Abstract
Purpose
Romidepsin is a potent histone deacetylase inhibitor (HDI) with activity in T-cell lymphoma. Given preclinical data demonstrating greater induction of gene expression with longer exposures to HDIs, a phase I study of a days 1, 3, and 5 romidepsin schedule was evaluated. A secondary objective was to assess the effect of romidepsin on radioactive iodine (RAI) uptake in thyroid cancers.
Experimental Design
Open label, single arm, phase I, 3 + 3 dose escalation study. Romidepsin was administered as a four-hour infusion on days 1, 3 and 5 of a 21-day cycle. Pharmacokinetics (PK) and pharmacodynamics (PD) were assessed, including histone acetylation in peripheral blood mononuclear cells (PBMCs); RAI uptake in refractory thyroid cancer; and HDI-related ECG changes.
Results
28 patients with solid tumors, including eleven patients with thyroid cancer were enrolled. Six dose levels were explored and 7 mg/m2 on days 1, 3, and 5 was identified as tolerable. No RECIST-defined objective responses were recorded although nine patients had stable disease a median 30 weeks (range 21 – 112) including six with thyroid cancer a median of 33 weeks. PD studies detected acetylated histones in PBMCs and ECG changes beginning at low dose levels. Follow-up RAI scans in patients with RAI refractory thyroid cancer did not detect meaningful increases.
Conclusions
A romidepsin dose of 7 mg/m2 administered on days 1, 3, and 5 was found tolerable and resulted in histone acetylation in PBMCs. Although there were no objective responses with romidepsin alone, this schedule may be useful for developing combination studies in solid tumors.
Keywords: phase I clinical trial, romidepsin, histone deacetylase inhibitor, cancer
INTRODUCTION
Romidepsin (FK228, FR901228, NSC630176, depsipeptide) is a potent, natural product histone deacetylase inhibitor (HDI) (1, 2). HDIs prevent the activity of histone deacetylases (HDACs), leading to unrestricted histone acetyltransferase activity and increased gene transcription (3). HDI exposure effects a global increase in histone acetylation as well as gene expression changes that lead to p21 induction and cell cycle arrest (4); increased expression of markers of differentiation such as fetal hemoglobin, P-glycoprotein, and the sodium-iodide symporter (5–7); and alterations in the expression of apoptotic proteins (8). The mechanism of action of HDIs is likely to be model specific, as several mechanisms have been suggested (9).
Romidepsin is effective in T-cell lymphoma. In two Phase II trials, 167 patients with cutaneous T-cell lymphoma (CTCL) treated with romidepsin had a 34–35% overall response rate (ORR), supporting approval in CTCL (10, 11). Similarly, an ORR of 25–38% in peripheral T-cell lymphoma (PTCL) in two Phase II trials supported approval for that indication (12, 13). Despite the clinical success of romidepsin in T-cell lymphoma, it has been disappointing that clinical trials conducted in solid tumors have failed to show significant clinical activity (14–18). The reason for this lack of activity is not well understood and studies that define mechanisms of resistance to HDIs are still early. It has been suggested that increased thioredoxin levels, increased levels of antiapoptotic proteins such as Bcl-XL or Bcl-2, or increased MAPK signaling may play a role (3, 19–21).
One potential strategy for developing romidepsin, or other HDIs in solid tumors is to exploit the “differentiation” capacity of the agents. While synergy can be observed between HDIs and cytotoxic agents (22–24), another approach is to use the HDIs to amplify a therapeutic target via their ability to increase gene expression. We previously demonstrated in preclinical studies that the sodium-iodide symporter (NIS), expressed at high levels in the normal thyroid, is induced in thyroid cancer cells with exposure to nontoxic doses of romidepsin for 48 – 72 hours (7). In vitro, romidepsin induced expression of thyroglobulin and NIS in both differentiated and anaplastic thyroid cancer cell lines. In turn, this increased radioactive iodine uptake suggesting that romidepsin could augment or induce radioactive iodine uptake in patients with thyroid cancer.
Earlier phase I studies of romidepsin tested two schedules of administration of romidepsin - day 1 and 5 every 21 days and day 1, 8, and 15 every 28 days; more dose intense schedules, such as daily dosing, were not developed due to greater toxicity in preclinical models. Because the day 1, 8, and 15 schedule that is active in T-cell lymphoma has not shown significant clinical activity in solid tumors, we sought an alternate schedule that might be more readily combined with other therapies and that would provide more continuous HDAC inhibition, even if for a limited time. We thus conducted an open label single arm phase I escalation study to establish the MTD and associated toxicities of romidepsin when administered as a four-hour infusion on days 1, 3 and 5 of a 21-day cycle. With the short half-life of romidepsin, no accumulation of the drug would be expected, but we hoped the more frequent exposure would augment the epigenetic effects of the agent and thereby increase NIS function and radioiodine uptake in thyroid cancers. Histone acetylation, ECG changes, and uptake of radioactive iodine in thyroid cancer were examined as pharmacodynamic markers of romidepsin effect.
PATIENTS AND METHODS
Patient Eligibility Criteria
The study was approved by National Cancer Institute Intuitional Board Review, and registered at www.clinicaltrials.gov NCT00048334. All patients were required to give a written informed consent. Eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤2, measurable disease, and an ejection fraction of >50% by echocardiogram or cardiac MRI, or 45% by MUGA scan. Patients with cardiac risk factors were excluded. Patients with thyroid cancer could not have medullary histology and had to have evidence of no or minimal (“faint”) radioactive iodine (RAI) uptake on RAI whole body scan, no RAI therapy within 3 months prior to study entry, and no history of administration of IV iodinated contrast or other large iodine loads (i.e. CT, amiodarone, Super Saturated Potassium Iodide (SSKI)) during the previous 3 months.
Trial Design and Dose Escalation
This was an open label, single arm, phase I, 3+3, dose escalation trial; infusion doses ranged from 1 to 9 mg/m2. Romidepsin was administered as a four-hour infusion on days 1, 3 and 5 of a 21-day cycle. Dose modifications and dose escalation beyond cycle 1 were allowed. MTD was to be defined as the highest dose level that resulted in a dose limiting toxicity (DLT) in fewer than 2/6 patients.
Toxicity Evaluation
All adverse events in this trial were graded using the NCI Common Toxicity Criteria version 2.0. DLT was defined as a hematologic toxicity of absolute granulocyte count (AGC) of <500 (Grade 4) for ≥5 days; platelet count of <10,000 (Grade 4) (both in patients without bone marrow involvement); Grade 3 or greater non-hematologic toxicity in patients without disease involvement of that particular organ system, excluding potassium (K), magnesium (Mg), calcium, phosphate, uric acid, nausea and vomiting. The latter were considered DLT if scored as grade 4 or if occurring despite maximal prophylaxis.
Efficacy Evaluation
Disease assessments (imaging) were performed every two cycles (each cycle being 21 days). The primary efficacy measure was objective disease response (complete responses + partial responses) according to the Response Evaluation Criteria in Solid Tumors Version 1 (RECIST v.1).
Cardiac Evaluation
Serum electrolytes (K, and Mg) were checked prior to treatment and repleted if K <4.0 mmol/L or Mg <0.85 mmol/L (25). Standard 12-lead electrocardiograms (ECGs) were obtained prior to the first dose of each cycle, within 1 hour before, 4 hours after each infusion, and on the day after each infusion. ECG abnormalities were assessed according to the AHA/ACCF/HRS recommendation for the standardization and interpretation of the electrocardiogram (26). T wave and ST segment abnormalities were graded based on definitions in the NCI Common Toxicity Criteria, version 2: grade 0 was a normal ECG, grade 1 was defined as nonspecific T-wave flattening or changes, and grade 2 was defined as ST segment or T wave changes suggestive of ischemia.
Pharmacokinetics
Blood for pharmacokinetic analysis was collected prior to the dose, immediately prior to the end of the 4-hr infusion, and 0.5, 7, and 14 hours post-infusion. Plasma was stored frozen at −80 °C until analysis. Plasma concentrations of romidepsin were determined by liquid chromatography with mass spectrometric (LC/MS) detection with a lower limit of quantitation of 2 ng/ml (27). Pharmacokinetic parameters were obtained using WinNonlin v5 (Pharsight Corp, Mountain View, CA), as previously reported.
Pharmacodynamics
Heparinized blood was obtained: prior to treatment (days 1, 3, and 5); at the end of infusion (days 1 and 5); and on the day following the last infusion. Histone acetylation was measured in peripheral blood mononuclear cells (PBMCs), using an immunodot-blot method previously validated (28). Radiolabeled sodium iodide (RAI) scans were performed in patients with thyroid cancer. Scans were performed using standard 2 mCi doses of 123I at baseline, and if disease was stable, after the third cycle and thereafter every 2 or 3 cycles.
RESULTS
Romidepsin Dose Escalation and Safety
Twenty-eight patients with solid tumors were enrolled. Table 1 summarizes their baseline characteristics. Patients had multiple prior therapies: 10 had four or more regimens; among 11 patients with thyroid cancer, 8 had two or more radioiodine ablations. Cycle 1 toxicities, shown in Table 2, were similar to those described in the previous phase I trial of romidepsin (29). Grade 3 toxicities included leucopenia (2), lymphopenia (4), neutropenia (2), thrombocytopenia (2), anorexia (4), nausea (3), and vomiting (2). Few grade 4 toxicities were observed, none in cycle 1. Of note, hematologic toxicities were transient and there were no episodes of febrile neutropenia.
Table 1.
On-study characteristics, gender, age, performance status and tumor types
| Baseline Characteristics (N= 28) | Patients, n (%) | |
|---|---|---|
| Mean age, years | 56.6 | |
| Prior therapy* | 28 (100) | |
| ≥ 4 systemic therapies | 10 (36) | |
| Performance Status | ||
| ECOG 0 | 3 | |
| ECOG 1 | 24 | |
| ECOG 2 | 1 | |
| Sex | ||
| Male | 17 (60.7) | |
| Female | 11 (39.3) | |
| Primary disease site | ||
| Thyroid | 11(39) | |
| Kidney | 7(25) | |
| Adrenal | 3(11) | |
| Lung | 2(7) | |
| Skin | 2(7) | |
| Cervical | 1(4) | |
| Ovarian | 1(4) | |
| Prostate | 1(4) | |
10/11 patients with thyroid cancer had prior 131I radioiodine, and 8/11 had ≥ 2 ablations
Table 2.
Adverse events reported in Cycle 1 and occurring at all dose levels in greater than 10% of patients*
| Adverse Event in C1 | # pts (%) | # pts Gr1 (%) | # pts Gr2 (%) | # pts Gr3 (%) |
|---|---|---|---|---|
| Clinical AE's | ||||
| Nausea | 17 (61%) | 10 (36%) | 4 (14%) | 3 (11%) |
| Anorexia | 14 (50%) | 8 (29%) | 2 (7%) | 4 (14%) |
| ECG Changes | 13 (46%) | 13 (46%) | 0 | 0 |
| Vomiting | 9 (32%) | 6 (21%) | 1 (4%) | 2 (7%) |
| Fatigue | 8 (29%) | 4 (14%) | 4 (14%) | 0 |
| Headache | 9 (32%) | 9 (32%) | 0 | 0 |
| Laboratory AE's | ||||
| Leukopenia | 11 (39%) | 1 (4%) | 8 (29%) | 2 (7%) |
| Thrombocytopenia | 13 (46%) | 10 (36%) | 1 (4%) | 2 (7%) |
| Neutropenia | 9 (32%) | 0 | 7 (25%) | 2 (7%) |
| Hypoalbuminemia | 10 (36%) | 3 (11%) | 6 (21%) | 1 (4%) |
| Hemoglobin | 11 (39%) | 7 (25%) | 4 (14%) | 0 |
| Lymphopenia | 10 (36%) | 3 (11%) | 1 (4%) | 6 (21%) |
| Hypocalcemia | 5 (18%) | 2 (7%) | 3 (11%) | 0 |
| Bilirubin | 5 (18%) | 5 (18%) | 0 | 0 |
| Hyponatremia | 5 (18%) | 5 (18%) | 0 | 0 |
| SGOT | 3 (11%) | 2 (7%) | 1 (4%) | 0 |
| Creatinine | 3 (11%) | 3 (11%) | 0 | 0 |
| ALK | 3 (11%) | 3 (11%) | 0 | 0 |
| Hypomagnesemia | 3 (11%) | 2 (7%) | 0 | 1 (4%) |
Clinical findings and laboratory abnormalities were reported as toxicities, regardless of clinical significance (n = 28)
Dose escalation proceeded according to protocol guidelines (Supplementary Table 1), with dose-limiting toxicities defined in the first cycle. Six dose levels were explored and 414 doses administered. Dose level 2 (2 mg/m2) was expanded after a patient with advanced renal cell cancer experienced grade 3 hypoxia and grade 3 atrial fibrillation, thought due to disease progression and increased pleural effusion. No additional DLTs were observed at the 2 mg/m2 dose level. Grade 3 nausea and vomiting observed on dose level 4 (5 mg/m2) was not considered dose-limiting because it corrected with antiemetic therapy. However, at dose level 6 (9 mg/m2), one patient experienced difficult-to-treat grade 3 nausea and anorexia and this prompted the enrollment of an additional six patients at this dose level. While no other patient had a DLT at 9 mg/m2, we did not attempt further dose escalation because the toxicities were consistent with those observed in patients treated with the approved romidepsin dose and schedule, and because most patients did not tolerate prolonged dosing at 9 mg/m2 (Table 3 and Supplementary Table 2). Specifically, 7 of 9 patients enrolled at 9 mg/m2 required dose reduction; completing eighteen cycles at 9 mg/m2 and twenty cycles at 7 mg/m2. Although a recommended phase II dose (RP2D) was not a defined protocol endpoint, we concluded that 7 mg/m2 with the option to increase to 9 mg/m2 if tolerable would be considered a RP2D.
Table 3.
Dose increase or reduction in patients enrolled at 7 and 9 mg/m2 romidepsin on day 1, 3, and 5 schedule
| Patient | Diagnosis | Entry Dose |
# Cycles at Entry Dose |
Dose Change [Cycle/Day] |
Final Dose |
# Cycles at New Dose |
AE Prompting Dose Change |
|---|---|---|---|---|---|---|---|
| 17 | Thyroid | 7 mg/m2 | 3 | C4D1 | 9 mg/m2 | 6 | -- |
| 18 | Thyroid | 7 mg/m2 | 1 | C2D1 | 9 mg/m2 | 9 | -- |
| 19 | Lung | 7 mg/m2 | 6 | C7D1 | 5 mg/m2 | 1 | G2 N&V 17d |
| 20 | Prostate | 9 mg/m2 | 1 | C2D1 | 7 mg/m2 | 1 | G1 LFT's and fever |
| 21 | ACC | 9 mg/m2 | 4 | C5D1 | 7 mg/m2 | 4 | G3/4 Platelets, 11d |
| 22 | Cervix | 9 mg/m2 | 1 | Off-study | -- | -- | G1 Anorexia, fatigue 37d |
| 23 | Ovarian | 9 mg/m2 | 1 | C2D1 | 7 mg/m2 | 1 | G3 N&V |
| 24 | Thyroid | 9 mg/m2 | 1 | Off-study | -- | -- | G1 Anorexia, 7d |
| 25 | ACC | 9 mg/m2 | 4 | C5D1 | 7 mg/m2 | 3 | G3 N&V |
| 26 | Thyroid | 9 mg/m2 | 1 | C2D1 | 7 mg/m2 | 8 | G2 Nausea 24d |
| 27 | Thyroid | 9 mg/m2 | 6 | C7D1 | 7 mg/m2 | 2 | G3 Atrial fibrillation |
| 28 | Thyroid | 9 mg/m2 | 1 | C2D1 | 7 mg/m2 | 1 | G2 Fatigue 23d |
Efficacy
Although no patient met criteria for RECIST-defined objective response, nine patients had stable disease a minimum of six cycles (18 weeks) with a median of 30 weeks (range 18–112) including six with thyroid cancer with a median of 33 weeks (range 26–112). The outcome in patients with thyroid cancer is summarized in Table 4. Three patients were considered non-evaluable: one had hypoxia, atrial fibrillation and disease progression (#6); one refused further therapy (#22); and another developed a thrombus at the site of the PICC line and refused further therapy (#24).
Table 4.
Outcome for patients with thyroid cancer enrolled on-study
| Pt | Classification | Variant | Prior Radiotherapy, 131I Ablations |
Time On Study (weeks) |
Best Response | RAI Scan Obtained (weeks)a |
Result |
|---|---|---|---|---|---|---|---|
| 1 | Papillary | Tall cell variant | RAI x2, EBb | 10 | PD | PD after cycle 3c | -- |
| 2 | Papillary | Follicular variant | RAI x2, EB | 12 | PD | NDd | -- |
| 5 | Papillary | Tall cell variant | RAI x3 | 112 | SD | 28, 53, 103 | Neg |
| 10 | Papillary | RAI x2 | 39 | SD | 36 | Faint | |
| 11 | Papillary | Tall cell, poorly differentiated | EB only | 3 | PD | PD after cycle 1c | -- |
| 17 | Follicular | Hürthle cell, poorly differentiated | RAI x1, EB | 33 | SD | 31 | Neg |
| 18 | Papillary | Tall cell, with Hashimoto’s thyroiditis |
RAI x1, EB | 33 | SD | 14, 30 | Neg |
| 24 | Papillary | RAI x3 | 3 | NE | Off Study after cycle 1e | -- | |
| 26 | Follicular | Hürthle cell | RAI x3 | 30 | SD | 16, 31 | Neg |
| 27 | Follicular | Hürthle cell | RAI x3 | 27 | SD | 14 | Faint |
| 28 | Papillary | RAI x3, EB | 6 | PD | PD after cycle 2c | -- |
Time when RAI scan obtained after study enrollment.
EB, External Beam radiotherapy also administered
RAI scans were not performed in patients with disease progression.
ND, Not done. Patient with abdominal mass unable to tolerate low iodine diet.
Patient refused further therapy
Pharmacokinetics
Romidepsin phamacokinetics demonstrated rapid clearance at all dose levels. Figure 1A depicts the log10-transformed mean plasma concentration vs. time (C X T) data at each dose level for cycle 1, day 1 (C1D1; n=28). Romidepsin demonstrated biphasic elimination, characterized by a fast distribution phase followed by a slower terminal elimination phase. Both CMAX and exposure (AUCLAST) increased with dose, suggesting linear pharmacokinetics (Figure 1B and 1C). In most patients, plasma romidepsin concentrations were below the lower limit of quantitation 14 hours after completing the infusion, preventing accurate calculations of terminal elimination rates (λZ), AUCINF, volume of distribution and clearance.
Figure 1.
Pharmacokinetics of romidepsin. The area under the concentration-time curves up to the last quantifiable point (AUCLAST) was calculated in WinNonlin v5 using the linear trapezoidal rule. Maximum plasma concentrations (CMAX) at the end of the 4-hr infusion were recorded as observed values. (A) Log-transformed C1D1 concentration-time profiles of all 28 patients. Relationship between dose and (B) CMAX or (C) exposure, defined as AUCLAST. A linear regression analysis was performed to determine the significance of the linear relationship. (D) Dose proportionality of romidepsin in dose-normalized romidepsin plasma exposure on C1D1, C1D3, and C1D5.
Mean cycle 1 pharmacokinetic parameters at each dose level were determined (Supplementary Table 3). Inter-day patient variability (%CV) for 27 of the 28 patients over days 1, 3, and 5 of cycle 1 ranged from 5–68% for CMAX and 2–59% for AUCLAST. Intra-patient comparisons of day 1 and day 3 or 5 showed no significant change in plasma exposure (Friedman Test, p=0.29) (Figure 1D); thus, as expected, romidepsin did not accumulate on this dosing schedule (29).
Pharmacodynamics
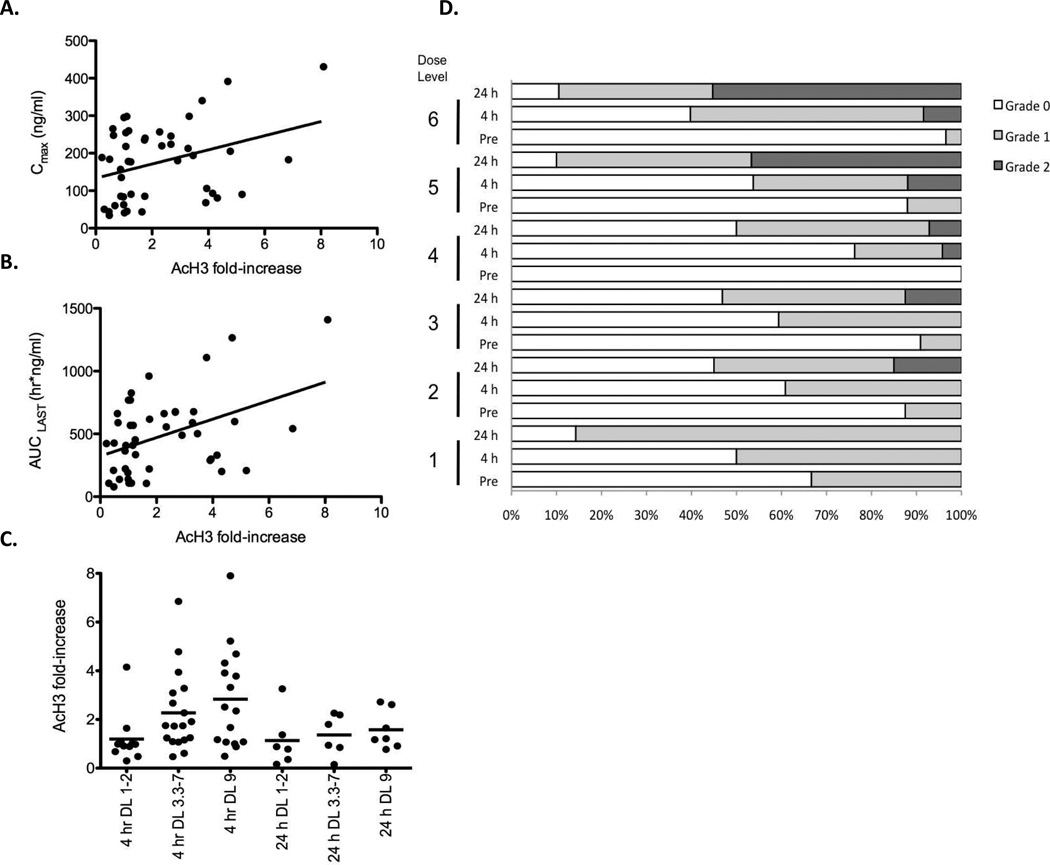
Evaluation of histone acetylation in PBMCs has been used as a surrogate marker to confirm that romidepsin or other HDIs block deacetylase activity (28, 30–34). Using a previously validated histone acetylation assay in PBMCs (28) we examined samples collected before and after each dose, and 24 hours after the final dose. As shown in Figure 2A and 2B, comparing the 4hr post-romidepsin samples in cycle 1 with blood concentrations (n = 45), a modest correlation with romidepsin exposure (Spearman, r = 0.34, p = 0.024) was observed for the 24 hour samples in the Phase II study. Fold-increases in acetylation over pre-dose levels are grouped according to cohort in Figure 2C. The data suggested an apparent threshold at 3.3 mg/m2, and no accumulation in acetylation over the 5 days of treatment.
Figure 2.
Pharmacodynamic endpoints of romidepsin. The fold-increase in acetylated histone H3 at the 4 h and 24 h timepoints compared to baseline in patient PBMCs was determined by an immunodot-blot. A linear regression analysis was used to detemine the relationship between fold-increase and (A) Cmax or (B) AUCLAST. (C) Fold-increase relative to baseline in acetylated histone H3 at the 4 and 24 h time points grouped by dose level as noted. Bar represents median value. Five of six patients studied at dose level 1 or 2 had no measurable increase in AcH3 (although increases over 2-fold were noted in these patients on subsequent cycles when higher doses were administered, data not shown). In contrast, 14 of 16 patients treated at 3.3 mg/m2 or greater had > 2-fold increase in histone acetylation. (D) Stacked bar graph denoting incidence of ST segment and T wave changes in 650 ECGs obtained in 120 cycles, according to dose administered. These are a subset of the ECGs reported in Supplementary Table 4. ECG data from patients whose dose was escalated to a higher dose level or de-escalated to a lower dose level than their entry dose are included at the actual dose administered, not at the entry dose. “Pre” indicates ECGs obtained prior to the first dose of a cycle, “4 h” and “24 h” indicate ECGs obtained at infusion end or on the following day, respectively, on days 1, 3 or 5. White segment depicts grade 0, gray segment depicts grade 1 and black segment depicts grade 2 ST and T wave changes.
Follow-up RAI scans were performed in six of eleven patients with thyroid cancer. None demonstrated significant uptake, although disease was detectable on FDG-PET scan (Table 3). Two patients showed faint or trace uptake in the post-romidepsin scans, one in mid-lung and one in the hilum, but neither increase was sufficient to merit therapy with radioactive iodine.
Another effect of romidepsin that can be viewed as a pharmacodynamic marker is reversible ST-T wave flattening and inversion without associated evidence of ischemia (10, 13, 35). Figure 2D plots a subset of and Supplementary Table 4 lists the graded ST segment and T wave changes in 905 ECGs obtained in 120 cycles. Although grade 1 ST and T wave changes were observed in some pre-romidepsin ECGs, the frequency increased after romidepsin administration. At dose levels above 5 mg/m2, grade 2 changes were observed frequently, demonstrating that ST-T wave changes occurred commonly and increased with increasing dose.
DISCUSSION
Promising pre-clinical data for HDIs and a mechanistic rationale prompted us to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of a new schedule of romidepsin administered on days 1, 3, and 5 of a 21-day cycle. We wished to explore a schedule that would provide more continuous HDAC inhibition and be easier to use in combinations. The cycle one toxicities included fatigue, nausea, vomiting, anorexia, and cytopenias. Dose escalation was discontinued when 9 mg/m2 was reached, as 7 of 9 patients enrolled at that dose level ultimately required a dose reduction to 7 mg/m2, mainly for prolonged grade 2 toxicities. We concluded that 7 mg/m2 could be sustained and can be considered, with dose escalation as tolerable, as the recommended Phase II dose for inclusion in combination therapies.
The data suggest that, relative to the day 1, 8, and 15 schedule, the day 1, 3 and 5 schedule gives comparable dose-normalized pharmacokinetic effects, and comparable pharmacodynamic effects including similar effects on histone acetylation and EGC changes. In the day 1, 8 and 15 schedule we found a median increase in histone acetylation in PBMCs of 3-fold at four hours, 1.85-fold at 24 h and 1.46-fold at 48 h (28). In the current study at the 4h time-point, an increase in histone acetylation of 2-fold or greater was consistently observed in PBMCs obtained from patients treated with a dose of 3.3 mg/m2 or higher; with a median 3-fold increase over baseline at 9.9 mg/m2. Furthermore, the data suggest an apparent threshold at 3.3 mg/m2, and no accumulation in acetylation over the 5 days of treatment. This magnitude of histone acetylation in PBMCs is comparable to that reported with other HDIs (31–34) and suggest histone acetylation may be a relatively sensitive indicator of drug effect, but that there may be a plateau in the magnitude of global histone acetylation induced by HDI therapy.
We also utilized the ECG changes, a class effect of the HDIs developed to date, as a pharmacodynamic marker (36). Grade 1 changes were noted from the first dose level onward, and Grade 2 changes were observed at the 24-hour timepoint in over half of patients treated at dose level 5 (7 mg/m2) and onward. This frequency is consistent with, if not greater than, that observed on the day 1, 8, and 15 schedule (35).
On the day 1, 8, and 15 schedule, there was evidence of a romidepsin-mediated differentiating effect with induction of fetal hemoglobin over time (28). However, in vitro studies suggested lower doses/longer exposures were needed to optimize the differentiating effects of HDIs. These in vitro studies also showed good induction of NIS in thyroid cells (7). Thus we had hoped that the day 1, 3 and 5 schedule of romidepsin would induce NIS in thyroid cancer cells leading to improved RAI uptake (7). However, serial RAI scans in six of our eleven patients with thyroid cancer did not demonstrate a significant increase in RAI uptake, with only small increases in areas of lung metastases in two patients after romidepsin. Thus we were unable to confirm the hypothesis that romidepsin would induce NIS in thyroid cancer. However, we would note that a recent report showed an increase in RAI avidity in 2 of 16 patients scanned following romidepsin (37) and that an earlier report described a patient in whom RAI in tumor tissue increased following vorinostat (38). We would note that the in vitro studies suggested longer exposures were needed to optimize the differentiating effects and that the day 1, 3 and 5 romidepsin schedule leaves two weeks in each cycle without histone deacetylase inhibition. The schedule was selected in part because the safety of romidepsin doses in close approximation was not known. And while the safety of administering romidepsin on alternating days was demonstrated, daily administration of an HDI orally or subcutaneously might be better at achieving the longer exposure that may be required for gene induction.
We would also note that eight of the eleven patients with thyroid cancer enrolled on this study had variant subtypes that often lose radioiodine uptake, a manifestation of de-differentiation. This patient population was selected because treatment options are lacking and radioiodine is inactive in these patients. However, inclusion of these tumor types may not have allowed a fair test of the hypothesis that HDIs can increase or reexpress NIS in thyroid cancer. The hypothesis should be tested in cancers with reduced rather than absent uptake of radioiodine, and not in a Phase I setting.
Laboratory and clinical observations suggest the activities of romidepsin and other HDIs can be divided into two classes. One is rapid induction of apoptosis that seems likely to be due to acetylation and replication-mediated DNA damage with an acute change in proliferation signaling (39). The other is the gene induction and differentiation effect that constituted some of the earliest observations with this class of agents. Recent studies in our laboratory suggest the susceptibility of T-cell lymphomas results from apoptosis induction rather than differentiation, a finding consistent with the rapid destruction of malignant Sezary cells in treated patients (13, 19). If this hypothesis is correct, the tested schedule would not provide additional benefit in the T-cell lymphoma setting, and we would not recommend its study in T-cell lymphoma. This may also explain why the “low dose/longer exposure” schedule inherent in the oral daily dosing of vorinostat does not have increased efficacy over romidepsin in T-cell lymphoma.
But the question that remains is how best to attempt to exploit HDIs in the therapy of solid tumors. Numerous ongoing trials combine HDIs with other anticancer agents (http://www.clinicaltrials.gov). Some trials attempt to exploit the ability of HDIs to relax chromatin or impair the DNA damage response, so as to increase the access and activity of drugs that target DNA. Other trials exploit the differentiating activities of HDIs to alter target expression, just as we attempted to do with the NIS in the study reported here. However, it is increasingly apparent that HDACs work in concert with histone methyltransferases or DNA methyltransferases to induce gene silencing and that this may constrain the response of genes to HDIs. In this regard we would note observations in clinical samples that the NIS promoter is frequently methylated in thyroid cancer, and that this may be associated with loss of mRNA expression and absence of radioiodine uptake (40, 41). Consequently, clinical approaches that attempt to alter gene expression will likely require a combined epigenetic approach, that administers an HDI with a demethylating agent or with novel agents in development such as inhibitors of the H3K27 methyltransferase, EZH2 (42). We feel that the schedule reported here is both safe and tolerable and could lend itself to such a combined epigenetic approach.
Supplementary Material
Statement of Translational Relevance.
Romidepsin has clinical activity in T-cell lymphomas but is minimally active in solid tumors. Like other histone deacetylase inhibitors, romidepsin activity in cancer cells can be classified as either pro-apoptotic or as differentiation induction. We had previously shown in pre-clinical studies that prolonged exposure of thyroid cancer cell lines with low, non-toxic doses of romidepsin induced expression of thyroglobulin and the sodium/iodide symporter (NIS). This resulted in an enhanced uptake of radioactive iodine that could potentially re-sensitize thyroid cancers to radioiodine. In this dose escalation phase I study, we assessed the safety and efficacy of a new schedule of romidepsin in patients with solid tumors, with the goal of using romidepsin to induce expression of targets for anticancer agents, such as the sodium iodine symporter to increase uptake of radioiodine in patients with thyroid cancer.
Acknowledgements
This work has been supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by a CRADA between the NCI and Celgene Corporation. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
We would like to thank Seth Steinberg and David Venzon for advice regarding biostatistics, Shawn Spencer for help with pharmacology analysis, Zhirong Zhan for clinical sample management, and Clara Chen for advice regarding nuclear medicine studies.
Footnotes
Conflicts of Interest: Dr. Susan E. Bates received research funding from Celgene Corporation through a Cooperative Research and Development Agreement with the National Cancer Institute. The other authors have no conflicts of interest to declare and no financial interests to report.
REFERENCES
- 1.Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968, on Ha-ras transformed NIH3T3 cells. Biosci Biotech Biochem. 1994;58:1579–1583. doi: 10.1271/bbb.58.1579. [DOI] [PubMed] [Google Scholar]
- 2.Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama M, Nakajima H, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 3.Robey RW, Chakraborty AR, Basseville A, Luchenko V, Bahr J, Zhan Z, et al. Histone deacetylase inhibitors: emerging mechanisms of resistance. Mol Pharm. 2011;8:2021–2031. doi: 10.1021/mp200329f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937–941. [PubMed] [Google Scholar]
- 5.Cao H, Stamatoyannopoulos G. Histone deacetylase inhibitor FK228 is a potent inducer of human fetal hemoglobin. Am J Hematol. 2006;81:981–983. doi: 10.1002/ajh.20676. [DOI] [PubMed] [Google Scholar]
- 6.Mickley LA, Bates SE, Richert ND, Currier S, Tanaka S, Foss F, et al. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989;264:18031–18040. [PubMed] [Google Scholar]
- 7.Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Tsang YH, Yu Q. c-Myc overexpression sensitizes Bim-mediated Bax activation for apoptosis induced by histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through regulating Bcl-2/Bcl-xL expression. Int J Biochem Cell Biol. 2007;39:1016–1025. doi: 10.1016/j.biocel.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Schrump D. Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: mechanisms and potential clinical implications. Clin Cancer Res. 2009;15:3947–3957. doi: 10.1158/1078-0432.CCR-08-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piekarz R, Frye R, Turner M, Wright J, Allen S, Kirschbaum M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 12.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 13.Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadler WM, Margolin K, Ferber S, McCulloch W, Thompson JA. A phase II study of depsipeptide in refractory metastatic renal cell cancer. Clinical Genitourinary Cancer. 2006;5:57–60. doi: 10.3816/CGC.2006.n.018. [DOI] [PubMed] [Google Scholar]
- 15.Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF, et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin Cancer Res. 2008;14:188–198. doi: 10.1158/1078-0432.CCR-07-0135. [DOI] [PubMed] [Google Scholar]
- 16.Molife L, Attard G, Fong P, Karavasilis V, Reid A, Patterson S, et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Ann Oncol. 2010;21:109–113. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]
- 17.Otterson GA, Hodgson L, Pang H, Vokes EE, B CaLG. Phase II study of the histone deacetylase inhibitor Romidepsin in relapsed small cell lung cancer (Cancer and Leukemia Group B 30304) J Thorac Oncol. 2010;5:1644–1648. doi: 10.1097/JTO.0b013e3181ec1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto FM, Lamborn KR, Kuhn JG, Wen PY, Yung WK, Gilbert MR, et al. A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03–03. Neuro Oncol. 2011;13:509–516. doi: 10.1093/neuonc/nor017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty AR, Robey RW, Luchenko VL, Zhan Z, Piekarz RL, Gillet JP, et al. MAPK pathway activation leads to Bim loss and histone deacetylase inhibitor resistance: rationale to combine romidepsin with a MEK inhibitor. Blood. 2013 doi: 10.1182/blood-2012-08-449140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Li A, Zhao M, Gao Y, Zhou T, Xu Y, et al. Proteomic analysis identifies protein targets responsible for depsipeptide sensitivity in tumor cells. J Proteome Res. 2008;7:2733–2742. doi: 10.1021/pr7008753. [DOI] [PubMed] [Google Scholar]
- 21.Newbold A, Lindemann RK, Cluse LA, Whitecross KF, Dear AE, Johnstone RW. Characterisation of the novel apoptotic and therapeutic activities of the histone deacetylase inhibitor romidepsin. Mol Cancer Ther. 2008;7:1066–1079. doi: 10.1158/1535-7163.MCT-07-2256. [DOI] [PubMed] [Google Scholar]
- 22.Luchenko VL, Salcido CD, Zhang Y, Agama K, Komlodi-Pasztor E, Murphy RF, et al. Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle. 2011;10:3119–3128. doi: 10.4161/cc.10.18.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–1343. doi: 10.1038/sj.leu.2402535. [DOI] [PubMed] [Google Scholar]
- 24.Hideshima T, Bradner J, Wong J, Chauhan D, Richardson P, Schreiber S, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noonan AM, Eisch RA, Liewehr DJ, Sissung TM, Venzon D, Flagg TP, et al. Electrocardiographic Studies of Romidepsin Demonstrate Its Safety and Identify a Potential Role for the KATP channel. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Gardner ER, Figg WD. Determination of the cyclic depsipeptide FK228 in human and mouse plasma by liquid chromatography with mass-spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865:153–158. doi: 10.1016/j.jchromb.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates S, Zhan Z, Steadman K, Obrzut T, Luchenko V, Frye R, et al. Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br J Haematol. 2010;148:256–267. doi: 10.1111/j.1365-2141.2009.07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, et al. Phase I Trial of the Histone Deacetylase Inhibitor, Depsipeptide (FR901228, NSC 630176), in Patients with Refractory Neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 30.Robey RW, Zhan Z, Piekarz RL, Kayastha GL, Fojo T, Bates SE. Increased MDR1 expression in normal and malignant peripheral blood mononuclear cells obtained from patients receiving depsipeptide (FR901228, FK228, NSC630176) Clin Cancer Res. 2006;12:1547–1555. doi: 10.1158/1078-0432.CCR-05-1423. [DOI] [PubMed] [Google Scholar]
- 31.Ellis L, Pan Y, Smyth G, George D, McCormack C, Williams-Truax R, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 32.Munster P, Marchion D, Thomas S, Egorin M, Minton S, Springett G, et al. Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br J Cancer. 2009;101:1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gojo I, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele N, Plumb J, Vidal L, Tjørnelund J, Knoblauch P, Rasmussen A, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 35.Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 36.Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J. HDAC inhibitors and cardiac safety. Clin Cancer Res. 2007;13:1068. doi: 10.1158/1078-0432.CCR-06-1715. [DOI] [PubMed] [Google Scholar]
- 37.Sherman EJ, Su YB, Lyall A, Schoder H, Fury MG, Ghossein RA, et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2012 doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti C, Leo E, Eichler GS, Sordet O, Martin MM, Fan A, et al. Inhibition of histone deacetylase in cancer cells slows down replication forks, activates dormant origins, and induces DNA damage. Cancer Res. 2010;70:4470–4480. doi: 10.1158/0008-5472.CAN-09-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galrão AL, Sodré AK, Camargo RY, Friguglietti CU, Kulcsar MA, Lima EU, et al. Methylation levels of sodium-iodide symporter (NIS) promoter in benign and malignant thyroid tumors with reduced NIS expression. Endocrine. 2013;43:225–229. doi: 10.1007/s12020-012-9779-8. [DOI] [PubMed] [Google Scholar]
- 41.Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Na+/I-symporter gene methylation status. J Clin Endocrinol Metab. 1999;84:2449–2457. doi: 10.1210/jcem.84.7.5815. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Liu Z, Woo CW, Li Z, Wang L, Wei JS, et al. EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72:315–324. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.