Abstract
Streptococcus mutans is a common Gram-positive bacterium and plays a significant role in dental caries. Tobacco and/or nicotine have documented effects on S. mutans growth and colonization. Sortase A is used by many Gram-positive bacteria, including S. mutans, to facilitate the insertion of certain cell surface proteins, containing an LPXTGX motif such as antigen I/II. This study examined the effect of nicotine on the function of sortase A to control the physiology and growth of S. mutans using wild-type S. mutans NG8, and its isogenic sortase-defective and -complemented strains. Briefly, the strains were treated with increasing amounts of nicotine in planktonic growth, biofilm metabolism, and sucrose-induced and saliva-induced antigen I/II-dependent biofilm formation assays. The strains exhibited no significant differences with different concentrations of nicotine in planktonic growth assays. However, they had significantly increased (P≤0.05) biofilm metabolic activity (2- to 3-fold increase) as the concentration of nicotine increased. Furthermore, the sortase-defective strain was more sensitive metabolically to nicotine than the wild-type or sortase-complemented strains. All strains had significantly increased sucrose-induced biofilm formation (2- to 3-fold increase) as a result of increasing concentrations of nicotine. However, the sortase-defective strain was not able to make as much sucrose- and saliva-induced biofilm as the wild-type NG8 did with increasing nicotine concentrations. These results indicated that nicotine increased metabolic activity and sucrose-induced biofilm formation. The saliva-induced biofilm formation assay and qPCR data suggested that antigen I/II was upregulated with nicotine but biofilm was not able to be formed as much as wild-type NG8 without functional sortase A.
Keywords: dental caries, metabolism, nicotine, sortase A, Streptococcus mutans
INTRODUCTION
Streptococcus mutans is a common Gram-positive oral bacterium and plays a key role in the formation of tooth decay.1 It is well documented that tobacco smoking affects health. Historically, the most often mentioned systemic diseases related to smoking are lung cancer and cardiovascular diseases.2 Although the relationship between caries and smoking has been documented,3,4,5 very few studies have focused on the mechanisms why smokers or children exposed to environmental tobacco smoke are prone to caries development. Recently we reported that nicotine increases S. mutans biofilm formation and metabolic activity6 as well as increased expression of antigen I/II, glucosyltransferases (GTF) and glucan-binding protein A (Gbp; manuscript in preparation). In addition, we previously reported that smokeless tobacco users had significantly higher levels of immunoglobulin A and J chains, but lower levels of secretory component, lactoferrin, and lysozyme levels in whole saliva than non-tobacco users, which indicated that smokers had impaired immune responses.7,8,9
Cell wall/membrane proteins of many Gram-positive bacteria contain repetitive domains which may serve as virulence determinants, or have a key role in protein–protein interactions. Several S. mutans cell surface proteins including GTF, Gbp and antigen I/II, are able to promote biofilm formation by adhering and accumulating cells together.10,11,12 Sucrose-independent adherence of S. mutans is provided primarily by antigen I/II, while sucrose-dependent adherence, which is mainly regulated by the enzymatic and glucan-binding action of Gbps such as GTF and other Gbp, plays a major role in the formation of dental biofilm.13 GbpC is a surface-bound enzyme, and GTFs and GbpA and GbpB are sucrose-dependent cell-associated enzymes. Antigen I/II polypeptides occupy a major role in initial attachment to the saliva-coated tooth surface. Surface-bound antigen I/II binds to salivary agglutinin in the salivary pellicle.14
Tobacco use has a documented effect on S. mutans growth and colonization.15 Sortase may be universal in Gram-positive bacteria. Sortase is a transpeptidase which covalently links important cell surface proteins containing a LPXTGX amino acid motif to bacterial cell walls.16,17,18 Sortase cleaves the bond between thr and gly residues in the LPXTGX motif of cell wall proteins18 and anchors these proteins to the cell wall of Gram-positive pathogens,19 including S. mutans. There are six cell surface proteins (antigen I/II, FruA, WapA, WapE, GbpC and DexA) containing the LPXTGX motif located at their C terminus in S. mutans UA159.20 GTF and GbpA do not have an LPXTGX motif and sortase should have no effect on expression and cell wall anchoring of these proteins. It has been demonstrated that sortase plays a significant role in sorting and anchoring antigen I/II to the cell wall of S. mutans NG8.21 Because of the increased caries in smokers22 and greater biofilm23 and colonization in tobacco-treated S. mutans cultures,6,15 we hypothesized that nicotine may increase antigen I/II expression through increased sortase activity. In this research, we provided a descriptive study of nicotine on several facets of S. mutans pathobiology to investigate the role of sortase in S. mutans.
MATERIALS AND METHODS
Bacteria and growth conditions
S. mutans NG8 (serotype c), sortase-defective mutant and sortase-complemented strain21 (both mutants derived from S. mutans NG8; kindly obtained from Song Lee, Dalhousie University, Halifax, NS, Canada) were stored at −80 °C in Todd-Hewitt broth (THB; Acumedia, Baltimore, MA, USA) with 10% glycerol until used for the study. For every experiment, we cultured the bacteria in THB in 5% CO2 at 37 °C without agitation. Briefly, the strains were used with various nicotine (Sigma-Aldrich, St Louis, MO, USA) concentrations in growth, metabolism and sucrose- and saliva-induced biofilm formation assays using microtiter plate technology.
Polymerase chain reaction
Polymerase chain reaction (PCR) was used to confirm the presence or absence of the sortase A gene in the three strains. One colony of each bacterium from an agar plate was used as the template. The sortase primers were SL177 (CCTCTAGATTA AAATGATATTTGATTATAGGACTG; XbaI site underlined) and SL176 (CTGAATTCATGAAAAAAGAACGTCAATCTAGGA; EcoRI site underlined).21 Each analysis was conducted using triplicate samples of each strain.
Planktonic growth assay
To determine minimum inhibitory concentration, an overnight bacterial culture was incubated in THB with twofold serial dilutions of nicotine concentrations from 0 to 32 mg⋅mL−1; 190 μL of the nicotine dilutions in THB and 10 μL of an overnight THB culture were placed into wells in a sterile 96-well microtiter plate and incubated overnight. The turbidity of planktonic bacteria was recorded at 595 nm using a spectrophotometer (Spectra Max 190; Molecular Devices, Sunnyvale, CA, USA) at the beginning of the study and after 24 h of incubation. The minimum inhibitory concentration was defined as after 24 h incubation, in the lowest concentration of nicotine that had no obvious optical density (OD) change in OD595 of less than 0.05 as being indicative of no bacterial growth compared to time point 0. To measure the minimum bactericidal concentration (MBC), bacterial media from the wells without an obvious optical density change were plated on tryptic soy agar (Difco, Detroit, MI, USA) plates and incubated for another 48 h. The MBC was defined as the lowest nicotine concentration that did not have any visible bacterial colony growth on the agar plates.
Biofilm metabolic activity assay
To measure metabolic activity, a 2,3-bis (2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay24 was used by growing each bacterium in THB containing 1% sucrose (THBS). THBS containing bacteria was incubated without nicotine in sterile 96-well microtiter plates for biofilm formation. After 24 h of incubation, the planktonic bacteria were removed and the formed biofilms washed with sterile 0.9% saline. Fresh THBS supplemented with different concentrations of nicotine (0–32 mg⋅mL−1) was incubated in the wells for an additional 24 h. To measure the metabolic activity of the nicotine-treated biofilm cells, 120 μL of an XTT/menadione solution was added to the washed bacterial biofilm, and the plate was incubated for another 2 h in the dark. The colored supernatant was transferred to a new plate, and the absorbance was measured at 490 nm.
Sucrose-induced biofilm formation
Crystal violet staining was used to measure biofilm formation.6,25 One hundred and ninety microliters of THBS containing different nicotine concentrations (0–32 mg⋅mL−1) with 10 μL of an overnight culture of bacteria were incubated in selected wells of sterile 96-well microtiter plates. After incubation for 24 h, the planktonic bacteria were removed and the wells were washed with 0.9% saline, then the biofilm was fixed with 10% formaldehyde, washed again and 5 g·L−1 crystal violet was added to stain the biofilm for 30 min. The wells were rinsed again twice with 0.9% saline and 200 μL of isopropanol was added to the wells to solubilize the dye, at last the isopropanol was transferred to a new plate and the absorbance at 490 nm was recorded.
Saliva-induced biofilm formation
Freshly obtained clarified pooled unstimulated whole saliva was centrifuged at 3 000g at 4 °C, filtered, aliquoted and stored at −20 °C before used. Two hundred microliters of undiluted filtered saliva was added to wells in sterile 96-well plates and stored at 4 °C for 24 h to allow the proteins to bind to the wells. The wells were washed with saline and 190 μL of THB containing different concentrations of nicotine (0–8 mg⋅mL−1) with 10 μL of an overnight culture of bacteria were incubated in the wells for 24 h. Biofilm formation was established as described above using crystal violet.
Quantitative real-time PCR
For real-time PCR (qRT-PCR) analysis, gene-specific primers (Table 1) were used and first-strand cDNAs were synthesized using TAKARA prime script RT reagent kit with gDNA Eraser, according to the manufacturer's instructions. Each PCR reagent (25 μL) contained SYBR premix Ex Taq II (TAKARA, Shiga, Japan), cDNA samples and forward and reverse gene-specific primers (10 μmol⋅L−1 each). The thermocycling conditions were 95 °C for 30 s, and 39 cycles at 95 °C for 30 s and 60 °C for 30 s. Amplification specificity was assessed using melting curve analysis. Different gene expressions were normalized to the levels of 16S rRNA gene transcripts. The degree of expression change was calculated using the 2−ΔΔCt method. The physiological concentration of nicotine in the saliva of smoker ranges is 70–1 560 μg⋅mL−1 (ref. 26). The levels of nicotine in saliva can be highly variable and depend upon the volume of saliva secreted, the duration of contact with tobacco, the amount of tobacco consumed, measuring time and the methods used for measurement.27,28,29 Accordingly, it is difficult to know the concentration of nicotine in the dental plaque which is actually exposed. Here we choose 1 mg⋅mL−1 nicotine concentration compared to the control without nicotine for the qRT-PCR.
Table 1. Specific primers used for real-time PCR.
| Primers | Sequences |
|---|---|
| 16S rRNA | 5′-AGCGTTGTCCGGATTTATTG-3′ |
| 5′-CTACGCATTTCACCGCTACA-3′ | |
| gtfB | 5′-CACTATCGGCGGTTACGAAT-3′ |
| 5′-CAATTTGGAGCAAGTCAGCA-3′ | |
| gtfC | 5′-GATGCTGCAAACTTCGAACA-3′ |
| 5′-TATTGACGCTGCGTTTCTTG-3′ | |
| gtfD | 5′-TTGACGGTGTTCGTGTTGAT-3′ |
| 5′-AAAGCGATAGGCGCAGTTTA-3′ | |
| gbpC | 5′-AATTCTGATACTGTTGCAGCACCTA-3′ |
| 5′-TTCTGTTGCAGCCGGTTCT-3′ | |
| antigen I/II | 5′-GGATCTGGCTGGGATAGTTCAG-3′ |
| 5′-GACCAGACATGCGGATAGCA-3′ | |
| dexA | 5′-GACAACTGCGGCCATTGC-3′ |
| 5′-CCAGAGCAGCCAGAGACATG-3′ |
Gbp, glucan-binding protein; GTF, glucosyltransferases; PCR, polymerase chain reaction.
Statistical analysis
Each of the experiments was conducted using triplicate wells and done at least three times. Differences between the nicotine-treated experimental and non-treated control groups were analyzed by SPSS software (version 16.0; SPSS, Chicago, IL, USA). Results are presented as means and standard deviations. One-way analysis of variance and post hoc Tukey multiple comparisons tests were performed to compare the multiple means. Significance was set at P≤0.05.
RESULTS
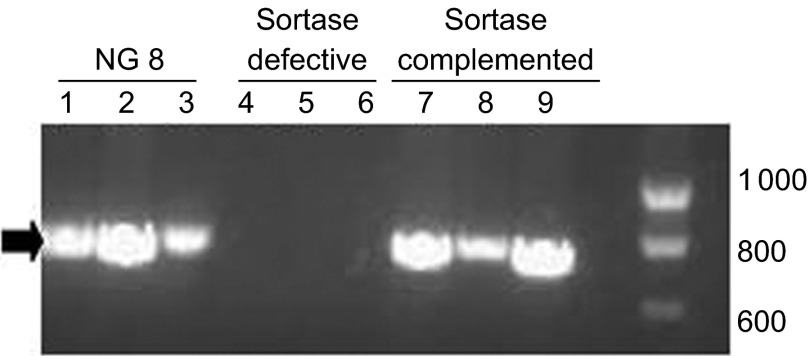
PCR demonstrated that both the wild-type NG8 and sortase-complemented strains carry the sortase gene (Figure 1). The sortase-defective mutant does not carry the sortase gene.
Figure 1.
PCR of sortase gene from S. mutans NG8, and the sortase-defective and sortase-complemented strains. Lanes 1–3, wild-type NG8; lanes 4–6, sortase-defective; and lanes 7–9, sortase-complemented. Each lane represents cells from a different experimental culture. The arrowhead (far left) indicates the location of the 741 bp sortase gene and the markers (far right) indicate the range of 600–1 000 bp. PCR, polymerase chain reaction.
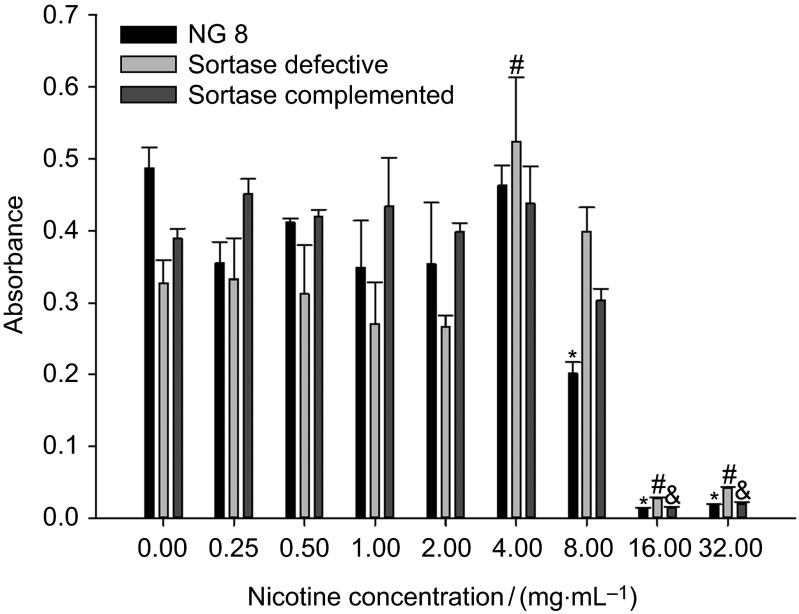
The three strains did not demonstrate a significant increase (P>0.05) in planktonic growth in lower concentrations of nicotine (<4 mg⋅mL−1; Figure 2), while higher concentrations (16–32 mg⋅mL−1) of nicotine inhibited the growth of the bacteria compared to samples without nicotine. However, the wild-type NG8 was significantly inhibited (P≤0.05) at 8 mg⋅mL−1, while the sortase-defective strain was significantly increased at 4 mg⋅mL−1.
Figure 2.
Sucrose-independent planktonic growth of three S. mutans strains (NG8, and sortase-defective and sortase-complemented NG8 mutants) after 24 h. The mean absorbance (OD at 595 nm) and standard deviation of S. mutans in different nicotine concentrations are shown. ‘*', ‘#' and ‘&' indicate significant differences of three strains, wild-type NG8, sortase-defective and sortase-complemented (P≤0.05), compared with the 0 nicotine control.
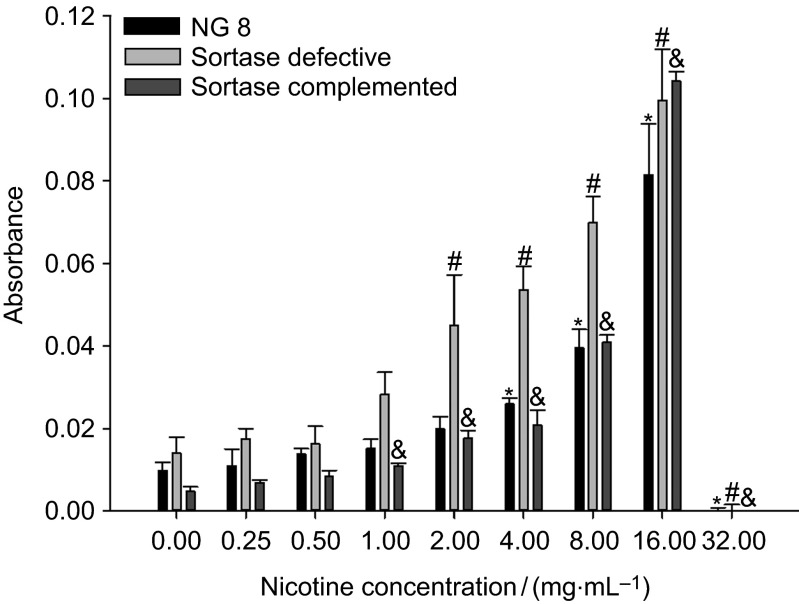
All bacteria had increased metabolic activity in THBS as the concentration of nicotine increased (Figure 3). One-way analysis of variance indicated that the sortase-defective mutant had significantly increased metabolism (up to 10-fold) at nicotine concentrations of 2–16 mg⋅mL−1 compared to the 0 mg⋅mL−1 nicotine control. NG8 had significantly increased metabolism at 4–16 mg⋅mL−1 (3- to 8-fold). The sortase-complemented strain had significantly increased metabolism at 1–16 mg⋅mL−1 (2- to 11-fold), while the sortase-defective strain overall had higher metabolic activity (2- to 3-fold) than that of the NG8 and sortase-complemented strains when there was no nicotine in THBS.
Figure 3.
Sucrose-dependent biofilm metabolic activity of three S. mutans strains (NG8, and sortase-defective and sortase-complemented NG8 mutants) after 24 h. The mean absorbance (OD at 490 nm) and standard deviation of S. mutans biofilm metabolic activity in different nicotine concentrations are shown. ‘*', ‘#' and ‘&' indicate significant differences of three strains, wild-type NG8, sortase-defective and sortase-complemented (P≤0.05), compared with the 0 nicotine control.
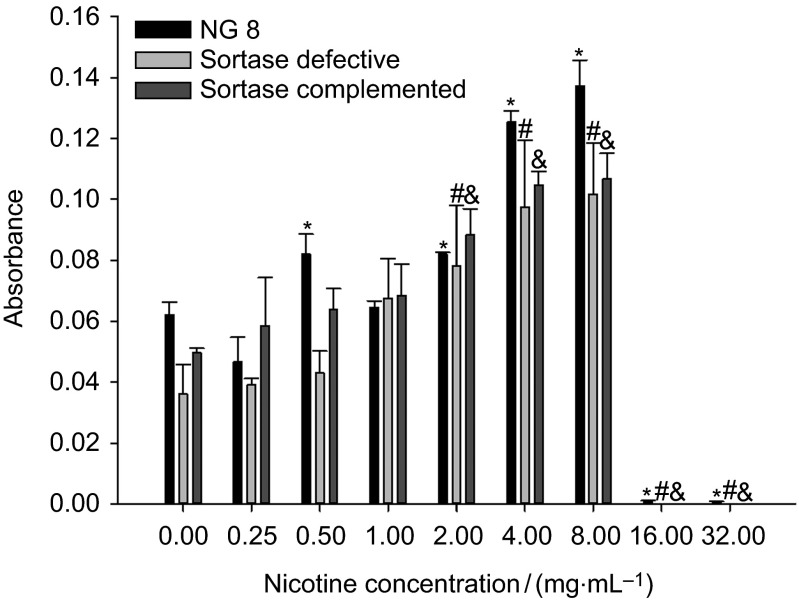
All strains had increased sucrose-induced biofilm formation as the concentration of nicotine increased (Figure 4). NG8 produced more biofilm than the sortase-defective strain without nicotine, and sortase-complemented strain also produced more biofilm than sortase-defective strain, but less than wild-type NG8, while NG8 had significantly increased biofilm at nicotine concentrations of 0.5–8 mg⋅mL−1 except 1 mg⋅mL−1 nicotine concentration. The biofilm formation of sortase-defective strain was less than wild-type NG8 at most of the nicotine concentrations, but at 1 mg⋅mL−1 nicotine, biofilm formation of sortase-defective strain was a little higher than wild-type NG8. We got the same result comparing sortase-defective to sortase-complemented strain. When different concentrations of nicotine were added to THBS, biofilm formation of the sortase-defective strain was increased, suggesting that nicotine induced an unknown non-sortase dependent adhesion possibly related to an increase in the sucrose-dependent reactions involving GTF.
Figure 4.
Sucrose-dependent biofilm formation of three S. mutans strains (NG8, and sortase-defective and sortase-complemented NG8 mutants) after 24 h. The mean absorbance (OD at 490 nm) and standard deviation of S. mutans biofilm in different nicotine concentrations are shown. ‘*', ‘#' and ‘&' indicate significant differences of three strains, wild-type NG8, sortase-defective and sortase-complemented (P≤0.05) compared with the 0 nicotine control.
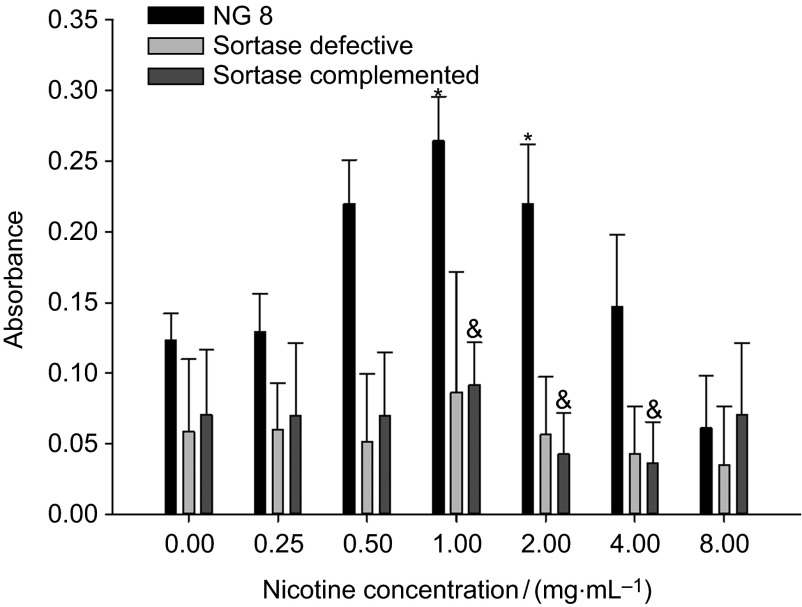
All of the three bacteria have reached the maximum biofilm formation at 1 mg⋅mL−1 nicotine (Figure 5). Biofilm was significantly decreased (or inhibited) when the nicotine concentration reached 16 mg⋅mL−1 (data not shown). Saliva-induced biofilm formation of wild-type NG8 was increased two- to threefold as nicotine concentration increased. NG8 exhibited a significant increase effect at 1 and 2 mg⋅mL−1. Nicotine has no significant effect on the saliva-induced biofilm formation of the sortase-defective strain. The sortase-complemented strain produced a significantly increase in biofilm at 1 mg⋅mL−1 nicotine concentration and significant decreases at 2 and 4 mg⋅mL−1. These results indicate that sortase is upregulatable with nicotine and no biofilm is able to be formed without functional sortase in saliva-induced biofilm formation.
Figure 5.
Saliva-dependent biofilm formation of three S. mutans strains (NG8, and sortase-defective and sortase-complemented NG8 mutants) after 24 h. The mean absorbance (OD at 490 nm) and standard deviation of S. mutans biofilm in different nicotine concentrations are shown. ‘*', ‘#' and ‘&' indicate significant differences of three strains, wild-type NG8, sortase-defective and sortase-complemented (P≤0.05) compared with the 0 nicotine control.
In order to identify the effect of nicotine on the gene expression associated with cells attachments and biofilm formation of sortase-defective strain, real-time PCR was used to quantify the mRNA expression of gtfB, gtfC, gtfD and gbpC, antigen I/II, dexA which are regulated by sortase, and took the 16S rRNA as an internal control. Melt curves revealed the absence of nonspecific products in all amplification reactions. For sortase-defective strain, nicotine at 1 mg⋅mL−1 concentration increased gtfB, gtfC and gbpC, antigen I/II, dexA by 1.3-, 1.6-, 2.7-, 1.8- and 1.1-fold respectively, but the expression of gtfD was decreased. And for wild-type NG8, gtfB, gtfD and gbpC were increased about 1.3-, 1.4- and 1.6-fold respectively, especially gtfC was increased about 600-fold. Both dexA and sortase A were increased 1.2-fold, while antigen I/II was decreased.
DISCUSSION
Sortase controls secretion and anchoring of important surface proteins pathways by a conserved mechanism in most Gram-positive bacteria.19 Sortase is an excellent target for new antimicrobial agents, since virulence is decreased when bacteria are deficient in these proteins.30 To explore the role of the sortase gene in S. mutans affected by nicotine in vitro, wild-type and sortase-defective strains were compared in this study. To date, this is the first report about the role of sortase in S. mutans under the effect of nicotine.
In this study, we observed that nicotine at high concentrations (16 and 32 mg⋅mL−1) had an antibacterial effect on all three strains studied (Figure 2). The study also indicated that lower concentrations of nicotine have no significant influence on planktonic growth. However, the sortase-defective strain had a sharp increase in planktonic growth at the nicotine concentration of 4 mg⋅mL−1, while NG8 had a significant reduction at 8 mg⋅mL−1 and a significant reduction is not seen for the other two strains until 16 mg⋅mL−1 nicotine. These results indicated that 8 mg⋅mL−1 of nicotine is sub-minimum inhibitory concentration for wild-type NG8, while 4 mg⋅mL−1 is optimal for the sortase-defective strain. However, at 8 mg⋅mL−1 of nicotine, the growth of the sortase-defective was decreased, but not significantly. We speculate that there is a threshold nicotine concentration between 4 and 8 mg⋅mL−1. There are no significant effects upon growth of the three strains in the presence of nicotine concentrations below 4 mg⋅mL−1 compared to control; on the other hand, the growth will be decreased, and even inhibited at 16 mg⋅mL−1.
The XTT reduction assay is a reliable technique to measure the metabolic activities of sessile cells in biofilm. It was originally used as a general tool for measuring Candida albicans and other Candida spp. biofilm.31 A water-soluble orange formazan compound is released by the intracellular reduction of XTT, the intensity of which can be quantified by colorimetric estimation using a microtiter-plate reader.32 The XTT reduction assay exhibits an excellent relationship between the metabolic activity and density of cells in biofilm. It is useful for the examination of multiple agents and parameters affecting the metabolic activity of biofilm cells. The XTT reduction assay indicated different metabolic activities among these three bacteria. Metabolic activity increased proportional to increasing concentrations of nicotine. In addition, this research demonstrated that the sortase-defective strain was much more sensitive than the wild-type strain to increasing nicotine concentrations with regard to metabolic activity.
Besides the direct influence of nicotine on planktonic growth and metabolic activity, this study also demonstrated that nicotine increased sucrose- and saliva-dependent biofilm formation. There are definitely growth differences across the three streptococcal strains tested, and differential growth rates will influence the biofilm-forming capabilities. Therefore, in this study, we took the 24 h incubation mature biofilm to make comparisons across the strains. The function of sortase in regulating the related surface proteins of S. mutans was investigated in sucrose- and saliva-induced biofilm formation. Proteins such as GTF and GbpA, GbpB, GbpC and GbpD are all sucrose-dependent surface-bound enzymes and are involved in sucrose-induced biofilm formation. All of the sucrose-dependent genes were up-regulated by nicotine in wild-type NG8, such as gtfB, gtfC, gtfD and gbpC (Figure 6b), especially the significantly increased of gtfC, about 600-fold. It confirmed the result that nicotine is able to increase the sucrose-induced biofilm formation (Figure 4). And GbpC contains the LPXTGX motif and is regulated by sortase. The elevated sucrose-dependent biofilm formation by sortase-defective mutant in the presence of nicotine is due to upregulated expression of GTFs (except gtfD) and gbpC (Figure 6a). gbpC would not be anchored to the cell wall in this mutant, despite significantly increased expression. Furthermore, nicotine-induced upregulation of gtfB or gtfC is not significant, and the result is the same as that at the nicotine concentration tested (1 mg⋅mL−1), there was no significant effect seen on sortase-defective mutant biofilm formation compared to control. Anyway, nicotine is able to increase the sucrose-dependent biofilm formation by sortase-defective mutant, despite not significant influence at the physiological concentration. What's more, with regard to the function of sortase, wild-type NG8 exhibited much more sensitivity to nicotine and produced much more biofilm than the sortase-defective strain. This suggests that nicotine enhanced the expression of those sucrose-dependent genes GTFs particularly gtfC and sortase A to anchor more gbpC, dexA to cell wall (Figure 6b).
Figure 6.
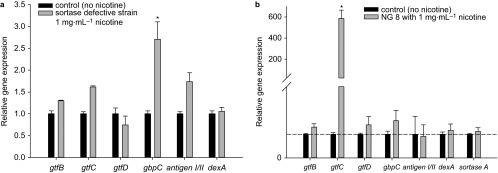
qRT-PCR assays for the gene expression of S. mutans affected by 1 g⋅L−1 nicotine. (a) Sortase-defective strain; (b) wild-type strain. The dotted line means ‘1'. qRT-PCR, quantitative real-time polymerase chain reaction.
Antigen I/II, known as cell surface protein P1, is also controlled by sortase through a LPXTGX motif.33 Antigen I/II plays a key role in saliva-induced biofilm formation;34,35 the sortase-defective strain could not produce as much biofilm as the wild-type NG8, even in the presence of nicotine. Figure 6a told us that nicotine was able to upregulate the expression of antigen I/II in sortase-defective strain, but it is not a significant effect. Without functional sortase, although the expression of antigen I/II was upregulated, it would not be anchored to the cell wall to form biofilm. The saliva-induced biofilm formation assay demonstrated that different concentrations of nicotine cannot affect saliva-induced biofilm formation of the sortase-defective strain, while wild-type NG8 was significantly affected by lower concentrations (1 and 2 mg⋅mL−1) of nicotine to form much more biofilms and the sortase-complemented strain biofilm was also significantly increased at 1 mg⋅mL−1 concentration of nicotine. In the saliva-induced experiment, the results demonstrated that the role of the sortase gene in the sortase-complemented strain is partly recovered; however, some of the function was not expressed completely as in the wild-type strain. The role of sortase in the sortase-complemented strain may be affected by some other agents we have not considered before.
Without a functional sortase and no nicotine in the culture, the sortase-defective strain was not able to demonstrate certain surface proteins-related activities, including sucrose-mediated and saliva-induced biofilm formation. However, without a functional sortase, the sortase-defective strain was able to produce much more biofilm with the addition of lower concentrations of nicotine in the sucrose-mediated biofilm formation compared to 0 mg⋅mL−1 nicotine control. Higher concentrations of nicotine had a significant influence on the sortase-defective strain in sucrose-induced biofilm formation. This confirmed that nicotine can promote sucrose-induced biofilm formation of the sortase-defective strain, and, in addition, affect biofilm formation in the presence of sucrose most likely by the increased expression of GTFs and sortase A. Sortase A plays an important role in nicotine-induced both sucrose- and saliva-induced biofilm formation.
Acknowledgments
We are indebted to Dr Song Lee (Dalhousie University) for providing some of the S. mutans strains. This work was partially supported by NIH grants HL098960 and DE020614, and the Indiana University Purdue University Indianapolis Tobacco Cessation and Biobehavioral Group (RLG). Publication of this manuscript is supported by Open Fund of State Key Laboratory of Oral Diseases, Sichuan University.
References
- Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44 2:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann KF. Smoking and health. On the report of the advisory committee to the surgeon general of the public health service. Dtsch Med Wochenschr. 1964;89:1085–1086. [PubMed] [Google Scholar]
- Carbajosa Garcia S, Llena Puy C. Relationship between tobacco smoke and dental caries in school children at the valencian country. Rev Esp Salud Publica. 2011;85 2:217–225. doi: 10.1590/S1135-57272011000200009. [DOI] [PubMed] [Google Scholar]
- Rooban T, Vidya K, Joshua E, et al. Tooth decay in alcohol and tobacco abusers. J Oral Maxillofac Pathol. 2011;15 1:14–21. doi: 10.4103/0973-029X.80032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL, Winn DM. Chewing tobacco use and dental caries among U.S. men. J Am Dent Assoc. 1999;130 11:1601–1610. doi: 10.14219/jada.archive.1999.0099. [DOI] [PubMed] [Google Scholar]
- Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci. 2012;120 4:319–325. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- Gregory RL, Gfell LE. Effect of nicotine on secretory component synthesis by secretory epithelial cells. Clin Diagn Lab Immunol. 1996;3 5:578–583. doi: 10.1128/cdli.3.5.578-583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RL, Kindle JC, Hobbs LC, et al. Effect of smokeless tobacco use in humans on mucosal immune factors. Arch Oral Biol. 1991;36 1:25–31. doi: 10.1016/0003-9969(91)90050-5. [DOI] [PubMed] [Google Scholar]
- Gregory RL, Kindle JC, Hobbs LC, et al. Effects of smokeless tobacco on the ability of secretory component to bind to the IgA/J chain complex. Hum Antibodies Hybridomas. 1990;1 3:126–131. [PubMed] [Google Scholar]
- Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988;56 8:2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Riedy CA, Weinstein P, et al. Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36-month-old children. Community Dent Oral Epidemiol. 2000;28 4:295–306. doi: 10.1034/j.1600-0528.2000.280408.x. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Bowen WH, Burne RA, et al. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61 9:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas JA, Vickerman MM. Glucan-binding proteins of the oral streptococci. Crit Rev Oral Biol Med. 2003;14 2:89–99. doi: 10.1177/154411130301400203. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Kerrigan SW, Nobbs AH, et al. Functions of cell surface-anchored Antigen I/II family and HSA polypeptides in interactions of Streptococcus gordonii with host receptors. Infect Immun. 2005;73 10:6629–6638. doi: 10.1128/IAI.73.10.6629-6638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonuz AT, Rahmati A, Mortazavi H, et al. Effect of cigarette smoke exposure on the growth of Streptococcus mutans and Streptococcus sanguis: an in vitro study. Nicotine Tobacco Res. 2008;10 1:63–67. doi: 10.1080/14622200701705035. [DOI] [PubMed] [Google Scholar]
- Cossart P, Jonquieres R. Sortase, a universal target for therapeutic agents against Gram-positive bacteria. Proc Natl Acad Sci U S A. 2000;97 10:5013–5015. doi: 10.1073/pnas.97.10.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285 5428:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- Ton-That H, Liu G, Mazmanian SK, et al. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci U S A. 1999;96 22:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen MJ, Lam AC, Antonio M, et al. An embarrassment of sortases—a richness of substrates. Trends Microbiol. 2001;9 3:97–102. doi: 10.1016/s0966-842x(01)01956-4. [DOI] [PubMed] [Google Scholar]
- Ajdic D. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99 22:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Boran TL. Roles of sortase in surface expression of the major protein adhesin P1, saliva-induced aggregation and adherence, and cariogenicity of Streptococcus mutans. Infect Immun. 2003;71 2:676–681. doi: 10.1128/IAI.71.2.676-681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer RG, Baum RH, Hsu SC, et al. In vitro effect of tobacco on the growth of oral cariogenic streptococci. J Am Dent Assoc. 1981;103 5:719–722. doi: 10.14219/jada.archive.1981.0372. [DOI] [PubMed] [Google Scholar]
- Vaananen MK, Markkanen HA, Tuovinen VJ, et al. Dental caries and mutans streptococci in relation to plasma ascorbic acid. Scand J Dent Res. 1994;102 2:103–108. doi: 10.1111/j.1600-0722.1994.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3 9:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182 5:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res. 1981;41 11:4305–4308. [PubMed] [Google Scholar]
- Caufield PW, Dasanayake AP, Li Y, et al. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68 7:4018–4023. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson N, Bond AJ, Wolff K. Salivary nicotine and cotinine concentrations in unstimulated and stimulated saliva. Afr J Pharm Pharmacol. 2010;4 2:61–65. [Google Scholar]
- Schneider NG, Jacob PR, Nilsson F, et al. Saliva cotinine levels as a function of collection method. Addiction (Abingdon, England) 1997;92 3:347–351. [PubMed] [Google Scholar]
- Bierne H, Mazmanian SK, Trost M, et al. Inactivation of the srtA gene in listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol Microbiol. 2002;43 4:869–881. doi: 10.1046/j.1365-2958.2002.02798.x. [DOI] [PubMed] [Google Scholar]
- Ramage G, Vande Walle K, Wickes BL, et al. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45 9:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm NW, Rodgers GH, Hatfield SM, et al. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142 2:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- Lee SF, Progulske-Fox A, Erdos GW, et al. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II) Infect Immun. 1989;57 11:3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N, Sasakawa C, Yoshikawa M, et al. Cloning of surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989;3 2:221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Koga T, Okahashi N, Takahashi I, et al. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58 2:289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]