Abstract
A novel method using ethanol was proposed for extracting lipids from wet microalga Picochlorum sp. at room temperature and pressure. In this study, Central Composite design (CCD) was applied to investigate the optimum conditions of lipid extraction. The results revealed that the solvent to biomass ratio had the largest effect on lipid extraction efficiency, followed by extraction time and temperature. A high lipid extraction yield (33.04% of the dry weight) was obtained under the following extraction conditions: 5 mL solvents per gram of wet biomass for 37 min with gentle stirring at room temperature. The extraction yield was comparable to that obtained by the widely used Bligh-Dyer method. Furthermore, no significant differences in the distribution of lipid classes and fatty acid composition were observed according to different extraction methods. In conclusion, these results indicated that the proposed procedure using ethanol could extract lipids from wet biomass efficiently and had giant potential for lipid extraction at large scale.
Keywords: lipid extraction, wet microalga, biofuel, ethanol, Picochlorum sp.
1. Introduction
Due to the serious energy crisis and environmental pollution associated with the using of fossil fuels, biofuel derived from microalgae has been advocated in recent years. Compared to other feedstocks like plant oils, animal fats, etc., microalgae have outstanding advantages: they are capable of growing rapidly and converting CO2 into substantial amounts of lipids [1,2,3]. Some microalgae species can absorb essential nutrients including the carbon, nitrogen and phosphorus from exhaust gas and waste water [4,5]. Additionally, many microalgae can grow well in unfavorable lands and saline water. That is, microalgae do not compete for the land required for producing food and overcome the discord between food and fuel [6,7,8].
Extracting lipids is one of the most key and limited processes for biofuel production based on microalgae at large scale. The conventional methods for lipid extraction generally involve dewatering before extracting lipids since residual water in wet microalgal biomass hindered mass transfer of the lipids from the cell and then lead to a decrease in the efficiency of lipids extraction. Lardon et al. [9] and Patil et al. [10] reported that the consumption energy of the drying accounted for the majority of the total process energy (84.9%). In addition, the organic solvents used in the conventional methods are regarded as highly-toxic, being environmentally unfriendly. These shortcomings hinder the application of conventional methods in industrial lipid extraction, despite of the high extraction efficiency. Therefore, it is essential to develop novel approach of lipid extraction, which is an effective eco-friendly process.
Compared to the traditional methods involving drying, extracting lipids from wet biomass is a more economic method, which requires no energy to dry the biomass. Various researchers have investigated the wet lipid extraction methods, including ultrasound-assisted extraction [11], simultaneous distillation and extraction process [12], microwave-assisted extraction [13] and supercritical fluid extraction [14]. Unfortunately, the most cases still require high temperature, long times or high energy inputs. Therefore, the technologies of lipid extraction are only limited to laboratory scale. The ideal method being suitable for industrial-scale extraction has not yet been settled.
The aim of the present study is to develop an eco-friendly solvent technique that will make it possible to extract lipid from wet microalgal biomass efficiently at large scale. Ethanol is considered as a cheap and safe solvent; additionally, ethanol has a strong affinity to the lipid complex, which implies that lipids can be extracted efficiently. Fajardo et al. [15] used ethanol, following by hexane, to extract and purify lipids from drying microalga Phaeodactylum tricornutum efficiently. Chen et al. [16] extracted lipids from wet microalga Nannochloropsis sp. at high temperature and pressure. However, no investigation about the application of ethanol in extracting lipids from wet microalgal biomass at room temperature and pressure has been reported.
In this study, we adopted a novel method using ethanol with gentle stirring for lipid extraction from wet microalga Picochlorum sp. directly. Then the effects of parameters including time, temperature, and the ratio of solvent to biomass on the lipid extraction yield were investigated by Central Composite design (CCD) to identify the optimum extraction conditions. Finally, the proposed methods were compared with the conventional Bligh-Dyer method in terms of lipid extraction yield, lipids quality.
2. Results and Discussion
2.1. Examine Ethanol for Lipid Extraction from Wet Biomass
A novel method, extracting lipids from wet biomass using ethanol at room temperature (27 °C) for 30 min, was proposed. The ratio of ethanol to wet biomass was 4:1 (mL/g). To examine the feasibility of the method, the lipid extraction yield was determined as described in Section 3.3. The lipids arising from microalgal biomass by ethanol, referred to as crude lipids, frequently contain several non-lipids (proteins bonding to lipids strongly and carbohydrates). To avoid the interference of non-lipid complex, the crude lipids were purified. Hexane was a low-toxicity solvent and employed to remove non-lipids complex from crude lipids. The purified lipids were quantified and used to calculate the lipid extraction yield. The extraction yield by ethanol was close to that of Bligh-Dyer’s method, namely, 31.89% and 33.18% of the dry weight, respectively. That is, the extraction rate of lipids was 96.1%. The result implied that ethanol had potential for extracting lipids from wet microalgae at room temperature.
2.2. Investigating the Optimum Procedure of Extracting Lipids Using Ethanol
In the experiment, Central Composite design (CCD) was employed to analyze comprehensively the influences of three extraction parameters on the lipid extraction yield and determine the optimum extraction conditions. Table 1 shown the actual factor levels corresponding to the coded factor levels. In total, 20 experiments were designated.
Table 1.
Levels and variables involved in Central Composite design.
| Variables | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| Extraction time (min) | 2.5 | 10 | 25 | 40 | 47.5 |
| Extraction temperature (°C) | 20 | 25 | 35 | 45 | 50 |
| the ratio of solvent to biomass (mL/g) | 1 | 2 | 4 | 6 | 7 |
The corresponding response value obtained from each run were illustrated in Table 2. By analyzing these data in Table 2, the following second order polynomial equation expressed in terms of coded values fitted to the results from the optimization experiments was obtained.
| Y = +30.98 + 1.27 X1 + 0.042X2 + 4.06X3 + 0.14X1X2 − 0.24X1X3 − 0.25X2X3 − 0.66X12 + 0.37X22 − 2.71X32 |
where, Y stood for lipid extraction yield (% of the dry weight); X1, X2 and X3 were extracting time (min), extracting temperature (°C) and the ratio of solvent to biomass (mL/g), respectively.
Table 2.
Results and experimental layout in Central Composite design.
| NO. | Extraction time (min) | Extraction temperature (°C) | Solvent to biomass ratio (mL/g) | Extraction yield (of the dry weight) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | 10.00 | 25.00 | 2.00 | 22.62 | 22.26 |
| 2 | 40.00 | 25.00 | 2.00 | 25.20 | 25.01 |
| 3 | 10.00 | 45.00 | 2.00 | 22.77 | 22.57 |
| 4 | 40.00 | 45.00 | 2.00 | 26.26 | 25.87 |
| 5 | 10.00 | 25.00 | 6.00 | 30.84 | 31.36 |
| 6 | 40.00 | 25.00 | 6.00 | 32.81 | 33.14 |
| 7 | 10.00 | 45.00 | 6.00 | 30.35 | 30.67 |
| 8 | 40.00 | 45.00 | 6.00 | 32.51 | 33.00 |
| 9 | 2.50 | 35.00 | 4.00 | 27.73 | 27.60 |
| 10 | 47.50 | 35.00 | 4.00 | 31.51 | 31.41 |
| 11 | 25.00 | 20.00 | 4.00 | 31.90 | 31.76 |
| 12 | 25.00 | 50.00 | 4.00 | 31.96 | 31.88 |
| 13 | 25.00 | 35.00 | 1.00 | 17.97 | 18.79 |
| 14 | 25.00 | 35.00 | 7.00 | 32.01 | 30.96 |
| 15 | 25.00 | 35.00 | 4.00 | 31.29 | 30.98 |
| 16 | 25.00 | 35.00 | 4.00 | 30.11 | 30.98 |
| 17 | 25.00 | 35.00 | 4.00 | 30.56 | 30.98 |
| 18 | 25.00 | 35.00 | 4.00 | 31.28 | 30.98 |
| 19 | 25.00 | 35.00 | 4.00 | 31.64 | 30.98 |
| 20 | 25.00 | 35.00 | 4.00 | 30.83 | 30.98 |
To check the adequacy of the quadratic polynomial model, the statistical significance of the above equation was calculated, illustrated in Table 3. Here, R2 was 0.9857, indicating that 98.57% of the data in CCD could be explained by the response surface model, that is, the model can be carried out to reveal the effects of variables on the response value and predict the maximum response value in subsequent optimization experiments. In addition, the F-value of 76.57 demonstrated that the model was significant, as indicated by the p-value less than 0.0001, which further supported the fitness of the proposed model. From the analysis of Radj2 and Rpred2, there was a high degree of agreement between them. In conclusion, these results clearly indicated that the model could be used to explain these data well.
Table 3.
Statistical analysis for experimental results of Central Composite design.
| Source | Sum of squares | Df | Mean square | F value | p-Value |
|---|---|---|---|---|---|
| Model | 308.98 | 9 | 34.33 | 76.57 | < 0.0001 |
| Linear | |||||
| X1 | 20.16 | 1 | 20.16 | 44.97 | < 0.0001 |
| X2 | 0.022 | 1 | 0.022 | 0.049 | 0.8301 |
| X3 | 205.72 | 1 | 205.72 | 458.83 | < 0.0001 |
| Quadratic | |||||
| X12 | 0.15 | 1 | 0.15 | 0.34 | 0.0105 |
| X22 | 0.47 | 1 | 0.47 | 1.05 | 0.1041 |
| X32 | 0.50 | 1 | 0.50 | 1.12 | < 0.0001 |
| Interaction | |||||
| X1X2 | 4.43 | 1 | 4.43 | 9.87 | 0.5714 |
| X1X3 | 1.43 | 1 | 1.43 | 3.20 | 0.3301 |
| X2X3 | 75.84 | 1 | 75.84 | 169.16 | 0.3148 |
| Residual | 4.48 | 10 | 0.45 | ||
| Lack of fit | 2.91 | 5 | 0.58 | 1.85 | 0.2578 |
| Pure error | 1.57 | 5 | 0.31 | ||
| Cor total | 313.47 | 19 |
R2 = 0.9857; RAdj2 = 0.9728; RPred2 = 0.9223.
As shown in Table 3, the linear coefficient indicated that the ratio of solvent to biomass (X3) was the most significant independent variable impacting on extraction yield with p-value less than 0.01. The higher ratio of solvent to biomass was, the more the extraction yield could be. The extraction time (X1) also exerted a positive individual influence on the extraction yield. That implied that an increase in the extraction time improved the lipid extraction amounts. In addition, the ratio of solvent to biomass and extraction time exerted the significant quadratic effects (Table 3). However, other terms (X2, X22, X1X2, X1X3, X2X3) were insignificant (Table 3). In particular, extraction temperature in the range of 20–50 °C had litter effect on extraction yield and the lipids could be extracted effectively at room temperature. It was therefore possible that Picochlorum sp. was cracked during the extracting process and ethanol get into the cell easily without the cell wall resistance. Additionally, gentle stirring could accelerate cells lysis and elevate extraction efficiencies.
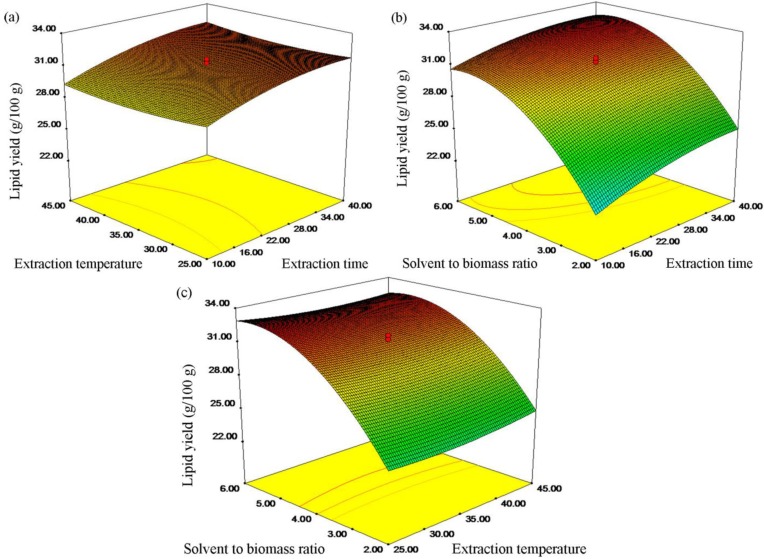
To understand the interaction of the corresponding parameters, the regression model was represented in terms of response surface plots, as shown in Figure 1. Figure 1a represented the mutual effect of the extraction time and temperature on the extraction yield. It was apparent that the interaction between the two selected variables had litter influence on the extraction yield. As seen in Figure 1b, it could be observed that the extraction yield increased significantly with an increase in the ratio of solvent to biomass at a given extraction time. However, excess solvent amount would not improve further the extraction yield. In addition, as extraction time elevating, the extraction efficiency enhanced, resulting in a higher lipid extraction yield. However, the interaction terms of the two variables possessed litter role as indicated by its p-value of 0.3301. Similarly, the interaction between extraction temperature and the ratio of solvent to biomass had insignificant effects on extraction yield, as illustrated in Figure 1c.
Figure 1.
Response surfaces and contour plots showing the mutual effect of (a) extraction temperature and time; (b) the ratio of solvent to biomass and extraction time; (c) the ratio of solvent to biomass and extraction temperature on the lipid extraction yield.
2.3. The Validation of the Model
According to the above results, the optimum extraction conditions were obtained as following: the ratio of solvent to biomass was 5:1 (mL/g) at room temperature (26 °C) for 37 min of extraction time. The extraction yield obtained from Picochlorum sp. was predicted to be 33.10% of the dry weight. In order to confirm these conclusions, extraction experiments based on the optimal extraction parameters were performed and the extraction yield was determined. The experimental value was 33.04% of the dry weight, which was agreement well with the predicted value calculated by the model equation, demonstrating the adequacy of the regression equation. The results also revealed that ethanol could employed successfully to extract lipids from wet microalga Picochlorum sp. However, the particular reason for this is not well understood and requires further research. Halim et al. [17] proposed a probable mechanism for lipid extraction from microalgae by solvent. The solvent penetrated through the cell membrane into the cytoplasm. Then the solvent interacted with the lipid complex and formed a complex. Finally, the solvent-lipids complex diffused out the cell and lipids were extracted. Ranjan et al. [18] revealed that the prominent mechanism of lipid extraction by organic solvent was diffusion across a cell wall. The extent of diffusion was attributed to the selectivity of the solvent. In the study, ethanol was used as extractant. Since ethanol had both polar and non-polar properties, it could interact with non-poplar and poplar lipids after entering into cells. This meant that ethanol could pull out neutral and polar lipids from cell efficiently [15,19]. Additionally, the gentler stirring in the extraction process could sweep away the extracted lipids from the microalgal cell surface and maintain a continuous diffusion of lipids from the cells. On the other hand, the disruption of microalgal cells was also a mechanism of lipid extraction [18]. The disruption depended greatly on cell morphology and the probability of interaction of the cell with cavitation bubbles [18,20]. The cell wall of Picochlorum sp. used in this study could be disrupted in the extracting procedure and cellular contents were probably released. Therefore, ethanol could extract lipids from wet microalga efficiently. However, the special mechanism requires further research.
2.4. Lipid Analysis and Fatty Acid Composition Comparisons
To evaluate the efficiency of our proposed method, extracting lipids from wet biomass using ethanol, the conventional Bligh-Dyer method was employed as a reference, due to its high lipid extraction efficiency. The moisture content of wet biomass was 90.02% wet weight.
Table 4 presented the lipid extraction yield for two extraction methods. When using ethanol as extractant, a high extraction yield of lipids (33.04% of the dry weight), which was similar with that of Bligh-Dyer method (33.18% of the dry weight), was obtained. This implied extraction rate of the proposed method was up to 99.6%. Additionally, we investigated the lipid extraction ratio of the fresh microalga. The results revealed that there were no significant changes in the extraction efficiency.
Table 4.
Fatty acid profile comparison between different extraction methods.
| Extraction method | ||
|---|---|---|
| Bligh-Dyer | Ethanol | |
| Lipid extraction yield (% of the dry weight) | 33.18 ± 0.24 | 33.04 ± 0.16 |
| Fatty acid composition (% of FAME) | ||
| Saturated | ||
| C16:0 | 32.49 ± 1.54 | 29.48 ± 3.12 |
| C18:0 | 2.82 ± 0.43 | 6.00 ± 1.89 |
| Unsaturated | ||
| C16:1 | 2.57 ± 0.62 | 2.05 ± 0.38 |
| C16:2 | 5.76 ± 0.16 | 5.65 ± 0.58 |
| C16:3 | 6.62 ± 0.54 | 6.09 ± 0.11 |
| C18:1 | 8.42 ± 0.01 | 9.06 ± 0.28 |
| C18:2 | 22.25 ± 0.21 | 22.47 ± 2.63 |
| C18:3 | 17.37 ± 0.65 | 17.04 ± 0.93 |
| Others | 1.71 ± 0.35 | 2.16 ± 0.40 |
The major fatty acid composition of lipids was determined by gas chromatography coupled to mass spectrometry (GC-MS). As shown in Table 4, no significant differences in fatty acid composition were observed between different extraction methods. The dominant fatty acids were palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3), which accounted for approximately 80% of total fatty acids. Other fatty acids, such as palmitolenic (C16:1), palmitolenic (C16:2), palmitoleidic (C16:3) and stearic (C18:0), were also present in smaller quantities. The fatty acid composition of Picochlorum sp. was similar with the profile presented by Tanzi et al. [12]. In generally, C16:0, C18:0, C18:1 and C18:2 were known to the most common components in biodiesel [14,21]. Therefore, the extracted lipids of Picochlorum sp. were suitable for biodiesel production.
The content of each fatty acid had no significant difference between two methods according to statistical analysis (P > 0.05). However, the ratio of some fatty profiles, especially C18:0, represented slight variation according to different extraction methods. When using ethanol as solvent, the lower proportion of C16:0, C16:1 and C16:3, with corresponding increasing in C18:0 and C18:1, compared to the Bligh-Dyer method (Table 4). The probable reason was that the change of extraction conditions resulted in the fatty acid profile of the extracted lipids [14]. The results revealed that ethanol could extract most of fatty acids efficiently. In addition, it must be highlighted that the microalga Picochlorum sp. had a high percentage of linolenic acid (around 17%), which was an essential and important fatty acid to human health [22].
The lipid class was determined and presented as % of lipid class in total lipids as shown in Table 5. The lipids were consisted mainly of neutral lipid, glycolipid and phospholipid. By statistical analysis, no significant difference in the content of each lipid class was observed (P > 0.05), although the content of phospholipid was higher than that obtained using Bligh-Dyer method. Additionally, the percentage of neutral lipid was highest (about 50% of total lipids) using the two extraction methods, suggesting the microalga Picochlorum sp. was a promising feedstock for biodiesel production. As a conclusion, most of essential lipids for biodiesel production could be extracted from wet biomass effectively using ethanol.
Table 5.
The lipid class comparison between different extraction methods. Values were given as means of total lipids percentage ± standard deviation.
| Lipid class | Extraction method | |
|---|---|---|
| Bligh-Dyer | Ethanol | |
| Neutral lipid | 54.73 ± 1.47 | 53.49 ± 2.11 |
| Glycolipid | 16.46 ± 0.76 | 15.62 ± 0.54 |
| Phospholipid | 28.81 ± 0.71 | 30.89 ± 1.57 |
2.5. Lipid Extraction at Larger Scale and Ethanol Recycling
To validate the applicability of the optimal method in lipid extraction at enlarged scale, 250 g wet biomass was employed to extract lipids. A summary of the protocol was shown in Figure 2. A high extraction ratio of 99.4% was obtained, implying that the optimum method was effective for extracting lipids at larger scale. Additionally, ethanol was recovered by using distillation tower in order to decrease the consumption of ethanol. The results revealed that the recovery of ethanol reached a yield of 95.24% with the purity of 93%. Furthermore, the experiments confirmed that the recycled ethanol had high efficiency for extracting lipids from wet biomass. Therefore, the extraction method with ethanol was suitable for extracting lipids from microalgae at large scale, with a high extraction efficiency and low environment pollution.
Figure 2.
A scheme illustration of lipid extraction procedure from wet microalga using ethanol.
2.6. Lipid Extraction Methods Comparison
The lipid extraction method using ethanol was evaluated in terms of extraction yield and lipid quality. There was no significant difference compared to the conventional Bligh-Dyer method, that is, ethanol could extract lipids from wet microalga effectively.
Comparing to the Bligh-Dyer method, the method, because it used ethanol, was environmentally friendly. Moreover, since the debris contained high contents of proteins, the debris would be reused for producing bait after extracting lipids. Conversely, the debris would be toxic and be not reused if the Bligh-Dyer method was employed to extract lipids. Therefore, the method was considered safe and suitable for the lipid extraction in the industrial scale.
So far, there have been a few reports related to the application of ethanol in extracting lipids [15,16]. However, in these cases, dewatering or high temperature still be required. In the study, ethanol was employed successfully to extract lipids from high-moisture microalgae at room temperature and pressure. It was probable that the Picochlorum sp. used in this study had different bio-characteristics and the cell wall was cracked easily. Hence, there was slight cell wall resistance and the ethanol could enter into cells for extracting lipids quickly. This was consistent with Prommuak et al. [20] which revealed that the extraction efficiency depended greatly on cell morphology. Compared to the conventional Bligh-Dyer method, the method did not require dewatering and heating, which implied the method was easier to operate at large scale.
In conclusion, the method based on ethanol possessed important advantages over Bligh-Dyer method, such as shorter treatment time, less environment pollution, high extraction efficiency, which was more applicable for lipid extraction at large scale than the conventional methods.
Despite this, the effectiveness of the method will be further evaluated using different types of microalgae at large scale to test the practical application of this method. Otherwise, it would be necessary to further investigate the applications of the residual biomass and linolenic acid as byproduct, to improve the overall economics of microalgal biofuel production.
3. Materials and Methods
3.1. Strain, Culture Conditions and Harvesting Method
Picochlorum sp., isolated from the India Ocean, was cultured in the modified f/2 media composed of sea water with an addition of phosphorus and nitrogen sources. The cultures were cultivated in an outdoor raceway system of up to 500 m2. Temperature and illumination intensity depended on the daily weather. Daily microscopic analysis revealed that Picochlorum sp. was not contaminated. At the same time, microalgal cultures were harvested and concentrated by the floatation method. A dilute aqueous suspension with water content of 90.02% wet weight was obtained and stored at 4 °C for subsequent analysis. In the experiments involving dried microalgae, the concentrated microalgal cultures were frozen-dried completely in a lyophilizer. In the experiments where wet microalgae were used, the concentrated microalgal cultures were used to extract lipid directly without further treatment.
3.2. Conventional Lipid Extraction Method
The lipids of drying biomass were extracted by Bligh-Dyer’s method with chloroform and methanol mixture [23]. The method was used as standard to evaluate our proposed method of extracting lipids from wet biomass. The chloroform and methanol mixture was added to 100 mg of drying biomass at 50 °C for 1 h. The extracted lipids were quantified and analyzed.
3.3. Lipid Extraction using Ethanol as Extractant
Ethanol was performed to extract lipids from wet biomass (approximately 1 g). The lipids arising from microalgal biomass by ethanol, referred to as total lipids or crude lipids, frequently contain several non-lipids (proteins bonding to lipids strongly and carbohydrates). Then the biomass residue was removed and the crude lipids obtained were later purified.
The lipid extraction ratio obtained by hexane was low since hexane preferably extracted non-polar lipids in the microalga. However, hexane was a low-toxicity solvent and could remove non-lipids from crude lipids efficiently [20]. In the study, to purify the crude lipids, the water and hexane were added into the crude lipids to form a liquid-liquid separation state according to the method illustrated by Fajardo et al. [15]. The upper phase (hexane and some ethanol) was loaded with most of the lipids while the lower phase (most ethanol with water) contained most non-lipids. The upper phase that contained lipids was transferred to a weighted tube and dried by stream of N2. The purified lipids were quantified and were later analyzed by gas chromatography and silica gel column chromatography. In all experiments, three parallels were set up for each treatment.
3.4. Experimental Design and Data Analysis
In order to investigate the optimum extraction conditions, a mathematical model-Central Composite design (CCD) was utilized when ethanol was used as extractant. By applying the CCD, the effect of each parameter, such as extraction time, extraction temperature and the ratio of solvent to biomass, on extraction yield were evaluated quickly and effectively. Table 1 illustrated the actual factor levels corresponding to the coded factor levels. In the experiment, the low and high levels of all variables, that is, star points were set up first. Depending on the number of parameters involved and desire of the design, the value of α, which expressed distance between star points and center points, was determined. In all, twenty experiments were employed with fifteen being the different combinations of three parameters and five being replications of center points. Each trial was performed in triplicate and lipid extraction yield was the mean values. According to the experimental results obtained, the second-degree polynomial equation was given below, which could calculate the predicted value of lipid extraction yield.
| Y = α0 + α1X1 + α2X2 + α3X3 + α4X1X2 + α5X1X3 + α6X2X3 + α7X12+ α8X22+ α9X32 |
where, Y represents the predicted value of extraction yield (% of the dry weight); X1, X2, X3 are the code values of extraction time (min), extraction temperature (°C) and the ratio of solvent to wet biomass (mL/g), respectively; α0 is quantity; α1–α9 stand for coefficient estimate.
The software (Design Expert, version 8.05) was conducted to analyze and calculate these results. Under the optimum conditions, the lipids were extracted. By comparing the experimental and predicted values, the model was verified.
3.5. Esterification and Analysis of Fatty Acids
The lipids were converted to fatty acid methyl ester (FAME) for gas chromatography. 1 mL of chloroform containing 0.2 mg of heptadecanoic acid (C17:0) was added to each of the purified lipid samples as an internal standard. Then 1 mL of NaOH-CH3OH was added at 75 °C for 10 min and 2 mL of BF3-CH3OH was added for transesterification reaction at 75 °C for 10 min. After the reaction, 3 mL of hexane and 1 mL of deionized water were added to the above samples. Finally, the samples were centrifuged and upper layer was separated for GC-MS analysis. Fatty acid compositions of the lipids were analyzed by GC-MS with an Omegawax 250 polyethylene glycol capillary column (length 30 m, diameter 0.25 mm and 0.25 μm film thickness) using the method reported by Goldberg et al. [24]. Samples of 1 µL were injected into the capillary column with a split ratio of 5:1. Helium was employed as the carrier gas with a flow rate of 1.5 mL/min. The temperatures of injector and detector both maintained at 250 °C. The column temperature was programmed from 130 °C at 5 °C/min ramp rate to 250 °C maintained for 5 min. Each component was identified by comparing retention time and fragmentation with standards using the GC-MS library. The fatty acid content was expressed as percentage of total fatty acids.
3.6. Lipid Analysis Using Silica Gel Column Chromatography
Lipid class separation was performed by silica gel column chromatography according to the method illustrated by Christie [25]. Typically, the samples of lipids re-suspended in chloroform were loaded onto silica gel column chromatography (Agela, Tianjin, China). Neutral lipid, phospholipid and glycolipid were successively eluted using chloroform, acetone and methanol, respectively. Each component was dried by stream of N2 and then weighed.
3.7. Lipid Extraction at Larger Scale and Ethanol Recycling
Ethanol was added to wet microalgal biomass (250 g) in an approximately 5:1 mass ratio. Under the optimum conditions, lipids were extracted. After extraction, the supernatant, loading with lipids and ethanol, was separated and then ethanol was recycled using distillation tower. The purity of ethanol was measured by the alcohol detector. The recycled ethanol was once again employed to extract lipids from wet biomass in order to evaluate its ability of extracting lipids. A summary of the protocol was shown in Figure 2.
4. Conclusions
This study demonstrated that the novel method using ethanol could be used to extract lipids from high-moisture microalgae at room temperature, with an extraction yield of 33.04% of the dry weight. The extraction yield was comparable to that of the conventional Bligh-Dyer method. Additionally, only minor variations in lipid profiles and fatty acid composition were observed according to different methods, suggesting that ethanol extracted the main components for biodiesel production effectively. Further research revealed that a high lipid extraction ratio of 99.4% was obtained using the proposed method at larger scale. Taken together, the results suggested that the method with ethanol was an easy, less environmentally polluting and high efficiency extraction process.
Acknowledgements
This research was supported by the Ocean Public Welfare Scientific Research Project (201305018-3), Funds for Marine Renewable Energy (GHME2011SW04), Main Direction Program of Chinese Academy of Sciences (KSCX2-YW-R-093).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Chisti Y. Biodiesel from microalgae. Biotechnol. Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Liam B., Philip O. Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;2:557–577. [Google Scholar]
- 3.Mairet F., Bernard O., Masci P., Lacour T., Sciandra A. Modelling neutral lipid production by the microalga Isochrysis aff. galbana under nitrogen limitation. Bioresour. Technol. 2011;1:142–149. doi: 10.1016/j.biortech.2010.06.138. [DOI] [PubMed] [Google Scholar]
- 4.Rawat I., Ranjith K.R., Mutanda T., Bux F. Dual role of microalgae: Phycoremediation of domestic wasterwater and biomass production for sustainable biofuels production. Appl. Energy. 2011;88:3411–3424. doi: 10.1016/j.apenergy.2010.11.025. [DOI] [Google Scholar]
- 5.Hende S.V.D., Vervaeren H., Boon N. Flue gas compounds and microalgae: (Bio-) chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012;30:1405–1424. doi: 10.1016/j.biotechadv.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Hu Q., Sommerfeld M., Jarvis E., Ghirardi M., Posewitz M., Seibert M., Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 7.Schenk P.M., Thomas-Hall S.R., Stephens E., Marx U.C., Mussgnug J.H., Posten C., Kruse O., Hankamer B. Second generation biofuels: High-Efficiency microalgae for biodiesel production. BioEnergy Res. 2008;1:20–43. doi: 10.1007/s12155-008-9008-8. [DOI] [Google Scholar]
- 8.Singh J., Gu S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010;9:2596–2610. doi: 10.1016/j.rser.2010.06.014. [DOI] [Google Scholar]
- 9.Lardon L., Helias A., Sialve B., Steyer J.P., Bernard O. Life-Cycle assessment of biodiesel production from microalgae. Sci. Technol. 2009;17:6475–6481. doi: 10.1021/es900705j. [DOI] [PubMed] [Google Scholar]
- 10.Patil P.D., Gude V.G., Mannarswamy A., Cooke P., Nirmalakhandan N., Lammers P., Deng S.G. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel. 2012;97:822–831. doi: 10.1016/j.fuel.2012.02.037. [DOI] [Google Scholar]
- 11.Adam F., Vian M.A., Peltier G., Chemat F. “Solvent-Free” ultrasound assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012;114:457–465. doi: 10.1016/j.biortech.2012.02.096. [DOI] [PubMed] [Google Scholar]
- 12.Tanzi C.D., Vian M.A., Chemat F. New procedure for extraction of algal lipids from wet biomass: A green clean and scalable process. Bioresour. Technol. 2013;134:271–275. doi: 10.1016/j.biortech.2013.01.168. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J., Yu T., Li T., Zhou J.H., Cen K.F. Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour. Technol. 2013;131:531–535. doi: 10.1016/j.biortech.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Halim R., Gladman B., Danquah M.K., Webley P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011;1:178–185. doi: 10.1016/j.biortech.2010.06.136. [DOI] [PubMed] [Google Scholar]
- 15.Fajardo A.R., Cerdán L.E., Medina A.R., Fernández F.G.A., Moreno P.A.G., Grima E.M. Lipid extraction from the microalga Phaeodactylum tricornutum. Eur. J. Lipid Sci. Technol. 2007;2:120–126. [Google Scholar]
- 16.Chen M., Chen X.L., Liu T.Z., Zhang W. Subcritical ethanol extraction of lipid from wet microalgae paste of Nannochloropsis sp. J. Biobased Mater. Bioenergy. 2011;5:385–389. doi: 10.1166/jbmb.2011.1157. [DOI] [Google Scholar]
- 17.Halim R., Danquah M.K., Webley P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012;30:709–732. doi: 10.1016/j.biotechadv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Ranjan A., Patil C., Moholkar V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010;49:2979–2985. doi: 10.1021/ie9016557. [DOI] [Google Scholar]
- 19.Brian M.C. Kinetics of Lipid Extraction from Microalgae. University of New Hampshire, University of New Hampshire Scholars’ Repository; Durham, NC, USA: 2013. [Google Scholar]
- 20.Prommuak C., Pavasant P., Quitain A.T., Goto M., Shotipruk A. Microalgal lipid extraction and evaluation of single-step biodiesel production. Eng. J. 2012;5:157–166. [Google Scholar]
- 21.Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86:1059–1070. doi: 10.1016/j.fuproc.2004.11.002. [DOI] [Google Scholar]
- 22.Hugo P., Luisa B., Filipe F., Luisa C., Catarina V.D., Cristina P., Eva R., Aschwin E., Joao V. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs. 2012;10:1920–1935. doi: 10.3390/md10091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:913–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg K.I., Shrestha P., Cohen Z. Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa. BBA Mol. Cell Biol. Lipids. 2005;1738:63–71. doi: 10.1016/j.bbalip.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Christie W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids. 3rd ed. The Oily Press; Bridgewater, UK: 2003. pp. 373–387. [Google Scholar]