Abstract
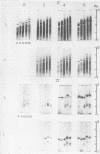
Nitrogen content and soluble protein and anodal peroxidase banding in acrylamide gel changed with leaf and internode development in the expanding leaf zone of eastern cottonwood (Populus deltoides Bartr.). Nitrogen per unit leaf area was high near the apex and decreased to a constant value at the sixth node below it. Soluble protein banding was qualitatively similar for leaves and internodes in this zone, but anodal peroxidases differed between leaves and internodes. The major leaf peroxidase band was absent from the second leaf below the apex but present in the fourth and sixth leaves; its appearance and intensification seemed to parallel the development of photosynthetic activity. The major internode peroxidase band was present in the apex, second, fourth, and sixth internodes, and intensified during internodal development. It is suggested that these two “isoenzymes” may have different functions in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. T., Steward F. C. The proteins of Tulipa and their relation to morphogenesis. Dev Biol. 1968 Mar;17(3):326–349. doi: 10.1016/0012-1606(68)90068-7. [DOI] [PubMed] [Google Scholar]
- Galston A. W., Davies P. J. Hormonal regulation in higher plants. Science. 1969 Mar 21;163(3873):1288–1297. doi: 10.1126/science.163.3873.1288. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavee S., Galston A. W. Structural physiological, and biochemical gradients in tobacco pith tissue. Plant Physiol. 1968 Nov;43(11):1760–1768. doi: 10.1104/pp.43.11.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Peroxidases of the Alaska pea (Pisum sativum L.). Enzymic properties and distribution within the plant. Arch Biochem Biophys. 1966 Nov;117(2):347–356. doi: 10.1016/0003-9861(66)90422-x. [DOI] [PubMed] [Google Scholar]
- SIEGEL S. M., GALSTON A. W. Peroxide genesis in plant tissues and its relation to indoleacetic acid destruction. Arch Biochem Biophys. 1955 Jan;54(1):102–113. doi: 10.1016/0003-9861(55)90012-6. [DOI] [PubMed] [Google Scholar]
- STEWARD F. C., LYNDON R. F., BARBER J. T. ACRYLAMIDE GEL ELECTROPHORESIS OF SOLUBLE PLANT PROTEINS: A STUDY ON PEA SEEDLINGS IN RELATION TO DEVELOPMENT. Am J Bot. 1965 Feb;52:155–164. [PubMed] [Google Scholar]
- Siegel B. Z., Galston A. W. The isoperoxidases of Pisum sativum. Plant Physiol. 1967 Feb;42(2):221–226. doi: 10.1104/pp.42.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]