Abstract
Preface
During the past two decades, the paradigm for cancer treatment has evolved from relatively non-specific cytotoxic agents to selective, mechanism-based therapeutics. Cancer chemotherapies were initially identified through screens for compounds that killed rapidly dividing cells. These drugs remain a backbone of current treatment, but are limited by a narrow therapeutic index, significant toxicities, and frequently acquired resistance. More recently, an improved understanding of cancer pathogenesis has given rise to new treatment options, including targeted agents and cancer immunotherapy. Targeted approaches aim to inhibit molecular pathways that are critical to tumor growth and maintenance, whereas immunotherapy endeavors to stimulate a host response that effectuates long-lived tumor destruction. Targeted therapies and cytotoxic agents also modulate immune responses, which raises the possibility that these treatment strategies might be effectively combined with immunotherapy to improve clinical outcomes.
Introduction
Targeted therapies act by blocking essential biochemical pathways or mutant proteins that are required for tumor cell growth and survival1. These drugs can arrest tumor progression and induce striking regressions in molecularly defined subsets of patients. Indeed, the first small molecule targeted agent, the BCR-ABL kinase inhibitor imatinib, rapidly induced complete cytogenetic responses in 76% of chronic myelogenous leukemia patients2. Further research into the underlying genetic pathways driving tumor proliferation uncovered additional oncoproteins that are critical for tumor maintenance, such as the epidermal growth factor receptor (EGFR), BRAF, KIT, HER2 (also known as neu and ERBB2) and anaplastic lymphoma kinase (ALK)3. Similar to imatinib, small molecule inhibitors of these kinases have effectuated impressive tumor responses in selected patients, although regressions are commonly followed by the development of progressive disease due to the emergence of drug-resistant variants. Resistance usually involves secondary mutations within the targeted protein or compensatory changes within the targeted pathway that bypass the drug-mediated inhibition. Accordingly, targeted therapies may elicit dramatic tumor regressions, but persistence is generally short-lived, limiting the overall clinical benefit.
In parallel to these advances in targeting oncogenic mechanisms, the recent successes of sipuleucel-T (Provenge®) and ipilimumab (Yervoy®) in Phase III clinical trials validated the principle that immunotherapy can extend cancer patient survival as well4. Sipuleucel-T, recently approved by the US food and drug administration (FDA) for use in metastatic, castration-resistant prostate cancer, is an autologous dendritic cell (DC) vaccine aimed at stimulating T cells specific for prostatic acid phosphatase (PAP), a protein that is overexpressed in prostate carcinoma cells5. Although the precise basis of action for sipuleucel-T remains under study, treatment with this drug increases median survival by four months with minimal toxicity. Ipilimumab, an antibody directed to cytotoxic T lymphocyte antigen 4 (CTLA4), blocks an important inhibitory signal for activated T cells, thereby bolstering T cell responses and potentiating tumor destruction6. Ipilimumab, recently approved by the FDA for use as first-line or second-line therapy for advanced melanoma patients, enhances overall survival compared to standard care and, most notably, achieves durable benefits (more than 2.5 years) for 15-20% of treated subjects7, 8. Blockade of CTLA4 with antibody drugs is associated with a significant incidence of inflammatory toxicities, albeit most are readily managed with medical treatment.
The clinical results with ipilimumab illustrate how immunotherapy may induce long-lasting responses due to the generation of anti-tumor memory. Although antibody treatment is typically completed within a few months, the stimulated immune response may accomplish disease control for extended periods. A dynamic host reaction may also underlie the unusual pattern of clinical response with ipilimumab, in which prolonged periods of stable disease or even an initial period of tumor growth prior to stabilization are sometimes observed9. Additionally, immune responses with ipilimumab may display an increase in breadth over time, diversifying reactivity to multiple tumor associated-antigens. Notwithstanding these advantages, an important limitation of ipilimumab is the relatively low proportion of patients who achieve clinical responses. This deficiency may reflect, at least in part, the potent immunosuppressive effects of well established tumors, particularly given that many cancers are detected at a late stage when bulky lesions are already present.
This analysis of the strengths and weaknesses of targeted agents and immunotherapy suggests that the two approaches may have complementary roles in cancer treatment, and that combinatorial therapy might prove synergistic. Because targeted therapies can induce rapid tumor regressions, with a consequent decrease in tumor-associated immunosuppression, they may afford a favorable window for immunotherapy to achieve more potent cytotoxicity. Additionally, targeted therapies may potentiate anti-tumor immune responses by breaking oncogene “addiction,” in turn triggering tumor senescence and facilitating immune clearance by T cells10, 11. Moreover, the release of large amounts of antigenic debris upon tumor cell death may contribute to vaccination in situ, particularly if concurrent DC activation can be triggered. Immunotherapy might thus consolidate the dramatic tumor responses achieved with targeted therapy into durable, long-lasting remissions in which sustained host responses targeting multiple antigens may reduce the risk of potentially lethal drug-resistant clones.
In addition to tumor cell killing, targeted therapies may also directly modulate immune responses. For example, some targeted therapies may attenuate the activities of specific immune cell populations that restrain cytotoxic T lymphocytes (CTLs), such as forkhead box P3 (FOXP3)+ regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs)12, 13. Other targeted agents may augment DC tumor antigen presentation and enhance the priming of tumor specific CTLs14, 15. Furthermore, some targeted therapies might sensitize tumor cells to immune-mediated destruction16, 17. The interplay of targeted agents and immunotherapy is complex, however, and parameters such as timing, dosage, and sequence are all likely to influence the overall anti-tumor effects and toxicity profiles of the combined treatments. A more complete understanding of the impact of targeted therapies upon specific molecular pathways in immune cells should help advance integrating these agents with immunotherapy.
Activating a multi-faceted immune response against tumor cells
To date, the clinical evaluation of immunotherapy has primarily involved single agents that target individual steps in the host anti-tumor reaction. Because the induction, potency, and persistence of host responses reflects the complex interplay of diverse immune cells with progressing tumors, monotherapies are unlikely to address the major mechanisms impeding anti-tumor immune responses in all patients. Indeed, the generation of clinically effective anti-tumor reactions likely requires the successful execution of several immune processes (Figure 1). Pre-clinical studies indicate that combinations of therapies that target distinct steps of tumor immunity may be synergistic, resulting in stronger and more sustained reactions that accomplish durable tumor destruction (Box 1).
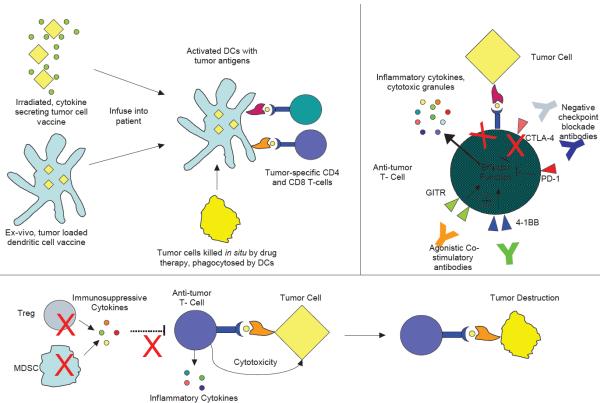
Figure 1. The generation of potent anti-tumor immune responses requires multiple steps.
a ǀ Dendritic cell (DC) priming can be accomplished in multiple ways. One example involves the injection of irradiated, cytokine secreting whole tumor cells, after which DCs phagocytose the tumor cells and present tumor antigens in vivo to T cells. In another strategy, DCs can be loaded with tumor cells and matured ex vivo, then re-infused back to the patient. Additionally, tumor cells killed by chemotherapy or targeted therapies may facilitate DC priming and activation in situ. All of these approaches promote in vivo DC priming of tumor specific T cells in a pro-inflammatory context. b ǀ T cell function may be enhanced through the delivery of exogenous costimulatory signals and blocking co-inhibitory signals. Agonistic antibodies to 4-1BB and glucocorticoid induced TNFR related protein (GITR) cross-link these activating receptors and bolster T cell effector responses, while blocking antibodies to the co-inhibitory receptors cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed death 1 (PD1) preclude transduction of negative signals and prevent T cell shutdown and anergy. Both strategies result in boosted T cell effector function, including cytokine release and cytotoxicity. c ǀ Immunosuppressive Tregs and myeloid-derived suppressor cells (MDSCs) secrete numerous tolerogenic cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGFβ), inhibiting anti-tumor immune responses. Strategies to inhibit immunosuppressive cytokine secretion or kill Tregs and MDSCs diminish immune suppression and promote T cell mediated tumor destruction.
Box 1. Combinatorial immunotherapy.
Optimizing immunotherapy requires treatments that affect multiple aspects of the immune response. One immunotherapy combination is the use of cancer vaccines to generate anti-tumor T cells and negative checkpoint blockade to prevent T cell anergy. Cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed death 1 (PD1) are two well-described co-inhibitory pathways on T cells decreasing anti-tumor responses (reviewed elsewhere in this issue). A small trial combining autologous granulocyte-macrophage colony stimulating factor (GM-CSF) secreting tumor cell vaccines with CTLA4 blockade found increased inflammatory infiltrates and tumor regression26, suggesting that vaccine induced anti-tumor T cells were present within the tumor but anergized due to CTLA4 co-inhibition. Similarly, in a pre-clinical study combining vaccines and PD1 blockade, mice receiving combination therapy had increased overall survival and decreased tumor growth25. Additionally, combining blockade of multiple inhibitory pathways decreases T-cell anergy and improves T cell responsiveness103. In one pre-clinical study, animals treated with cancer vaccines were found to have significantly higher overall survival if they were concurrently treated with antibodies to PD1 and CTLA4 compared to animals treated with vaccination and either antibody alone103.
A different strategy to overcome T cell anergy is through providing an exogenous costimulatory signal while simultaneously blocking co-inhibitory signaling. 4-1BB (CD137) is a costimulatory receptor expressed on activated T cells, and cross-linking 4-1BB with agonistic antibodies augments anti-tumor T cell function in many different mouse models20-22. In one study, combining CTLA4 blocking antibodies and 4-1BB agonistic antibodies induced complete tumor regression in the combination treatment group, whereas mice receiving either single agent had continued tumor growth102. Combinatorial therapy using an agonistic antibody to another costimulatory molecule, the glucocorticoid induced TNFR related protein (GITR) and CTLA4 blockade shows similar results23. These data suggest combining agonistic antibodies delivering a costimulatory signal with antibodies blocking inhibitory ones promotes potent anti-tumor immunity. Additionally, the development of new combinatorial therapeutics for cancer immunology is ongoing, with multiple agents, such as bispecific T cell engagers (BiTEs)104, immunotoxins105, and Fc-fusion proteins, entering clinical testing106.
In order to generate potent anti-tumor immunity, antigen presenting cells, most notably DCs, must first capture tumor antigens, process them into major histocompatibility complex (MHC) class I and II pathways, and display the resulting peptide epitopes in an immunogenic context to stimulate CD4+ T cells and CD8+ T cells, usually in a regional lymph node18. This process appears to be relatively inefficient during tumor formation, but can be effectively stimulated with a variety of cancer vaccination strategies. Second, tumor-specific T cells must differentiate into effector cells, which requires a combination of signals from both the T cell receptor (TCR; which establishes specificity) and several costimulatory molecules19. The TCR (and associated CD4 or CD8 molecules) engages MHC-peptide complexes presented on DCs, while costimulatory signals are delivered through multiple transmembrane proteins of the B7 and tumor necrosis factor receptor (TNFR) families, as well as receptors for some cytokines, such as interleukin-12 (IL-12). In addition to the costimulatory molecule CD28, which binds B7-1 (also known as CD80) and B7-2 (also known as CD86) on DCs, other activating receptors under active study include 4-1BB (also known as CD137 and TNFRSF9), OX40 (also known as TNFRSF4), and glucocorticoid induced TNFR related protein (GITR; also known as TNFRSF18). Agonistic antibodies to these molecules can enhance costimulation to augment anti-tumor immunity20-23.
Third, T cells must avoid negative regulatory signals that dampen their activation or induce tolerance programs such as anergy or exhaustion24. In this regard, CTLA4 and programmed death 1 (PD1) are major negative costimulatory molecules that are expressed on activated T cells. Similar to ipilimumab, antibody blockade of PD1 amplifies T cell function and has demonstrated encouraging anti-tumor effects in initial clinical testing25, 26. Lastly, the induced anti-tumor response must be capable of overcoming diverse immunosuppressive networks within the tumor microenvironment, which may be driven by both soluble factors and regulatory cell populations.
Targeted therapies display immunomodulatory effects
The molecular understanding of cancer biology has advanced substantially over the past two decades. Many investigations have uncovered critical pathways driving tumor growth and maintenance, yielding rationally designed drugs that may attenuate these circuits3. Interestingly, a number of the targeted pathways are also involved in the development, activation, differentiation, and function of immune cells. Thus, some of the targeted agents manifest important immunomodulatory properties. Similar to more typical immunotherapies, targeted agents may influence diverse aspects of the immune response, including bolstering tumor antigen presentation and T cell priming; enhancing T cell activation, effector function, and differentiation; sensitizing tumor cells to killing by immune effector cells; and antagonizing tumor-mediated immune suppression. These activities raise the intriguing possibility that some component of the anti-tumor efficacy of targeted therapies might involve participation of the host (Table 1).
Table 1.
Effects of approved and experimental targeted agents on tumor cells and immune cells
| Drug | Effect on Tumor | Effect on Immune System | Current and experimental immunotherapy combinations | Refs |
|---|---|---|---|---|
| Sunitinib | Blocks multiple tumor associated tyrosine kinases, including VEGFR and PDGFR | Blocks STAT3, decreases numbers and effectiveness of MDSCs and Tregs, blocks VEGF signaling | Preclinical trial of adoptive T-cell transfer plus sunitinib in HCC and RCC; preclinical trial of combination of sunitinib, agonistic anti-CD137, plus IL- 12 in colon adenocarcinoma | 12, 13, 79, 80, 82, 83 |
| Imatinib | Blocks multiple tumor associated tyrosine kinases, including ABL and KIT | Blocks IDO, decreases numbers and effectiveness of Tregs, promotes DC- NK cell cross talk, induces B1 B-cells and ‘natural’ antitumor carbohydrate antibodies | Phase III trial of Interferon alfa-2a imatinib in CML; Phase II trial of imatinib plus BCR-ABL vaccine in CML; preclinical trial of imatinib plus anti-CTLA4 in GIST | 86, 87, 113-116 |
| Vemurafenib | Blocks BRAF-V600E | Increases expression of gp100, MARTI, and other antigens; decreased tumor secretion of immune-suppressive cytokines | Phase I trial of vemurafenib plus ipilumimab (NCT01400451) | 64, 90 |
| Trastuzumab | Blocks growth signaling through HER2 | Primes anti-tumor CTLs, boosts NK cell secretion of IFNγ, antibody-dependent cellular cytotoxicity | Phase II trial of trastuzumab plus HER2 peptide vaccine; Phase I trial of trastuzumab plus IL-12 plus paclitaxel in HER2+ breast cancer; Preclinical trial of anti-HER2 plus anti-PDl; preclinical trial of anti-HER2 plus 4-lBB agonistic antibody |
32, 34-37 |
| Bevacizumab | Neutralizing antibody against VEGF, blocks angiogenesis | Increases DC maturation, shifts DC differentiation towards mature DC instead of MDSC, increases DC priming of T-cells | Phase III trial of bevacizumab plus interferon alfa-2a in metastatic RCC Phase I trial of bevacizumab plus ipilimumab (NCT00790010); preclinical trial of anti-VEGF plus adoptive T cell transfer therapy | 77, 78, 117, 118 |
| Cetuximab | Neutralizing antibody against EGFR, blocks growth signals | Immune activating: Complement fixation, ADCC, increases MHC I and MHC II expression, augments DC priming of tumor specific CTLs, Immunosuppressive: activates M2 macrophage | Phase II trial of cetuximab plus EGFR vaccine (NCT 00305760) | 29, 38-40, 119, 120 |
| Temsirolimus , rapamycin and other mTOR inhibitors | Blocks mTOR pathway | Immunostimulatory: Enhances CD8+ T-cell activation and IFNγ production, augments CD8+ T-cell differentiation into memory cells, impairs Treg homeostasis, decreases IDO expression Immunosuppressive: Augments Treg responsiveness to antigen | Preclinical trial of temsirolimus plus HSP90 and tumor specific antigen vaccines in melanoma and RCC; preclinical trial of temsiroliums plus agonist CD40 antibody in RCC | 46-50, 86 |
| Bortezomib | Blocks 26S subunit of proteasome | Sensitizes tumor cells to CTL lysis. Sensitizes tumor cells to NK cell lysis by downregulating MHC I, boosts antigen-specific T-cell response to vaccination | Pre-clinical trial of bortezomib plus vaccination with DNA encoding a tumor specific protein | 16, 59, 60, 62 |
| JAK2 inhibitors | Block JAK2 signahng in tumor cells | Enhances DC maturation, bolsters DC antigen presentation and T-cell priming, decreases immunosuppressive STAT3 signaling, decreases IAP expression, decreases tumor PDLl expression | Preclinical trial of JAK2 inhibitor plus DC vaccines | 14, 15, 84, 121 |
| HSP90 inhibitors | Blocks HSP90, which increases unfolded protein associated stress in tumor cells | Immune-activating: Increases expression of NKG2D ligands, boosts CTL recognition of tumor cells Immune-suppressing: Decreases cytokine secretion from macrophages and T-cells; decreases expression of costimulatory molecules on DCs, decreases antigen presentation by DCs | None | 67-70, 96, 97 |
| PI3K-AKT inhibitors | Decrease PI3K-AKT signaling in tumour cells | Increases tumor susceptibility to perforin-granzyme mediated lysis (mediated by CTLs and NK cells), decreases pro-survival signaling, decreases tumor-promoting inflammation | Preclinical trial of AKT inhibitor plus vaccine | 17, 63, 89 |
| Lenalidomide | Not well understood | Pleiotropic: Increases costimulatory molecules on tumor cells; modulates SOCSl expression to increase cytokine secretion; decreases PDLl expression on tumor cells; increases NK cell cytotoxicity and cytokine secretion; and increases NKG2D ligand expression | Phase II trial of lenalidomide as maintenance after BMT; pre-clinical trial of lenalidomide plus KIR antibody; pre-clinical trial of lenalidomide plus CD38 antibody (daratumomab) | 122-126 |
| GSK3β inhibitors | Blocks GSK3β mediated signaling of tumor cell growth | Facilitates differentiation towards T-cell ‘stem-cell’ population (Tscm); augments TLR4 signaling | None | 51-53, 127 |
| IAP inhibitors | Sensitizes tumor cells to apoptosis | Increases T-cell, NK cell and NKT cell responses to stimulation | Pre-clinical trial of GM-CSF secreting autologous tumor vaccine plus IAP inhibitor | 55 |
ADCC, antibody-dependent cellular cytotoxicity; BMT, bone marrow transplant; CML, chronic myeloid leukaemia; CTL, cytotoxic T lymphocyte; CTLA4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; EGFR, epidermal growth factor receptor; GIST, gastrointestinal stromal tumour; GM-CSF, granulocyte-macrophage colony stimulating factor; GSK3β, glycogen synthase kinase 3Β; HCC, hepatocellular carcinoma; HSP, heat shock protein; lAP, inhibitor of apoptosis protein; IDO, indoleamine-pyrrole 2,3-dioxygenase; IFN, interferon; IL-12, interleukin-12; JAK2, janus kinase 2; KIR, killer cell immunoglobulin-like receptor; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; NK, natural killer; PDl, programmed death 1; PDGFR, platelet-derived growth factor receptor; PDLl, programmed death 1 ligand 1; RCC, renal cell carcinoma; STAT3, signal transducer and activator of transcription 3; SOCSl, suppressor of cytokine signalling 1; TLR, toll-like receptor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Targeted therapies may augment tumor antigen presentation and T cell priming
As mentioned above, DCs are critical for priming anti-tumor T cells18. DCs express an array of Fc receptors that bind and internalize antibody-antigen immune complexes, which contributes to the activation and maturation of DCs27. Monoclonal antibodies facilitate DC uptake of tumor antigens, helping to stimulate tumor-specific CD4+ and CD8+ T cells (Figure 2). Trastuzumab and cetuximab are clinically efficacious monoclonal antibodies directed towards the tumor-associated receptor tyrosine kinases HER2 and EGFR, respectively28. In addition to their abilities to attenuate oncogenic signaling, these antibodies augment tumor antigen presentation through the formation of immune complexes, enhancing the induction of tumor-specific T cells29, 30. In patients that received trastuzumab, a potent T cell response was correlated with improved clinical outcomes31. Animal models further established that the therapeutic efficacy of HER2 antibodies requires Fc receptors and CD8+ T cells in vivo; animals treated with these antibodies generated more tumor-specific T cells compared to controls32.
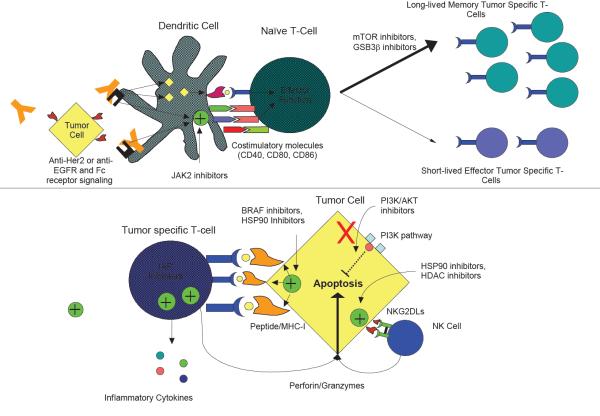
Figure 2. Targeted agents may boost DC priming and the activities of tumor specific T cells.
a ǀ Multiple targeted agents affect dendritic cell (DC)-mediated priming of T cells. Monoclonal antibodies coat tumor cells and promote phagocytosis through Fc receptors, increasing DC presentation of tumor antigens. Fc receptor-mediated opsonization also enhances expression of costimulatory molecules such as CD40, B7-1 (CD80), and B7-2 (CD86) on the DC surface, promoting T cell activation. Janus kinase 2 (JAK2) inhibitors inhibit signal transducer and activator of transcription 3 (STAT3), an immunosuppressive pathway, boosting expression of co-stimulatory molecules. mTOR and glycogen synthase kinase 3β (GSK3β) inhibitors drive T cells towards long-lived memory T cell phenotypes that generate a large pool of enduring anti-tumor T cells, even after completion of immunotherapies. b ǀAgents that increase anti-tumor activity of T cells are shown. Numerous therapies increase the expression of tumor antigens on the tumor cell surface, increasing T cell receptor signaling and T cell activation. Inhibitor of apoptosis protein (IAP) inhibitors reinforce T cell signaling and provide exogenous costimulatory signals, increasing production of inflammatory cytokines. PI3K inhibitors eliminate some pro-survival signals, increasing tumor cell lysis to perforin and granzymes released from cytotoxic T lymphocytes (CTLs). Therapies also increase expression of NKG2D ligands, which serve as additional costimulatory molecules for CTLs as well as activators of natural killer (NK) cells, which also kill tumor targets through the perforin-granzyme pathway. EGFR, epidermal growth factor receptor; HDAC, histone deacetylase; HSP90, heat shock protein 90; MHC I, major histocompatibility class I.
These effects of monoclonal antibodies on antigen presentation suggest a potential synergy with cancer vaccines. In a transgenic mouse model of breast cancer, mice that received a combination of HER2 antibodies and a cancer cell vaccine demonstrated significantly longer survival than either therapy alone30. The combined therapy increased DC uptake of the cells from the cancer vaccine, enhanced DC expression of costimulatory molecules such as CD40, CD80 and CD86, and triggered greater tumor-specific T cell responses compared to either agent alone33. Consistent with these findings, a recent Phase I/II clinical trial of a HER2 peptide vaccine combined with trastuzumab showed that 69% of patients developed T cell immunity to tumour cells expressing HER2, and that 70% of these subjects manifested ‘epitope spreading’ to target HER2 peptides that were not represented in the vaccine, as well as other tumor-associated antigens34; these findings suggest that vaccine activated T-cells may release cytokines facilitating DC trafficking, phagocytosis, activation, and priming of additional tumor-specific T-cells. Augmented T cell reactivity as measured with an enzyme-linked immunosorbent spot (ELISPOT) assay correlated with increased survival as well.
Because HER2 antibody therapy boosts T cell priming, combinatorial strategies with other approaches to enhance T cell activation appear promising. In experimental models, the combination of HER2 antibodies and either blocking PD1 or agonistic 4-1BB antibodies accomplished greater tumor growth control compared to either monotherapy35. The combination of HER2 antibodies with systemic IL-12, a Th1 polarizing cytokine that activates cytotoxic T cells and natural killer (NK) cells, similarly decreased tumor progression and increased tumor necrosis compared to either treatment alone36. In accordance with these results, a Phase I clinical trial combining systemic IL-12 with trastuzumab and paclitaxel showed enhanced production of interferon-γ (IFNγ), a pro-inflammatory cytokine that activates many immune cell types, as well as increases NK cell activation, both of which were correlated with clinical responses37.
The EGFR antibody cetuximab facilitates DC priming to augment tumor immunity as well29. In an in vitro study using colon cancer cell lines, cetuximab promoted DC opsonization of tumor cells and associated maturation, with increased expression of MHC class II, CD40, CD80 and CD86. DCs incubated with tumor cells and cetuximab more effectively primed tumor-specific T cells compared to DCs that were incubated with tumor cells alone. Additionally, cetuximab facilitates NK cell antibody-dependent cellular cytotoxicity (ADCC)38 and complement-dependent cytotoxicity (CDC)39, 40, which may further enhance tumor cell killing. Based in part on these results, cetuximab is currently being evaluated in a Phase II clinical trial in combination with an allogeneic pancreatic cancer cell vaccine secreting granulocyte-macrophage colony stimulating factor (GM-CSF), an immune stimulatory cytokine (NCT 00305760).
Analogous to monoclonal antibodies, small molecule kinase inhibitors may have important effects on DCs and antigen presentation. Janus kinase 2 (JAK2) inhibitors not only interfere with tumor cell survival signals, but also augment DC function through the blockade of signal transducer and activator of transcription 3 (STAT3) signaling, a key immunosuppressive pathway41. DCs treated with JAK2 inhibitors in vitro show increased MHC class II, CD40, and CD86 expression14, whereas ex vivo DCs isolated from tumor bearing mice treated with a JAK2 inhibitor displayed enhanced induction of allogeneic CD4+ T cell responses 15. JAK2 inhibitors may elicit the accumulation of mature DCs and decrease the number of immature DCs, which typically trigger dysfunctional or suppressive T cells14. As a consequence of these stimulatory effects, combined treatment with a JAK2 inhibitor and a DC vaccine accomplished greater anti-tumor effects compared to either monotherapy15. The mechanisms by which targeted therapies modulate DC function will be addressed in more detail later in the Review.
Enhancing T cell responses and differentiation with targeted therapies
While DCs are crucial for priming the initial anti-tumor T cell response, maintaining activation of T cells and promoting their differentiation into memory T cells is important for achieving a long-lasting anti-tumor effect42 and is also correlated with prolonged overall survival in patients43, 44. Many targeted therapies modulate T cell proliferation and responsiveness to tumor antigens, making them attractive candidates for potential synergy with immunotherapy (Figure 2).
The mTOR pathway has been well characterized in the regulation of multiple immune cells, and rapamycin (an inhibitor of mTOR) has been used clinically as an immunosuppressant for over a decade45. However, recent evidence suggests that under some conditions mTOR inhibitors may have a stimulatory effect on T cells 46. In a lymphocytic choriomeningitis virus (LCMV) mouse model of acute viral infection, animals treated with rapamycin showed an increased early expansion of CD8+ viral specific T cells, increased effector-to-memory T cell differentiation, and enhanced re-activation, function, and survival of memory T cells46. Comparable responses were obtained in mice using a non-replicating virus vaccine and in non-human primates using a live-attenuated viral vaccine46, suggesting a novel therapeutic indication for mTOR inhibitors.
Based on these findings, recent investigations have evaluated the use of mTOR inhibitors in the context of cancer immunotherapy. The combination of temsirolimus (a derivative of rapamycin) and cancer vaccination with an HSP90 adjuvant and tumor specific proteins gp100 and CA9 strongly inhibited tumor growth in the B16 melanoma and RENCA renal cell carcinoma mouse models, respectively47. Notably, animals that received temsirolimus and the vaccines developed significantly more tumor-specific T cells than animals receiving either treatment alone. In another mouse model of metastatic renal cancer, the mTOR inhibitor AZD8055 combined with an agonistic CD40 antibody triggered greater intra-tumoral CD8+ T cell, DC, and macrophage infiltration and superior disease control compared to either monotherapy48. On a cautionary note though, this synergy was not observed when a second mTOR inhibitor (rapamycin) was combined with the agonistic CD40 antibody, suggesting that alterations in drug design or perhaps dosing may result in different immune properties.
In addition to these stimulatory actions on effector and memory T cells, mTOR inhibitors impact FOXP3+ Tregs49, 50. mTOR inhibitors may expand the population of Tregs when given prior to an immune stimulus such as a vaccine49, whereas continuous mTOR inhibition after immune stimulation may impede both Tregs and T cell effectors equally50. Furthermore, release of mTOR inhibition after tonic blockade may cause a rapid rebound in the population of Tregs, skewing the ratio of T effectors and Tregs towards immunosuppression. These results indicate that the timing, dose, sequence, and cycling of mTOR inhibitors with immunotherapy need to be carefully investigated in order to maximize anti-tumor effects.
Wnt signaling is another important oncogenic pathway that may impact T cell differentiation. One important component of the Wnt signaling pathway is glycogen synthase kinase 3β (GSK3β). Because this protein has been implicated in promoting tumor growth in many gastrointestinal malignancies, inhibitors of GSK3β may decrease tumor cell viability in vitro and in vivo51, 52. Interestingly, a recent study linked this pathway to memory T cell differentiation53. Transgenic T cells that were stimulated with peptide-loaded DCs and exposed to the GSK3β inhibitor TWS119 differentiated into a ‘stem-cell’ like phenotype, called ‘Tscm’ cells. These ‘Tscm’ are distinct from classical central memory (Tcm) and effector memory T-cells (Tem). Tscm cells manifested low basal rates of division, but were self-renewing and promptly differentiated into central and effector memory T cells upon stimulation. After adoptive transfer into tumor bearing mice, Tscm cells mediated potent tumor regression, whereas transferred effector or central memory T cells showed lesser anti-tumor activity, likely due to inferior survival of these cells53. Given the importance of generating long-lasting T cells to achieve durable tumor control, GSK3β inhibitors should be investigated further in combination with immunotherapies.
Targeted therapies may also function as costimulatory agents, analogous to agonistic antibodies directed to 4-1BB, GITR, OX40, and other TNFR superfamily members expressed on T cells. Of particular interest in this regard are antagonists of the inhibitor of apoptosis proteins (IAPs). IAP inhibitors were originally developed to counteract the role of IAPs in blocking caspase activation, thereby sensitizing cancer cells to death stimuli54. Unexpectedly, IAP inhibitors were found to mimic costimulatory signaling in T cells55. Although these compounds had minimal effects on resting T cells, they dramatically enhanced T cell activation upon TCR engagement, resulting in increased proliferation and cytokine production. These stimulatory effects involved triggering non-canonical nuclear factor-κB (NF-κB) signaling, as cIAP1 (also known as BIRC2) and cIAP2 (also known as BIRC3) are major negative regulators of NF-κB inducing kinase (NIK; also known as MAP3K14)56. In a pre-clinical mouse model of melanoma, the combination of IAP antagonists and tumor cell vaccines evoked greater reduction in tumor growth and stronger anti-tumor T cell responses compared to either monotherapy55. These findings suggest that IAP inhibitors might potentiate the effects of vaccination and possibly other T cell immunotherapies.
Targeted therapies may sensitize tumor cells to immune-mediated destruction
While T cells have the capacity to kill tumor cells efficiently, the execution of the lytic pathway requires engagement of cytotoxic T cells with their cognate tumor targets. Consequently, tumor cells may develop escape mechanisms to evade T cell killing, such as lowering MHC class I expression to avoid detection or increasing anti-apoptotic proteins to resist cytotoxic programs57. Targeted agents may counteract these strategies to improve immune-mediated tumor destruction (Figure 3).
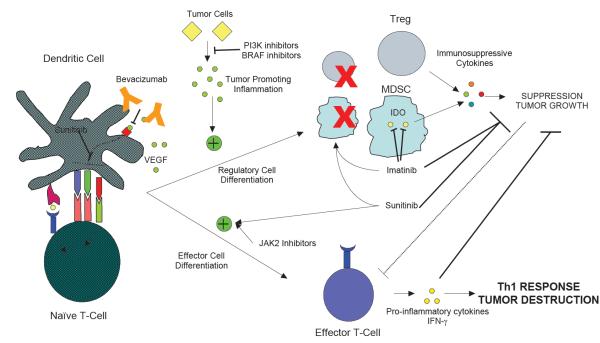
Figure 3. Targeted agents may antagonize immunosuppression in the tumor microenvironment.
Multiple factors within tumors promote immune tolerance and curb the anti-tumor immune response. Tumor cells secrete vascular endothelial growth factor A (VEGFA), and VEGF signaling decreases dendritic cell (DC) costimulatory molecule expression and T cell priming, and encourages the formation of myeloid derived suppressor cells (MDSCs). VEGF antagonists, either as a monoclonal antibody (mAb; such as bevacizumab) or small molecule inhibitors (for example, sunitinib) reverse these deleterious effects and promote formation of potent anti-tumor T cells. Tumor cells also produce inflammatory mediators that promote tumorigenesis as well as encourage suppressor cell formation; these can be inhibited with PI3K and BRAF inhibitors respectively. Tregs and MDSCs are two immunosuppressive cell types that dampen immune responses. Tregs secrete immunosuppressive cytokines, whereas MDSCs use indoleamine-pyrrole 2,3-dioxygenase (IDO) to deplete tryptophan and kill effector T cells. Sunitinib and imatinib both decrease the number and effectiveness of these suppressor cell types. Imatinib also directly inhibits IDO, decreasing MDSC suppressive capacity. Sunitinib and janus kinase 2 (JAK2) inhibitors also block the signal transducer and activator of transcription 3 (STAT3) pathway, an immunosuppressive pathway favoring differentiation into regulatory cells and tumor growth. Decreasing STAT3 signaling diminishes formation of regulatory T cells and promotes the formation of effector Th1 T cells secreting interferon-γ (IFNγ).
Bortezomib is a potent inhibitor of the proteasome that is approved for use in multiple myeloma, and treatment with this drug compromises tumor cell viability, at least in part, through the accumulation of misfolded proteins that in turn engage the unfolded protein response58. In addition to these cell autonomous effects, bortezomib modulates the sensitivity of tumor cells to immune attack. Because the proteasome generates peptide epitopes for MHC class I molecules, bortezomib may decrease peptide loading and MHC class I expression, rendering tumor cells more susceptible to NK cells, the activation of which is normally blocked by MHC class I molecules59. Bortezomib may also increase tumor cell surface expression of FAS (also known as TNFRSF6) and the TNF-related apoptosis-inducing ligand (TRAIL) receptor DR5 (also known as TNFRSF10B), further augmenting NK cell cytotoxicity60. Moreover, bortezomib may sensitize tumor cells to lysis through enhanced expression of NOXA (also known as PMAIP1)16, a BH3 protein that sequesters potent anti-apoptotic proteins like myeloid cell leukemia sequence 1 (MCL1)61. Lastly, bortezomib may augment the baseline activity of granzyme B and caspase 8 in tumor cells16, providing additional sensitization towards apoptosis.
In a pre-clinical model of human papilloma virus (HPV) induced disease, the combination of bortezomib and an HPV E7 based vaccine achieved prolonged stable disease, in contrast to either monotherapy, which displayed minimal effects62. The increased survival correlated with enhanced sensitivity of bortezomib treated tumor cells to killing by E7 specific CTLs. Splenocytes from mice that received the combination therapy also showed increased E7 specific CTL responses compared to control-treated animals, suggesting that bortezomib might augment T cell priming and activation as well.
Inhibitors of the PI3K-AKT pathway are a second class of targeted agents that sensitize tumor cells to immune-mediated destruction, in part through compromising anti-apoptotic signaling17, 63. Tumor cells with an inducible AKT expression cassette manifested enhanced resistance to T cell killing upon AKT activation compared to the non-induced cells, and this involved an increase in MCL1 expression17. In complementary work, a tumor cell line selected for resistance to immunotherapy displayed AKT upregulation compared to wild-type sensitive cells, and this was associated with elevated levels of cIAP1 and 2, BCL-2 and BCL-XL (also known as BCL2L1)63. AKT inhibitors diminished the expression of these anti-apoptotic proteins, sensitizing the tumor cells to T cell killing in vivo. Together, these findings suggest that PI3K-AKT inhibitors might prove synergistic with some immunotherapies, although the impact of these agents on T cell and NK cell function will need to be evaluated carefully.
Another approach to boost T cell tumor killing is to increase the levels of tumor antigens. In this context, an unexpected link between BRAF inhibitors and the expression of melanoma T cell antigens was recently uncovered64. In malignant melanoma, the BRAF-V600E mutant, present in approximately 50% of patients with metastatic disease, promotes constitutive activation of the MAPK pathway, contributing to tumor growth. Vemurafenib, a specific BRAF inhibitor recently approved for therapy of BRAF mutant melanomas65, enhances the expression of well-defined T cell targets including gp100 (also known as PMEL), tyrosinase-related protein 1 (TYRP1), TYRP2, and MART164. Consistent with these results, MART1 and gp100 specific T cells showed increased responsiveness and IFNγ production against BRAF inhibitor treated melanoma cells compared to controls. The BRAF inhibitors did not appear to interfere with T cell differentiation and activation, at least in vitro64; MEK inhibitors, which might be used in combination with vemurafenib as a strategy to reduce drug resistance mediated through MEK activation, require further study to assess their effects on T cell function and melanoma antigen expression.
Heat shock protein 90 (HSP90) inhibitors provide an additional strategy to augment tumor cell antigen expression. HSP90 is a major chaperone that helps to maintain correct folding of multiple client proteins, including several oncoproteins66. HSP90 inhibitors promote the accumulation of misfolded proteins, which may be digested to short peptides and then loaded onto MHC class I molecules67. These effects of HSP90 inhibitors on antigen presentation increase tumor cell sensitivity to CTL-mediated lysis in vitro68 and to adoptive T cell transfer in vivo67. In experimental models, HSP90 inhibitors were also effectively combined with DNA vaccines to decrease tumor growth and improve survival; these effects were abrogated upon treatment with an antibody that depletes CD8+ T cells, establishing a critical role for this cell type in tumor destruction67.
HSP90 inhibitors also augment the expression of ‘stress molecules’ such as NKG2D ligands in both Hodgkin’s lymphoma69 and multiple myeloma70. These surface proteins may directly activate NK cells and costimulate CD8+ T cells, serving as a major mechanism for protective tumor immunity71. Histone deacetylase (HDAC) inhibitors similarly promote NKG2D ligand expression, suggesting that these drugs may sensitize tumor cells to immune cell killing through a shared mechanism72, 73.
Targeted therapies may dampen tumor-induced immunosuppression
A major impediment to the therapeutic efficacy of anti-tumor T cells is the immunosuppressive tumor microenvironment74. Diverse mechanisms may be operative, including the production of inhibitory cytokines such as IL-10 and transforming growth factor-β (TGFβ), the expression of negative costimulatory ligands such as programmed death 1 ligand 1 (PDL1), and the presence of regulatory lymphocyte and myeloid cell populations. The identification of agents that attenuate these suppressive networks might substantially increase the efficacy of immunotherapies (Figure 3).
Vascular endothelial growth factor A (VEGF-A) is best known for its impact on angiogenesis75, but this cytokine manifests potent immunoregulatory actions as well. These effects include blocking the maturation of DCs and promoting the expansion of MDSCs76. The addition of bevacizumab, a monoclonal antibody that neutralizes VEGF, during DC differentiation enhanced the induction of T cell responses. In contrast, bevacizumab had limited effects when added to matured DCs, suggesting that there may be a specific window during DC development in which VEGF functions. In accordance with these results, DCs loaded with multiple myeloma cell lysates (containing VEGF-A) displayed lower levels of costimulatory molecules and a reduced ability to stimulate T cells compared to unloaded DCs, whereas bevacizumab diminished these suppressive effects77. VEGF-A blockade augmented NF-κB activation but blocked STAT3 signaling, resulting in increased IL-12 but decreased IL-10 levels. In the B16 melanoma model, a VEGF-A antibody combined with adoptive T cell transfer intensified tumor infiltration, decreased tumor growth, and prolonged survival compared to either monotherapy78. The effects of VEGF-A blockade were greater if animals were pre-treated prior to cell transfer, perhaps reflecting a more efficient localization of transferred T cells to the tumor microenvironment.
Small molecule inhibitors targeting VEGF receptor (VEGFR) signaling display comparable immunostimulatory effects. In a mouse model of colon cancer, the administration of sunitinib, a multi-tyrosine kinase inhibitor that blocks VEGFR function, decreased MDSC and Treg numbers and function, both systemically and in the tumor microenvironment79. Consequently, sunitinib augmented tumor-infiltrating T cell IFN-γ production and cytotoxicity, but diminished expression of CTLA4, PD1, and PDL-1. Furthermore, the combination of sunitinib, an agonistic 4-1BB antibody, and IL-12 resulted in improved overall survival compared to regimens containing only one or two of these agents. In a B16 melanoma model, the combination of sunitinib and a DC vaccine similarly enhanced survival compared to either agent alone80. Sunitinib was most effective when administered concurrently with vaccination, suggesting that VEGF blockade with small molecules, analogous to antibodies, may be most important during the initial DC priming of anti-tumor T cells. One cautionary note, though, is that seemingly related small molecule inhibitors might not have identical activities; sorafenib is a multi-kinase inhibitor that in part targets VEGFR, but this agent appears to be immunosuppressive, possibly related to inhibitory effects on MEK signaling81.
In addition to inhibiting VEGFR function, sunitinib may block STAT3 activation, which is another major immunosuppressive pathway41. In the B16 melanoma model, animals with a T cell specific STAT3-null mutation showed enhanced overall survival and increased tumor specific T cells compared to wild-type animals82. In a RENCA renal cancer model, mice treated with sunitinib and adoptive T cell transfer showed better responses compared to either monotherapy. However, if STAT3 deficient T cells were transferred, sunitinib showed no additional therapeutic effects, suggesting that STAT3 is the major target in this system82. Sunitinib-mediated STAT3 inhibition similarly enhanced the therapeutic effects of adoptive T cell transfer and vaccination in a mouse model of hepatocellular carcinoma83.
Because JAK2 phosphorylates STAT3 in many signaling cascades, JAK2 inhibitors might also be an effective strategy to dampen STAT3 activation15. Comparable to the investigations with sunitinib, tumor bearing mice that were treated with JAK2 inhibitors displayed increased DC maturation and T cell polarization towards a Th1 phenotype, with enhanced IL-2 and IFN-γ production15. JAK2 inhibitors augment the therapeutic effects of DC vaccination15 and decrease tumor cell PDL-1 expression at a transcriptional level84.
Another immunosuppressive pathway mediated by tumor associated myeloid cells involves indoleamine-pyrrole 2,3-dioxygenase (IDO) catalyzed degradation of tryptophan, an amino acid required for optimal T cell function, into metabolites that are directly toxic to T cells and important for immune tolerance85. Unexpectedly, imatinib was found to decrease IDO expression in myeloid cells86. In a murine model of gastrointestinal stromal tumors, imatinib led to an increased ratio of intra-tumoral CD8+ CTLs to Tregs, promoting tumor destruction. Concurrent administration of imatinib and CTLA4 antibodies resulted in greater increases in CD8+ T cell IFN-γ production and cytotoxicity compared to either agent alone. Imatinib might also have direct inhibitory effects on Tregs, decreasing their number and suppressive capacity87. Notably, the combination of imatinib and a DC vaccine in a BCR-ABL negative lymphoma model resulted in decreased Tregs, fewer metastases, and increased T cell IFN-γ production compared to either monotherapy. Given the potent effects of imatinib in this BCR-ABL negative model, further studies should be undertaken to determine if this targeted therapy might be employed more broadly as a component of combination immunotherapy.
Tumor-promoting inflammatory reactions are an additional major component of the tumor microenvironment88. Myeloid cells play a central role in these reactions and, in a PI3K catalytic subunit-γ (p110γ)-dependent fashion, respond to tumor-derived factors, infiltrate the lesions, and release pro-tumorigenic cytokines89. Accordingly, genetic and pharmacologic inhibition of p110γ decreased myeloid cell infiltration, with a concomitant reduction in tumor growth and metastases. Because the p110γ isoform is expressed predominantly in immune cells and not tumor cells, the primary target for these inhibitors appears to be the host. A further link between oncogenic pathways and immunoregulation involves mutant BRAF, which drives the secretion of suppressive cytokines such as IL-10 and VEGF-A from melanoma cells90. The inhibitor of mutant BRAF, vemurafenib, blocks these effects and thereby increases DC responsiveness, indicating that targeted therapies can directly antagonize tumor-derived suppressive factors.
This Review focuses on targeted agents, but conventional cytotoxic chemotherapy also impacts many aspects of the anti-tumor immune response (Box 2). For example, chemotherapy triggers the release of ‘danger’ molecules from tumor cells, such as high mobility group protein B1 (HMGB1) and ATP, increasing DC tumor antigen presentation and maturation91. Chemotherapy may sensitize tumor cells to T cell mediated killing by increasing the expression of death receptors such as DR5 and costimulatory molecules such as NKG2D ligands92. Furthermore, chemotherapy may kill Tregs and MDSCs, allowing for partial amelioration of tumor-driven systemic immunosuppression93, 94. Interestingly, external beam radiation therapy (XRT) appears to have similar effects, facilitating the release of danger signals like HMGB1 as well as sensitizing tumor cells by increasing expression of Fas and NKG2D ligands95. Given the properties both of these therapeutic modalities, incorporating immunotherapeutic strategies into conventional treatment protocols provides many opportunities for potential synergies.
Challenges for combining targeted therapies and immunotherapies
Targeted therapies offer great promise for boosting responses with immunotherapy, but the appropriate timing, dosage, and sequencing of these agents will likely be crucial to the success of combinatorial approaches. In this context, some targeted therapies with immunostimulatory potential also display immunosuppressive activities. For example, whereas HSP90 inhibitors may increase CTL-mediated tumor lysis through enhanced MHC class I presentation, they may also decrease some macrophage and DC functions96, 97. Similarly, the mTOR inhibitor temsirolimus may diminish the ability of DCs to prime tumor-specific T cells47, whereas bortezomib98, 99 and HDAC inhibitors100 may impede some NK cell actions.
A second key issue for combinatorial therapies is whether intensified anti-tumor effects can be achieved without a corresponding increase in serious toxicities. The major adverse events with immunomodulatory agents such as ipilumimab and PD1 antibodies are inflammatory pathologies, and combining treatments that affect different steps in an immune response might be anticipated to increase the likelihood of these side effects. Although pre-clinical studies investigating these toxicities are clearly warranted, they may not fully predict the spectrum of pathology observed in humans; for example, inflammation in the pituitary gland (hypophysitis) is a relatively common side effect of ipilimumab, but this was not anticipated from experimental studies in mice101. Unexpectedly, some pre-clinical experiments raise the possibility that particular combination treatments, such as CTLA4 blockade and 4-1BB agonist antibody, might diminish, rather than exacerbate inflammatory toxicities102.
The optimal timing and sequence of particular combinations of targeted agents and immunotherapy will need to be determined experimentally, but some guidance might be provided through considering the stage of immune response most likely impacted (Figure 4). Broadly speaking, different therapies affect one of four areas of immune activation: DC presentation and T cell priming, T cell activation and anti-tumor effector functions, T cell differentiation into memory cells, and tumor microenvironment antagonism.
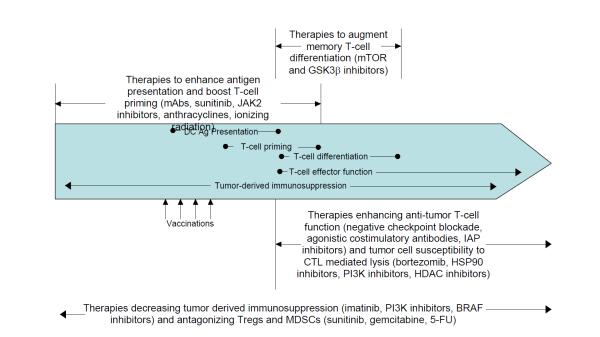
Figure 4. Critical variables in combining targeted agents and immunotherapy.
Immune responses against tumors occur in a step-wise fashion. First, dendritic cells (DCs) must capture tumor antigens and present them to naïve T cells under inflammatory conditions. Naïve T cells then differentiate into effector T cells, which may take up to one week, prior to leaving the lymph node and entering the blood. At this time, some T cells further differentiate into long-lived memory T cells, providing a pool of renewable anti-tumor T cells for an extended period after immunotherapy has ceased. Once in the periphery, tumor cells activate T cells, causing them to secrete inflammatory cytokines and/or cytotoxic granules. Throughout this process, T cells must overcome tumor-derived immunosuppression from myeloid-derived suppressor cells (MDSCs), Tregs, and tumor cell-secreted suppressive molecules. Drugs modulating each of these areas should be delivered just prior to and during the respective critical steps of immune maturation. Therapies boosting DC antigen presentation and initial T cell priming should be delivered prior to vaccines and continued through initial T cell priming. Agents promoting T cell memory formation should be giving during T cell priming and then discontinued after differentiation is complete in order to avoid deleterious effects on effector function. Therapies enhancing T cell function and tumor cell lysis should be given after T cell priming is complete and continued throughout treatment to maximize effector function. Tumor derived immunosuppression constantly antagonizes anti-tumor immune responses; accordingly, therapies designed to mitigate this should be given prior to vaccination and continued throughout treatment. 5-FU, 5-fluorouracil; CTL, cytotoxic T lymphocyte; GSK3β, glycogen synthase kinase 3β; HDAC, histone deacetylase; HSP90, heat shock protein 90; IAP, inhibitor of apoptosis protein; JAK2, janus kinase 2; mABs, monoclonal antibodies.
Targeted therapies affecting DC priming might be given first, pre-treating a patient prior to any immunotherapies such as vaccination or adoptive cell transfer, and then continued throughout the treatment course. This might skew the actions of DCs towards promoting T cell effector responses rather than Tregs. For example, initiating sunitinib prior to tumor vaccination yielded superior anti-tumor responses compared to concurrent administration; in contrast, cycling of sunitinib (4 weeks on-2 weeks off) favored the accumulation of MDSCs and Tregs in the ‘off’ period, perhaps reflecting a rebound effect of unopposed VEGF-A12. Pre-treatment with monoclonal antibodies such as cetuximab or trastuzumab may similarly prove favorable, in this case through the enhanced phagocytosis of tumor antigens by DCs, which may promote DC maturation and priming29, 33. Additionally, agents that might antagonize DC function, such as HSP90 inhibitors96 or sorafenib81, might be avoided at this time in order to maximize tumor specific T cell priming.
Targeted therapies that augment T cell differentiation, such as GSK3β or mTOR inhibitors, might be administered after vaccination to promote the induction of memory T cells. Relatively short courses might be delivered, though, as GSK3β inhibitors may block Tscm differentiation into effector T cells53 and mTOR inhibitors may prevent maximal T cell proliferation47. Immunostimulatory agents, such as antibodies that block negative checkpoint activation, agonistic costimulatory antibodies, and IAP inhibitors might be given after T cell priming and continued for a period thereafter (perhaps intermittently) to maintain effector T cell activation and prevent exhaustion.
Lastly, targeted therapies that antagonize the immunosuppressive tumor microenvironment might find broad applicability, from preceding tumor vaccination through treatment consolidation. Imatinib (which inhibits IDO), sunitinib (which antagonizes MDSCs and Tregs), cyclophosphamide (which kills Tregs), gemcitabine and 5-fluorouracil (5-FU; which both kill MDSCs), and p110γ inhibitors (which eliminate tumor-promoting inflammation) might be in this category, although a potential impact on T cell effector function needs to be carefully investigated.
Some of these principles are being tested in a recently initiated Phase I/II clinical trial that combines the mutant BRAF inhibitor vemurafenib with the immune checkpoint blocking antibody ipilimumab in patients with metastatic melanoma (NCT01400451). This important study will aim to convert the frequent tumor regressions achieved with vemurafenib into long-lasting clinical responses through the induction of a potent immunological memory. The abilities of vemurafenib to increase CTL recognition of tumor cells64 and to decrease tumor immunosuppression90 may amplify the capacity of CTLA4 blockade to boost T cell responses and prevent anergy. This trial will both evaluate the safety and tolerability of the combination therapy and provide a preliminary assessment of overall survival. Given the complementary modes of achieving tumor control with the two modalities, the patterns of clinical response will be of particular interest. This combinatorial strategy shows great potential based on mechanistic considerations, and will likely establish a paradigm for many similar studies to follow.
Conclusions
The discovery of critical molecular pathways that promote tumor growth and maintenance together with the development of drugs that specifically inhibit these pathways has ushered in a new era of cancer medicine. Analogously, an improved understanding of the mechanisms of protective tumor immunity and the translation of these concepts into efficacious immunotherapies that prolong patient survival has validated the long-standing idea that immunity plays an important role in cancer pathogenesis. The complementary modes of action with these two promising modalities suggest intriguing possibilities for therapeutic synergy with combination treatment. A strong foundation has been established to advance the crafting of both pre-clinical and early stage patient investigations to determine the best ways to integrate targeted agents and immunotherapy.
Box 2. Conventional cytotoxic therapies in conjunction with immunotherapies.
Numerous lines of evidence suggest that anti-tumor immunity is crucial for clinical responses toward conventional cancer treatments. For instance, in a broad array of tumor types, patients with denser T cell infiltrates within tumors have a better clinical response to cytotoxic therapy compared to patients with smaller infiltrates92, 107, 108. In animal models, tumors regress more quickly to chemotherapy in immunocompetent animals compared to immunodeficient ones92. Surprisingly, although initially thought to be immunosuppressive, anti-cancer therapies can promote tumor immunity. Some cytotoxic therapies, such as anthracyclines or ionizing radiation, promote ‘immunogenic cell death’, which includes the release of danger molecules from tumor cells such as calreticulin, high mobility group box protein B1 (HMGB1), and ATP (reviewed in 91). These danger molecules polarize dendritic cells (DCs) towards a pro-inflammatory phenotype and increase priming towards Th1 anti-tumor T cells and away from Tregs. Additionally, some chemotherapies such as cyclophosphamide are directly toxic to immunosuppressive Tregs, and combining low-dose cyclophosphamide with tumor vaccines induces anti-tumor immunity in animal models109. Similarly, gemcitabine93 and 5-fluorouracil (5-FU)94 both selectively kill myeloid-derived suppressor cells (MDSCs) in vitro and in vivo, and animal models show that gemcitabine substantially increases the activity of immunotherapy when the two are given in combination. Surgical removal of primary tumor masses has also been shown to reverse tumor-derived immunosuppression110, suggesting that immunotherapies may have even greater potency in adjuvant settings compared to first-line therapy.
Numerous clinical trials are currently examining combinatorial strategies with immunotherapy and chemotherapy. In metastatic pancreatic adenocarcinoma, patients receiving gemcitabine and a DC vaccine had a median survival of 360 days111, a substantial prolongation compared to other cohorts treated with chemotherapy alone; in metastatic non-small cell lung cancer combining a pox-virus based interleukin-2 (IL-2) and mucin 1 (MUC1) immunotherapy with chemotherapy found a trend towards increased progression free survival at 6 months112; and in melanoma, combining CTLA4 blockade with standard chemotherapy increased overall survival in a recent Phase III trial7.
Glossary
Complete cytogenetic responses-the lack of any detectable tumor burden by conventional cytogenetic studies such as karyotype analysis or FISH
Dendritic cell vaccine-a process where dendritic cells are removed from the patient, loaded with tumor or tumor antigens, matured, and then re-infused back into the patient in order to stimulate immune responses in vivo
Oncogene “addiction”-a process where a single mutated gene or signaling pathway drives tumor proliferation; inhibition results in rapid tumor response
Vaccination in situ –as tumor cells die and release danger molecules, tumor antigens are phagocytosed and presented by DCs to prime anti-tumor responses
Cytotoxic CD8+ T lymphocytes –a T-cell subtype that recognizes a specific peptide on targets cells and kills those cells
Regulatory T cells –a T-cell subtype that releases suppressive cytokines and serves to silence immune responses
Myeloid derived suppressor cells-a myeloid cell subtype that silences responses of cytotoxic CD8+ T-cells and helper CD4+ T-cells while simultaneously promoting the formation of regulatory T-cells
Major histocompatibility complex-proteins responsible for displaying varied peptide antigens on the cell surface
Helper CD4+ T cells-a T-cell subtype that recognizes peptides on target cells and secretes signaling molecules (called cytokines) to direct an appropriate immune response
Anergy-a state whereby T-cells do not respond to antigenic stimulation even when presented in the appropriate context
Exhaustion-after chronic stimulation, T-cell responses become diminished or non-existent to repeated antigenic encounters
Epitope spreading-after peptide vaccination, T-cells are generated that respond to peptides not in the original vaccine formulation, indicating that a secondary round of T-cell priming has occurred with antigens taken directly from tumor cells
Th1-a helper T-cell response characterized by IFNγ production and stimulation of CD8+ cytotoxic T-cells
Natural killer cells-a cytotoxic cell of the innate immune system that does not recognize target cells in an antigen-specific manner and kills its targets using similar mechanisms as the CD8+ CTL
Opsonization-phagocytosis of antigens from the external environment by dendritic cells or other antigen presenting cells
Antibody-dependent cellular cytotoxicity-destruction of target cells that are coated with antibodies by innate immune cells with Fc receptors such as NK cells, monocytes, or macrophages using cytotoxic substances such as perforin and granzymes, reactive oxygen species, and reactive nitrogen species
Complement-dependent cytotoxicity-destruction of target cells coated with antibodies by a series of serum proteins that undergo a cascade of enzymatic cleavage and culminate in the formation of the pores within the target cell membranes, permeabilizing the cells
Memory T cells-T-cells that have undergone antigenic stimulation at least once and are capable of rapidly responding to additional antigen encounters
Adoptive transfer-the infusion of cells into animals or patients that have been taken directly from another source or expanded and modified ex vivo
Central memory T cells-long-lived memory T-cells residing in peripheral blood, lymph nodes, or spleen capable of undergoing rapid differentiation into effector T cells upon antigenic stimulation
Lytic pathway-the release of cytotoxic molecules such as perforin and granzymes from cytotoxic T-cells to kill their cognate targets
Unfolded protein response (UPR)-an endoplasmic reticulum stress response triggered by the accumulation of misfolded proteins that initially results in increased protein chaperone synthesis and decreased total protein synthesis in an attempt to remove the misfolded proteins; however, if misfolded proteins continue to persist this continued activation of this pathway ultimately results in cell apoptosis
Biography
Matthew Vanneman is a medical student at Harvard Medical School who currently studies the NKG2D pathway in human malignancies. He is interested in developing novel antibody-based therapeutics in treating cancer and other human diseases.
Glenn Dranoff is Professor of Medicine at Dana-Farber Cancer Institute and Harvard Medical School and Leader of the Dana-Farber/Harvard Cancer Center Program in Cancer Immunology. His research focuses on the molecular and cellular mechanisms underlying anti-tumor immunity and the development of novel cancer immunotherapies.
Footnotes
At-a-glance summary
--So called “targeted therapies” and cancer immunotherapies are two novel treatment modalities that have recently begun to enter the oncology clinic. Targeted therapies and immunotherapy offer a number of possible synergies in treatment when used together, however these combinations have not been well studied.
--Many targeted therapies against tumors affect pathways that are also critical to immune development and function, suggesting possibilities that targeted therapies may help optimize anti-tumor immune responses from immunotherapies. Similarly, immunotherapies may serve to consolidate impressive clinical responses from targeted therapies into long lasting clinical remissions.
--Targeted therapies promote effective DC maturation, T-cell priming, activation, and differentiation into long-lived memory cells, suggesting possible combinations of cancer vaccines along with targeted therapies to bolster vaccine response as well as effector T-cell function.
--Targeted therapies may sensitize tumor cells to immune mediated killing by increasing expression of death receptors or “distress” ligands while simultaneously diminishing expression of pro-survival signals, increasing the efficiency of immune mediated tumor clearance once immune cells are activated in vivo.
-Targeted therapies may diminish tumor-mediated immunosuppression by abrogating production of tumorigenic inflammation and through inhibition of immunosuppressive cell types. Impairing immune suppression improves effector cell function and increases immune destruction of tumor targets, suggesting possible synergies with immunotherapies designed to generate anti-tumor T-cells or bolster their effector function.
--Important considerations regarding optimizing dose, sequence, and timing of targeted therapies will be required in rationally designing future clinical trials in order to maximize anti-tumor efficacy while minimizing any immunosuppressive side effects.
References
- 1.Druker BJ, David A. Karnofsky Award lecture. Imatinib as a paradigm of targeted therapies. J Clin Oncol. 2003;21:239s–245s. doi: 10.1200/JCO.2003.10.589. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien SG, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Haber DA, Gray NS, Baselga J. The evolving war on cancer. Cell. 2011;145:19–24. doi: 10.1016/j.cell.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Korman A, Peggs K, Allison JP. Checkpoint blockade in cancer immunotherapy. Advances in Immunology. 2006;90:293–335. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 10.Rakhra K, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarle R, et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med. 2008;14:676–80. doi: 10.1038/nm1769. [DOI] [PubMed] [Google Scholar]
- 12.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2011 doi: 10.1002/ijc.26219. -This paper details how alterations in scheduling of the targeted therapy sunitinib significantly alter Treg populations, and that in animal models pre-treating with sunitinib improves vaccine efficacy while co-administration had no effect on vaccine efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 14.Nefedova Y, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175:4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nefedova Y, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35. doi: 10.1158/0008-5472.CAN-05-0529. -This paper discusses the use of the JAK2 inhibitor targeted therapy to improve maturation of dendritic cells, showing that animals treated with JAK2 inhibitors have increased numbers of mature DCs, increased T-cell priming by DCs, and increased surival when the inhibitor was combined with a dendritic cell vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeger JM, et al. The proteasome inhibitor bortezomib sensitizes melanoma cells toward adoptive CTL attack. Cancer Res. 2010;70:1825–34. doi: 10.1158/0008-5472.CAN-09-3175. [DOI] [PubMed] [Google Scholar]
- 17.Hahnel PS, et al. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 2008;68:3899–906. doi: 10.1158/0008-5472.CAN-07-6286. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 20.May KF, Jr., Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62:3459–65. [PubMed] [Google Scholar]
- 21.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 22.Miller RE, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 23.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–91. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 24.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 26.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boruchov AM, et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 29.Correale P, et al. Cetuximab +/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2011 doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 30.Wolpoe ME, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol. 2003;171:2161–9. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 31.Ladoire S, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer. 2011;105:366–71. doi: 10.1038/bjc.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim PS, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Disis ML, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. -This paper demonstrates how targeted monoclonal antibody therapies such as HER2 antibodies require immune-mediated tumor destruction for clinical responses and synergize with both costimulatory 4-1BB agonistic antibody as well as blockade of an inhibitory signal through a PD1 antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaime-Ramirez AC, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. J Immunol. 2011;186:3401–9. doi: 10.4049/jimmunol.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekaii-Saab TS, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–91. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marechal R, et al. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer. 2010;10:340. doi: 10.1186/1471-2407-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dechant M, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 40.Hsu YF, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: a target to enhance antitumor immune response. Curr Top Microbiol Immunol. 2011;344:41–59. doi: 10.1007/82_2010_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilinc MO, Gu T, Harden JL, Virtuoso LP, Egilmez NK. Central role of tumor-associated CD8+ T effector/memory cells in restoring systemic antitumor immunity. J Immunol. 2009;182:4217–25. doi: 10.4049/jimmunol.0802793. [DOI] [PubMed] [Google Scholar]
- 43.Pages F, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 44.Leffers N, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–15. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. -This paper illustrates how inhibitors of the mTOR pathway, such as rapamycin, enhance memory T-cell differenation as well as augments their function in multiple different animal models of viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–52. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Q, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–84. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Procaccini C, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–41. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, et al. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J Immunol. 2011;186:2809–18. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mai W, et al. Deregulated GSK3{beta} sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin Cancer Res. 2009;15:6810–9. doi: 10.1158/1078-0432.CCR-09-0973. [DOI] [PubMed] [Google Scholar]
- 52.Shakoori A, et al. Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 2007;98:1388–93. doi: 10.1111/j.1349-7006.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. -This paper demonstrates how targeted therapies such as GSK3β inhibitors are able to drive T-cell differentiation to retain long-lasting, self-renewing, "Tscm" memory T-cells that provide potent tumor protection in adoptive transfer models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 55.Dougan M, et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J Exp Med. 2010;207:2195–206. doi: 10.1084/jem.20101123. -This paper shows that inhibitors of the inhibitor of apoptosis proteins (IAPs) increase T-cell responses to multiple different immune stimuli in vitro and that combining IAP inhibitors with tumor vaccination decreases tumor growth kinetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varfolomeev E, Vucic D. (Un)expected roles of c-IAPs in apoptotic and NFkappaB signaling pathways. Cell Cycle. 2008;7:1511–21. doi: 10.4161/cc.7.11.5959. [DOI] [PubMed] [Google Scholar]
- 57.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Advances in Immunology. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 58.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- 59.Shi J, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–17. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallett WH, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–70. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Tseng CW, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl) 2008;86:899–908. doi: 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noh KH, et al. Activation of Akt as a mechanism for tumor immune evasion. Mol Ther. 2009;17:439–47. doi: 10.1038/mt.2008.255. -This paper demonstrates that one mechanism of tumor resistance to vaccination therapy is through up-regulation of the AKT pathway, which mediates resistance to apoptosis; inhibiting this pathway increased CTL mediated killing of AKT-upregulated tumor cells in vitro and that AKT inhibition in combination with vaccination augmented responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boni A, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. -This paper shows how targeted inhibition of mutant BRAF augments expression of tumor antigens on the tumor cell surface, increasing T cell responses against tumor cells while showing no deletrious effect on T cell proliferation or function. [DOI] [PubMed] [Google Scholar]
- 65.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taipale M., Jarosz, D.F., Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 67.Lin CC, et al. Inhibitor of heat-shock protein 90 enhances the antitumor effect of DNA vaccine targeting clients of heat-shock protein. Mol Ther. 2007;15:404–10. doi: 10.1038/sj.mt.6300014. [DOI] [PubMed] [Google Scholar]
- 68.Kawabe M, et al. Heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin enhances EphA2+ tumor cell recognition by specific CD8+ T cells. Cancer Res. 2009;69:6995–7003. doi: 10.1158/0008-5472.CAN-08-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boll B, et al. Heat shock protein 90 inhibitor BIIB021 (CNF2024) depletes NF-kappaB and sensitizes Hodgkin's lymphoma cells for natural killer cell-mediated cytotoxicity. Clin Cancer Res. 2009;15:5108–16. doi: 10.1158/1078-0432.CCR-09-0213. [DOI] [PubMed] [Google Scholar]
- 70.Fionda C, et al. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J Immunol. 2009;183:4385–94. doi: 10.4049/jimmunol.0901797. [DOI] [PubMed] [Google Scholar]
- 71.Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66:3959–62. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 72.Poggi A, et al. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23:641–8. doi: 10.1038/leu.2008.354. [DOI] [PubMed] [Google Scholar]
- 73.Skov S, et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–45. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 74.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 76.Alfaro C, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–9. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang DH, et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009;33:665–70. doi: 10.1016/j.leukres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Shrimali RK, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozao-Choy J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. -This paper demonstrates how a targeted thereapy, sunitinib, is able to decrease both the number and function of suppressive cells Tregs and MDSCs in tumor infiltration lymphocytes in an in vivo mouse model of colon cancer, and that combining sunitinib with agonistic 4-1BB antibody and IL12 improved responses to therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bose A, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2010 doi: 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hipp MM, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–20. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 82.Kujawski M, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010;70:9599–610. doi: 10.1158/0008-5472.CAN-10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avella DM, et al. Regression of established hepatocellular carcinoma is induced by chemo-immunotherapy in an orthotopic murine model. Hepatology. 2011 doi: 10.1002/hep.24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green MR, et al. Integrative analysis reveals selective 9p24. amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hwu P, et al. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 86.Balachandran VP, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. -This paper shows how imatinib blocks the supressor function of MDSCs through the inhibition of IDO, and that combining imatinib with CTLA4 antibody improves clinical responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larmonier N, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181:6955–63. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grivennikov S.I., Greten, F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmid MC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–27. doi: 10.1016/j.ccr.2011.04.016. -This paper demonstrates how pharmacologic and genetic inhibition of the PI3K pathway impedes immune-mediated tumor-promoting inflammation, slowing tumor growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zitvogel L, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 94.Vincent J, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 95.Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol Biosyst. 2009;5:1262–70. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bae J, et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178:7730–7. doi: 10.4049/jimmunol.178.12.7730. [DOI] [PubMed] [Google Scholar]
- 97.Yun TJ, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–75. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- 98.Feng X, et al. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol Immunol. 2010;47:2388–96. doi: 10.1016/j.molimm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, et al. Proteasome inhibition induces apoptosis in primary human natural killer cells and suppresses NKp46-mediated cytotoxicity. Haematologica. 2009;94:470–8. doi: 10.3324/haematol.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rossi LE, et al. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J Leukoc Biol. 2011 doi: 10.1189/jlb.0711339. [DOI] [PubMed] [Google Scholar]
- 101.Dranoff G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nature Reviews Immunology. 2011;12:61–6. doi: 10.1038/nri3129. [DOI] [PubMed] [Google Scholar]
- 102.Kocak E, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–84. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 103.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Topp MS, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 105.Burris HA, 3rd, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 106.Zhang B, et al. Immune Surveillance and Therapy of Lymphomas Driven by Ebstein-Barr-Virus Protein LMP1 in a Mouse Model. Cell. doi: 10.1016/j.cell.2011.12.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halama N, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 108.Denkert C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 109.Ghiringhelli F, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 110.Danna EA, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 111.Kimura Y, et al. Clinical and Immunologic Evaluation of Dendritic Cell-Based Immunotherapy in Combination With Gemcitabine and/or S-1 in Patients With Advanced Pancreatic Carcinoma. Pancreas. 2011 doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 112.Quoix E, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–33. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 113.Catellani S, Pierri I, Gobbi M, Poggi A, Zocchi MR. Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia. PLoS One. 2011;6:e18925. doi: 10.1371/journal.pone.0018925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Preudhomme C, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511–21. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 115.Jain N, et al. Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: a phase 2 trial. Cancer. 2009;115:3924–34. doi: 10.1002/cncr.24468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Menard C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–9. doi: 10.1158/0008-5472.CAN-08-3807. -This paper demonstrates that the presence of NK cells in patients with GIST treated with imatinib serves as an independent prognostic factor in clinical response, suggesting that off-target effects of imatinib stimulating NK-cells may partially account for its therapeutic success. [DOI] [PubMed] [Google Scholar]
- 117.Escudier B, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 118.Manzoni M, et al. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology. 2010;79:187–96. doi: 10.1159/000320609. [DOI] [PubMed] [Google Scholar]
- 119.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK):dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–54. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pander J, et al. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin Cancer Res. 2011;17:5668–73. doi: 10.1158/1078-0432.CCR-11-0239. [DOI] [PubMed] [Google Scholar]
- 121.Lanuti P, et al. Enhancement of TRAIL cytotoxicity by AG-490 in human ALL cells is characterized by downregulation of cIAP-1 and cIAP-2 through inhibition of Jak2/Stat3. Cell Res. 2009;19:1079–89. doi: 10.1038/cr.2009.80. [DOI] [PubMed] [Google Scholar]
- 122.Benson DM, Jr, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chanan-Khan AA, et al. Biological effects and clinical significance of lenalidomide-induced tumour flare reaction in patients with chronic lymphocytic leukaemia: in vivo evidence of immune activation and antitumour response. Br J Haematol. 2011;155:457–67. doi: 10.1111/j.1365-2141.2011.08882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gorgun G, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–37. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herman SE, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117:4323–7. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benson DM, Jr, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011 doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang H, et al. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. 2008;181:6797–802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]