Ds-Red-positive cells or adipocytes were counted, and number of DsRed-positive cells or adipocytes was adjusted to the total cell number and expressed per 10,000 cells. In (A–H), data are expressed as the mean ± SD (n > 3). *p < 0.05, **p < 0.01, n.s.; not significant, N.D.; not detected.
To efficiently express Lrp5 in calvarial cells, we prepared an adenovirus possessing the full length of Lrp5 cDNA. Real-time PCR and Western blot analysis confirmed the overexpression of Lrp5 in calvarial cells (Fig. 4D and Supplementary Fig. S4A). Furthermore, a luciferase assay using the TOP-Flash reporter also confirmed that the overexpression of Lrp5 enhanced Wnt3a-induced Tcf/Lef activity in calvarial cells (Supplementary Fig. S4B). Adenovirus-mediated gene transfer of Lrp5 into Wnt5a−/− cells rescued the Tcf/Lef activity (Fig. 4E) and the mineralization (Fig. 4F). These results suggest that Wnt5a promotes osteoblastogenesis through the expression of Lrp5 and Lrp6.
We have previously shown that adipogenesis was enhanced in Wnt5a−/− calvarial cell cultures, even under osteogenic culture conditions14. We next investigated whether the overexpression of Lrp5 suppressed adipogenesis in Wnt5a−/− calvarial cells. The number of adipocytes was significantly decreased in Wnt5a−/− calvarial cell cultures in which Lrp5 was expressed (n = 3, P = 0.01) (Fig. 4G). The percentage of ZsGreen-positive adipocytes (indicating an adenoviral infection) was lower in Wnt5a−/− calvarial cell cultures infected with the Lrp5-adenovirus (Fig. 4H). These results suggest that Wnt5a suppresses adipogenic differentiation in calvarial cells through the up-regulation of Lrp5 and Lrp6.
Effects of Sp7 on the expression of Lrp5/6
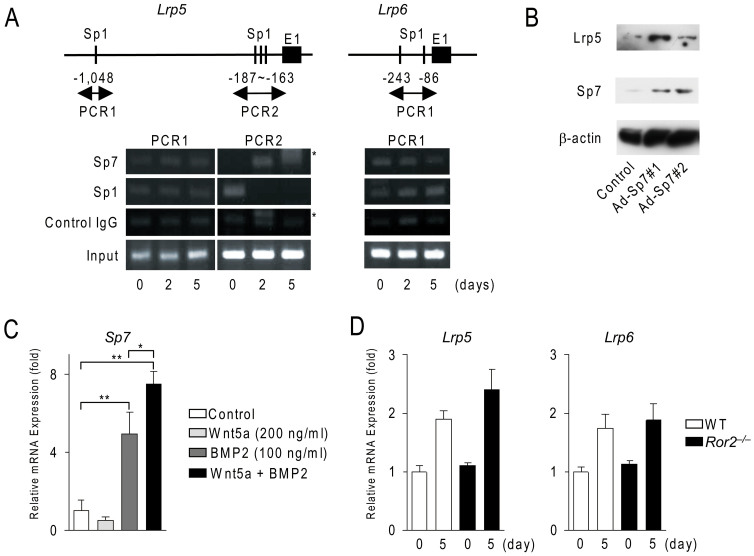
We attempted to identify the possible mechanism by which Wnt5a enhanced Lrp5/6 expression in calvarial cells (Fig. 5). Using the TRANSFAC retrieval program, we searched for putative transcription binding sites on the 2.0-kb Lrp5 promoter and 2.0-kb Lrp6 promoter, and found that both promoters contained putative Sp1 binding sites (Fig. 5A). Sp7 was previously shown to bind to Sp1-binding sites17. The expression of Sp7 was significantly lower in Wnt5a−/− calvarial cells (see Fig. 2D). These results suggest that Sp7 may up-regulate Lrp5/6 expression during osteoblast differentiation. The chromatin immunoprecipitation assay showed that Sp1 already bound the proximal Sp1 sites on the Lrp5 and Lrp6 promoters in wild-type calvarial cells. Sp7 was recruited to the proximal Sp1 sites on the Lrp5 promoter within 2 days, while the recruitment of Sp1 was decreased on the proximal Sp1 sites during the culture period. We previously reported that c-Jun was recruited to Sp1 sites on the tnfrsf11a promoter under Wnt5a-Ror2 signaling14. However, the recruitment of c-Jun was not observed on the proximal Sp1 sites on the Lrp5 promoter (data not shown). The recruitment of Sp1, but not Sp7, was increased on the proximal Sp1 sites on the Lrp6 promoter (Fig. 5A). These results suggest that Lrp5 expression may be regulated by Sp7 in calvarial cells.
Figure 5. Wnt5a and BMP2 cooperatively enhance Lrp5 expression through Sp7 in calvarial cells.
(A) Chromatin immunoprecipitation assay of the Lrp5 and Lrp6 genes in wild-type calvarial cells. Calvarial cells were cultured in osteogenic medium for the indicated time and subjected to ChiP analysis. Upper schemas show the promoter of the Lrp 5 and Lrp6 genes. Putative Sp1 binding sites were indicated. E1; exon 1. Asterisks indicate non-specific bands. The full length gels were presented in Supplementary Fig. S5. (B) Effects of Sp7 on Lrp 5 expression in wild-type calvarial cells. After the adenovirus-mediated gene transfer of LacZ (Control) or Sp7 cDNA, cells were cultured in osteogenic medium for 2 days and subjected to Western blot analysis. The full length blots were presented in Supplementary Fig. S5. (C) Effects of Wnt5a and BMP2 on Sp7 expression in Wnt5a−/− calvarial cells. Cells were cultured in the presence or absence of Wnt5a (200 ng/ml) with or without BMP2 (100 ng/ml). The expression of Sp7 mRNA was examined in these cultures. *p < 0.05, **p < 0.01 (D) The expression of Lrp5 and Lrp6 mRNA in Ror2−/− calvarial cells. Wild-type (WT) and Ror2−/− calvarial cells were cultured under osteogenic conditions and subjected to real-time PCR analysis. In (C, D), data are expressed as the mean ± SD (n = 3).
We next examined whether the overexpression of Sp7 up-regulated Lrp5 expression in calvarial cells (Fig. 5B). The adenovirus-mediated gene transfer of Sp7 into wild-type calvarial cells increased the expression of the Lrp5 protein.
Bone morphogenetic protein 2 (BMP2) was previously reported to induce Sp7 expression in C2C12 cells19. Wnt5a enhanced BMP2 signals in C2C12 cells20. Therefore, we examined the effects of Wnt5a and BMP2 on the expression of Sp7 in Wnt5a−/− calvarial cells (Fig. 5C). Although Wnt5a by itself failed to induce the expression of Sp7 in calvarial cells, Wnt5a enhanced the BMP2-induced expression of Sp7. These results suggested that BMP2 and Wnt5a co-operatively regulate Sp7 expression in calvarial cells.
We finally examined the involvement of Ror2 co-receptors in Lrp5/6 expression in calvarial cells. Real-time-PCR analysis revealed that the expression of Lrp5/6 in Ror2−/− calvarial cells was similar to that in wild-type calvarial cells (Fig. 5D). We previously demonstrated that mineralized nodule formations commonly occurred in Ror2−/− calvarial cell cultures14. These results suggest that Ror2 co-receptors were not involved in Lrp5/6 expression in osteoblastogenesis.
Discussion
In this study, we demonstrated that noncanonical Wnt5a up-regulated the expression of Lrp5/6 in osteoblast-lineage cells, which promoted osteoblastogenesis and inhibited adipogenesis via Wnt/β-catenin signaling.
Several lines of evidence have shown that Wnt5a antagonizes Wnt/β-catenin signaling through the Wnt/Ca2+ pathway21 and β-catenin degradation by seven in absentia homolog 2, an E3 ubiquitin ligase22. Wnt5a was shown to compete with Wnt3a for binding to Fzd2, which mediates Wnt5a- and Wnt3a-induced signals23. Wnt/β-catenin signaling was consistently lower in HEK293 cells treated with Wnt3a and Wnt5a than in cultures treated with Wnt3a (data not shown). On the other hand, pretreatment of ST2 cells with Wnt5a enhanced canonical Wnt ligand-induced Wnt/β-catenin signaling. Not only the expression of Wnt10b, Lrp5, and Lrp6, but also the expression of Wnt5a was increased during osteoblastogenesis. Wnt/β-catenin signaling, as detected by the expression of DsRed protein, was higher in wild-type calvarial cells than in Wnt5a−/− cells under osteogenic culture conditions. Therefore, our results suggest that Wnt5a is not just an inhibitor of Wnt/β-catenin signaling, but rather enhances it. We and others have shown that osteoblast differentiation in Wnt5a+/− and osteoblast-lineage-specific Wnt5a conditional knockout mice was impaired13,14. Thus, Wnt5a is involved in osteoblast differentiation by the up-regulating Lrp5/6.
Wu et al.24 have shown that Wnt3a activated Rac1-c-Jun N-terminal kinase2 pathway, a noncanonical Wnt signaling, which can promote the nuclear accumulation of β-catenin. Noncanonical Wnt signaling controls Wnt/β-catenin signaling, which is involved in the development of limb skeletons. In contrast, Wnt/β-catenin signaling is not involved in the up-regulation of Lrp5/6 since Dkk1 failed to inhibit their expression during osteoblast differentiation. Thus, the present study provides a novel finding that Wnt5a-induced noncanonical signaling up-regulated Lrp5/6 expression in osteoblast-lineage cells to increase their sensitivity for canonical Wnt ligands. Thus, Wnt5a regulates osteoblast differentiation in collaboration with canonical Wnt ligands such as Wnt3a and Wnt10b.
By using mice crossing tetO-Wnt5a; Rosa26rtTA-double transgenic mice (in which Wnt5a is expressed by the administration of doxycycline) with reporter mice (in which lacZ is expressed under the Axin2 promoter), Wnt5a was shown to activate Wnt/β-catenin signaling in the developing skull, especially the meninges, although the calvarial bone of these mice exhibited impaired ossification25. On the other hand, Wnt/β-catenin signaling was reduced in their skin. These findings indicate that Wnt5a can both activate and inhibit Wnt/β-catenin signaling in a tissue- and temporal-specific manner. We have previously shown that Wnt5a was highly expressed in calvarial cells under growth culture conditions, and its expression was further increased under osteogenic culture conditions14. Our present study showed that Wnt5a promoted Wnt/β-catenin signaling during osteoblast differentiation. Thus, Wnt5a signals modulate a receptor context of the cells to properly respond to canonical Wnt ligands during osteoblastogenesis.
Wnt5a has been shown to bind a receptor complex of Fzd4 and Lrp5 and activate Wnt/β-catenin signaling in HEK293 cells, in which Fzd4 and Lrp5 were overexpressed26. Our observation of the increased expression of Wnt5a, Lrp5, and Lrp6 during osteoblastogenesis implies that Wnt5a by itself may activate Wnt/β-catenin signaling through Lrp5 or Lrp6. However, the overexpression of Lrp5 in Wnt5a−/− calvarial cells enhanced Wnt/β-catenin signaling. Moreover, the expression of Fzd4 in calvarial cells was not increased in osteogenic medium (data not shown). Based on these findings, it is unlikely that Wnt5a directly binds to a receptor complex of Lrp5 and Fzd4 and activates Wnt/β-catenin signaling in calvarial cells. Endogenous Wnt ligands such as Wnt10b may activate Wnt/β-catenin signaling in these cells under osteogenic conditions.
Wnt5a was shown to activate noncanonical Wnt signaling in a Ror receptor-dependent manner27,28. We previously showed that Ror2+/− mice failed to exhibit impaired bone formation, and mineralization was indeed normal in calvarial cell cultures isolated from Ror2−/− mice14. Furthermore, the present study showed that the expression of Lrp5/6 in Ror2−/− calvarial cells was similar to that in wild-type cells. These results indicate that Ror2-mediated signaling is not involved in the Wnt5a-induced up-regulation of Lrp5/6 in osteoblast-lineage cells.
In the present study, we demonstrated that Sp7 may be involved in the enhanced expression of Lrp5 by Wnt5a in calvarial cells for the following reasons. First, the expression of Lrp5 was lower in Wnt5a−/− calvarial cells with a reduction in the expression of Sp7. Second, Sp7 was recruited to Sp1 binding sites on the proximal Lrp5 promoter in calvarial cells in osteogenic medium. Third, the overexpression of Sp7 enhanced Lrp5 expression in calvarial cells. Fourth, Wnt5a enhanced BMP2-induced expression of Sp7 mRNA in calvarial cells, but not Wnt5a by itself. These results indicated that Wnt5a and BMP2 may co-operatively regulate Lrp5 expression in calvarial cells. This is important for osteoblast lineage cells to activate Wnt/β-catenin signaling effectively in conditions that canonical Wnt ligands work. Thus, Wnt5a may regulate osteoblast differentiation in collaboration with BMP2 as well as canonical Wnt ligands. Further studies are needed to clarify how Wnt5a enhances Lrp6 expression and enhances BMP2 signaling during osteoblast differentiation.
Wnt/β-catenin signaling was shown to inhibit the differentiation of stromal cells into adipocytes through the suppression of Cebp/α and Ppar-γ expression29,30. Wnt5a also inhibited adipogenesis through the suppression of transcriptional activity of Ppar-γ13. The number of adipocytes was higher in the bone marrow from 20-week-old Wnt5a+/− mice. We previously reported that calvarial cells from Wnt5a−/− mice preferably differentiated into adipocytes14. Ex vivo experiments showed that the enforced expression of Lrp5 suppressed adipogenesis in Wnt5a−/− calvarial cell cultures by promoting Wnt/β-catenin signaling. Thus, Wnt5a suppresses adipogenesis through two mechanisms, suppression of the transcriptional activity of Ppar-γ and an enhancement in Wnt/β-catenin signaling through Lrp5/6 expression. TAZ has also been shown to function as an inducer for osteoblastogenesis and a suppressor for adipogenesis during canonical Wnt signaling6. Thus, it can be envisaged that Wnt5a enhances Wnt/β-catenin and Wnt/TAZ signals through the up-regulation of Lrp5/6 during osteoblast differentiation.
We showed that Wnt5a autonomously regulated the expression of Lrp5 and Lrp6, which promoted Wnt/β-catenin signaling during osteoblast differentiation. This signaling axis was also critical for the suppression of adipogenesis. Thus, Wnt5a regulates osteoblastogenesis and adipogenesis through the up-regulation of Wnt/β-catenin signaling.
Methods
Mice and reagents
Wnt5a+/− mice were maintained as described previously31. Ror2+/− mice were maintained as described previously32. In in vitro experiments, cells isolated from embryos were used regardless of their gender. All procedures using mice were approved by the Animal Management Committee of Matsumoto Dental University. All experiments using mice were performed in accordance with the guidelines of the Animal Management Committee of Matsumoto Dental University. Recombinant mouse Wnt5a, Wnt3a, Wnt7a, Wnt 9b and Wnt10b were purchased from R&D systems Co. Ltd. (Minneapolis, MN). Chemicals and reagents used in this study were of analytical grade.
Cell cultures
Calvarial cells were prepared from the calvariae of Wnt5a−/− and wild-type embryos at E18.5 as described previously14. Cells were maintained in α-MEM with 10% fetal bovine serum (growth culture conditions). To induce osteoblastic differentiation, cells (2 × 104 cells/cm2) were cultured in osteogenic medium containing 5 mM β-glycerophosphate and 100 μg/ml ascorbic acid (osteogenic culture conditions) with or without 200 ng/ml BMP2 (R&D systems) using collagen-coated plates. Approximately 2 weeks after the cultivation, cells were fixed. These cells were stained with alizarin red S or stained for alkaline phosphatase activity as described previously33. Mineralized area (%) in wells was calculated using NIH image software. Confluent calvarial cells were cultured for RNA analysis for the indicated time in osteogenic medium.
Wnt5a−/− calvarial cells were cultured for 10 days in the presence or absence of 500 ng/ml of recombinant mouse Wnt5a, and used for the subsequent analysis.
ST2 cells were cultured for 24 hours in the presence or absence of 200 ng/ml of recombinant mouse Wnt5a. Cells were washed with fresh culture medium and further cultured for 48 hours in the presence of 100 ng/ml of Wnt3a, Wnt7a or Wnt10b.
PCR and Real time PCR
Total RNA was isolated from calvarial cells using RNA isolation kits (PureLink RNA mini kit, Life Technology, Carlsbad, CA) and RNase-free DNase I (Qiagen, Hilden, Germany). cDNA synthesis was performed on 1–2 μg of total RNA using reverse transcriptase (Revatra Ace; Toyobo, Tokyo, Japan) and oligo dT primers. All PCR products were verified by direct DNA sequencing. Real time PCR was performed using SYBR Green Master Mixes (Life Technologies) with the StepOnePlus system (Life Technologies) as described previously14. Briefly, mRNA levels were calculated by normalizing to the house keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the ΔCT method. Changes in gene expression of samples were expressed relative to that of untreated samples. Sequences of the primers used for PCR and real-time PCR analyses are described in Supplementary Table S1 online.
Adenovirus-mediated gene transfer
Full length Lrp5 cDNA, full length Sp7 cDNA (Thermo Scientific Open Biosystems, Yokohama, Japan), the 8xTcf/Lef promoter, and DsRed cDNA were amplified using high fidelity DNA polymerases (PrimeSTAR GXL, Takara Bio Inc, Otsu, Japan). PCR fragments of Lrp5 and of 8xTcf/Lef-DsRed were ligated into adenoviral vectors pAdenoX-ZsGreen1 and pAdenoX-PRLS-ZsGreen1, respectively (Takara Bio Inc). The linearized vectors were transfected into HEK293T cells to produce an adenovirus according to the manufacturers' instructions. Purified adenoviruses were infected into cells at a dose of 50–100 multiplicity of infection (MOI). cDNA fragments were amplified using the following primers. Lrp5 forward; acgacatggaaacggcgccga, Lrp5 reverse; tcaggacgagtccgtcgagg, Sp7 forward; atggcgtcctctctgcttga, Sp7 reverse; atcagatgtgtagcaggaag, 8xTcf/Lef promoter forward; gtactaacatacgtcgctctcca, 8xTcf/Lef promoter reverse; cggaatgccaagctttttac, DsRed forward; tttagtgaaccgtcagatcc, DsRed reverse; tgagtttggacaaaccacaac.
Tcf/Lef reporter cells
Lentivirus (Cignal Lenti reporter with Tcf/Lef binding sites, Qiagen, Hilden, Germany) was infected into ST2 cells at a dose of 50 MOI. To select stable clones expressing the reporter, cells were treated with 2 μg/ml puromycin dihydrochloride (Sigma, St Louis, MO). Resistant colonies were used for subsequent experiments.
Immunohistochemical and immunoblotting studies
Paraffin sections of the scapulae from wild-type and Wnt5a−/− embryo at E18.5 were subjected to immunostaining using anti-β-catenin antibodies (R&D systems). Immunocomplexes were visualized with diaminobenzidine (Dako). The sections were counterstained with hematoxylin.
Western blot analyses were performed using anti-GFP (Abcam) and anti-β-catenin (Cell Signaling Technology), anti-β-actin (Sigma), anti-Wnt5a (R&D Systems), Anti-Wnt7b (Santa Cruz), Anti-Wnt10b (Abcam), Anti-Lrp5 (Cell Signaling Technology), anti-Lrp6 (Abcam) antibodies as described previously14. Lysates (40 μg protein/lane) from cells and from scapulae of E18.5 mice embryos were electrophoresed on SDS-PAGE gels, transferred to polyvinylidene difluoride membranes, and subjected to immunoblotting using the ECL plus chemiluminescence detection system (GE Healthcare, Buckinghamshire, UK).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed with the ChIP Assay kit (Upstate Biotechnology), using antibodies against Sp7 (Abcam), Sp1 (Santa Cruz), c-Jun (Cell Signaling Technology) and normal IgG (Santa Cruz) as described previously14. The purified DNA was analyzed by PCR using following primers PCR1 forward; cctcctgagtgctgggatta, PCR1 reverse; agagatgggcactgtcttgc, PCR2 forward; acgtgcctcagtttcctcac, PCR2 reverse; cagtacagcaccagcagcag on the Lrp5 and PCR1 forward; cgagaggaagaggctgaatg, PCR1 reverse; cctcacctctcagcagcac, on the Lrp6 promoter.
shRNA-mediated knock down
Retroviral vectors expressing short hairpin RNA against mouse Wnt5a (pSIREN-shRNA against Wnt5a) were constructed by inserting double-stranded oligonucleotides of the target into pSIREN (Clonetech). Retroviral packaging was performed by transfection of plasmids into Plat-E cells as described previously14. Calvarial cells were incubated for 48 hours with virus-containing supernatants from Plat-E cultures, and used for real-time PCR analysis. The target sequences of Wnt5a are described. sh-Wnt5a#1; 5′CGCUAGAGAAAGGGAACGAAU3′, sh-Wnt5a#2; 5′CCACUUGUAUCAGGACCACAU3′.
Statistical analyses
All cell culture experiments were performed at least three times and similar results were obtained. Statistical analysis of the data was performed by the Student's t test.
Author Contributions
M.O. conducted most of the experiments and prepared the manuscript. K.M., T.Y. and S.U. supported the in vitro experiments. N.U., Y.N., H.K., N.S. and Y.M. contributed the data analysis. Y.K. designed and supervised the project. N.T. and Y.K. wrote the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS, Kakenhi) [Grant number; 25221310 (N.T.), 25293423 (Y.K.), 24390417 (N.U.), 24241045 (N.S.)], and by a grant from Naito Foundation Natural Science (Y.K.).
References
- Baron R. & Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013). [DOI] [PubMed] [Google Scholar]
- Krishnan V., Bryant H. U. & Macdougald O. A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories R. J., Corr M. & Lane N. E. To Wnt or not to Wnt: the bone and joint health dilemma. Nat. Rev. Rheumatol. 9, 328–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. & Varmus H. E. Wnt genes. Cell 69, 1073–1087 (1992). [DOI] [PubMed] [Google Scholar]
- Angers S. & Moon R. T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- Azzolin L. et al. Role of TAZ as mediator of Wnt signaling. Cell 151, 1443–1456 (2012). [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Yamamoto H. & Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 19, 119–129 (2009). [DOI] [PubMed] [Google Scholar]
- Gong Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001). [DOI] [PubMed] [Google Scholar]
- Kato M. et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157, 303–314 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y. et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 17, 684–691 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen E. et al. WNT-LRP5 Signaling Induces Warburg Effect through mTORC2 Activation during Osteoblast Differentiation. Cell Metab. 17, 745–755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada I. et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat. Cell Biol. 9, 1273–1285 (2007). [DOI] [PubMed] [Google Scholar]
- Maeda K. et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 18, 405–412 (2012). [DOI] [PubMed] [Google Scholar]
- Mao B. et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417, 664–667 (2002). [DOI] [PubMed] [Google Scholar]
- Komori T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997). [DOI] [PubMed] [Google Scholar]
- Nakashima K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002). [DOI] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A. L. & Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 (1997). [DOI] [PubMed] [Google Scholar]
- Ulsamer A. et al. BMP-2 induces Osterix expression through up-regulation of Dlx5 and its phosphorylation by p38. J. Biol. Chem. 283, 3816–3826 (2008). [DOI] [PubMed] [Google Scholar]
- Nakashima A. et al. Cross-talk between Wnt and Bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J. Biol. Chem. 280, 37660–37668 (2005). [DOI] [PubMed] [Google Scholar]
- Ishitani T. et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell Biol. 23, 131–139 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L. et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 162, 899–908 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Yamamoto H., Sakane H., Koyama H. & Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 29, 41–54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133, 340–353 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Fuerer C., Mizutani M. & Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev. Biol. 369, 101–114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A. J. & Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I. et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8, 645–654 (2003). [DOI] [PubMed] [Google Scholar]
- Minami Y., Oishi I., Endo M. & Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev. Dyn. 239, 1–15 (2010). [DOI] [PubMed] [Google Scholar]
- Ross S. E. et al. Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 (2000). [DOI] [PubMed] [Google Scholar]
- Bennett C. N. et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 102, 3324–3329 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., McMahon A. P. & Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223 (1999). [DOI] [PubMed] [Google Scholar]
- Takeuchi S. et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5, 71–78 (2000). [DOI] [PubMed] [Google Scholar]
- Tu X. et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell 12, 113–127 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information