Abstract
In this review, we summarize recent advances in understanding vitamin A-dependent regulation of sex-specific differences in metabolic diseases, inflammation, and certain cancers. We focus on the characterization of the aldehyde dehydrogenase-1 family of enzymes (ALDH1A1, ALDH1A2, ALDH1A3) that catalyze conversion of retinaldehyde to retinoic acid. Additionally, we propose a “horizontal transfer of signaling” from estrogen to retinoids through the action of ALDH1A1. Although estrogen does not directly influence expression of Aldh1a1, it has the ability to suppress Aldh1a2 and Aldh1a3, thereby establishing a female-specific mechanism for retinoic acid generation in target tissues. ALDH1A1 regulates adipogenesis, abdominal fat formation, glucose tolerance, and suppression of thermogenesis in adipocytes; in B cells, ALDH1A1 plays a protective role by inducing oncogene suppressors Rara and Pparg. Considering the conflicting responses of Aldh1a1 in a multitude of physiological processes, only tissue-specific regulation of Aldh1a1 can result in therapeutic effects. We have shown through successful implantation of tissue-specific Aldh1a1−/− preadipocytes that thermogenesis can be induced in wild-type adipose tissues to resolve diet-induced visceral obesity in females. We will briefly discuss the emerging role of ALDH1A1 in multiple myeloma, the regulation of reproduction, and immune responses, and conclude by discussing the role of ALDH1A1 in future therapeutic applications.
Keywords: oestrogen, Raldh, alcohol dehydrogenase, lymphotactin, retinoids, tissue factor, PCOS, gender differences, Esr1, android obesity
1. Introduction
In recent decades, interests have shifted from studies in reproductive biology to a global assessment of sex-specific differences in metabolism and pathogenesis [1]. Numerous studies have demonstrated gender specific differences in susceptibility to immune and metabolic disorders [1,2,3]. Researchers began to recognize that health benefits in men and women were attributed to different types of nutrients. For example, yellow vegetables rich in carotenoids and retinoids are associated with sex-specific patterns of disease prevention [2,4]. Furthermore, yellow vegetables are associated with reduced risk of lung cancer in women [4], whereas in men a diet rich in green xanthophyll carotenoids vegetables does not promote reductions in cardiovascular disease [2]. Thus, there is a vital need to develop prevention and treatment programs dependent upon the mechanisms responsible for divergent metabolic responses between genders. In this review, we discuss the female-specific aspects of vitamin A metabolism and its contributions to the regulation of obesity, immunity, and reproduction.
The fundamental understanding of sex-divergent responses evolved from major discoveries related to the female sex hormones. This first occurred between 1930 and 1950 when Edward Adelbery Doisy isolated the female sex hormones: estrogen (17-β-estradiol, E2, oestrogen), estron (E1), and estriol (E3) [5]. Years later, nuclear estrogen receptors were cloned and discovered to be responsible for estrogen’s actions [6]. In 1962 estrogen receptor 1 (alpha) (gene nomenclature term Esr1, also known as Era, Erα) was described by Elwood V. Jensen [7]. Following this discovery, in 1996, Dr. Jan-Ake Gustafsson’s laboratory cloned estrogen receptor 2 (beta) (gene nomenclature term Esr2, also known as Erb, Erβ) [8]. E2 is a specific high affinity ligand for these receptors, whereas E3 acts to antagonize E2’s activation of ER [9]. The expression pattern of estrogen receptor Erα, and especially Erβ, is suggestive of their role in the regulation of metabolic, immune, and reproductive functions [6] (Figure 1). The genome-wide location of transcription factor binding-sites or histone modifications (cistrome) have proven that ERα responses are cell-type specific in tissue. This is the result of ERα localization in response to different epigenetically marked cis-regulatory regions of target genes [10]. Furthermore, suppression of genes in these regions by ERα regulates tissue-specific responses to E2 that are dependent on concurrent cooperation among transcription factors and coactivators [11,12]. Together, these E2-regulated events contribute to tissue-specific responses.
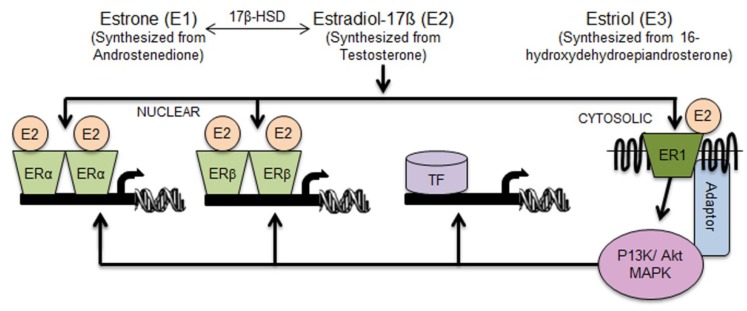
Figure 1.
Direct and indirect mechanisms for the regulation of gene expression by estrogen. E1, E2, and E3 are the three predominant naturally occurring forms of estrogen. 17β-HSD catalyzes the reversible conversion of E1 and E2. Three types of estrogen receptors were identified: nuclear receptors ERα and ERβ, as well as transmembrane G-protein coupled estrogen receptor ER1 (alias GPER). The classical pathway for direct gene transcription is the binding of E2 to ERα and ERβ and activation of target genes containing ER response elements (ERE) (Vertical signaling). Activation of ER1 by E2 can in turn activate the classical ERα and ERβ pathways through the signaling cascade governed by PI3K/AKT protein kinases (Vertical signaling). Alternatively, PI3K/AKT triggers activation of other transcription factors (TF) that do not contain ERE (Horizontal signaling). 17β-HSD, 17β-Hydroxysteroid dehydrogenase; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; GPER, G-protein-coupled estrogen receptor one (alias ER1, GPER or GPR30); P13K, phosphatidylinositide 3-kinase/protein kinase B; AKT, protein kinase B.
The dependence of metabolic [13,14] and inflammatory [15] processes on ERs in both genders was later confirmed in mouse models deficient in Erα, Erβ, or both [16]. The absence of Erα in both male and female mice resulted in increased white adipose tissue mass (WAT) and impaired insulin resistance [16,17,18]. In ovariectomized mice, both E2 and Erα are necessary to overcome insulin resistance induced by a high-fat diet [19]. In contrast, Erβ−/− mice are resistant to age-induced obesity ([16], reviewed in [6]), suggesting differences between the metabolic roles of ERα and ERβ. In addition, E2 can induce potent anti-inflammatory and neuroprotective effects via ERα-mediated mechanisms in female mice with pronounced autoimmune encephalomyelitis [20,21]. Previous experiments examining E2 in Erα−/− and Erβ−/− mice showed a spectrum of responses that occur as a result of both E2-dependent and ER-independent mechanisms. Furthermore, later investigation revealed that E2 can also operate through a transmembrane G protein-coupled receptor 1 (ER1, GPER; formerly known as GPR30) to induce rapid signaling events [22] (Figure 1). The ER1-mediated phosphorylation cascade leads to activation of the nuclear ERα and ERβ (vertical signal transfer) and other families of transcription factors (horizontal signal transfer).
To understand sex-specific tissue effects, varying susceptibility to diseases, and distinctive responses to nutrients in males and females, other genomic and non-genomic mechanisms of E2 action must be explored. In this review, we discuss the relationship between E2 and vitamin A pathways in the context of our recent work investigating female-specific mechanisms for: “thrifty” metabolism; central-to-peripheral fat distribution; cytokine production; and immune responses [23,24,25,26,27].
We propose a novel mechanism for horizontal auto-/paracrine signal transfer from E2 to retinoic acid (RA) and RA-dependent transcriptional network that regulates tissue responses in a sex- and tissue-specific fashion.
2. Review Section
2.1. Aldh1 Family of Enzymes: Function and Regulation by E2
Retinoic acid (RA) production begins with the conversion of the vitamin A metabolite, retinol (ROL), to retinaldehyde (Rald) by the alcohol dehydrogenase (ADH) family of enzymes (reviewed in [28]). Several microsomal enzymes of the short chain dehydrogenase (SDR/RDH) family convert ROL to Rald in vitro [28,29] This conversion of retinol to Rald is a reversible step, carried out by ADHs, RDHs, DHRS9, and some aldo-keto reductases like AKR1B1 [30,31]. The cytosolic aldehyde dehydrogenase 1 enzyme family (ALDH1, alias RALDH) catalyzes the final irreversible step in RA production by oxidation of Rald to RA [28,32]. These enzymes in the ALDH1 family possess the same biochemical functions (retinoic acid production), but display different physiological functions [28,32]. Dissimilar functions of ALDH1 enzymes in adipose tissue will be discussed in the following section.
RA induces differentiation of cells and regulates gene transcription, signaling events, and post-translational modification of proteins (reviewed in [26,33]). All isomers of RA are high affinity ligands for the retinoic acid receptor (RAR) (reviewed in [34]). RAR can heterodimerize with retinoic X receptor (RXR), another nuclear receptor that binds to 9-cis RA. This heterodimer then binds to RA response elements (RARE) and regulates gene expression [35]. Furthermore, RA regulates expression of peroxisome proliferator–activated receptor γ (Pparg) [29], the master regulator of adipogenesis, via activation of transcription factor Zfp423 [24,36] (Figure 2). In contrast, Rald suppresses adipogenesis by inhibiting PPARγ and RXR activation [37]. PPARγ, RAR, and RXR regulate numerous differentiation processes and immune response [26,33,34,35]. Considering the role of ALDH1in controlling RA and Rald concentrations, it is possible that ALDH1 plays a role in the auto-/paracrine switch regulating adipogenesis and various other differentiation processes. RA production must be regulated in a spatiotemporal fashion due to the abundant yet versatile physiological activity of RA [38]. The ALDH1 family is comprised of three enzymes: ALDH1A1, ALDH1A2, ALDH1A3 [32,38]. All the three isoforms exist in mice, chickens, and humans and their sequences are conserved between species [38]. Murine ALDH1A1 is a 511 amino acid protein that is 87% identical to human ALDH1A1, murine ALDH1A2 is a 518 amino acid protein that is 97% identical to its human counterpart, and lastly, murine ALDH1A3 contains 512 amino acids and is 94% identical to the human protein (NCBI/BLAST database). ALDH1 enzymes share similar properties, such as conversion of Rald to RA but have unique biochemical and physiological functions (reviewed in [38]).
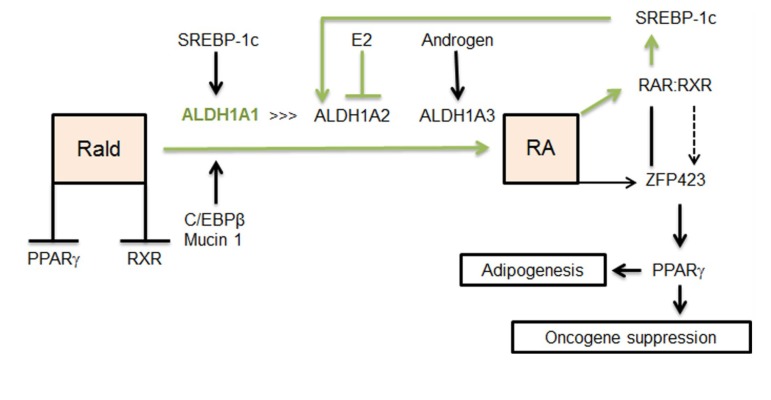
Figure 2.
Sex hormones and SREBP selectively regulate expression of Aldh1. ALDH1A1, ALDH1A2, and ALDH1A3 are members of the ALDH1 enzyme family that convert Rald to RA. E2 suppresses Aldh1a2 and Aldh1a3 in adipose tissue. Androgens can stimulate Aldh1a3 expression. Aldh1a3 expression is elevated in the visceral fat of ovariectomized mice. Hypothetical feed-forward pathway for Aldh1a1 regulation in females (green line). SREBP-1c can up-regulate Aldh1a1 and Aldh1a2 in animals fed a high-fat diet. However, in females E2 can suppress Aldh1a2. RA produced by ALDH1A1 can activate the RAR/RXR transcriptional complex, which in turn activates SREBP-1c. C/EBPβ and mucin1 can regulate Aldh1a1 in breast cancer cells. RA production can stimulate adipogenesis through ZFP423 and PPARγ. In multiple myeloma B cell lines, RA suppresses oncogenes (Hoxa10 and Ap1) through ZFP423 and PPARγ activation. Rald suppresses adipogenesis through inhibition of RXR and PPARγ. Rald, retinaldehyde; ALDH1A1, retinaldehyde dehydrogenase 1 family, member A1; ALDH1A2, retinaldehyde dehydrogenase 1 family, member A2; ALDH1A3, retinaldehyde dehydrogenase 1 family, member A3; SREBP-1c, sterol regulatory binding protein 1; C/EBPβ, CCAAT/enhancer-binding protein beta; RA, retinoic acid; ZFP423, zinc finger protein 423; PPARγ, peroxisome proliferator-activated receptor gamma; RXR, retinoid X receptor.
All ALDH1 enzymes catalyze the formation of Rald to RA [38]. Hierarchy of Aldh1 activation, their expression levels in different tissues, and contribution to RA production is regulated by sex hormones and nutrients, such as cholesterol and fatty acids [27,39,40,41]. Estrogen response element sites exist in the promoter of Aldh1a2 [39], while Aldh1a3 is regulated by androgens via androgen receptors [41] (Figure 3). In our studies, ovariectomy enhanced Aldh1a3 expression in retroperitoneal adipose tissue; while both Aldh1a2 and Aldh1a3 were expressed at lower levels than Aldh1a1 in a control female group [27]. The E2-dependent regulation of Aldh1 occurs in a tissue specific manner. For example, in the uterus of ovariectomized rats, E2 stimulation markedly increases Aldh1a2 expression but suppresses Aldh1a1 expression [42]. The dissimilar response of tissues to E2 depends on the presence of transcription factors regulating Aldh1. In breast cancer cells, the Aldh1a1 gene promoter is activated by a transcriptional complex of phosphorylated CCAAT/enhancer-binding protein β (C/EBPβ) and mucin 1 [43]. In addition, the promoter of Aldh1a1 and Aldh1a2 can be activated by sterol regulatory element binding protein 1 (SREBP-1c; nomenclature SREBF1) and to a lesser extent by SREBP2 that binds to SREBP regulatory element sites (SRE) [27,40] (Figure 2). The up-regulation of RAsynthesis [27] and Aldh1 expression in response to high-fat and high-cholesterol diets [40] can involve SREBP. In turn, RA, Rald, and ROL regulate the expression of Srebp-1c [44] through RXR [45], and possibly, RAR dependent mechanisms. This suggests feed-forward regulation of Aldh1a1 and Aldh1a2 through SREBP-1c. In consonance with this hypothesis, Aldh1a1 deficiency in mice reduces RA production and the expression of Aldh1a2 and Aldh1a3 [27,46]. Examination of the relative contribution of all transcriptional pathways to regulation of Aldh1 expression could provide further insight about Aldh1 regulation by nutrients in males and females.
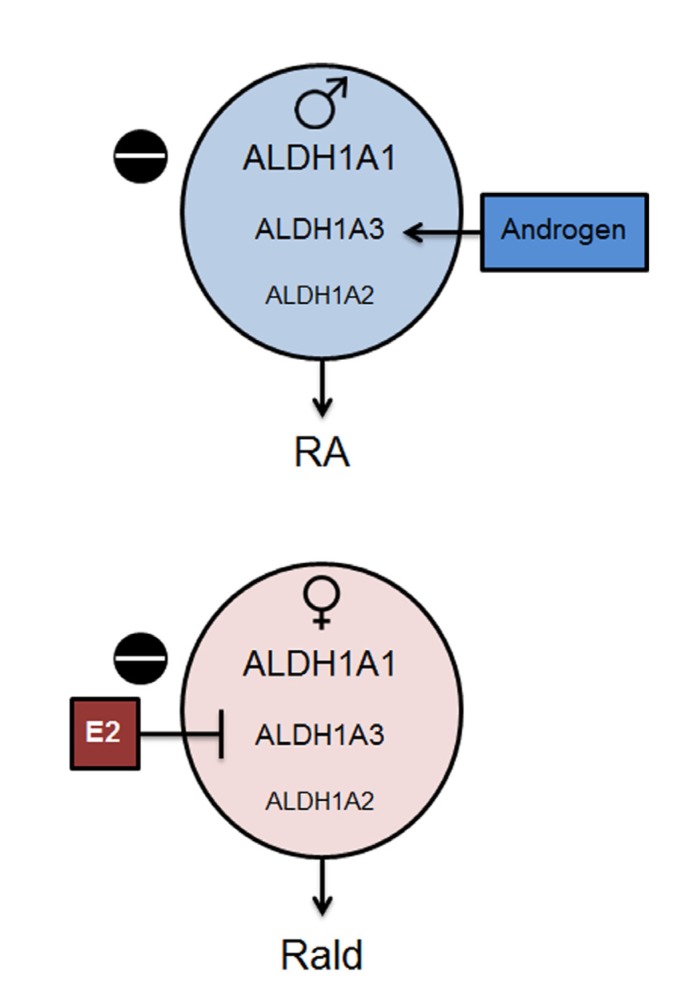
Figure 3.
Schematic presentation of the sex-specific change in the retinoid balance in male and female VF. In VF of mice, Aldh1a1 was predominantly expressed compared to Aldh1a2 and Aldh1a3 (indicated by size of the letters). Aldh1a2 and Aldh1a3 levels are lower in females (pink circle) compared to those seen in males (blue circle) due to suppression of Aldh1a3 by estrogen. Deficiency in Aldh1a1 (black circle with “−” symbol) further reduces expression of Aldh1a2 and Aldh1a3 in females, which leads to decreased Rald catabolism. Rald was detected in the VF of female mice. In Aldh1a1−/− male mice, Aldh1a3 expression is under androgen control; it can catalyze RA production from Rald in Aldh1a1−/− males.
Although the gene members of the Aldh1 family are expressed quantitatively in a tissue-specific manner [38], in the majority of adult tissues, all three isoforms of Aldh1 are expressed. Specifically, Aldh1a1 is ubiquitously expressed at higher levels than Aldh1a2 and Aldh1a3 in all tissues except the arachnoid membrane in the brain and some other cell types (Ziouzenkova, unpublished observations). The functional importance of expression of the three Aldh1 family members at different levels is poorly understood, and thought to be reserved for the compensatory RA production. In males, RA production is dependent on both Aldh1a1 and Aldh1a3. However, in females, Aldh1a1 is the predominant enzyme for RA production [24,27], because both Aldh1a2 and Aldh1a3 are suppressed by E2 [27] (Figure 3). Aldh1a1 becomes a critical downstream pathway mediating metabolic sex-specific differences since obesogenic and inflammatory responses to a high-fat diet are averted in Aldh1a1−/− females but not in Aldh1a1−/− males [27]. Based on our studies in adipose tissues, we propose a sex hormone-dependent “exclusion model” for the regulation of the ALDH1 family of enzymes that alter Rald and RA concentrations in a sex-specific manner (Figure 3). We expect this pattern to be present in tissues in which expression of nuclear and cytosolic ER and other transcription factors enables expression of only one Aldh1 enzyme. The predominant expression of Aldh1a1 in female adipose tissue [27] offers an opportunity to test the contributions of the vitamin A pathway to the regulation of female-specific responses in VF.
2.2. Aldh1 Regulation of Female-Specific Fat Distribution
Women tend to have higher fat mass than men throughout their entire lifespan [47,48]. Statistically, there are higher rates of obesity in women than men (61.3% vs. 42%). Obesity in women is correlated with an increased risk of diabetes mellitus, cardiovascular disease, cancer, and premature death [49,50,51]. The differences in metabolic rate [52,53,54] and energy homeostasis between males and females is dependent on actions of sex hormones [55,56,57] and expression of ER receptors in different tissues, including muscle [56,58] and adipose [59,60,61,62,63,64,65]. While the influence of ALDH1 enzymes in muscle remains unclear, current research has demonstrated that they have a critical regulatory role in adipose tissue [24,27].
Fatty Acids (FA) are stored as triglycerides in humans in three types of WAT: subcutaneous (SF), omental, and mesenteric. Given the central location of omental fat around viscera, this fat depot is also referred to as visceral (VF), central, abdominal, and trunk fat. Based on both heritable developmental gene expression and their striking physiological differences, these fat depots are proposed to be differentiated as separate tissues [66]. Additionally, WAT has a rare population of thermogenic cells known as thermocytes, bright, or beige cells [67]. Thermocytes, which directly contribute to basal energy expenditure, can reduce propensity for obesity [67].
It has been well established that fat distribution in the abdominal visceral region is typically attributed to males and it is often termed “android obesity” [60]. Young healthy females usually exhibit a different fat distribution pattern, which is characterized by increased SF relative to VF, especially in the lower body [48,68,69,70,71,72]. This pattern of fat distribution is known as “gynoid obesity”. On a regular diet, WAT is predominantly formed in SF depot in females and VF depot in males [73]. In women with polycystic ovary syndrome (PCOS), excessive androgen production leads to the development of “android” visceral obesity [48,74,75,76,77]. However, development of android obesity in women can be reverted through administration of E2 [48,78,79,80,81,82], whereas androgen replacement is associated with increased VF formation in these patients [83]. The sulfoconjugation of E2 in female mice overexpressing estrogen sulfotransferase does not influence VF; however, it does decrease SF depots by suppressing adipogenesis and impairing insulin sensitivity [84]. Physiologically, the postmenopausal period is marked by E2 deprivation and a significant increase in VF accumulation [4,85]. In experimental rodent studies, ovariectomy is the most commonly used method to study the effects of E2 deprivation [4]. We have found that ovariectomy is associated with increased expression of Aldh1a3 in the VF, although Aldh1a1 remained the most predominantly expressed member of the ALDH1 family. Deficiency in Aldh1a1 effectively protects female mice against VF formation induced by ovariectomy [27]. In healthy women, Aldh1a1 mRNA expression is the most abundant compared to other Aldh1 genes, in both VF and SF [27]. Considering this, it appears that Aldh1a1 is a central downstream pathway through which E2 regulates VF formation in female mice and possibly postmenopausal women.
2.3. Aldh1 Role in Sex-Specific Diet-Induced Thermogenic Regulation
Diet modifies sex-specific fat distribution [86]. On a high-fat diet, females exceedingly accumulate VF, which increases their risks for type 2 diabetes and cardiovascular disease. Imbalance of energy storage and expenditure is the accepted cause of obesity [87]. This is commonly interpreted as increased food intake vs. decreased physical activity. However, this imbalance in energy storage and expenditure can also affect regulatory mechanisms in adipose tissue leading to suppression of thermogenic energy expenditure and activation of lipogenic and lipid storage processes [87]. In females, this type of deregulation contributes to the decrease in basal metabolic rate and increase in visceral obesity on high-fat and Western diets [52]. ERβ is involved in the regulation of metabolic rate and can suppress diet- and ovariectomy-induced obesity in mice [88]. In obese ovariectomized mice, E2 effects depend on Ppara [89]. Downstream mechanisms by which ERα regulates these processes are also poorly understood and may involve regulation of the transcriptional co-activator of PPARγ, PGC-1α (nomenclature PPARGC1) [90]. Our recent studies [27] have demonstrated that ALDH1 enzymes can act downstream of sex-hormone responses and switch the lipogenic processes to thermogenic.
In vitro, Aldh1a1 expression is increased during adipocyte differentiation and contributes to ~70% RARE activation [24]. Knocking out Aldh1a1 in mice demonstrates how prevention of lipogenic adipogenesis in adipocyte cultures results in the acquisition of thermogenic characteristics [25]. Additionally, differentiated Aldh1a1−/− cells compared to WT or 3T3-L1 adipocytes display higher expression of Ucp1, Pgc-1α, and CoxIV as well as down-regulation of lipogenic genes Pparg, Fas and Fabp4 [24,25]. These results are in agreement with findings in vivo, where thermogenic remodeling occurs in the VF of Aldh1a1−/− females [27]. Thermogenesis does not occur in the VF of Aldh1a1−/− males expressing the two other RA producing enzymes, Aldh1a2 and Aldh1a3. This sex-specific phenomenon results in greater weight loss in the VF depots of Aldh1a1−/− females compared to their male counterparts [27]. RA production and effects are also depot-specific in females. In females, RA is produced in VF primarily through the action of Aldh1a1, whereas RA in SF is produced by Aldh1a1 and Aldh1a3 [24,27]. Due to depot-specific expression of Aldh1 enzymes, Aldh1a1 deficiency influences formation of the VF depot more than SF [24,26,27]. Moderate supplementation with vitamin A does not result in alterations to this fat distribution, even when concentration is increased up to 5-fold [23]. However, the implantation of Aldh1a1−/− cells into the VF of WT female mice induces similar thermogenic modification of adipocytes as seen in whole body Aldh1a1 deficient female mice [25]. This increased thermogenesis in VF results in the loss of VF mass in treated females despite consumption of a high-fat diet [25]. Thus, the lack of a single Aldh1a1 gene in a minor population of adipocytes is capable of abrogating the “thrifty” female metabolism via increased thermogenesis in VF.
The mechanism of this profound thermogenic remodeling of adipose tissue in Aldh1a1−/− females appears to stem from autocrine displacement of RA with Rald (Figure 3). In Aldh1a1−/− females, deficient expression of all ALDH1 enzymes abolishes Rald catabolism and RA production [37]. As a result, this modified signaling landscape leads to dysregulation of both genomic and non-genomic events. In Aldh1 deficient female adipocytes, Rald rapidly (within 30 min) increases translation of ATGL protein and induces lipolysis [27]. ATGL-mediated hydrolysis of fatty acids activates PPARα and expression of its target genes, Ucp1, Pgc-1α, and CoxVI [91,92]. In Aldh1a1−/− male adipose tissue explants, stimulation with Rald can recapitulate increase in ATGL-mediated lipolysis [27]. Thus, non-genomic effects on lipolysis appear to be dependent on the presence of Rald. Genomic Aldh1 effects in adipose tissue depend on: (1) RA regulating expression of Zfp423 and Pparg [24]; and (2) Rald inhibiting PPARγ and RXR activation [37]. An alternative explanation for thermogenic mechanisms in Aldh1a1−/− females, as proposed by Kiefer et al. [93], would be through the binding of Rald to RAR. However, Rald is known as a weak agonist for the RAR [94] and is inferior to RA in mediating RAR-dependent transcription. RA is abundantly produced by all three ALDH1 enzymes in WT, but is not produced in Aldh1a1−/− VF. In addition, it is unclear how this proposed Rald/RAR receptor interaction could explain increases in ATGL protein levels in the presence of cycloheximide that occur without changes in mRNA expression [27]. RA can activate RAR and induce thermogenesis in animals treated with high concentrations of RA [95].
Humans, similar to mice, exhibit enhanced lipolysis in the presence of Rald than RA in vitro [27]. Stromal vascular cells isolated from obese women, but not men, had significantly greater expression of Aldh1a1 suggesting that ALDH1A1 function in the regulation of adipocyte biology is conserved between species [27]. It seems plausible that Aldh1a1 deficiency in human omental fat or implantation of Aldh1a1−/− deficient pre-adipocytes (as in [25]) could serve as a potential sex-specific therapeutic strategy to combat android obesity in women. Cells encapsulated in alginate poly-l-lysine have been tested and implanted and used for long-term (9.5 years) xenotransplantation of pancreatic β-cells producing insulin in human patients [96]. The successful application of encapsulated Aldh1a1−/− cells for induction of thermogenesis in mice then raises the question: can these implants be similarly effective for the reduction of deleterious VF in women?
2.4. Aldh1 Sexual Dimorphism of WAT and Risks for Chronic Diseases
Fat depot distribution is an important risk factor for chronic metabolic diseases and particular cancers. Research has shown that VF (omental in humans and retroperitoneal in mice) is associated with agreater risk of developing metabolic syndrome [48,70,97]. VF and SF produce dissimilar levels of signaling molecules, including hormones and adipokines, that can further modify fat distribution patterns [98,99,100]. Thus, sex-specific fat distribution and adipose tissue mass in each depot contribute to sexual dimorphism of adipokines in blood [48]. The retinoid-relevant cytokines include retinol binding protein 4 (RBP4), dipeptidyl-peptidase 4 (DPP4), chemerin (formerly known as tazarotene-induced gene 2 protein (TIG2) and retinoic acid receptor responder protein 2 (RARRES2)), and lipocalin 2 (LCN2) [48]. Cytokines from VF act in a pro-inflammatory manner and contribute to insulin resistance. Females tend to have higher SF mass and insulin sensitivity [97,101] possibly due to the adiponectin production in SF [102,103]. Recently, many comprehensive reviews have discussed the collective influence of sex hormones and retinoids in the regulation of cytokine production and secretion in various tissues, including adipose (reviewed in [97,104,105,106]). The extent of ALDH1A1’s influence can be better explained through the differences in plasma cytokine profiles of WT and Aldh1a1−/− mice on a high-fat diet [23].
It appears that Aldh1a1 contributes to a sex-specific adipokine/cytokine profile in plasma by mechanisms dependent and independent of adipose tissue remodeling. The observed reduction in levels of SF and VF in Aldh1a1−/− compared to obese WT mice were associated with reduced adipokine secretion of adiponectin, leptin, and various pro-inflammatory cytokines including monocyte chemoattractant protein-1 (MCP1) and chemokine (C–X–C motif) ligand 10 (CXCL10; also known as IP-10). However, these changes were similar between genders and showed a positive correlation between MCP1 and IP10 cytokines and adipose tissue mass [23]. In contrast, tissue factor (TF) and factor VII reduction was specific to the Aldh1a1−/− female group, and did not correlate with adipose tissue mass [23]. The lack of correlation between these factors and adipose tissue mass suggest participation of other tissues. Therefore, in other tissues ALDH1A1 can regulate production or secretion of coagulation proteins in female mice and possibly control thrombogenic events. The relevance of these observations for wound healing or cardiovascular events requires further examination to understand the mechanism by which ALDH1A1 regulates TF and factor VII. For example, Aldh1a1 is expressed and responsible for the high-fat diet-induced steatosis in the liver [37]. TF and factor VII synthesis occurs in hepatic cells. This link between ALDH1 enzymes and acute response proteins could elucidate the role of Aldh1a1 in liver disease.
The Aldh1a1-dependent regulation of cytokines also contributes to improved glucose sensitivity in Aldh1a1−/− on a high-fat diet [23]. Previous research has suggested that there is a pivotal hepatic role in establishing insulin sensitivity in Aldh1a1−/− mice [107]. However, our recent studies have shown that engrafted Aldh1a1−/− cells in VF also improve glucose tolerance in obese WT females [25]. This finding, in line with numerous others, implicates VF in the development of insulin resistance [4,82]. Paradoxically, HOMA index assessments revealed that Aldh1a1−/− males exhibit more pronounced improvement of glucose metabolism than Aldh1a1−/− females, as a result of lower plasma insulin levels observed in Aldh1a1−/− females compared to Aldh1a1−/− males [23]. Recent studies have found 9-cis RA in insulin-producing pancreatic β-cells [108]. In light of these findings, the influence of RA and ALDH1 enzymes over insulin production and secretion in males and females needs to be examined. Although more work needs to be performed in the future, the central role of ALDH1 in sex-specific auto-/paracrine control of obesity, glucose metabolism, thrombogenic factors, and inflammation is evident.
2.5. Aldh1 Control of Sex-Specific Reproduction
Meiosis is a key process in mammalian reproduction that leads to formation of spermatogoniam and oocgonia. Meiosis signifies the first sex-specific differentiation of primordial germ cells (PGC). PGC’s development into gametes at the completion of meiosis is dependent on sex-chromosome composition [109,110,111,112]. Additionally, the time sensitive induction of PGC sexual differentiation is influenced by the environment and localization of cells in gonads [113]. Meiosis occurs prenatally in females and postnatally in males. Early studies have shown that regardless of XX or XY chromosomal composition in PGC, their localization in the ovary, but not in the testes can initiate meiosis [114,115]. Thus, the environment in the gonad (mesonephroi) plays a regulatory role and produces paracrine factors that influence PCG initiation into meiosis. However, the question remains: why does meiosis occur prenatally in females, but postnatally in males?
PGC contains meiosis commitment genes, including Stra8 in vertebrates [116]. Stra8 is expressed in both the cytoplasm and nucleus [117]. In response to RA, STRA8 initiates meiosis in stimulated male and female PGC cells in vitro [116,118]. In vivo, Stra8 mRNA expression coincides with the onset of meiosis in postnatal testes and prenatal ovaries [118]. The specific mechanism by which STRA8 regulates meiosis has not yet been identified. Stra8 regulates chromosomal condensation and formation of synaptonemal complexes. A search for an enzymatic switch releasing RA to initiate meiosis led to the identification of ALDH1A2 (RALDH2) as the principal enzyme regulating RA synthesis in the gonadal mesonephroi [119,120,121,122]. Studies conducted with Aldh1a2 and Aldh1a3 double knockout mice crossed with RARE reporter mice have revealed that meiosis in females can occur in the absence of detectable RA production by these enzymes. In postnatal testes, the critical role of RA for meiosis has been supported by numerous genetic and pharmacological studies. In male mesonephros, ALDH1A2 governs RA production. Expression of Cyp26B in prenatal PGCs catabolizes RA. In the postnatal period, paracrine RA signals are required for meiosis. The unexpected lack of RA during meiosis in females leaves unanswered questions about alternative paracrine initiating factors (reviewed in [119]) and/or alternative vitamin A metabolites activating Stra8 in the ovary.
The analytical sensitivity for the detection of RA production in mesonephroi for PGC activation can be a technical issue responsible for the paradoxical finding in Aldh1a2 and Aldh1a3 double knockouts. However, the indirect role of RA in female meiosis is supported by a robust inhibition of meiosis in fetal ovaries by an antagonist of RAR [123,124]. An important shortcoming of previous genetic studies was the low expression levels of Aldh1a1 enzymes producing nanomolar concentrations of RA. At these levels, RA is not detectable in RARE reporter models, but capable of meiotic induction. Meiosis in the human ovary is accompanied by increased Aldh1a1 expression in agreement with a suggested requirement of RA synthesis for meiosis [125]. Recently, Aldh1a1 has been shown to have sex specific influences on female biology [51], possibly warranting further investigation into its contribution to female reproduction.
3. Conclusions
(1) Sex-hormones regulate a tissue-specific pattern of Aldh1 enzyme expression.
(2) ALDH1A1 is the predominant enzyme for the regulation of RA and Rald concentrations in female tissues.
(3) The regulation of sex differences could be mediated by E2 and/or be transferred to retinoid-dependent pathways.
(4) Aldh1a1 deficiency in female adipose tissue improves cytokine profile and metabolic disorders such as visceral obesity and glucose tolerance (Figure 4).
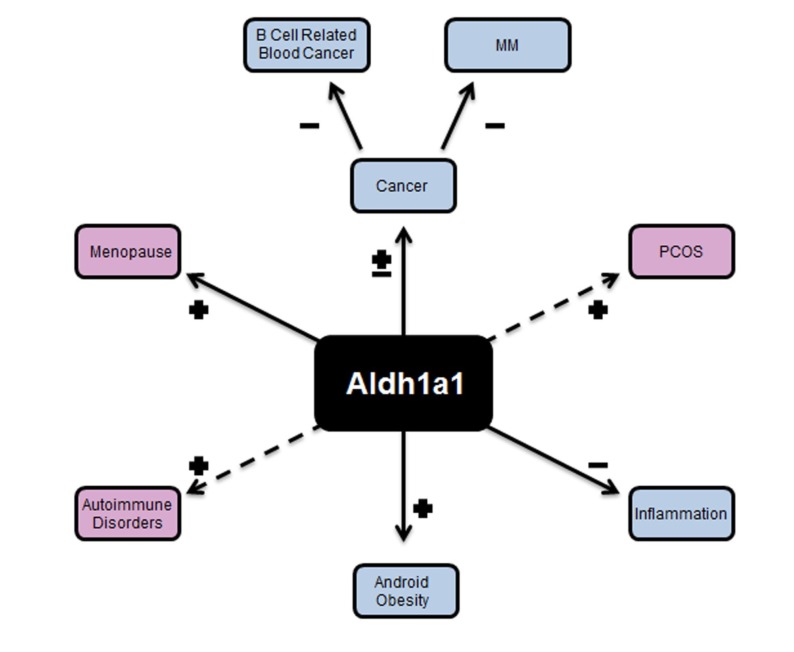
Figure 4.
The spectrum of disorders that are influenced by ALDH1A1. Android obesity, which is marked by the accumulation of fat around the inner organs (viscera) is dependent on Aldh1a1. In the figure, (+) represents upregulation of the gene in a specific disease, where as (−) represents down-regulation of this gene and elucidates that up-regulation or down-regulation of the gene contributes to development of the disease or condition. Visceral fat is decreased in Aldh1a1−/− female mice and also in obese WT female mice treated with Aldh1a1−/− preadipocytes. Visceral obesity is developed in the absence of estrogen in postmenopausal women and in patients with PCOS. Inflammation and B cell differentiation is regulated by RA, and depends on ALDH1A1. RA regulates tumorigenesis. Aldh1a1 expression is markedly reduced ((−) in the figure) in multiple myeloma (MM) and other blood cancers. In contrast, breast cancer cells have elevated Aldh1a1 expression.
The genetic deficiency in Aldh1a1 may have potential therapeutic applications in adipose tissue [25]. However, physiological levels of body fat need to be maintained to avoid insulin resistance, leptin deficiency and dyslipidemia that is associated with lipodistrophy [126]. However, the disruption of Aldh1a1 signaling in other tissues can have deleterious effects. Our recent studies showed that deficiency in Aldh1a1, but not in Aldh1a2 and Aldh1a3, is the characteristic feature of B cell cancers in humans, especially multiple myeloma (MM) [127]. In B cells, the lack of Aldh1a1 results in decreased Rara and Pparg expression that leads to the up-regulation of oncogenes Hoxa10 and Ap1 [127].
RA regulates a network of transcription factors and is a powerful therapeutic agent [33]. However, administration of RA is unspecific and causes numerous side effects known as “RA syndrome”. The targeted regulation of ALDH1 enzymes in females or implantation of Aldh1a1−/− cells [25] could create an opportunity to regulate RA synthesis in a physiological tissue-specific manner for therapeutic purposes.
Perspectives
PCOS affects approximately 6.5% of reproductive age women [128,129] and results in development of secondary male sex characteristics, including hyperandrogenism and anovulation. This often leads to amenorrhea, oligomenorrhea, dysfunctional uterine bleeding, and infertility [130]. In addition, PCOS shares many common traits of metabolic syndrome including: visceral obesity, insulin resistance, hyperinsulemia, greaterrisk of diabetes mellitus, and cardiovascular disease [131,132]. Obesity in PCOS patients can present in adolescence prior to menarche [130] suggesting that PCOS dysregulation occurred long before puberty.
Postmenopausal obesity is also a predictor of poor survival rates in women with breast cancer [133]. A strong association was found between family history of breast cancer and a high frequency of Aldh1a1 expression in breast ductules in women, regardless of a hereditary breast-ovarian cancer syndrome status (Brca1 and Brca2 mutations), age, parity, or occurrence of cancer [134]. The role of Aldh1a1 in these diseases warrants investigation, considering the central role of Aldh1a1 in ovariectomy-induced visceral obesity [27]. We have explored the therapeutic application of Aldh1a1−/− preadipocytes and observed delayed onset of visceral obesity in WT female mice after implantation of biocompatible encapsulated Aldh1a1−/− cell [25]. Potentially, similar therapies could be developed for PCOS patients.
PCOS manifestation often includes development of hypertrophied ovarian theca cells [135] that express steroidogenic enzymes for androgen biosynthesis: CYP11A1, CYP17, HSD3B2 [136] 5a-reductase, and 5a-androstane-3,17-dione [135,137]. Additionally, 5a-reductase increases in PCOS granulosa cells leads to elevated 5a-androstane-3,17 dione concentrations which then inhibit aromatase and reduces estrogen production in granulosa cells (Figure 5) [137]. Hyperandrogenism in PCOS may also be a function of increased production of biologically active retinoids by theca cells [135]. PCOS theca cells express a set of vitamin A enzymes that are sufficient for atRA biosynthesis, including retinol dehydrogenase 2 (Rdh2), retinol dehydrogenase (RoDH4,2), cellular retinoic acid binding protein II (Crabp II), and prostate short chain dehydrogenase/reductase (Psdr2) [138], and Aldh1a3 [128]. However, retinol is converted to retinaldehyde at an increased rate in PCOS vs. normal theca cells [138], suggesting enzymatic dysregulation. In these cells, atRA increases expression of 17 alpha-hydroxylase and androgen production via GATA6 dependent mechanisms [138]. RA also induces expressions of CYP17 and CYP11A1 that produce DHEA [139]. Interestingly, in PCOS theca cells, promoters of steroidogenic enzyme expression are up-regulated by retinol, while in healthy women these cells responded only to RA [135]. Thus, ALDH enzymes may have a major role in the imbalance of retinoid concentrations that induce androgen production and steroidogenic enzyme expression in theca cells. Many factors, including production of luteinizing hormones, seem to be activating, mediating and facilitating PCOS, yet RA remains to be a co-regulator of all these processes. Thus, the role of endogenous retinoids in these processes needs to be examined as a potential candidate pathway regulating hormonal balance. A critical understudied aspect of ALDH1A1 function includes its possible catabolic role in different other pathways. While ALDH1A2 and ALDH1A3 specifically utilize Rald as a substrate [32,140], ALDH1A1 utilizes Rald as a preferred substrate at nanomolar concentrations. However, ALDH1A1 also utilizes other aldehyde substrates if they are present at micromolar concentrations in vitro [46,141]. In vivo, Aldh1a1−/− created reductions of physiological levels of retinoic acid and 3-deoxyglucosone [46,142], but it remains unclear whether this metabolic product of glucose oxidation contributes to the metabolic phenotype observed in Aldh1a1−/− mice. In adipocyte cultures, 70% of the RA-dependent RARE activation depends on Aldh1a1 expression. Thus, it appears that ALDH1A1 is a major enzyme contributing to endogenous RA production in adipogenesis in females. However, the environmental condition increasing concentrations of other aldehydes can potentially disrupt RA production in females and increase their susceptibility to autoimmune disorders and blood cancers (Figure 4).
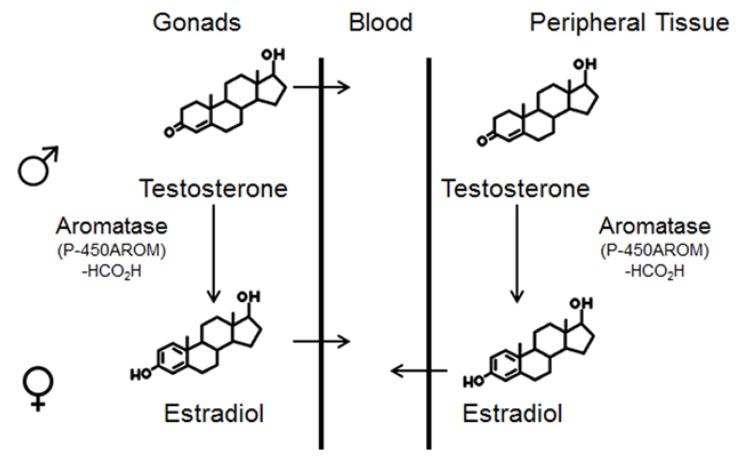
Figure 5.
Schematic presentation of sex hormone production in gonads and peripheral tissues. Sex hormones are secreted from the male and female gonads. The testes secrete testosterone into the blood stream while in the ovaries aromatase converts testosterone to estradiol prior to secretion into the blood stream. In the peripheral adipose tissue, aromatase converts testosterone to estradiol.
Acknowledgments
We thank R. Yasmeen (The Ohio State University) for the helpful discussion. Funding: This research was supported by Egg Nutrition Center (American Egg Board); Food Innovation Center, Office for International Affairs and Center for Advanced Functional Foods Research and Entrepreneurship at The Ohio State University; and Daskal Foundation (O.Z., R.Y.). The project described was supported by Award Number Grant UL1TR000090 from the National Center for Advancing Translational Sciences and the Cancer Center Support Grant (CA016058) (O.Z). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- 3β-HSD
3β-Hydroxysteroid dehydrogenase type II enzymes
- ADH
Alcohol dehydrogenase
- Adh1
Alcohol dehydrogenase 1
- Aldh1a1−/−
Knockout in Aldh1a1
- Aldh1a1
Aldehyde dehydrogenase 1 family, member a1
- Aldh1a2
Aldehyde dehydrogenase 1 family, member a2
- Aldh1a3
Aldehyde dehydrogenase 1 family, member a3
- AP-1
Activator protein 1
- ATGL
Adipose triglyceride lipase
- BAT
Brown adipose tissue
- BMI
Body mass index
- COX2
Prostaglandin-endoperoxide synthase 2
- CRABP
Cellular retinoic acid binding protein
- CYP17
Cytochrome P450 17
- CYP1711A1
Cytochrome P450 family 17 subfamily A polypeptide 11
- CYP26B1
Cytochrome P450 26B1
- CYP450
Cytochrome P450
- CXC
CXCL Chemokine group of cytokines
- C/EBPβ
CCAAT/enhancer-binding Protein β
- DHEA
Dehydroepiandrosterone
- DPP4
Dipeptidyl peptidase-4 also known as CD26
- E1
Estron
- E2
Estradiol
- E3
Estriol
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- Erα−/−
Knockout in Erα
- Erβ−/−
Knockout in Erβ
- ERE
Estrogen response element
- FABP
Fatty acid binding protein
- FSH
Follicle stimulating hormone
- GATA
GATA-binding factor 1
- GPER
G protein-coupled estrogen receptor 1
- HSD3B2
3beta-hydroxysteroid dehydrogenase-delta 5-delta 4 isomerase type II
- IL-6
Interleukin-6
- IFNγ(IFNg)
Interferon gamma
- IP-10
Inducible Protein-10
- LH
Luteinizing hormone
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein-1
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PCOS
Polycystic ovary syndrome
- PGC
Primordial germ cell
- PGC-1A (PPARGC1A)
Peroxisome proliferator-activated receptor, gamma, Coactivator 1 alpha
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPARγ
Peroxisome proliferator-activated receptor gamma, Pparg
- RAR
Retinoic acid receptor, Rara
- RARE
Retinoic acid response elements
- Rald
Retinaldehyde, retinal
- RA
Retinoic acid
- RoDH
Retinol dehydrogenase
- ROL
Retinol
- RXR
Retinoid X receptor
- SF
Subcutaneous fat
- SREBP-1c (SREBF1)
Sterol regulatory element-binding protein isoform 1 C
- STRA8
Stimulated by retinoic acid 8
- STAR
Steroidogenic acute regulatory protein
- STAT
Signal transducer and activator of transcription
- TF
Tissue factor
- UCP1
Uncoupling protein 1
- VF
Visceral fat (in this paper mouse retroperitoneal and human omental)
- WAT
White adipose tissue
- ZFP423
Zinc-finger transcription factor 423
- 5a-r
5a-Reductase, and 5a-Androstane-3,17-dione
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wittnich C., Tan L., Wallen J., Belanger M. Sex differences in myocardial metabolism and cardiac function: an emerging concept. Pflug. Arch. 2013;465:719–729. doi: 10.1007/s00424-013-1232-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhupathiraju S.N., Wedick N.M., Pan A., Manson J.E., Rexrode K.M., Willett W.C., Rimm E.B., Hu F.B. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am. J. Clin. Nutr. 2013;98:1514–1523. doi: 10.3945/ajcn.113.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawasaki T., Chaudry I.H. The effects of estrogen on various organs: Therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: Liver, intestine, spleen, and kidney. J. Anesth. 2012;26:892–899. doi: 10.1007/s00540-012-1426-2. [DOI] [PubMed] [Google Scholar]
- 4.Meyer M.R., Clegg D.J., Prossnitz E.R., Barton M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoni R.D., Hill R.L., Vaughan M. The discovery of estrone, estriol, and estradiol and the biochemical study of reproduction. The work of Edward Adelbert Doisy. J. Biol. Chem. 2002;277:e17. [Google Scholar]
- 6.Barros R.P., Gustafsson J.A. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Jensen E.V. On the mechanism of estrogen action. Perspect. Biol. Med. 1962;6:47–59. doi: 10.1353/pbm.1963.0005. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed M., Castano E., Notides A.C., Sasson S. Molecular and kinetic basis for the mixed agonist/antagonist activity of estriol. Mol. Endocrinol. 1997;11:1868–1878. doi: 10.1210/mend.11.12.0025. [DOI] [PubMed] [Google Scholar]
- 10.Krum S.A., Miranda-Carboni G.A., Lupien M., Eeckhoute J., Carroll J.S., Brown M. Unique ERalpha cistromes control cell type-specific gene regulation. Mol. Endocrinol. 2008;22:2393–2406. doi: 10.1210/me.2008-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll J.S., Meyer C.A., Song J., Li W., Geistlinger T.R., Eeckhoute J., Brodsky A.S., Keeton E.K., Fertuck K.C., Hall G.F., et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 12.Lupien M., Eeckhoute J., Meyer C.A., Krum S.A., Rhodes D.R., Liu X.S., Brown M. Coactivator function defines the active estrogen receptor alpha cistrome. Mol. Cell. Biol. 2009;29:3413–3423. doi: 10.1128/MCB.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velickovic K., Cvoro A., Srdic B., Stokic E., Markelic M., Golic I., Otasevic V., Stancic A., Jankovic A., Vucetic M., et al. Expression and subcellular localization of estrogen receptors alpha and beta in human fetal brown adipose tissue. J. Clin. Endocrinol. Metab. 2014;99:151–159. doi: 10.1210/jc.2013-2017. [DOI] [PubMed] [Google Scholar]
- 14.Kooptiwut S., Mahawong P., Hanchang W., Semprasert N., Kaewin S., Limjindaporn T., Yenchitsomanus P.T. Estrogen reduces endoplasmic reticulum stress to protect against glucotoxicity induced-pancreatic beta-cell death. J. Steroid. Biochem. Mol. Biol. 2013;139C:25–32. doi: 10.1016/j.jsbmb.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Dulos J., Vijn P., van Doorn C., Hofstra C.L., Veening-Griffioen D., de Graaf J., Dijcks F.A., Boots A.M. Suppression of the inflammatory response in experimental arthritis is mediated via estrogen receptor alpha but not estrogen receptor beta. Arthritis Res. Ther. 2010;12:R101. doi: 10.1186/ar3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohlsson C., Hellberg N., Parini P., Vidal O., Bohlooly-Y M., Rudling M., Lindberg M.K., Warner M., Angelin B., Gustafsson J.A. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 17.Heine P.A., Taylor J.A., Iwamoto G.A., Lubahn D.B., Cooke P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manrique C., Lastra G., Habibi J., Mugerfeld I., Garro M., Sowers J.R. Loss of estrogen receptor alpha signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal Med. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riant E., Waget A., Cogo H., Arnal J.F., Burcelin R., Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 20.Morales L.B., Loo K.K., Liu H.B., Peterson C., Tiwari-Woodruff S., Voskuhl R.R. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J. Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H.B., Loo K.K., Palaszynski K., Ashouri J., Lubahn D.B., Voskuhl R.R. Estrogen receptor alpha mediates estrogen’s immune protection in autoimmune disease. J. Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 22.Prossnitz E.R., Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gushchina L.V., Yasmeen R., Ziouzenkova O. Moderate vitamin A supplementation in obese mice regulates tissue factor and cytokine production in a sex-specific manner. Arch. Biochem. Biophys. 2013;539:239–247. doi: 10.1016/j.abb.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichert B., Yasmeen R., Jeyakumar S.M., Yang F., Thomou T., Alder H., Duester G., Maiseyeu A., Mihai G., Harrison E.H., et al. Concerted action of aldehyde dehydrogenases influences depot-specific fat formation. Mol. Endocrinol. 2011;25:799–809. doi: 10.1210/me.2010-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F., Zhang X., Maiseyeu A., Mihai G., Yasmeen R., DiSilvestro D., Maurya S.K., Periasamy M., Bergdall K.V., Duester G., et al. The prolonged survival of fibroblasts with forced lipid catabolism in visceral fat following encapsulation in alginate-poly-l-lysine. Biomaterials. 2012;33:5638–5649. doi: 10.1016/j.biomaterials.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasmeen R., Jeyakumar S.M., Reichert B., Yang F., Ziouzenkova O. The contribution of vitamin A to autocrine regulation of fat depots. Biochim. Biophys. Acta. 2012;1821:190–197. doi: 10.1016/j.bbalip.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasmeen R., Reichert B., Deiuliis J., Yang F., Lynch A., Meyers J., Sharlach M., Shin S., Volz K.S., Green K.B., et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62:124–136. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S., Sandell L.L., Trainor P.A., Koentgen F., Duester G. Alcohol and aldehyde dehydrogenases: Retinoid metabolic effects in mouse knockout models. Biochim. Biophys. Acta. 2012;1821:198–205. doi: 10.1016/j.bbalip.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napoli J.L. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta. 2012;1821:152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz F.X., Gallego O., Ardèvol A., Moro A., Domínguez M., Alvarez S., Alvarez R., de Lera A.R., Rovira C., Fita I., et al. Aldo-keto reductases from the AKR1B subfamily: Retinoid specificity and control of cellular retinoic acid levels. Chem. Biol. Interact. 2009;178:171–177. doi: 10.1016/j.cbi.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Billings S.E., Pierzchalski K., Butler Tjaden N.E., Pang X.Y., Trainor P.A., Kane M.A., Moise A.R. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013;27:4877–4889. doi: 10.1096/fj.13-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziouzenkova O. Vitamin A metabolism: Challenges and perspectives. Vitam. Trace Elem. 2012;1:e106. [Google Scholar]
- 34.Germain P., Chambon P., Eichele G., Evans R.M., Lazar M.A., Leid M., De Lera A.R., Lotan R., Mangelsdorf D.J., Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 35.Germain P., Chambon P., Eichele G., Evans R.M., Lazar M.A., Leid M., De Lera A.R., Lotan R., Mangelsdorf D.J., Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R.K., Arany Z., Seale P., Mepani R.J., Ye L., Conroe H.M., Roby Y.A., Kulaga H., Reed R.R., Spiegelman B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziouzenkova O., Orasanu G., Sharlach M., Akiyama T.E., Berger J.P., Viereck J., Hamilton J.A., Tang G., Dolnikowski G.G., Vogel S. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duester G., Mic F.A., Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 2003;143–144:201–210. doi: 10.1016/S0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Sperkova Z., Napoli J.L. Analysis of mouse retinal dehydrogenase type 2 promoter and expression. Genomics. 2001;74:245–250. doi: 10.1006/geno.2001.6546. [DOI] [PubMed] [Google Scholar]
- 40.Huq M.D., Tsai N.P., Gupta P., Wei L.N. Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. EMBO J. 2006;25:3203–3213. doi: 10.1038/sj.emboj.7601181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trasino S.E., Harrison E.H., Wang T.T. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp. Biol. Med. 2007;232:762–771. [PubMed] [Google Scholar]
- 42.Li X.H., Kakkad B., Ong D.E. Estrogen directly induces expression of retinoic acid biosynthetic enzymes, compartmentalized between the epithelium and underlying stromal cells in rat uterus. Endocrinology. 2004;145:4756–4762. doi: 10.1210/en.2004-0514. [DOI] [PubMed] [Google Scholar]
- 43.Alam M., Ahmad R., Rajabi H., Kharbanda A., Kufe D. MUC1-C oncoprotein activates ERK→C/EBPβ signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J. Biol. Chem. 2013;288:30892–30903. doi: 10.1074/jbc.M113.477158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Zhang Y., Li R., Chen W., Howell M., Zhang R., Chen G. The hepatic Raldh1 expression is elevated in Zucker fatty rats and its over-expression introduced the retinal-induced Srebp-1c expression in INS-1 cells. PLoS One. 2012;7:e45210. doi: 10.1371/journal.pone.0045210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshikawa T., Shimano H., Amemiya-Kudo M., Yahagi N., Hasty A.H., Matsuzaka T., Okazaki H., Tamura Y., Iizuka Y., Ohashi K., et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 2001;21:2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molotkov A., Duester G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 2003;278:36085–36090. doi: 10.1074/jbc.M303709200. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher D., Visser M., Sepulveda D., Pierson R.N., Harris T., Heymsfield S.B. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am. J. Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 48.White U.A., Tchoukalova Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta. 2014;1842:377–392. doi: 10.1016/j.bbadis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C., Ford E.S., McGuire L.C., Mokdad A.H. Increasing trends in waist circumference and abdominal obesity among U.S. adults. Obesity. 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 50.Empana J.P., Ducimetiere P., Charles M.A., Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: The Paris Prospective Study I. Circulation. 2004;110:2781–2785. doi: 10.1161/01.CIR.0000146395.64065.BA. [DOI] [PubMed] [Google Scholar]
- 51.Canoy D., Boekholdt S.M., Wareham N., Luben R., Welch A., Bingham S., Buchan I., Day N., Khaw K.T. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation into Cancer and Nutrition in Norfolk cohort: A population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 52.Arciero P.J., Goran M.I., Poehlman E.T. Resting metabolic rate is lower in women than in men. J. Appl. Physiol. 1993;75:2514–2520. doi: 10.1152/jappl.1993.75.6.2514. [DOI] [PubMed] [Google Scholar]
- 53.Tarnopolsky M.A., Atkinson S.A., Phillips S.M., MacDougall J.D. Carbohydrate loading and metabolism during exercise in men and women. J. Appl. Physiol. 1995;78:1360–1368. doi: 10.1152/jappl.1995.78.4.1360. [DOI] [PubMed] [Google Scholar]
- 54.Komi P.V., Karlsson J. Skeletal muscle fibre types, enzyme activities and physical performance in young males and females. Acta Physiol. Scand. 1978;103:210–218. doi: 10.1111/j.1748-1716.1978.tb06208.x. [DOI] [PubMed] [Google Scholar]
- 55.Michaud A., Drolet R., Noel S., Paris G., Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012;61:689–698. doi: 10.1016/j.metabol.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Smith S.R., Lovejoy J.C., Greenway F., Ryan D., deJonge L., de la Bretonne J., Volafova J., Bray G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 57.Snijder M.B., Dekker J.M., Visser M., Yudkin J.S., Stehouwer C.D., Bouter L.M., Heine R.J., Nijpels G., Seidell J.C. Larger thigh and hip circumferences are associated with better glucose tolerance: The Hoorn study. Obes. Res. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 58.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 59.Zillikens M.C., Yazdanpanah M., Pardo L.M., Rivadeneira F., Aulchenko Y.S., Oostra B.A., Uitterlinden A.G., Pols H.A., van Duijn C.M. Sex-specific genetic effects influence variation in body composition. Diabetologia. 2008;51:2233–2241. doi: 10.1007/s00125-008-1163-0. [DOI] [PubMed] [Google Scholar]
- 60.Blouin K., Boivin A., Tchernof A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008;108:272–280. doi: 10.1016/j.jsbmb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen T.T., Hernandez Mijares A., Johnson C.M., Jensen M.D. Postprandial leg and splanchnic fatty acid metabolism in nonobese men and women. Am. J. Physiol. 1996;271:E965–E972. doi: 10.1152/ajpendo.1996.271.6.E965. [DOI] [PubMed] [Google Scholar]
- 62.Blaak E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Lladó I., Rodríguez-Cuenca S., Pujol E., Monjo M., Estrany M.E., Roca P., Palou A. Gender effects on adrenergic receptor expression and lipolysis in white adipose tissue of rats. Obes. Res. 2002;10:296–305. doi: 10.1038/oby.2002.41. [DOI] [PubMed] [Google Scholar]
- 64.Schousboe K., Willemsen G., Kyvik K.O., Mortensen J., Boomsma D.I., Cornes B.K., Davis C.J., Fagnani C., Hjelmborg J., Kaprio J., et al. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Res. 2003;6:409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 65.Shi H., Seeley R.J., Clegg D.J. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tran T.T., Kahn C.R. Transplantation of adipose tissue and stem cells: Role in metabolism and disease. Nat. Rev. Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodpaster B.H., Krishnaswami S., Harris T.B., Katsiaras A., Kritchevsky S.B., Simonsick E.M., Nevitt M., Holvoet P., Newman A.B. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch. Intern. Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 69.Yim J.E., Heshka S., Albu J.B., Heymsfield S., Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J. Appl. Physiol. 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demerath E.W., Sun S.S., Rogers N., Lee M., Reed D., Choh A.C., Couch W., Czerwinski S.A., Chumlea W.C., Siervogel R.M., et al. Anatomical patterning of visceral adipose tissue: Race, sex, and age variation. Obesity. 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schreiner P.J., Terry J.G., Evans G.W., Hinson W.H., Crouse J.R., III, Heiss G. Sex-specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric indices. The atherosclerosis risk in communities study. Am. J. Epidemiol. 1996;144:335–345. doi: 10.1093/oxfordjournals.aje.a008934. [DOI] [PubMed] [Google Scholar]
- 72.Camhi S.M., Bray G.A., Bouchard C., Greenway F.L., Johnson W.D., Newton R.L., Ravussin E., Ryan D.H., Smith S.R., Katzmarzyk P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity. 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tchoukalova Y.D., Votruba S.B., Tchkonia T., Giorgadze N., Kirkland J.L., Jensen M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ley C.J., Lees B., Stevenson J.C. Sex- and menopause-associated changes in body-fat distribution. Am. J. Clin. Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 75.Svendsen O.L., Hassager C., Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 76.Toth M.J., Tchernof A., Sites C.K., Poehlman E.T. Effect of menopausal status on body composition and abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 2000;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 77.Lovejoy J.C., Champagne C.M., de Jonge L., Xie H., Smith S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotani K., Tokunaga K., Fujioka S., Kobatake T., Keno Y., Yoshida S., Shimomura I., Tarui S., Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994;18:207–202. [PubMed] [Google Scholar]
- 79.Kanaley J.A., Sames C., Swisher L., Swick A.G., Ploutz-Snyder L.L., Steppan C.M., Sagendorf K.S., Feiglin D., Jaynes E.B., Meyer R.A., et al. Abdominal fat distribution in pre- and postmenopausal women: The impact of physical activity, age, and menopausal status. Metabolism. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 80.Perrone G., Liu Y., Capri O., Critelli C., Barillaro F., Galoppi P., Zichella L. Evaluation of the body composition and fat distribution in long-term users of hormone replacement therapy. Gynecol. Obstet. Investig. 1999;48:52–55. doi: 10.1159/000010134. [DOI] [PubMed] [Google Scholar]
- 81.Espeland M.A., Stefanick M.L., Kritz-Silverstein D., Fineberg S.E., Waclawiw M.A., James M.K., Greendale G.A. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal estrogen-progestin interventions study investigators. J. Clin. Endocrinol. Metab. 1997;82:1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 82.Munoz J., Derstine A., Gower B.A. Fat distribution and insulin sensitivity in postmenopausal women: Influence of hormone replacement. Obes. Res. 2002;10:424–431. doi: 10.1038/oby.2002.59. [DOI] [PubMed] [Google Scholar]
- 83.Escobar-Morreale H.F., San Millan J.L. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2007;18:266–272. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Khor V.K., Dhir R., Yin X., Ahima R.S., Song W.C. Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice. Am. J. Physiol. Endocrinol. Metab. 2010;299:E657–E664. doi: 10.1152/ajpendo.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burger H.G., Dudley E.C., Robertson D.M., Dennerstein L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 86.Halkjaer J., Tjonneland A., Overvad K., Sorensen T.I. Dietary predictors of 5-year changes in waist circumference. J. Am. Diet. Assoc. 2009;109:1356–1366. doi: 10.1016/j.jada.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 87.Spiegelman B.M., Flier J.S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 88.Yepuru M., Eswaraka J., Kearbey J.D., Barrett C.M., Raghow S., Veverka K.A., Miller D.D., Dalton J.T., Narayanan R. Estrogen receptor-β-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J. Biol. Chem. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim B.H., Won Y.S., Kim D.Y., Kim B., Kim E.Y., Yoon M., Oh G.T. Signal crosstalk between estrogen and peroxisome proliferator-activated receptor alpha on adiposity. BMB Rep. 2009;42:91–95. doi: 10.5483/BMBRep.2009.42.2.091. [DOI] [PubMed] [Google Scholar]
- 90.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 91.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K., et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmadian M., Abbott M.J., Tang T., Hudak C.S., Kim Y., Bruss M., Hellerstein M.K., Lee H.Y., Samuel V.T., Shulman G.I., et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiefer F.W., Vernochet C., O’Brien P., Spoerl S., Brown J.D., Nallamshetty S., Zeyda M., Stulnig T.M., Cohen D.E., Kahn C.R., et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 2012;18:918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Repa J.J., Hanson K.K., Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc. Natl. Acad. Sci. USA. 1993;90:7293–7297. doi: 10.1073/pnas.90.15.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mercader J., Palou A., Bonet M.L. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity. 2010;18:655–662. doi: 10.1038/oby.2009.330. [DOI] [PubMed] [Google Scholar]
- 96.Elliott R.B., Escobar L., Tan P.L., Muzina M., Zwain S., Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 97.Geer E.B., Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perrini S., Leonardini A., Laviola L., Giorgino F. Biological specificity of visceral adipose tissue and therapeutic intervention. Arch. Physiol. Biochem. 2008;114:277–286. doi: 10.1080/13813450802334752. [DOI] [PubMed] [Google Scholar]
- 99.Masuzaki H., Paterson J., Shinyama H., Morton N.M., Mullins J.J., Seckl J.R., Flier J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 100.Kim J.Y., van de Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M., Schraw T., Durand J.L., Li H., Li G., et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ansar Ahmed S., Penhale W.J., Talal N. Sex hormones, immune responses, and autoimmune disease. Mechanisms of sex hormone action. Am. J. Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 102.Bidulescu A., Liu J., Hickson D.A., Hairston K.G., Fox E.R., Arnett D.K., Sumner A.E., Taylor H.A., Gibbons G.H. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: The Jackson Heart Study. BMC Cardiovasc. Disord. 2013;13:9. doi: 10.1186/1471-2261-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turer A.T., Scherer P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 104.Whitacre C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 105.Garcia O.P. Effect of vitamin A deficiency on the immune response in obesity. Proc. Nutr. Soc. 2012;71:290–297. doi: 10.1017/S0029665112000079. [DOI] [PubMed] [Google Scholar]
- 106.Lee Y.S., Jeong W.I. Retinoic acids and hepatic stellate cells in liver disease. J. Gastroenterol. Hepatol. 2012;27:75–79. doi: 10.1111/j.1440-1746.2011.07007.x. [DOI] [PubMed] [Google Scholar]
- 107.Kiefer F.W., Orasanu G., Nallamshetty S., Brown J.D., Wang H., Luger P., Qi N.R., Burant C.F., Duester G., Plutzky J. Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology. 2012;153:3089–3099. doi: 10.1210/en.2011-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kane M.A., Folias A.E., Pingitore A., Perri M., Krois C.R., Ryu J.Y., Cione E., Napoli J.L. CrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrations. Mol. Cell. Biol. 2011;31:3277–3285. doi: 10.1128/MCB.05516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amleh A., Smith L., Chen H., Taketo T. Both nuclear and cytoplasmic components are defective in oocytes of the B6.Y(TIR) sex-reversed female mouse. Dev. Biol. 2000;219:277–286. doi: 10.1006/dbio.1999.9600. [DOI] [PubMed] [Google Scholar]
- 110.Bradbury M.W. Functional capacity of sex-reversed (XX, Sxr/+) mouse germ cells as shown by progeny derived from XX, Sxr/+ oocytes of a female chimera. J. Exp. Zool. 1983;226:315–320. doi: 10.1002/jez.1402260218. [DOI] [PubMed] [Google Scholar]
- 111.Burgoyne P.S. The role of the mammalian Y chromosome in spermatogenesis. Development. 1987;101:133–141. doi: 10.1242/dev.101.Supplement.133. [DOI] [PubMed] [Google Scholar]
- 112.McLaren A. The fate of germ cells in the testis of fetal Sex-reversed mice. J. Reprod. Fertil. 1981;61:461–467. doi: 10.1530/jrf.0.0610461. [DOI] [PubMed] [Google Scholar]
- 113.McLaren A. Germ cells and germ cell sex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995;350:229–233. doi: 10.1098/rstb.1995.0156. [DOI] [PubMed] [Google Scholar]
- 114.Evans E.P., Ford C.E., Lyon M.F. Direct evidence of the capacity of the XY germ cell in the mouse to become an oocyte. Nature. 1977;267:430–431. doi: 10.1038/267430a0. [DOI] [PubMed] [Google Scholar]
- 115.Palmer S.J., Burgoyne P.S. In situ analysis of fetal, prepuberal and adult XX↔XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- 116.Oulad-Abdelghani M., Bouillet P., Décimo D., Gansmuller A., Heyberger S., Dollé P., Bronner S., Lutz Y., Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J. Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tedesco M., La Sala G., Barbagallo F., De Felici M., Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J. Biol. Chem. 2009;284:35781–35793. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou Q., Li Y., Nie R., Friel P., Mitchell D., Evanoff R.M., Pouchnik D., Banasik B., McCarrey J.R., Small C., et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar S., Cunningham T.J., Duester G. Resolving molecular events in the regulation of meiosis in male and female germ cells. Sci. Signal. 2013;6:pe25. doi: 10.1126/scisignal.2004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krentz A.D., Murphy M.W., Sarver A.L., Griswold M.D., Bardwell V.J., Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev. Biol. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naillat F., Prunskaite-Hyyryläinen R., Pietilä I., Sormunen R., Jokela T., Shan J., Vainio S.J. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum. Mol. Genet. 2010;19:1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- 122.Bowles J., Feng C.W., Spiller C., Davidson T.L., Jackson A., Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev. Cell. 2010;19:440–449. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 123.Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M.J., Rossant J., et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 124.Koubova J., Menke D.B., Zhou Q., Capel B., Griswold M.D., Page D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Bouffant R., Guerquin M.J., Duquenne C., Frydman N., Coffigny H., Rouiller-Fabre V., Frydman R., Habert R., Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum. Reprod. 2010;25:2579–2590. doi: 10.1093/humrep/deq195. [DOI] [PubMed] [Google Scholar]
- 126.Mantzoros C.S. Leptin in relation to the lipodystrophy-associated metabolic syndrome. Diabetes Metab. J. 2012;36:181–189. doi: 10.4093/dmj.2012.36.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yasmeen R., Meyers J.M., Alvarez C.E., Thomas J.L., Bonnegarde-Bernard A., Alder H., Papenfuss T.L., Benson D.M., Jr., Boyaka P.N., Ziouzenkova O. Aldehyde dehydrogenase-1a1 induces oncogene suppressor genes in B cell populations. Biochim. Biophys. Acta. 2013;1833:3218–3227. doi: 10.1016/j.bbamcr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Azziz R., Woods K.S., Reyna R., Key T.J., Knochenhauer E.S., Yildiz B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 129.Asuncion M., Calvo R.M., San Millan J.L., Sancho J., Avila S., Escobar-Morreale H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J. Clin. Endocrinol. Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 130.Franks S. Polycystic ovary syndrome. N. Engl. J. Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 131.Chang R.J. A practical approach to the diagnosis of polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2004;191:713–717. doi: 10.1016/j.ajog.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 132.Stovall D.W., Bailey A.P., Pastore L.M. Assessment of insulin resistance and impaired glucose tolerance in lean women with polycystic ovary syndrome. J. Women’s Health. 2011;20:37–43. doi: 10.1089/jwh.2010.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petrelli J.M., Calle E.E., Rodriguez C., Thun M.J. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/A:1015288615472. [DOI] [PubMed] [Google Scholar]
- 134.Isfoss B.L., Holmqvist B., Jernstrom H., Alm P., Olsson H. Women with familial risk for breast cancer have an increased frequency of aldehyde dehydrogenase expressing cells in breast ductules. BMC Clin. Pathol. 2013;13:28. doi: 10.1186/1472-6890-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wickenheisser J.K., Nelson-DeGrave V.L., Hendricks K.L., Legro R.S., Strauss J.F., III, McAllister J.M. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- 136.Millman D.S., Kisslo J. Health Care Financing Administration release of final physician payment reform regulation. J. Am. Soc. Echocardiogr. 1992;5:103–106. doi: 10.1016/s0894-7317(14)80114-4. [DOI] [PubMed] [Google Scholar]
- 137.Magoffin D.A. Ovarian enzyme activities in women with polycystic ovary syndrome. Fertil. Steril. 2006;86:S9–S11. doi: 10.1016/j.fertnstert.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 138.Wood J.R., Nelson V.L., Ho C., Jansen E., Wang C.Y., Urbanek M., McAllister J.M., Mosselman S., Strauss J.F., III. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J. Biol. Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 139.Sladek N.E., Dockham P.A., Lee M.O. Human and mouse hepatic aldehyde dehydrogenases important in the biotransformation of cyclophosphamide and the retinoids. Adv. Exp. Med. Biol. 1991;284:97–104. doi: 10.1007/978-1-4684-5901-2_12. [DOI] [PubMed] [Google Scholar]
- 140.Shimamura M., Karasawa H., Sakakibara S., Shinagawa A. Raldh3 expression in diabetic islets reciprocally regulates secretion of insulin and glucagon from pancreatic islets. Biochem. Biophys. Res. Commun. 2010;401:79–84. doi: 10.1016/j.bbrc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 141.Sladek N.E. Aldehyde dehydrogenase-mediated cellular relative insensitivity to the oxazaphosphorines. Curr. Pharm. Des. 1999;5:607–625. [PubMed] [Google Scholar]
- 142.Collard F., Vertommen D., Fortpied J., Duester G., van Schaftingen E. Identification of 3-deoxyglucosone dehydrogenase as aldehyde dehydrogenase 1A1 (retinaldehyde dehydrogenase 1) Biochimie. 2007;89:369–373. doi: 10.1016/j.biochi.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]