Abstract
8-14C-Zeatin is taken up rapidly and is extensively metabolized by excised bean axes during a 12-hour incubation at 26 C. Most of the radioactivity is found in the 80% ethanol soluble fraction and consists of zeatin, zeatin riboside, zeatin-5′-ribotide, as well as corresponding dihydrozeatin derivatives. The characterization of 14C-dihydrozeatin included crystallization to constant specific radioactivity. No cleavage of the zeatin side chain to adenine, hypoxanthine, their ribosides, or glycylpurine was detected. Dihydrozeatin has been previously isolated from yellow lupin seeds, and our experiments indicate that it can be derived through reduction of the side chain from preexisting cytokinin. While the total amount of zeatin metabolized is not affected by growth-inhibiting concentrations of abscisic acid or cycloheximide, the conversion to dihydrozeatin derivatives is curtailed. Although somewhat less effective than zeatin and zeatin riboside, dihydrozeatin and dihydrozeatin riboside also counteract the abscisic acid-induced growth inhibition.
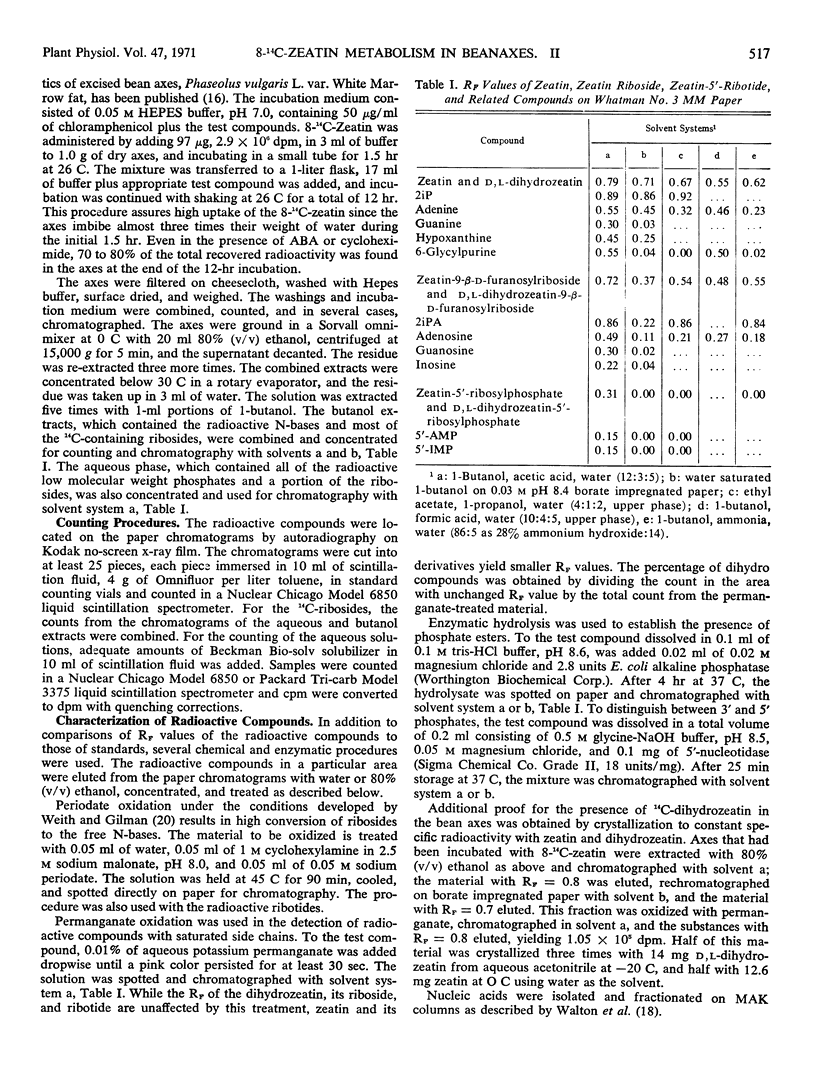
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARTER C. E. Synthesis of 6-succinoaminopurine. J Biol Chem. 1956 Nov;223(1):139–146. [PubMed] [Google Scholar]
- Hall R. H., Csonka L., David H., McLennan B. Cytokinins in the soluble RNA of plant tissues. Science. 1967 Apr 7;156(3771):69–71. doi: 10.1126/science.156.3771.69. [DOI] [PubMed] [Google Scholar]
- LEONARD N. J., FUJI T. THE SYNTHESIS OF COMPOUNDS POSSESSING KINETIN ACTIVITY. THE USE OF A BLOCKING GROUP AT THE 9-POSITION OF ADENINE FOR THE SYNTHESIS OF 1-SUBSTITUTED ADENINES. Proc Natl Acad Sci U S A. 1964 Jan;51:73–75. doi: 10.1073/pnas.51.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard N. J., Hecht S. M., Skoog F., Schmitz R. Y. Cytokinins: synthesis, mass spectra, and biological activity of compounds related to zeatin. Proc Natl Acad Sci U S A. 1969 May;63(1):175–182. doi: 10.1073/pnas.63.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura G. A., Miller C. O. 6-(gamma,gamma-Dimethylallylamino)Purine As A Precursor of Zeatin. Plant Physiol. 1969 Mar;44(3):372–376. doi: 10.1104/pp.44.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C. Germination of phaseolus vulgaris I. Resumption of axis growth. Plant Physiol. 1966 Feb;41(2):298–302. doi: 10.1104/pp.41.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Soofi G. S., Sondheimer E. The effects of abscisic Acid on growth and nucleic Acid synthesis in excised embryonic bean axes. Plant Physiol. 1970 Jan;45(1):37–40. doi: 10.1104/pp.45.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weith H. L., Gilham P. T. Structural analysis of polynucleotides by sequential base elimination. The sequence of the terminal decanucleotide fragment of the ribonucleic acid from bacteriophage f2. J Am Chem Soc. 1967 Oct 11;89(21):5473–5474. doi: 10.1021/ja00997a042. [DOI] [PubMed] [Google Scholar]



