Abstract
The purpose of this research was to study the effects of age and genetic alterations in key connective tissue proteins on susceptibility to experimental glaucoma in mice. We used mice haploinsufficient in the elastin gene (EH) and mice without both alleles of the fibromodulin gene (FM KO) and their wild type (WT) littermates of B6 and CD1 strains, respectively. FM KO mice were tested at two ages: 2 months and 12 months. Intraocular pressure (IOP) was measured by Tonolab tonometer, axial lengths and widths measured by digital caliper post-enucleation, and chronic glaucoma damage was measured using a bead injection model and optic nerve axon counts. IOP in EH mice was not significantly different from WT, but FM KO were slightly lower than their controls (p = 0.04). Loss of retinal ganglion cell (RGC) axons was somewhat, but not significantly greater in young EH and younger or older FM KO strains than in age-matched controls (p = 0.48, 0.34, 0.20, respectively, multivariable regression adjusting for IOP exposure). Older CD1 mice lost significantly more RGC axons than younger CD1 (p = 0.01, multivariable regression). The CD1 mouse strain showed age-dependence of experimental glaucoma damage to RGC in the opposite, and more expected, direction than in B6 mice in which older mice are more resistant to damage. Genetic alteration in two genes that are constituents of sclera, fibromodulin and elastin do not significantly affect RGC loss.
Keywords: glaucoma, mouse, experimental model, sclera, biomechanics, retinal ganglion cell, fibromodulin, elastin
1. Introduction
Glaucoma is the second leading cause of world blindness (Quigley and Broman, 2006) and older age (Burgoyne and Downs, 2007) and myopia (Boland and Quigley, 2007) are found to be risk factors for its prevalence. There is substantial evidence that the state of ocular connective tissues and their response to the stress of intraocular pressure (IOP) are key determinants of susceptibility to glaucoma damage to retinal ganglion cells (RGC) (Burgoyne et al., 2005; Quigley and Addicks, 1981). If we can determine the features of ocular connective tissues that make glaucoma more likely or more damaging, new treatments could be directed to altering those features (Quigley and Cone, 2013; Strouthidis and Girard, 2013). With increasing age, human eyes develop changes in the connective tissues of the sclera (Friberg and Lace, 1988) and lamina cribrosa of the optic nerve head (Quigley, 1977). Scleral thickness decreases with age in humans (Coudrillier et al., 2012) and monkeys (Girard et al., 2009), though older reports suggested an increase with age in humans (Watson and Young, 2004). There is an associated increase in scleral stiffness with age as measured by ex vivo strip (Friberg and Lace, 1988) and inflation testing (Coudrillier et al., 2012). Initial testing of the effect of age on scleral stiffness in mice confirmed that the sclera also was stiffer in older than in younger B6 mice (Myers et al., 2010). The determinants of this age-related change have been studied in animals and include alterations in proteoglycans (Rada et al., 2000) or increased cross-linkage of extracellular matrix components (Schultz et al., 2008). In initial studies of the effect of experimental glaucoma in mice, we have reported and confirmed the surprising finding that older B6 mice are less susceptible to RGC loss with chronic elevated IOP than younger B6 mice (Cone et al., 2010; Cone et al., 2012).
Myopic eyes have, in general, not only larger than normal axial length, but thinner sclera and decreased stiffness (Curtin and Teng, 1958; Curtin, 1969; McBrien et al., 2009). While there have been many studies of induced myopia/axial length increase in a variety of animal models (Rada et al., 2006), the relation between myopia and its scleral alterations on the one hand and susceptibility to glaucoma injury on the other has not been studied in detail in animals. If the sclera were considered as a theoretical thin-walled sphere, axially longer eyes would be at a disadvantage in withstanding the same IOP as a smaller eye, since it would be expected that the stress would be greater. However, this simple relation does not take into account scleral thickness, baseline behavior of the sclera biomechanically, and the dynamic response of the sclera, all of which are probably important. Reasoning that axially longer mouse eyes might have different susceptibility to experimental glaucoma, we have tested several strains and compared scleral thickness, scleral inflation behavior, and RGC loss with comparable chronic IOP elevation. In the first such comparison, we found that albino CD1 mice, which have larger eyes than B6 mice, are more susceptible to RGC loss than B6 (Cone et al., 2010; Nguyen et al., 2013). Next, we studied another mouse strain with axially longer eyes, the Aca23 mutant, with a mutation in Collagen 8α2 (Steinhart et al., 2012). Interestingly, these mutants were significantly less susceptible to glaucoma damage than wild type littermates.
These initial findings suggest that the common beliefs that glaucoma damage would occur more easily in all older or in all larger eyes are not supportable, at least in a murine glaucoma model. We need to understand better what features of age and myopia may contribute to susceptibility to glaucoma injury. The state of scleral connective tissues may be one area that affects this susceptibility. In the present study, we include study of two further mouse strains with genetic deficiency in key components of scleral connective tissue. One of the strains is haploinsufficient for elastin (Aszodi et al., 2006) (designated EH and developed on a B6 background) and the other is a knockout of fibromodulin (Chakravarti et al., 2003; Jepsen et al., 2002; Svensson et al., 1999) (designated FM KO and produced in CD1 mice). EH mice have abnormal biomechanical responses in major connective tissues, such as arterial walls (Carta et al., 2009). Fibromodulin is a small interstitial proteoglycan thought to participate in the assembly of the extra-cellular matrix as it interacts with type I and type II collagen fibrils and inhibits fibrillogenesis in vitro. It is reported to regulate TGF-β activity (Kalamajski and Oldberg, 2007). The FM KO mice have thinner sclera and smaller collagen fibril diameter, potentially altering their susceptibility to IOP-induced damage. In the FM KO strain, we studied mice at two distinct ages. Neither strain, EH or FM KO, showed significantly different RGC loss from their wild type controls, but there was significantly greater damage in older than in younger CD1 mice – the opposite of our prior finding in B6.
2. Methods
2.1. Animals
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, using protocols approved and monitored by the Johns Hopkins University School of Medicine Animal Care and Use Committee. Wild type (WT) CD1 albino mice were obtained from Charles River Laboratories, Wilmington, MA, USA, and WT B6 mice were obtained from Jackson Laboratories, Bar Harbor, ME, USA. The fibromodulin knockout mice (FM KO) mice on a CD1 background were obtained from co-investigator, Dr. Shukti Chakravarti, and have collagen structural defects including reduced strength of skin and tendon. These animals also showed distinct sclera architecture abnormalities including a reduction of sclera thickness and a decreased number of lamellae. The lamellae which were present were less organized and defined than in WT. The absence of fibromodulin from the sclera of these animals has been confirmed by Western blot. Also noted during that analysis was a marked increase in lumican in the sclera of these animals (Chakravarti et al., 2003). A second mouse strain (on a B6 background) used were haploinsufficient for elastin (EH) and develop supravalvular aortic stenosis as well as larger than normal eyes. Northern blot analysis showed a 47% decrease in elastin mRNA in EH mice as compared to WT. Also, it was found that elastic lamellae were about 50% thinner than in WT in the aorta indicating a structural abnormality (Li et al., 1998). Masked histological analysis in the peripapillary sclera showed no difference in the circumferential pattern or content of elastin between EH and WT mice (data not shown). The homozygous knockout for elastin does not survive more than a few days after birth.
2.2. Bead glaucoma
We induced experimental glaucoma by employing a protocol previously published by this laboratory (Cone et al., 2012). First, we anesthetized the animal intraperitoneally with 50 mg/kg of keta-mine, 10 mg/kg of xylazine, and 2/kg mg of acepromazine. Prop-aracaine hydrochloride eye drops (Akorn Inc., Buffalo Grove, IL) were used for additional ocular topical anesthesia. Then, we injected 2 μl of 6 μm diameter beads (Polybead Microspheres®; Polysciences, Inc., Warrington, PA), then 2 μl of 1 μm diameter beads, followed by 1 μl of viscoelastic compound (10 mg/ml sodium hyaluronate, Healon; Advanced Medical Optics Inc., Santa Ana, CA). The injection was made with a glass cannula with tip diameter of 50 μm connected by polyethylene tubing to a Hamilton syringe (Hamilton, Inc., Reno, NV) into which all 3 components of the injection were loaded in series.
2.3. IOP Measurements
IOP measurements were taken prior to bead injection, immediately after the injection, and at 3 days, 1 week, and weekly thereafter until sacrifice 6 weeks after injection. For IOP measurements, mice were sedated with inhalation of isoflurane delivered by means of the RC2 – Rodent Circuit Controller (VetEquip, Inc., Pleasanton, CA), delivering 2.5% isoflurane in oxygen to the animal via a sealed immersion box, then through a nose cone. The TonoLab tonometer (TioLat, Inc., Helsinki, Finland) was used to perform IOP measurements. The tonometer performs 6 separate pressure measurements and then provides the mean of those measurements for optimal reproducibility. Only measurements indicated as optimal reproducibility were utilized.
2.4. Axial length/width measurements
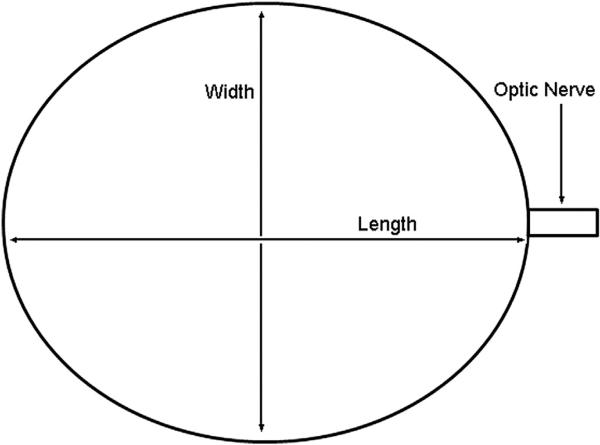
In this study, we measured length in aldehyde-fixed eyes. Normal mice and experimental glaucoma mice were sacrificed by exsanguination and perfused transcardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.2). The optic nerve was collected 1 mm distal to the globe. IOP was set at 15 mmHg with a needle connected to a fluid-filled reservoir to produce standard conditions for axial length and width measurement. The measurements were performed with a digital caliper (Instant Read Out Digital Caliper, Electron Microscopy Sciences, Hatfield, PA, USA). The axial length was measured from the center of the cornea to a position just temporal to the optic nerve insertion. The axial width was measured nasal—temporal and superior—inferior at the largest dimension at the equator, midway between the cornea and optic nerve (Fig. 1).
Fig. 1.
Axial Length and Width Measurements Schematic The axial length was measured from the center of the cornea to a position just temporal to the optic nerve insertion. The axial width was measured at the largest dimension at the equator, midway between the cornea and optic nerve, both in the superior—inferior and in the nasal—temporal directions.
2.5. Optic nerve axon count method
To quantify RGC loss, we quantified axon loss in optic nerve cross-sections using a validated sampling method (Levkovitch-Verbin et al., 2002). The optic nerves were fixed either by perfusion or immersion as described above, then post-fixed in 1% osmium tetroxide, dehydrated in alcohol, and stained with 1% uranyl acetate in 100% ethanol for 1 h. They were then embedded in epoxy resin, sectioned at 1 μm, and stained with Toluidine Blue. The cross-sections were digitally imaged (Cool Snap camera, Metamorph Image Analysis software; Molecular Devices, Downington, PA) at 10× to assess optic nerve area. Five higher power images (40 μm square, 100×) were used to measure axon density by observers masked to the protocol used for the eye. To ensure that non-axonal elements were not mistaken for axons, the high power images were individually viewed and edited to include only axon profiles. The mean axon density was multiplied by the nerve area for each sample to give total axon number. In order to yield percent axon loss, we compared this estimated total axon number for each sample against the mean of control or fellow eye axon counts.
2.6. Statistical methods
The parameters that were measured and compared in this study included IOP at baseline and during chronic glaucoma, including IOP average level, IOP exposure over time, axial lengths and widths, and axon counts. The IOP exposure over time is measured in two ways, IOP integral and positive IOP integral. The IOP integral is calculated as the area under the curve for the IOP difference between left and right eye over time. The positive IOP integral is the same area under the curve of the function of IOP difference over time, but the values included are only those in which the treated eye's IOP exceeded the untreated eye's IOP. Mean values were compared using t tests and Mann–Whitney tests. Multivariable regression models were used to adjust certain comparisons for variables of interest including age, glaucoma status, and strain.
3. Results
3.1. Elastin haploinsufficient mice and B6 controls
3.1.1. IOP and axial length
The IOP of EH and WT mice did not differ significantly, whether considered as only younger mice (3–7 months of age), older mice (11–18 months of age), or all ages combined (all p > 0.2, t test; Table 1). However, younger mice had higher IOP than older mice (EH and WT differences: p = 0.01; combined EH + WT difference: p = 0.0003). The EH mice also had similarly long eyes compared to WT when measured in aldehyde-fixed eyes, 3.51 ± 0.09 mm compared to 3.52 ± 0.12 mm (p = 0.52; n = 40 and 47 eyes).
Table 1.
IOP data in Elastin Haploinsufficient Mice and Age-Matched B6 Controls All p > 0.2 when compared separately; Data are in mmHg; N = number of mice; EH = elastin haploinsufficient; WT = matched wild type B6 mice; SD = standard deviation.
| Young |
Young |
Old |
Old |
|
|---|---|---|---|---|
| EH | WT | EH | WT | |
| Mean ± SD | 12.0 ± 2.4 | 12.6 ± 2.4 | 10.7 ± 2.8 | 11.1 ± 3.6 |
| Median | 12.0 | 13.0 | 10.0 | 10.0 |
| N | 90 | 92 | 32 | 30 |
3.1.2. Glaucoma experiments
We performed unilateral IOP elevation in the left eye of 88 EH mice at 2 months of age. Not all nerves were suitable for quantitative analysis. The untreated right eyes of young (2 month-old) EH mice had a mean RGC axon number of 45,295 ± 5189 (n = 38) while the B6 littermate controls had a mean of 42,860 ± 7291 (n = 46), a difference that was not significant (p = 0.08, t test). The mean baseline IOP was not significantly different between the two groups (p = 0.2), nor was the cumulative IOP exposure different between the groups in either mean IOP elevation, positive integral or total integral IOP (p = 0.2, 0.5, 0.4, respectively, t tests; Table 2). The increase in length and width in glaucoma eyes compared to fellow controls in both EH and B6 eyes was significant compared to their fellow, untreated eyes: length increase = 10.4% ± 6.1% (EH) and 9.1% ± 7.2% (B6) (mean ± standard deviation, p ≤ 0.001, t but the difference between EH and B6 was not significant (p = 0.5, t test). Axon loss due to glaucoma was calculated as percent decrease compared to control. The EH mice lost 8.9 ± 13.7%, while B6 lost 6.7 ± 20.6% (p = 0.57, t test). In a multivariable model with percent axon loss as dependent variable and mouse type (EH vs B6) and IOP positive integral as independent variables, there was no significant difference between EH and B6 in percent axon loss (r2 = 0.028, p = 0.48). The older EH mice did not undergo chronic glaucoma testing.
Table 2.
Elastin Glaucoma Data N = number of mice; SD = standard deviation; IOP = intraocular pressure; EH = elastin haploinsufficient; WT = wild type B6 mice; # = number; IOP integral = Positive IOP Integral; % Axon Loss = compared to pooled, untreated and matched controls.
| Control IOP (mmHg) | IOP increase (mmHg) | IOP integral (mmHg-days) | Control length (mm) | Length increase (mm) | Control axon# | Experimental axon# | % Axon Loss | ||
|---|---|---|---|---|---|---|---|---|---|
| EH | Mean ± SD | 13.7 ± 1.2 | 6.1 ± 4.5 | 135 ± 150 | 3.51 ± 0.09 | 0.38 ± 0.24 | 45,295 ± 5190 | 41,283 ± 6228 | 8.9 ± 14.0 |
| N = 42 | Median | 13.7 | 5.3 | 76 | 3.5 | 0.38 | 45,319 | 41,414 | 8.6 |
| WT | Mean ± SD | 14.3 ± 1.3 | 6.8 ± 4.1 | 162 ± 161 | 3.52 ± 0.12 | 0.37 ± 0.29 | 42,860 ± 7291 | 39,977 ± 8834 | 6.7 ± 20.6 |
| N = 46 | Median | 14.3 | 6.5 | 95 | 3.5 | 0.4 | 42,279 | 41,876 | 2.6 |
| p = 0.2 | p = 0.2 | p = 0.4 | p = 0.52 | p = 0.86 | p = 0.08 | p = 0.4 | p = 0.57 |
3.2. Fibromodulin knockout mice and CD1 littermate controls
3.2.1. IOP and axial length
Mean IOP was slightly lower in FM KO than in WT overall (p = 0.04, t test; Table 3). Older mice (11– to 18 months of age) had a lower mean IOP than younger mice (3–7 months of age) in FM KO, WT and combined FM KO + WT data (all p < 0.0001, t test). The mean IOP at baseline in the sample of older (12-month old) FM KO animals was significantly lower than both the equal-aged WT and the 2 month FM KO (both p < 0.001, t test; Table 4). The mean increase in IOP and the two measures of exposure to IOP over time (positive and total integral IOP) were not significantly different between FM KO and WT after experimental glaucoma (p = 0.8, 0.3, and 0.1, respectively, t test). The mean IOP increase between older FM KO and WT age-matched control mice was not significantly different (p = 0.4), and there was no significant difference in positive or total integral IOP between older FM KO and older WT (p = 0.7, 0.9, respectively).
Table 3.
Intraocular Pressure in Fibromodulin Mice N = number of mice; FM KO = fibromodulin knockout; WT = littermate wild type CD1 mice; SD = standard deviation; data are in mmHg.
| Young |
Young |
Old |
Old |
|
|---|---|---|---|---|
| FM KO | WT | FM KO | WT | |
| Mean ± SD | 13.0 + 2.0 | 13.4 + 2.3 | 9.8 + 3.1 | 11.4 + 3.3 |
| Median | 13.0 | 13.0 | 8.5 | 11.0 |
| N | 86 | 86 | 42 | 42 |
Table 4.
Fibromodulin Glaucoma Experiment N = number of mice; SD = standard deviation; IOP = intraocular pressure; FM KO = fibromodulin knockout mice; WT = littermate wild type CD1 mice; # = number; IOP integral = Positive IOP Integral; % Axon Loss = compared to pooled, untreated and matched controls.
| Control IOP (mmHg) | IOP increase (mmHg) | IOP integral (mmHg-days) | Control length (mm) | Length increase (mm) | Control axon# | Experimental axon# | % Axon Loss | ||
|---|---|---|---|---|---|---|---|---|---|
| FM KO Young | Mean ± SD | 13.7 ± 1.7 | 5.1 ± 3.0 | 91 ± 80 | 3.72 ± 0.17 | 0.22 ± 0.28 | 55,362 ± 8016 | 41,313 ± 17,211 | 24.7 ± 31.7 |
| N = 43 | Median | 14 | 5 | 68 | 3.71 | 0.24 | 55,872 | 45,129 | 18.5 |
| WT Young | Mean ± SD | 13.7 ± 1.2 | 5.9 ± 2.2 | 107 ± 73 | 3.70 ± 0.18 | 0.18 ± 0.19 | 61,188 ± 6918 | 49.476 ± 18,485 | 19.1 ± 30.2 |
| N = 43 | Median | 14 | 6 | 105 | 3.66 | 0.19 | 61,183 | 54,987 | 10.1 |
| p = 1.0 | p = 0.2 | p = 0.3 | p = 0.6 | p = 0.4 | p = 0.0006 | p = 0.03 | p = 0.4 | ||
| FM KO Old | Mean ± SD | 11.5 ± 1.6 | 7.8 ± 3.6 | 149 ± 103 | 3.9 ± 0.11 | 0.14 ± 0.14 | 59,822 ± 6970 | 33,839 ± 15,682 | 43.4 ± 26.2 |
| N = 21 | Median | 12 | 8 | 120 | 3.9 | 0.1 | 59,329 | 28,876 | 51.73 |
| WT Old | Mean ± SD | 13.5 ± 1.5 | 6.9 ± 4.6 | 133 ± 162 | 3.8 ± 0.08 | 0.17 ± 0.19 | 61,989 ± 5607 | 41,195 ± 16,639 | 33.5 ± 26.8 |
| N = 21 | Median | 13 | 6 | 74 | 3.8 | 0.1 | 61,024 | 40,627 | 34.46 |
| p = 0.0002 | p = 0.5 | p = 0.7 | p = 0.002 | p = 0.6 | p = 0.7 | p = 0.2 | p = 0.2 |
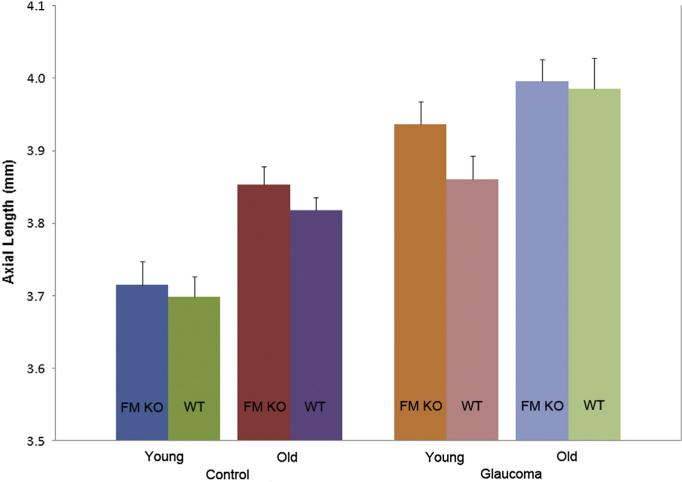
The mean axial length measured in fixed globes inflated to 15 mmHg was similar between FM KO and WT littermate mice, both at 3–7 months of age and at 11–18 months of age (p = 0.6 and 0.3, respectively; Fig. 2). Untreated older FM KO and WT mice had significantly longer eyes than younger mice of the same group (p = 0.002, 0.005, t test). After exposure to experimental glaucoma, both younger and older mice in the FM KO and in the WT groups increased axial length significantly: length increase = 5.5% ± 6.7% in FM KO and 4.6% ± 4.8% in WT (p < 0.001, t test), but there were no significant differences between the glaucoma-induced length increase in FM KO compared to WT either in younger or older animals (all p > 0.05, t test). The width data yielded similar results to those for length (data not shown).
Fig. 2.
Mean Axial Length, Fibromodulin Mice by Age and Treatment Error bars indicate standard error of the mean; FM KO = fibromodulin knockout; WT = littermate wild type CD1 mice; mm = millimeters.
3.2.2. Glaucoma experiments
Overall, the combined FM KO and WT mice in the fibromodulin group had no significant difference in RGC axon number between younger and older mice: 58,309 ± 7993 compared to 60,905 ± 6343 (p = 0.07; 86 and 42 nerves, respectively). However, the younger FM KO mice had significantly fewer RGC axons than younger WT: 55,362 ± 8016 compared to 61,188 ± 6918 (p = 0.0006, t test; n = 42 and 43 nerves). Older untreated FM KO nerves did not significantly differ from older untreated WT nerves (p = 0.2).
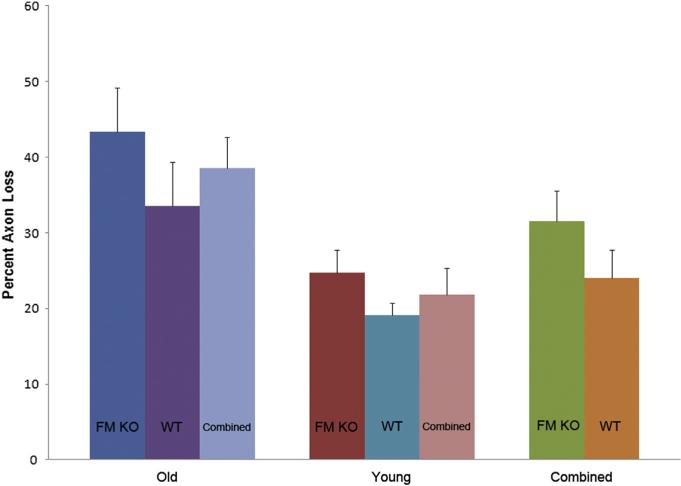
In 2 month old FM KO and littermate WT controls, 86 animals underwent exposure to chronic IOP elevation by microbead injection (Table 4). After chronic IOP increase, the FM KO group lost 25% of axons compared to fellow eyes, while the WT group lost 19% of axons compared to fellow eyes. In a multivariable model with percent axon loss as the dependent variable and mouse type (FM KO vs WT), IOP positive integral, and axial length increase as independent variables, this difference between FM KO and WT was not significant (p = 0.34; Fig. 3).
Fig. 3.
Mean Percent Axon Loss by Mouse Type and Age With Glaucoma Error bars indicate standard error of the mean; FM KO = fibromodulin knockout; WT = littermate wild type CD1 mice.
Chronic bead-induced glaucoma was produced in 42 older mice (12 months of age), equally divided between FM KO and matched WT. The untreated FM KO mice eyes had somewhat fewer RGC axons than WT control nerves, but the difference was not statistically significant (p = 0.7). After chronic IOP increase, there was highly significant loss of RGC axons in both FM KO and WT (p < 0.0001, t test). Similar to the 2 month old mice, the FM KO group had greater RGC axon loss, 43% compared to 34% in WT but the difference did not achieve statistical significance (p = 0.2).
When we compared the 12 month old to the 2 month old CD1 animals, there was a significantly greater loss in older versus younger mice in FM KO, in WT and in the combined CD1 groups (p = 0.02, 0.05 and 0.003; Mann Whitney; Fig. 3). In a multivariable model with percent axon loss as dependent variable and independent variables age and IOP exposure (positive integral), older animals showed significantly more damage than younger ones (p = 0.01, r2 = 0.07).
4. Discussion
We found that older CD1 mice suffered more RGC damage than younger mice, whether in those with knockout of the fibromodulin gene or their controls. Since many diseases are more prevalent in older persons, including both open angle and angle closure glaucoma, it might be expected that susceptibility would increase with age. Presumably, the many ocular and systemic mechanisms protecting RGC from death degrade over time. Prior to the present experiments, however, we repeatedly found that older B6 mice were paradoxically less susceptible to experimental glaucoma damage than younger B6 mice (Cone et al., 2010). Since the opposite is true in FM KO mice and WT CD1 controls, other factors must modify the effect of age on susceptibility to glaucoma among mouse strains.
Other than our own cited work, to our knowledge, there is no prior study published on susceptibility to chronic glaucoma damage by age in rats, mice, or monkeys. An unpublished ARVO presentation compared 8 month old to 28 month old Norway brown rats after hypertonic saline injection and suggested greater loss in the older group (Morrison et al., 2007). Crowston and colleagues have acutely raised IOP to 50 mmHg for 30 min and find that 18 month old B6 mice recover their retinal electrical responses more slowly than 3 month old mice, but 12 month old mice were generally similar to young mice (Kong et al., 2012). Older rats were found more susceptible to ischemic RGC damage (Kawai et al., 2001; Kim et al., 2004). On the other hand, in dissociated RGC and retinal explant experiments in rats and mice, neonatal RGC were more easily damaged than 2 month old RGC (Guerin et al., 2011; McKernan et al., 2006). This raises the possibility that the age-related sensitivity may change more than once during life, becoming less perinatally, then increasing again in old age. Furthermore, study of RGC in isolation may yield different age relationships with damage than models that involve the intact eye and animal.
While myopia is an identified risk factor for open angle glaucoma in humans, we have now studied mouse strains with longer axial lengths and compared their susceptibility to glaucoma to mice with normal eye length and width. In the present report, we confirm that young CD1 mice, whose eyes are larger than B6 mice, suffer 3 times greater loss of RGC in the glaucoma model than do B6 mice. At baseline, axial length is shortest in B6 mice, longer in CD1 (Nguyen et al., 2013), and longest in Aca23 mutants. However, Aca23 mice are quite resistant to glaucoma damage (Steinhart et al., 2012). Thus, simple eye length alone is not the dominant factor in susceptibility to experimental murine glaucoma, since the two strains that most resist damage are at opposite ends of the axial length spectrum (B6 WT and Aca23 mutant). Older mice in both B6 and CD1 strains have longer and wider eyes than younger mice (Nguyen et al., 2013). In each mouse strain, globe size enlarges with chronic glaucoma, as in infant humans (Quigley, 1977) and in experimental rats and monkeys (Yang et al., 2011). We found that axial elongation of the globe with chronic IOP elevation was significantly greater in CD1 mice than in B6 in this experiment. It seems then that mouse strains that better resist elongation during experimental glaucoma are more able to prevent RGC loss. The more resistant strains B6 and Aca23 also exhibit stiffer inflation responses in mechanical testing than CD1 mice (Nguyen et al., 2013). Also associated with greater resistance to experimental RGC damage in mice is the presence of a thicker peripapillary sclera at baseline. These findings reinforce the concept that it is the dynamic response of the eye to glaucoma that determines its effect, perhaps more than the initial state of the tissues. In addition to measuring baseline and glaucoma-induced mechanical behavior across strains, we will soon report responses of the scleral fibroblasts, fibril diameter distribution, and proteomic scleral content as potential contributors to the susceptibility to glaucoma damage among mouse strains.
We found a significantly higher IOP in untreated 10 month old B6 mice compared to 2 month old B6 mice, while there was no similar age effect on IOP in CD1 mice (Cone et al., 2012). Likewise, there are fewer RGCs at baseline in older compared to younger mice; 10 month old B6 had 8% fewer RGC somas and axons than 2 month B6, a minimal loss similar to that found by Danias et al. (2003) in B6 mice. In rats and strains of mice not included here, there are reports of modest losses of RGC with age (Cepurna et al., 2005; Neufeld and Gachie, 2003), while in humans the RGC number declines moderately to age 50, then decreases more rapidly thereafter (Jonas et al., 1992, Kerrigan-Baumrind et al., 2000; Mikelberg et al., 1989; Repka and Quigley, 1989). It is unlikely that baseline differences in IOP or RGC number relate to the difference in susceptibility by age to glaucoma damage when calculated as percent loss from baseline. However, persons who begin with fewer RGCs prior to development of glaucoma would, of course, be at greater risk to reach functional impairment, since they have less neuronal reserve.
While we have studied the connective tissues of mouse strains as potential features affecting the response to chronic IOP elevation, we recognize that there are many other potential factors that can influence susceptibility that have not yet been studied, either in standard strains or in particular genetically altered strains. We have determined that one year old CD1 mice are significantly more likely to lose RGC in experimental glaucoma than 2 month old CD1 mice. By comparison, one year old B6 mice are less susceptible to glaucoma damage than young B6. Age alone appears not to be the single dominant determining factor in glaucoma damage in mice. The age of experimental animals should be taken into account in investigations of experimental glaucoma.
Acknowledgments
This work was supported in part by PHS research grants EY 02120 and EY 01765 (Dr Quigley, and Wilmer Institute Core grant), by the research grant G 2010042 from the American Health Assistance Foundation (Dr. Nguyen), by the research grant EY 11654 (Dr. Chakravarti), and by unrestricted support from Saranne and Livingston Kosberg and from William T. Forrester. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. http://dx.doi.org/10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: concepts and applications. J. Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. http://dx.doi.org/10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. http://dx.doi.org/10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC. Premise and prediction e how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J. Glaucoma. 2007;17:318–328. doi: 10.1097/IJG.0b013e31815a343b. http://dx.doi.org/10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Wagenseil JE, Knutsen RH, Mariko B, Faury G, Davis EC, Starcher B, Mecham RP, Ramirez F. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arterioscler. Thromb. Vasc. Biol. 2009;29:2083–2089. doi: 10.1161/ATVBAHA.109.193227. http://dx.doi.org/10.1161/ATVBAHA.109.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepurna WO, Kayton RJ, Johnson EC, Morrison JC. Age related optic nerve axonal loss in adult Brown Norway rats. Exp. Eye Res. 2005;80:877–884. doi: 10.1016/j.exer.2004.12.021. http://dx.doi.org/10.1016/j.exer.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Investig. Ophthalmol. Vis. Sci. 2003;44:2422–2432. doi: 10.1167/iovs.02-0783. http://dx.doi.org/10.1167/iovs.02-0783. [DOI] [PubMed] [Google Scholar]
- Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp. Eye Res. 2010;91:415–424. doi: 10.1016/j.exer.2010.06.018. http://dx.doi.org/10.1016/j.exer.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp. Eye Res. 2012;99:27–35. doi: 10.1016/j.exer.2012.04.006. http://dx.doi.org/10.1016/j.exer.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Investig. Ophthalmol. Vis. Sci. 2012;53:1714–1728. doi: 10.1167/iovs.11-8009. http://dx.doi.org/10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1958;62:777–790. [PubMed] [Google Scholar]
- Curtin BJ. Physiopathologic aspects of scleral stress-strain. Trans. Am. Ophthalmol. Soc. 1969;67:417–461. [PMC free article] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57BL/6 mice. Investig. Ophthalmol. Vis. Sci. 2003;44:5151–5162. doi: 10.1167/iovs.02-1101. http://dx.doi.org/10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- Friberg TR, Lace JW. A comparison of the elastic properties of human choroid and sclera. Exp. Eye Res. 1988;47:429–436. doi: 10.1016/0014-4835(88)90053-x. http://dx.doi.org/10.1016/0014-4835(88)90053-X. [DOI] [PubMed] [Google Scholar]
- Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Investig. Ophthalmol. Vis. Sci. 2009;50:5226–5237. doi: 10.1167/iovs.08-3363. http://dx.doi.org/10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin MB, Donovan M, McKernan DP, O'Brien CJ, Cotter TG. Age-dependent rat retinal ganglion cell susceptibility to apoptotic stimuli: implications for glaucoma. Clin. Exper. Ophthalmol. 2011;39:243–251. doi: 10.1111/j.1442-9071.2011.02496.x. http://dx.doi.org/ 10.1111/j.1442-9071.2011.02496.x. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J. Biol. Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. http://dx.doi.org/10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Investig. Ophthalmol. Vis. Sci. 1992;33:2012–2020. [PubMed] [Google Scholar]
- Kalamajski S, Oldberg A. Fibromodulin binds collagen type I via Glu-353 and Lys-355 in leucine-rich repeat 11. J. Biol. Chem. 2007;282:26740–26745. doi: 10.1074/jbc.M704026200. http://dx.doi.org/10.1074/jbc.M704026200. [DOI] [PubMed] [Google Scholar]
- Kawai SI, Vora S, Das S, Gachie E, Becker B, Neufeld AH. Modeling of risk factors for the degeneration of retinal ganglion cells after ischemia/reper-fusion in rats: effects of age, caloric restriction, diabetes, pigmentation, and glaucoma. FASEB J. 2001;15:1285–1287. doi: 10.1096/fj.00-0666fje. http://dx.doi.org/10.1096/fj.00-0666fje. [DOI] [PubMed] [Google Scholar]
- Kim KY, Ju WK, Neufeld AH. Neuronal susceptibility to damage: comparison of the retinas of young, old and old/caloric restricted rats before and after transient ischemia. Neurobiol. Aging. 2004;25:491–500. doi: 10.1016/j.neurobiolaging.2003.07.005. http://dx.doi.org/10.1016/j.neurobiolaging.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Kong YX, van Bergen N, Bui BV, Chrysostomou V, Vingrys AJ, Trounce IA, Crowston JG. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol. Aging. 2012;33:1126.e15–1126.e25. doi: 10.1016/j.neurobiolaging.2011.11.026. http://dx.doi.org/10.1016/j.neurobiolaging.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. The number of ganglion cells in glaucoma eyes compared to threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular mesh-work as a model of glaucoma in rats. Investig. Ophthalmol. Vis. Sci. 2002;43:402–410. [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Investig. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optometry Vis. Sci. 2009;86:E23–E30. doi: 10.1097/OPX.0b013e3181940669. http://dx.doi.org/10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Caplis C, Donovan M, O'Brien CJ, Cotter TG. Age-dependent susceptibility of the retinal ganglion cell layer to cell death. Investig. Ophthalmol. Vis. Sci. 2006;47:807–814. doi: 10.1167/iovs.05-0520. http://dx.doi.org/10.1167/iovs.05-0520. [DOI] [PubMed] [Google Scholar]
- Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Cepurna WO, Jia L, Johnson EC. Characteristics of modeling pressure-induced optic nerve damage in elderly rat eyes. Investig. Ophthalmol. Vis. Sci. 2007;48 E-Abstract 3662. [Google Scholar]
- Myers KM, Cone FE, Quigley HA, Gelman SE, Pease ME, Nguyen TD. The in vitro inflation response of mouse sclera. Exp. Eye Res. 2010;91:866–875. doi: 10.1016/j.exer.2010.09.009. http://dx.doi.org/10.1016/j.exer.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, Gachie EN. The inherent, age-dependent loss of retinal ganglion cells is related to the lifespan of the species. Neurobiol. Aging. 2003;24:167–172. doi: 10.1016/s0197-4580(02)00059-3. http://dx.doi.org/10.1016/S0197-4580(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Quigley HA. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Investig. Ophthalmol. Vis. Sci. 2013;54:1767–1780. doi: 10.1167/iovs.12-10952. http://dx.doi.org/10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am. J. Ophthalmol. 1977;84:358–370. doi: 10.1016/0002-9394(77)90680-8. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch. Ophthalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman A. The number of persons with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:151–156. doi: 10.1136/bjo.2005.081224. http://dx.doi.org/10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Cone FE. Development of diagnostic and treatment strategies for glaucoma through understanding and modification of scleral and lamina cribrosa connective tissue. Cell Tissue Res. 2013;353:231–244. doi: 10.1007/s00441-013-1603-0. http://dx.doi.org/10.1007/s00441-013-1603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Achen VR, Penugonda S, Schmidt RW, Mount BA. Proteoglycan composition in the human sclera during growth and aging. Investig. Ophthalmol. Vis. Sci. 2000;41:1639–1648. [PubMed] [Google Scholar]
- Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp. Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. http://dx.doi.org/10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. Structural factors that mediate scleral stiffness. Investig. Ophthalmol. Vis. Sci. 2008;49:4232–4236. doi: 10.1167/iovs.08-1970. http://dx.doi.org/10.1167/iovs.08-1970. [DOI] [PubMed] [Google Scholar]
- Steinhart MR, Cone FE, Nguyen C, Nguyen TD, Pease ME, Puk O, Graw J, Oglesby E, Quigley HA. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol. Vis. 2012;18:1093–1106. [PMC free article] [PubMed] [Google Scholar]
- Strouthidis NG, Girard MJ. Altering the way the optic nerve head responds to intraocular pressure-a potential approach to glaucoma therapy. Curr. Opin. Pharmacol. 2013;13:83–89. doi: 10.1016/j.coph.2012.09.001. http://dx.doi.org/10.1016/j.coph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. http://dx.doi.org/10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Watson PG, Young RD. Scleral structure, organization and disease. A review. Exp. Eye Res. 2004;78:609–623. doi: 10.1016/s0014-4835(03)00212-4. http://dx.doi.org/10.1016/S0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Yang H, Thompson H, Roberts MD, Sigal IA, Downs JC, Burgoyne CF. Deformation of the early glaucomatous monkey optic nerve head connective tissue after acute IOP elevation in 3-D histomorphometric reconstructions. Investig. Ophthalmol. Vis. Sci. 2011;52:345–363. doi: 10.1167/iovs.09-5122. http://dx.doi.org/10.1167/iovs.09-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]