Abstract
Sea angels, Clione limacina and Paedoclione doliiformis, are small, floating sea slugs belonging to Gastropoda, and their gonads are a bright orange-red color. Sea angels feed exclusively on a small herbivorous sea snail, Limacina helicina. Carotenoids in C. limacina, P. doliiformis, and L. helicina were investigated for comparative biochemical points of view. β-Carotene, zeaxanthin, and diatoxanthin were found to be major carotenoids in L. helicina. L. helicina accumulated dietary algal carotenoids without modification. On the other hand, keto-carotenoids, such as pectenolone, 7,8-didehydroastaxanthin, and adonixanthin were identified as major carotenoids in the sea angels C. limacina and P. doliiformis. Sea angels oxidatively metabolize dietary carotenoids and accumulate them in their gonads. Carotenoids in the gonads of sea angels might protect against oxidative stress and enhance reproduction.
Keywords: carotenoids, sea angels, food chain, metabolism
1. Introduction
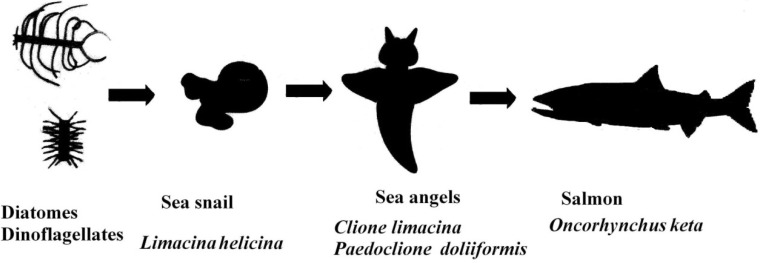
Clione limacina is a small, floating sea slug (0.5~3 cm body length) belonging to the family Clionidae, which is a group of pelagic marine gastropods. Paedoclione doliiformis is a very small, floating sea slug (<0.5 cm body length) that also belongs to the family Clionidae. Their shells are lost during development and their body is gelatinous and transparent. On the other hand, their gonads and viscera are a bright orange-red color. They float by flapping their “wings”. Their floating styles resemble angels and so they are called “sea angels” [1]. From spring to autumn, sea angels live at a depth of 200 m in the Sea of Okhotsk. In winter, they migrate to the coast of north Hokkaido with drift ice. The sea angels, C. limacina and P. doliiformis, are carnivorous and feed exclusively on Limacina helicina, which is a small, swimming predatory sea snail belonging to the family Limacinidae (Gastropoda) which feed on micro algae such as diatoms and dinoflagellates [2]. Chum salmon, Oncorhynchus keta, is one of the major predators of sea angels in the Okhotsk Sea of north Hokkaido [3,4].
Marine animals, especially marine invertebrates, contain various carotenoids, showing structural diversity [5,6,7,8]. New carotenoids are still being discovered in marine animals [9]. In general, animals do not synthesize carotenoids de novo, and so those found in animals are either directly accumulated from food or partly modified through metabolic reactions [6,7,8]. The major metabolic conversions of carotenoids found in marine animals are oxidation, reduction, the translation of double bonds, oxidative cleavage of double bonds, and cleavage of epoxy bonds. Therefore, structural diversity is found in carotenoids of marine animals [6,7,8].
We have studied carotenoids in several marine animals from chemical and comparative biochemical points of view [8,9,10]. We have been interested in the orange-red pigments, which were assumed to be carotenoids, of sea angels. Thus, we studied the carotenoids of the sea angels C. limacina and P. doliiformis. Furthermore, carotenoids in the small snail L. helicina and chum salmon O. keta were studied from the perspective of the food chain (Figure 1). In the present paper, we describe the carotenoids of these marine animals from the viewpoints of comparative biochemistry and the food chain.
Figure 1.
Food chains from phytoplankton to salmon via sea angels in the Okhotsk Sea of north Hokkaido.
2. Results
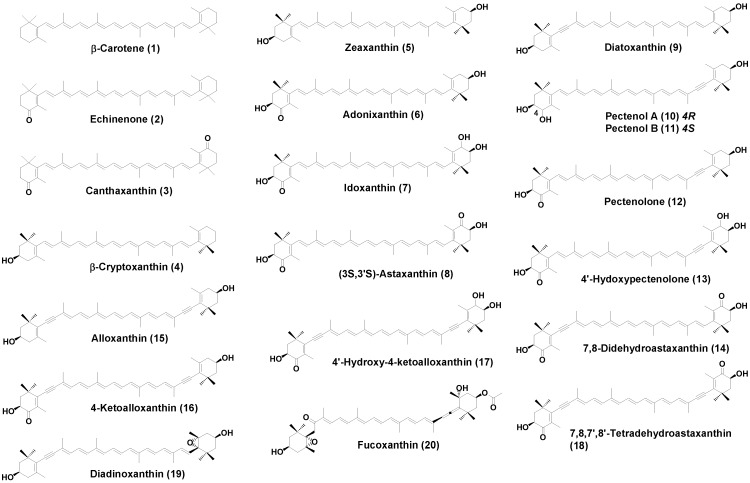
Structural formulae of carotenoids identified from the sea angels C. limacina and P. doliiformis and the small herbivorous sea snail L. helicina are shown in Figure 2.
Figure 2.
Structure of carotenoids found in C. limacina, P. doliiformis, and L. helicina.
2.1. Carotenoids of L. helicina
The carotenoid content and composition of the small herbivorous sea snail L. helicina are shown in Table 1. The total carotenoid content of L. helicina was 21.0 μg/g wet weight. β-Carotene (32.2%), zeaxanthin (24.2%), diatoxanthin (11.1%), and β-cryptoxanthin (10.4%) were found to be major carotenoids. Characteristic algal carotenoids, fucoxanthin (5.2%) and diadinoxanthin (2.4%), were also found.
Table 1.
Carotenoids content and composition of L. helicina, C. limacina, and P. doliiformis.
| L. helicina | C. limacina | P. doliiformis | |
|---|---|---|---|
| Carotenoid content (μg/g wet weight) | 21.0 | 47.0 | 159.8 |
| (μg/specimen) | 0.70 | 0.75 | 2.68 |
| Composition | % | % | % |
| β-Carotene (1) | 32.2 | 27.1 | 10.2 |
| Echinenone (2) | 9.2 | 6.4 | |
| Canthaxanthin (3) | 3.3 | ||
| β-Cryptoxanthin (4) | 10.4 | 13.1 | 12.8 |
| Zeaxanthin (5) | 24.2 | 1.1 | 1.2 |
| Adonixanthin (6) | 9.1 | 1.4 | |
| Idoxanthin (7) | 2.5 | ||
| (3S,3′S)-Astaxanthin (8) | 1.1 | 5.5 | |
| Diatoxanthin (9) | 11.1 | 3.5 | 3.6 |
| Pectenol A (10) | 1.2 | 2.2 | |
| Pectenol B (11) | 1.2 | 2.2 | |
| Pectenolone (12) | 9.2 | 30.5 | |
| 4′-Hydroxypectenolone (13) | 4.2 | 2.5 | |
| 7,8-Didehydroastaxanthin (14) | 4.5 | 6.4 | |
| Alloxanthin (15) | 6.4 | 2.1 | 1.1 |
| 4-Ketoalloxanthin (16) | 3.5 | 2.3 | |
| 4′-Hydroxy-4-ketoalloxanthin (17) | 3.2 | 2.5 | |
| 7,8,7′,8′-Tetradehydroastaxanthin (18) | 4.5 | 1.2 | |
| Diadinoxanthin (19) | 2.4 | ||
| Fucoxanthin (20) | 5.2 | ||
| Others | 8.1 | 2.2 | 2.2 |
2.2. Carotenoids of C. limacinea
The carotenoid content and composition of the sea angel C. limacina are shown in Table 1. The total carotenoid content of C. limacina was 47.0 μg/g wet weight. Fifteen carotenoids were identified. β-Carotene (27.6%), β-cryptoxanthin (13.5%), and echinenone (9.2%) were found to be major components. Monoacetylenic carotenoids, such as diatoxanthin, 7,8-didehydroastaxanthin, pectenolone, pectenol A, pectenol B, and 4′-hydroxypectenolone, comprised 25.9% of the total carotenoids. Diacetylenic carotenoids, such as alloxanthin, 7,8,7′,8′-tetradehydroastaxanthin, 4-ketoalloxanthin, and 4′-hydroxy-4-ketoalloxanthin, comprised 13.3% of the total carotenoids.
2.3. Carotenoids of P. doliiformis
The carotenoid content and composition of the sea angel P. doliiformis are shown in Table 1. P. doliiformis contained 159.8 μg/g wet weight carotenoid in the body. This was about three times higher than that of C. limacina. It was uncertain why P. doliiformis accumulated carotenoids three times higher than C. limacina. P. doliiformis showed more bright red color than C. limacina. This might reflect difference of species. The carotenoid composition of P. doliiformis was similar to that of C. limacina. Pectenolone (30.5%) was found to be a major component, followed by β-cryptoxanthin (12.8%) and β-carotene (10.2%). The monoacetylenic carotenoid diatoxanthin and its oxidative metabolites, 7,8-didehydroastaxanthin, pectinolone, pectenol A, pectenol B, and 4′-hydroxypectenolone, comprised with 25.9% of the total carotenoids. Diacetylenic carotenoids, alloxanthin, 7,8,7′,8′-tetradehydroastaxanthin, 4-ketoalloxanthin, and 4′-hydroxy-4-ketoalloxanthin, comprised 13.3% of the total carotenoids.
2.4. Carotenoids of the Chum Salmon O. keta
The carotenoid content and composition of flesh of the chum salmon O. keta, collected in Monbetsu bay, are shown in Table 2. Acetylenic carotenoids, pectenolone and 7,8-didehydroastaxanthin, were found in O. keta as minor carotenoids, along with astaxanthin.
Table 2.
Carotenoids content and composition of flesh of the chum salmon O. keta collected in Monbetsu bay.
| Carotenoids Content and Composition of Flesh of the Chum Salmon O. keta | |
|---|---|
| Carotenoid content (μg/g wet weight) | 0.89 |
| Composition | % |
| Astaxanthin * | 83.5 |
| 9-cis-Astaxanthin * | 5.1 |
| 13-cis-Astaxanthin * | 2.5 |
| 7,8-Didehydroastaxanthin | 0.5 |
| Adonixanthin | 1.1 |
| Pectenolone | 2.5 |
| Others | 4.8 |
* Astaxanthin consisted of three optical isomers (3R,3′R),(3R,3′S), and (3S,3′S) at the ratio of 82:2:16.
3. Discussion
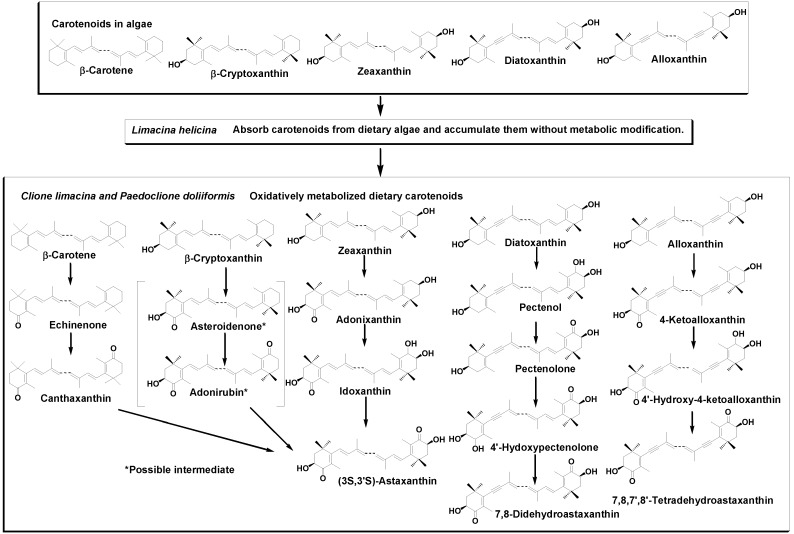
It has been reported that animals do not synthesize carotenoids de novo, and so those found in animals are either directly accumulated from food or partly modified through metabolic reactions [6,7,8]. L. helicina is a herbivorous animal that feeds on micro algae such as diatoms and dinoflagellates [2]. Sea angels, C. limacina and P. doliiformis are carnivorous animals that exclusively feed on the small mollusk L. helicina [1]. Therefore, carotenoids produced by micro algae are made available to sea angels through L. helicina in the food chain. As shown in Table 1, β-carotene, zeaxanthin, diatoxanthin, and β-cryptoxanthin were found to be major carotenoids along with alloxanthin, fucoxanthin, and diadinoxanthin in L. helicina. They are characteristic carotenoids in diatoms and microalgae belonging to Cyanophyceae, Rhodophyceae, etc. [5,6]. The results indicate that L. helicina directly absorbs carotenoids from dietary algae and accumulates them without metabolic modification. On the other hand, keto-carotenoids such as pectenolone, 7,8-didehydroastaxanthin, 4-ketoalloxanthin, and echinenone were found to be major components in sea angels. The results clearly indicate that sea angels oxidatively metabolize ingested carotenoids from L. helicina. So, β-carotene was oxidatively converted to astaxanthin via echinenone and canthaxanthin. β-Cryptoxanthin was also metabolized to astaxanthin via asteroidenone and adonirubin, as shown in Figure 3. There are three optical isomers of astaxanthin in nature. However, sea angels contain only one (3S,3′S) isomer. This shows that hydroxylation at C-3 and/or C-3′ of 4-keto and/or 4′-keto β-end group of carotenoid in sea angels is stereo-selective to form (3S,3′S)-astaxanthin. This stereo-selective hydroxylation has also been reported in other snails: Fushinus perplexus, F. perplexus ferrugineus, F. forceps [11,12], Cipangopaludina chinensis laeta, Semisulcospia libertina [13], and Pomacea canaliculata [14].
Figure 3.
Accumulation and metabolic pathways of carotenoids that originated from phytoplankton in the sea angels C. limacina and P. doliiformis.
Sea angels also introduced a carbonyl group at C-4 and/or C-4′ in the 3-hydroxy- and/or 3′-hydroxy-β-end group. Namely, zeaxanthin was metabolized to astaxanthin via adonixanthin and idoxanthin. Similarly, an acetylenic carotenoid, diatoxanthin, was metabolized to 7,8-didehydroastaxanthin via pectenol, pectenolone, and 4′-hydroxypectenolone. Alloxanthin was also oxidatively metabolized to 7,8,7′,8′-tetradehydroastaxanthin via 4′-hydroxy-4-ketoalloxanthin, and 4-ketoalloxanthin, as shown in Figure 3. By introducing a carbonyl group at C-4 and/or C-4′ in the 3-hydroxy- and/or 3′-hydroxy-β-end group, carotenoids changed their color from yellow to red. Therefore, the red color of the gonads of sea angels is due to the presence of keto-carotenoids such as pectenolone, 7,8-didehydroastaxanthin, and 7,8,7′,8′-tetradehydroastaxanthin. Epoxy carotenoids, diadinoxanthin and fucoxanthin, which are present in L. helicina, were not found in sea angels. It is suggested that sea angels cannot absorb epoxy carotenoids.
Chum salmon, O. keta, feeds not only on micro crustaceans but also on sea angels [3,4,5]. Astaxanthin, which consists of three optical isomers, was found to be a major carotenoid, along with the acetylenic carotenoids pectenolone and 7,8-didehydroastaxanthin, in O. keta. It is well-known that astaxanthin in crustaceans such as krill also consists of three optical isomers [6,7,8,15]. Therefore it is clear that astaxanthin in salmon originates from crustaceans. On the other hand, the acetylenic carotenoids pectenolone and 7,8-didehydroastaxanthin were not found in these crustaceans [6,7,8,15]. So, they are suggested to originate from sea angels.
It has been reported that marine animals accumulate carotenoids in their gonads, such as astaxanthin in salmon, pectenolone in scallops, and echinenone in sea urchins and that carotenoids are essential for reproduction in marine animals [8]. For example, astaxanthin supplementation in cultured salmon and red sea bream increased ovary development, fertilization, hatching, and larval growth [16]. In the case of sea urchins, supplementation with β-carotene, which was metabolized to echinenone, also increased reproduction and the survival of larvae [17].
As described above, sea angels converted dietary carotenoids to corresponding keto-carotenoids by introducing a carobonyl group and accumulated these keto-carotenoids in their gonads. Several investigators have reported that introducing a carobonyl group at C-4 and/or C-4′ of the β-end group of carotenoids enhanced their antioxidant effects, such as the quenching of singlet oxygen (1O2), inhibiting lipid peroxidation, and protection from photo-oxidation [18,19,20,21]. As well as astaxanthin, pectenolone, an oxidative metabolite of diatoxanthin, showed excellent antioxidative activity by inhibiting lipid peroxidation [22] and quenching singlet oxygen (1O2). Therefore, keto-carotenoids such as pectenolone may contribute to protection against oxidative stress and promote the reproduction of sea angels through antioxidative activity.
4. Experimental Section
4.1. General
The UV-visible (UV-VIS) spectra were recorded with a Hitachi U-2001 (Hitachi High-Technologies Corporation, Tokyo, Japan) in diethyl ether (Et2O). The positive ion electro spray ionization time of flight mass (ESI-TOF MS) spectra were recorded using a Waters Xevo G2S Q TOF mass spectrometer (Waters Corporation, Milford, CT, USA). The 1H-NMR (500 MHz) spectra were measured with a Varian UNITY INOVA 500 spectrometer (Agilent Technologies, Santa Clara, CA, USA) in CDCl3 with TMS as an internal standard. HPLC was performed on a Shimadzu LC-6AD with a Shimadzu SPD-6AV spectrophotometer (Shimadzu Corporation, Kyoto, Japan) set at 470 nm. The column used was a 250 × 10 mm i.d., 10 μm Cosmosil 5C18-II (Nacalai Tesque, Kyoto, Japan) with acetone:hexane (3:7, v/v) at a flow rate of 1.0 mL/min, run time of 60 min. The optical purity of astaxanthin was analyzed by chiral HPLC using a 300 × 8 mm i.d., 5 μm Sumichiral OA-2000 (Sumitomo Chemicals, Osaka, Japan) with n-hexane/CHCl3/ethanol (48:16:0.8, v/v) at a flow rate of 1.0 mL/min [23].
4.2. Animal Specimens
The sea angel C. limacina (30 specimens, 464 mg wet weight) was collected at Monbetsu bay, Monbetsu City, Hokkaido, Japan in December 2011. Another sea angel, P. doliiformis (60 specimens, 1041 mg wet weight), was also collected at Monbetsu bay in April 2013. The small sea snail L. helicina (6 specimens, 200 mg wet weight) was collected at Monbetsu bay in May 2013. Chum salmon, O. keta (3 specimens, five to six years of age), was collected at Monbetsu in September 2013.
4.3. Analysis of Carotenoids
The extraction and identification of carotenoids were carried out according to our routine methods [24]. Carotenoids were extracted from living or fresh animal specimens with acetone. The acetone extract was translated to an ether-hexane (1:1) layer by the addition of water. The total carotenoid contents were calculated employing an extinction coefficient of 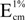 = 2100 [25] at λ max. The ether-hexane solution was evaporated. The residue was subjected to HPLC on silica gel. Carotenoid compositions were estimated by the peak area of the HPLC on silica gel with acetone–hexane (2:8)–(4:6) monitored at 450 nm.
= 2100 [25] at λ max. The ether-hexane solution was evaporated. The residue was subjected to HPLC on silica gel. Carotenoid compositions were estimated by the peak area of the HPLC on silica gel with acetone–hexane (2:8)–(4:6) monitored at 450 nm.
Individual carotenoids were identified by retention time in HPLC, UV-vis (ether), ESI-TOF MS, and 1H NMR (500 MHz, CDCl3) in the case of pecetenolone.
4.4. Identification of Carotenoids
β-Carotene (1). ESI-TOF MS: m/z 536.4372 [M]+ (calcd for C40H56, 536.4382); UV-VIS: 425, 449, 475 nm.
Echinenone (2). ESI-TOF MS: m/z 551.4271 [M + H]+ (calcd for C40H53O, 551.4253); UV-VIS: 460 nm.
Canthaxanthin (3). ESI-TOF MS: m/z 565.4044 [M + H]+ (calcd for C40H53O2, 565.4046); UV-VIS 470 nm.
β-Cryptoxanthin (4). ESI-TOF MS: m/z 553.4511 [M + H]+ (calcd for C40H53O, 553.4409); UV-VIS: (425), 450, 475 nm.
Zeaxanthin (5). ESI-TOF MS: m/z 569.4353 [M + H]+ (calcd for C40H57O2,569.4359); UV-VIS: (425) 450, 475 nm.
Adonixanthin (6). ESI-TOF MS: m/z 583.4139 [M + H]+ (calcd for C40H55O3, 583.4151); UV-VIS 460 nm.
Idoxanthin (7). ESI-TOF MS: m/z 599.4090 [M + H]+ (calcd for C40H55O4, 599.4100); UV-VIS 460 nm.
Astaxanthin (8). ESI-TOF MS: m/z 597.3942 [M + H]+ (calcd for C40H53O4, 597.3944); UV-VIS 472 nm, Chiral HPLC [13] revealed that astaxanthin fraction in sea angels was consisted of only (3S,3′S) optical isomers.
Diatoxanthin (9). ESI-TOF MS: m/z 567.4225 [M + H]+ (calcd for C40H55O2, 567.4202); UV-VIS: (426), 451, 478 nm.
Pectenol A (10). ESI-TOF MS: m/z 583.4173 [M + H]+ (calcd for C40H55O3, 583.4152); UV-VIS: (426), 451 478 nm.
Pectenol B (11). ESI-TOF MS: m/z 583.4170 [M + H]+ (calcd for C40H55O3, 583.4152); UV-VIS: (426), 451, 478 nm.
Pectenolone (12). ESI-TOF MS: m/z 581.3983 [M + H]+ (calcd for C40H53O3, 581.3995); UV-VIS: 460 nm; 1H-NMR (CDCl3, 500 MHz) δ 1.15 (H3-16′, s), 1.20 (H3-17′, s), 1.21 (H3-17, s), 1.32 (H3-16, s), 1.45 (H-2′β, dd, J = 12, 11), 1.82 (H-2β, d, J = 13, 13), 1.84 (H-2′α, ddd, J = 12, 4, 1.5), 1.92 (H3-19′, s), 1.95 (H3-19, s), 2.07 (H-2′β, dd, J = 18, 10), 2.15 (H-2α, dd, J = 13, 6), 2.43 (H-4′α, ddd, J = 18, 6, 1.5), 3.68 (OH-3, d, J = 2), 3.99 (H-3′, m), 4.32 (H-3, ddd, J = 13, 6, 2), 6.22 (H-7, d, J = 16), 6,28 (H-14′, d, J = 11), 6.30 (H-10, d, J = 11), 6.30 (H-14, d, J = 11), 6.36 (H-12′, d, J = 15), 6.43 (H-8, d, J = 16), 6.45 (H-12, d, J = 15), 6.45 (H-10′, d, J = 11), 6.53 (H-11′, dd, J = 15, 11), 6.63 (H-15 and H-15′, m), 6.65 (H-11, dd, J = 15, 11).
4′-Hydroxypectenolone (13). ESI-TOF MS: m/z 597.3942 [M + H]+ (calcd for C40H53O4, 597.3944); UV-VIS: 460 nm.
7,8-Didehydroastaxanthin (14). ESI-TOF MS: m/z 595.3789 [M + H]+ (calcd for C40H51O4, 595.3787); UV-VIS: 474 nm.
Alloxanthin (15). ESI-TOF MS: m/z 565.4028 [M + H]+ (calcd for C40H53O2, 565.4046); UV-VIS: (426), 451 478 nm.
4-Ketoalloxanthin (16). ESI-TOF MS: m/z 579.3851 [M + H]+ (calcd for C40H51O3, 579.3838); UV-VIS: 460 nm.
4′-Hydroxy-4-Ketoalloxanthin (17). ESI-TOF MS: m/z 595.3801 [M + H]+ (calcd for C40H51O4,595.3787); UV-VIS: 469 nm.
7,8,7′,8′-Tetradehydroastaxanthin (18). ESI-TOF MS: m/z 593.3649 [M + H]+ (calcd for C40H49O4,593.3631); UV-VIS: 476 nm.
Diadinoxanthin (19). ESI-TOF MS: m/z 583.4173 [M + H]+ (calcd for C40H55O3, 583.4151); UV-VIS: 420, 433, 470 nm.
Fucoxanthin (20). ESI-TOF MS: m/z 659.4333 [M + H]+ (calcd for C42H59O6,659.4312); UV-VIS: 445, 470 nm.
4.5. 1O2 Quenching Activity of Carotenoids
Quenching activity of 1O2 was measured according to the method described in the literature [26]. 1O2 quenching activities (IC50 values) of pectenolone and astaxanthin were 7.9 and 6.5 μM, respectively.
5. Conclusions
Carotenoids originating from phytoplankton are accumulated in the sea angels, C. limacina and P. doliiformis, through eating the herbivorous sea snail, L. helicina, in the food chain. In sea angels, dietary carotenoids were oxidatively metabolized, as shown in Figure 3. Sea angels mainly accumulate carotenoids in their gonads. Carotenoids in the gonads of sea angels might protect against oxidative stress and enhance reproduction. Furthermore, carotenoids in sea angels can then be found in salmon through the food chain.
Acknowledgements
We wish to thank Kazutoshi Shindo and Ayako Osawa; Department of Food and Nutrition; Japan Women’s University for measurement of the 1O2 quenching activities of pectenolone and astaxanthin.
Authors Contributions
T.M. analyzed carotenoids of marine animals. T.K. and M.N. collected marine animals and studied their ecology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Carol M., Ronald L., Gilmer W. Pelagic Snails. Stanford University Press; Stanford, CA, USA: 1989. [Google Scholar]
- 2.Gilmer R.W., Harbison G.R. Diet of Limacina helicina (Gastropoda: Thecosomata) in arctic waters in midsummer. Mar. Ecol. Prog. Ser. 1991;77:125–134. doi: 10.3354/meps077125. [DOI] [Google Scholar]
- 3.Azuma T. Biological mechanisms enabling sympatry between salmoids with special reference to sockeye and chum salmon in oceanic water. Fish. Res. 1995;24:291–300. doi: 10.1016/0165-7836(95)00383-3. [DOI] [Google Scholar]
- 4.Davis N.D., Volkov A.V., Efimkin A.Y., Kuznetsova N.A., Armstrong J.L., Sakai O. Review of basis salmon food habits studies. N. Pac. Anadr. Fish Comm. Bull. 2009;5:197–208. [Google Scholar]
- 5.Britton G., Liaaen-Jensen S., Pfander H. Carotenoids Hand Book. Birkhäuser; Basel, Switzerland: 2004. [Google Scholar]
- 6.Liaaen-Jensen S. Carotenoids in Food Chain. In: Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids Volume 3: Biosynthesis and Metabolism. Birkhäuser; Basel, Switzerland: 1998. pp. 359–371. [Google Scholar]
- 7.Matsuno T. Aquatic animal carotenoids. Fish. Sci. 2001;67:771–789. doi: 10.1046/j.1444-2906.2001.00323.x. [DOI] [Google Scholar]
- 8.Maoka T. Carotenoids in marine animals. Mar. Drugs. 2011;9:278–293. doi: 10.3390/md9020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maoka T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009;483:191–195. doi: 10.1016/j.abb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Maoka T., Akimoto N., Tsushima M., Komemushi S., Mezaki T., Iwase F., Takahashi Y., Sameshima N., Mori M., Sakagami Y. Carotenoids in marine invertebrates living along the Kuroshio current coast. Mar. Drugs. 2011;9:1419–1427. doi: 10.3390/md9081419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuno T., Katagiri K., Maoka T., Komori T. Novel reductive metabolic pathways of 4-oxo-β-end group in carotenoids of the spindle shell Fushinus perplexus. Comp. Biochem. Physiol. 1985;81B:905–908. [Google Scholar]
- 12.Katagiri K., Maoka T., Matsuno T. Carotenoids of shellfishes VIII. Comparative biochemical studies of carotenoids in three species of spindle shell, Fushinus perplexus, F. perplexus ferrugineus and F. forceps. Comp. Biochem. Physiol. 1986;84B:473–476. [Google Scholar]
- 13.Maoka T., Ochi J., Mori M., Sakagami Y. Identification of Carotenoids in the Freshwater Shellfish Unio douglasiae nipponensis, Anodonta lauta, Cipangopaludina chinensis laeta, and Semisulcospira libertia. J. Oleo Sci. 2012;61:69–74. doi: 10.5650/jos.61.69. [DOI] [PubMed] [Google Scholar]
- 14.Tsushima M., Katsuyama M., Matsuno T. Metabolism of carotenoids in the apple snail Pomacea canaliculata. Comp. Biochem. Physiol. 1997;118B:431–436. doi: 10.1016/S0305-0491(97)00215-0. [DOI] [Google Scholar]
- 15.Maoka T., Katsuyama M., Kaneko N., Matsuno T. Stereochemical investigation of carotenoids in the antarctic krill Euphausia superba. Bull. Jpn. Soc. Sci. Fish. 1985;51:1671–1673. doi: 10.2331/suisan.51.1671. [DOI] [Google Scholar]
- 16.Torrissen O.J., Christiansen R. Requirements for carotenoids in fish diets. J. Appl. Ichthyol. 1995;11:225–230. doi: 10.1111/j.1439-0426.1995.tb00022.x. [DOI] [Google Scholar]
- 17.Tsushima M., Kawakami T., Mine M., Matsuno T. The role of carotenoids in the development of the sea urchin Pseudocentrotus depressus. Invertebr. Reprod. Dev. 1997;32:149–153. doi: 10.1080/07924259.1997.9672616. [DOI] [Google Scholar]
- 18.Terao J. Antioxidative activity of beta-carotene-related carotenoids in solution. Lipids. 1989;24:659–661. doi: 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]
- 19.Shimidzu N., Goto M., Miki W. Carotenoids as singlet oxygen quenchers in marine organism. Fish. Sci. 1996;62:134–137. doi: 10.2331/suisan.62.134. [DOI] [Google Scholar]
- 20.Miki W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991;63:141–146. doi: 10.1351/pac199163010141. [DOI] [Google Scholar]
- 21.Maoka T., Goto Y., Isobe K., Fujiwara Y., Hashimoto K., Mochida K. Antioxidative activity of capsorubin and related compounds from paprika (Capsicum annuum) J. Oleo Sci. 2001;50:663–665. doi: 10.5650/jos.50.663. [DOI] [Google Scholar]
- 22.Narita M., Maoka T., Ebitani K., Nishino H. Characteristics of chemical constituents and red pigment of orange adductor muscle of scallop Mizuhopecten yessoensis in the Okhotsk Sea and anti-oxidative activity of the pigment. Nippon Suisan Gakkaishi. 2013;79:48–54. doi: 10.2331/suisan.79.48. [DOI] [Google Scholar]
- 23.Maoka T., Komori T., Matsuno T. Direct resolution of diastereomeric carotenoid-I. 3-oxo-β-end group. J. Chromatogr. 1985;318:122–124. doi: 10.1016/S0021-9673(01)90671-5. [DOI] [Google Scholar]
- 24.Maoka T., Akimoto N. Natural product chemistry in carotenoid, some experimental techniques for structural elucidation and analysis of natural carotenoids. Carotenoid Sci. 2008;13:10–17. [Google Scholar]
- 25.Britton G. UV/Visible Spectroscopy. In: Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids. Birkhäuser Verlag; Basel, Switzerland: 1995. pp. 13–62. [Google Scholar]
- 26.Maoka T., Yasui H., Ohmori A., Tokuda H., Suzuki N., Osawa A., Shindo K., Ishibashi T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013;62:181–186. doi: 10.5650/jos.62.181. [DOI] [PubMed] [Google Scholar]