Abstract
Objective: Research on D-cycloserine (DCS), a partial N-methyl-d-aspartic acid (NMDA) agonist, has suggested that it may enhance exposure-based therapies for anxiety disorders. Results with DCS in adult posttraumatic stress disorder (PTSD) have been conflicting; however, no data have been reported on children with PTSD. Although many individuals with PTSD respond to exposure-based cognitive behavioral therapy (CBT), there are subgroups of individuals who are nonresponders, and many responders still have substantial residual symptoms. This randomized, triple-blind, placebo-controlled study tested DCS as an adjunct to CBT to improve and speed treatment response for PTSD in youth.
Methods: Seven to 18 year-old youth with exposure to trauma and PTSD were offered a 12 session, manualized CBT treatment. Those who remained in treatment at the fifth session were randomly allocated (n=57) to either CBT and DCS or CBT and placebo.
Results: Youth in the CBT and DCS group had significant reductions in symptoms, but these reductions were not greater than those in the CBT and placebo group. There was a trend toward DCS speeding PTSD symptom recovery during the exposure-based sessions, and evidence that the CBT and DCS group better maintained stability of gains on inattention ratings from posttreatment to the 3 month follow-up.
Conclusions: This initial study of CBT and DCS to treat pediatric PTSD provided suggestive and preliminary evidence for more rapid symptom recovery and beneficial effects on attention, but did not show an overall greater effect for reducing PTSD symptoms. It appears that augmentation with DCS represents unique challenges in PTSD. Because PTSD involves complex, life-threatening trauma memories, as opposed to the imagined dreadful outcomes of other anxiety disorders, the use of DCS may require greater attention to how its use is coupled with exposure-based techniques. DCS may have inadvertently enhanced reconsolidation of trauma memories rather than more positive and adaptive memories. In addition, the results suggest that future research could focus on the longer-term benefits of DCS on attention and ways to capitalize on attention-enhancing therapies.
ClinicalTrials.gov registry: Effect of D-cycloserine on Treatment of Posttraumatic Stress Disorder (PTSD) in Youth, #NCT01157416, http://clinicaltrials.gov/ct2/results?term=NCT01157416&Search=Search, and D-cycloserine Adjunctive Treatment for Posttraumatic Stress Disorder (PTSD) in Adolescents, #NCT01157429, http://clinicaltrials.gov/ct2/results?term=NCT01157429&Search=Search.
Introduction
Randomized controlled trials have demonstrated the effectiveness of exposure-based cognitive behavioral therapy (CBT) for posttraumatic stress disorder (PTSD) in children and adolescents who sustained sexual abuse (Cohen and Mannarino 1998; King et al. 2000; Cohen et al. 2004; Smith et al. 2007; Gilboa-Schechtman et al. 2010). When conducted in group formats in schools, CBT has also been effective with youth exposed to violence, (Kataoka et al. 2003; Stein et al. 2003) and to Hurricane Katrina (Jaycox et al. 2010). Most studies have compared CBT with wait list control groups, but CBT has appeared superior even when compared with other active treatments such as supportive (Cohen and Mannarino 1998; Cohen et al. 2004) and dynamic therapy (Gilboa-Schechtman et al. 2010). Developmentally modified CBT has also been effective for 3–6-year-old children who sustained sexual abuse (Cohen and Mannarino 1996; Deblinger et al. 2001) or a wide variety of traumas (Scheeringa et al. 2011).
However, rapid and full remission is uncommon after a typical 10–12 session CBT course (Silverman et al. 2008). Most children who remit below the level of full diagnosis still have some enduring symptoms and impairment (Silverman et al. 2008). In a multisite study of children who experienced sexual abuse, which was also the largest pediatric study to date, Cohen et al. (2004) reported that 21% of the CBT group continued to have full PTSD diagnosis with a mean of more than five PTSD symptoms posttreatment (Cohen et al. 2004). Similarly, in a test of school-based CBT with children who experienced Hurricane Katrina, PTSD severity scores, as measured by the Children's PTSD Symptom Scale, decreased from 22.0 pretreatment to only 15.8 posttreatment (Jaycox et al. 2010). A score of ≥15 on this scale is consistent with a diagnosis of PTSD (Foa et al. 2001). Accordingly, there is a need for treatment advances and a highly promising adjunct to CBT may be the drug D-cycloserine (DCS).
DCS as an Adjunct to Psychotherapy for PTSD
DCS is an antibiotic that was introduced to treat tuberculosis >50 years ago. Since its early use, the antianxiety properties of DCS were serendipitously discovered (Crane 1961). DCS is a partial agonist at the glycine modulatory site of the N-methyl-d-aspartic acid (NMDA) receptor. It appears to act by facilitating the opening of calcium channels attached to NMDA receptors. DCS also produces a dose-dependent facilitation of extinction of fear-potentiated startle in the rat (Walker et al. 2002). However, DCS only shows an extinction effect as an adjunct, that is, when paired with behavioral training, not when given alone. DCS readily crosses the blood–brain barrier, and peak blood levels occur 1 hour after oral administration (van Berckel et al. 1997). In order to pair DCS with a behavioral component to extinguish fear responses, DCS is typically administered 1 hour before therapy sessions (Norberg et al. 2008).
A meta-analysis of preclinical and clinical studies concluded that adjunctive use of DCS enhanced fear extinction when coupled with extinction trials in animals or enhanced exposure-based psychotherapy in humans (Norberg et al. 2008). DCS increased efficacy generally, but also might have been associated with more rapid improvements by facilitating extinction learning during exposure sessions (Norberg et al. 2008). The successful clinical human studies, however, have all been in non-PTSD anxiety disorders, and all of the positive studies have been in adults.
The only published DCS studies have involved children and adolescents with obsessive-compulsive disorder (Storch et al. 2010; Farrell et al. 2013). Storch and colleagues randomized 30 8–17-year-old youth to either CBT and DCS or CBT and placebo for seven exposure sessions. They found initial support for DCS augmentation because the CBT and DCS group showed small to moderate treatment effects (d=0.31–0.47) on primary outcomes. The differences between groups were not statistically significant, but the sample size was small. Farrell and colleagues randomized 17 8–18-year-old youth who were deemed difficult to treat to either CBT and DCS or CBT and placebo for five exposure sessions. There were no significant differences between groups from pre- to posttreatment. Interestingly however, the CBT and DCS group showed significantly greater improvement from posttreatment to 1 month follow-up compared with the CBT and placebo group. These pediatric studies provided additional empirical findings to support further exploration of DCS for other conditions.
The aim of the present study was to test the effectiveness of DCS as adjunct to CBT for the first time in pediatric PTSD. When the present study began, there had been one positive trial in adults with PTSD, but the DCS was not paired with an exposure therapy (Heresco-Levy et al. 2002); therefore, it was not a true test of using DCS as an adjunct. Since the present study began, two studies that used DCS as an adjunct produced conflicting evidence of relative efficacy for DCS (de Kleine et al. 2012; Litz et al. 2012). de Kleine et al. randomized 67 adults to either placebo or DCS for eight exposure therapy sessions (de Kleine et al. 2012). There was no significant overall effect for DCS relative to placebo on the outcome measures. When outcome was dichotomized as response (a decrease of ≥10 points on the Clinician Administered PTSD Scale), the DCS was significantly more likely to show a response (64%) compared with the placebo group (34%). In addition, post-hoc analyses suggested that participants with more severe pretreatment PTSD who needed longer treatment improved more with DCS. Litz et al. randomized 26 combat veterans to either placebo or DCS for six exposure therapy sessions (Litz et al. 2012). Contrary to expectations, the veterans who received DCS plus therapy experienced significantly less symptom reduction than those who received placebo plus therapy.
As prior studies have demonstrated substantial remaining symptoms after full courses of evidence-based treatments for PTSD from different types of traumatic exposures, and DCS has shown the potential to augment CBT efficacy in adults, we proposed to conduct a preliminary test of DCS adjunctive treatment in children and adolescents. The first aim of this study was to test the relative efficacy of CBT and DCS versus CBT and placebo for reducing the number of PTSD symptoms and related secondary outcomes. Both groups received the same manualized 12 session CBT; therefore, an initial expectation would be that both groups would show significant reductions in symptoms. Hypothesis 1 stated that the CBT and DCS group would show a significantly greater reduction in the primary outcome variable – the severity of PTSD symptoms – compared with the CBT and placebo group. Secondary outcome variables were the common comorbid syndromes of depression, anxiety, and externalizing behaviors. A second aim was to test if DCS produced more rapid declines in PTSD symptoms. Hypothesis 2 stated that the CBT and DCS group would show a more rapid reduction in symptoms compared with the CBT and placebo group.
Methods and Materials
Participants
Investigators attempted to contact a total of 644 potential participants: 30% were referred by other professionals (from 15 clinicians, five social service agencies, three child advocacy centers, and 349 schools that had been made aware of the project), 14% referred themselves from radio and television advertisements, and 56% were contacted from the local level I trauma center registry. Of the 644 potential participants, 243 were ineligible because of exclusion criteria (38%), 195 could not be reached (30%), and 206 were contacted and eligible (32%). Of those 206, 65 declined to participate (33%) and 141 were evaluated in the laboratory (72%).
Inclusion criteria were: 1) Experienced or witnessed at least one life-threatening event; 2) age 7–18 years; or 3) five or more PTSD symptoms plus functional impairment. Exclusion criteria were: 1) Glascow Coma Scale score of ≤5 in the emergency room; 2) moderate mental retardation (standard scores <50 on the Peabody Picture Vocabulary Test), autistic disorder (from clinical observations by the first author), blindness, deafness, or coming from foreign language speaking families; 3) being suicidal, homicidal, or severely disabled; 4) concurrent counseling outside of the study; 5) any kidney or liver ailment; 6) epilepsy or history of seizures; or 7) bipolar disorder or schizophrenia. Psychoactive medications were allowed as long as the dose had been stable at least 4 weeks prior to treatment, and had remained stable.
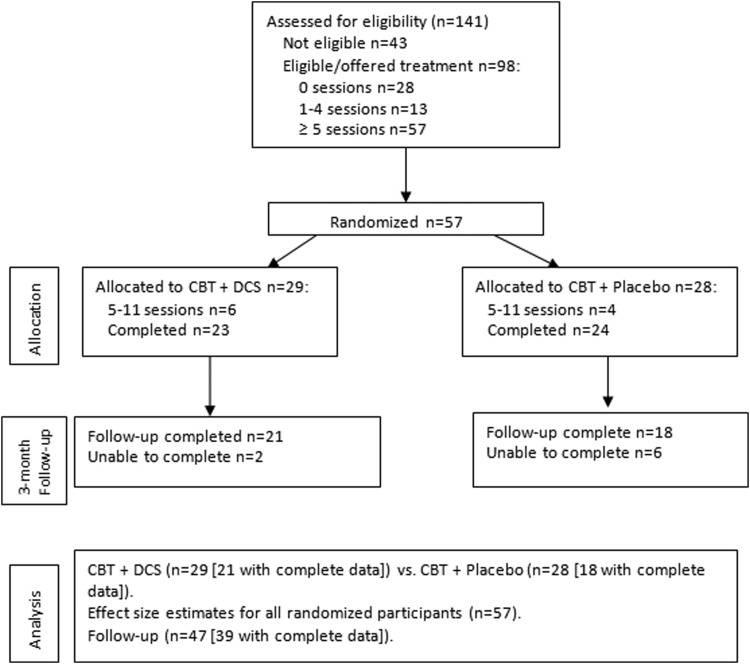
Of the 141 evaluated, 98 continued to be eligible and were offered treatment, 28 did not return for any therapy sessions, and 13 dropped out prior to the fifth session when randomization occurred, leaving 57 who were randomly allocated (CONSORT diagram in Fig. 1).
FIG. 1.
Flow chart of group assignment, attrition, treatment completers, and 3 month follow-up assessment.
Measures
National Institute of Mental Health Diagnostic Interview Schedule for Children-IV (DISC-IV)
The modules for PTSD from the Child and Parent Versions were used to determine the number of PTSD symptoms. Test–retest reliability with 82 youth ranged from κ=0.25 (social phobia) to 0.92 (major depressive disorder) (Shaffer et al. 2000). Despite the absence of psychometric data on the PTSD module, we chose it because the DISC is the most widely used diagnostic instrument for youth, and the questions map in a very straightforward way on the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria (American Psychiatric Association 1994). Given the established discordance between youth and parent reports of PTSD symptoms (Scheeringa et al. 2006), a joint rating was created using the either/or rule: If either the child or the parent reported a symptom, it was counted. Cronbach's α in this study was 0.87 for the youth ratings and 0.83 for the parent ratings.
Child PTSD Symptom Scale (CPSS)
The CPSS is a self-administered measure that maps onto the 17 DSM-IV symptoms rated on four point (0–3) Likert scales. This yields a broader range of scores that reflects intensity and frequency that may be more sensitive to change than the number of PTSD symptoms. This measure was administered weekly. Child and parent versions were used, and a joint rating was created using the either/or rule. In a study of 8–15 year-old youth, test–retest reliability 1–2 weeks after the first administration was moderate, with a κ of 0.55 for PTSD diagnosis (Foa et al. 2001). Cronbach's α in this study was 0.93 for the youth ratings and 0.90 for the parent ratings.
Child Depression Inventory (CDI)
The child version of the CDI is 27 items and the parent version is 17 items, and each item is scored on a three point (0–2) Likert scale. A cut-point of 12 has distinguished between depressed and nondepressed patients, and normal subjects scored a mean of 6.1 (Kovacs 1992). Test–retest reliabilities have been good with an intraclass correlation coefficient (ICC) of 0.74–0.77 (Carle et al. 2008). Cronbach's α in this study was 0.90 for the youth ratings and 0.89 for the parent ratings.
Screen for Child Anxiety Related Disorders (SCARED)
Both the child and caregiver versions were used (41 items each), and a joint rating was created using the either/or rule. Items are scored on three point (0–2) Likert scales. Both parent and child forms have demonstrated good internal consistency, test–retest reliability, discriminative validity, and sensitivity to treatment effects (Birmaher et al. 2003). Cronbach's α in this study was 0.94 for the child ratings and 0.95 for the parent ratings.
Swanson, Nolan and Pelham (SNAP) Questionnaire
In this study, attention-deficit/hyperactivity disorder (ADHD) was measured with 10 inattention items and 10 hyperactivity items. Oppositional defiant disorder (ODD) was measured with 10 items. Caregivers completed the checklists, because the literature indicates that youth are relatively unreliable reporters and caregivers are valid sole reporters about youth externalizing problems. Items were scored on four point (0–3) Likert scales. The SNAP has shown good test–retest reliability (ICC=0.59–0.72) (Gau et al. 2008). In this study, Cronbach's α for the 10 item inattention subscale was 0.95, for the 10 item hyperactivity subscale was 0.92, and for the 10 item ODD subscale was 0.94.
Adverse Event Checklist (AEC)
This is a 27 item checklist created for this study that tracks development of new problems during the course of treatment that are either psychological (e.g., being suicidal, homicidal, or severely disabled or having hallucinations) or physical (seizures, bruising, diarrhea, constipation, discolored urine, numbness, tics, or rash). It was filled out by caregivers at every treatment visit after pills were started, posttreatment assessment, and 3 month follow-up.
Treatment
Both groups received individual CBT treatment with a 12 session manualized protocol, Youth PTSD Treatment (YPT), created for this study. YPT includes traditional components of CBT for pediatric trauma including psychoeducation, skill-building in identification and expression of feelings, relaxation exercises, exploration of negative thoughts, narrative processing of trauma events, graded exposure exercises in and out of the office, safety plans, and involvement of parents in every session. The exposures in the office, which would have been most augmented by the DCS, were primarily cognitive. Youth were given the option of exposing themselves by drawing, writing, or verbally retelling anxiety-provoking events from their traumas. The youth repeated the exposures several times within every session, while being coached to recall aspects that would continue to increase their anxiety, and then using relaxation techniques at key points to allow the new coping skills to help extinguish their fear reactions. The exposures were not timed, but typically lasted 10–30 minutes. Less formal exposure usually continued for another 10–20 minutes as the clients worked through inaccurate thought exercises, developed safety plans, and planned homework assignments. The YPT manual is an older-age extension of the Preschool PTSD Treatment manual that has shown good efficacy in a previous trial with young children (Scheeringa et al. 2011). Therapy was delivered by two masters level therapists trained in CBT, and supervised by the authors.
The dosing of DCS was seven doses of 50 mg given before sessions 5–11. Subjects swallowed either DCS or placebo pills in the presence of staff 1 hour before exposure started. The choice of 50 mg was consistent with that used in previous controlled trials. The research pharmacist at Tulane Hospital assisted in procuring, preparing, and dispensing the pills.
Fidelity check
A 121-item fidelity checklist (∼10 items for each of the 12 sessions) was created for this study. Therapists rated themselves after every session. They achieved 93% fidelity. Also, two independent raters viewed 27% of therapy sessions (n=181 out of 661 sessions) on videotape. Cases were assigned in consecutive order so that the two raters had equal distributions of younger (7–12 years) and older (13–18 years) patients, and each of the 12 sessions was equally represented. Out of 1736 tasks rated from sessions of 68 different patients, the therapists showed 91% fidelity, and the independent raters agreed with the therapists' self-ratings 95% of the time.
Procedure
The Tulane University Committee on the Use of Human Subjects approved this study. When participants arrived at the laboratory, the study was explained to the caregivers and youth orally and in writing. Written informed consent was obtained from the caregivers. Written assent was obtained from the youth.
Randomized occurred after session 4 because the first pills were administered prior to session 5. The 7–12-year-old and 13–18-year-old participants were randomized separately to ensure balanced representation of ages. For each age group, we created a list of randomized numbers using the Microsoft Excel 2007 random number generator. Block randomization in sets of four was used. Within the first set of four numbers, two were randomly assigned to CBT and DCS and two to CBT and placebo. This procedure was repeated for the second set of four numbers, and so on. This was used to prevent long runs of unequal assignment. All research personnel were blinded except the pharmacist, who had no contact with subjects.
An independent Data and Safety Monitoring Board of three experts from outside institutions was formed to monitor adverse effects and effectiveness. The study was triple-blind as the Board, the participants, and the investigators were blind to allocation status.
Data Analysis
The effectiveness of CBT and DCS versus CBT and placebo (hypothesis 1) was tested with mixed-model random intercept analyses for repeated measures (pre- and posttreatment, and at 3 month follow-up) using the PROC MIXED statement with unstructured covariance matrix in SAS 9.3 (Cary, NC). The outcome of interest was the group-by-time interaction. Mixed models can handle missing data without excluding participants. The main outcome was joint CPSS scores. Age, race, sex, and type of trauma were examined as potential covariates. Secondary outcomes were examined with similar tests. For all outcome scores, higher scores indicated more maladaptive outcomes.
More rapid effectiveness of CBT and DCS compared with CBT and placebo (hypothesis 2) was tested using the weekly joint CPSS scores from sessions 5–12. A more rapid effect of CBT and DCS followed by leveling off would be indicated by a significant quadratic effect. An additional test was a simple linear effect between groups in the subset of data from sessions 5–8.
Power
Power analyses (Lenth 2006–9) using effect size data from a previous randomized trial of CBT in a group of youth from the same geographical region (Scheeringa et al. 2011) (for fixed factors and differences in slopes) indicated that power with this sample size for all treatment and follow-up analyses was >0.90.
Results
Preliminary analyses
Table 1 shows the sample characteristics of the 57 participants who were randomized. In both the CBT and DCS and CBT and placebo groups, the participants were evenly distributed among black/African American and white; the parents were not highly educated, and most fathers did not live in the homes. The youth had experienced a median of two types of trauma, and they were heterogeneous in the number of trauma occurrences. By chance, the CBT and DCS group had higher joint CPSS (z=−2.5, p<0.05), joint SCARED (z=−2.3, p<0.05), and joint CDI ratings (z=−2.1, p<0.05) compared with the CBT and placebo group. The groups did not differ on baseline inattention, hyperactivity, or ODD baseline measures. The number of subjects with lower intelligence estimates was low and equal in each group; one subject (Peabody Picture Vocabulary Test [PPVT] standard score 66) completed treatment in the CBT and DCS group and one subject (standard score 67) completed treatment in the CBT and placebo group.
Table 1.
Demographics of n=57 Participants Who Remained at the Fifth Session and Were Randomized
| CBT+DCS (n=29) | CBT+placebo (n=28) | |
|---|---|---|
| Age (year), mean±SD | 12.4, ±3.3 | 12.6, ±3.4 |
| Sex (female), n (%) | 19 (66) | 13 (46) |
| Race | ||
| Black, n (%) | 12 (41) | 12 (43) |
| White, n (%) | 12 (41) | 11 (39) |
| Mixed, n (%) | 4 (14) | 4 (14) |
| Other, n (%) | 1 (3) | 1 (4) |
| Maternal age (year), mean±SD | 46.1, ±10.9 | 45.9, ±12.1 |
| Maternal education (year), mean±SD | 14.4, ±2.5 | 14.6, ±3.4 |
| Paternal age (year), mean±SD | 40.7, ±16.3 | 39.9, ±12.9 |
| Paternal education (year), mean±SD | 10.3, ±8.1 | 12.0, ±4.6 |
| Father lives with child, n (%) | 5 (17) | 5 (19) |
| Primary type of trauma | ||
| Disaster, n (%) | 4 (14) | 4 (14) |
| Domestic violence, n (%) | 7 (24) | 8 (29) |
| Assaulted, n (%) | 2 (7) | 2 (7) |
| Sexual, n (%) | 12 (41) | 6 (21) |
| Threatened with weapon, n (%) | 0 (0) | 0 (0) |
| Accident, n (%) | 0 (0) | 1 (4) |
| Seen/heard someone killed/hurt badly, n (%) | 0 (0) | 3 (11) |
| Seen unexpected dead body, n (%) | 4 (14) | 4 (14) |
| Number of types of traumas – child report | 2.9, ±1.7 | 2.8, ±1.5 |
| median 2 | median 2 | |
| range 1–7 | range 1–6 | |
| Number of types of traumas – parent report | 2.6, ±1.6 | 2.6, ±1.4 |
| median 2 | median 2 | |
| range 1–8 | range 1–6 | |
| Number of occurrences of traumas – child report | 37.2, ±137.0 | 281.0, ±1024.0 |
| median 4 | median 5 | |
| range 1–738 | range 1–5,205 | |
| Number of occurrences of traumas –parent report | 108.1, ±244.4 | 209.4, ±520.5 |
| median 3 | median 10.5 | |
| range 1–815 | range 1–2,505 | |
| PTSD symptoms – child report, mean±SD | 7.0, ±4.4 | 6.0, ±3.9 |
| PTSD symptoms – parent report, mean±SD | 8.8, ±3.8 | 8.0, ±2.6 |
CBT, cognitive behavioral therapy; DCS, D-cycloserine; PTSD, posttraumatic stress disorder.
Dropouts were significantly older (mean=13.8, SD=3.5 vs. mean=12.0, SD=3.1), had younger mothers (mean=40.3, SD=8.1 vs. (mean=46.5, SD=11.9), had mothers with fewer years of education (mean=13.0, SD=2.4 vs. mean=14.6, SD=3.0), and were more severely depressed by child report (mean=16.4, SD=9.8 vs. mean=12.0, 7.9) compared with the completers (all Wilcoxon rank sum tests p<0.05, two sided). Also, dropouts were more often black/African American (67% vs. 40%) (χ2=10.0, df=3, p<0.05). There were no significant differences between completers (n=47) and dropouts (n=51) on sex, maternal employment status, paternal age, paternal years of education, paternal employment status, whether a father (or stepfather/boyfriend) lived in the home, family income, number of trauma types (by either child or parent reports), number of trauma episodes (by either child or parent reports), severity of PTSD symptoms (by either child or parent reports), severity of depression by parental report, anxiety (by either child or parent reports), inattention, hyperactivity, or ODD.
Hypothesis 1: Overall effectiveness
Posttraumatic stress symptoms
There was a significant effect of drug (F=155.9, df=2, p<0.0001). The CBT and DCS group had elevated CPSS scores compared with the CBT and placebo group at pretreatment (z=−2.5, p<0.05) and posttreatment (z=−2.2, p<0.05), but not at 3 month follow-up (p=0.2); see Table 2 for means.
Table 2.
Means and Effect Sizes for CBT+DCS and CBT+Placebo Groups, Intent to Treat Sample
| Pretreatment | Posttreatment | 3 mo. follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Mean | SD | Mean | SD | Pre to post effect size | Mean | SD | Pre to 3 mo. effect size | Test | |
| CPSSa | CBT+DCS | 33.3 | 10.9 | 16.8 | 12.5 | 1.5 | 16.3 | 12.5 | 1.5 | Drug p<0.0001 |
| CBT+P | 26.3 | 9.2 | 9.3 | 8.0 | 1.9 | 10.0 | 6.7 | 2.0 | Time p<0.0001 | |
| Drug×time NS | ||||||||||
| CDIa | CBT+DCS | 33.0 | 11.3 | 21.7 | 10.2 | 1.0 | 19.5 | 12.7 | 1.1 | Drug p<0.0001 |
| CBT+P | 28.1 | 7.8 | 16.2 | 7.8 | 1.5 | 15.8 | 5.7 | 1.8 | Time p<0.0001 | |
| Drug×time NS | ||||||||||
| SCAREDa | CBT+DCS | 50.2 | 16.0 | 36.4 | 18.3 | 0.9 | 31.7 | 19.0 | 1.0 | Drug p<0.0001 |
| CBT+P | 40.7 | 14.3 | 23.4 | 13.4 | 1.2 | 21.6 | 12.0 | 1.5 | Time p<0.0001 | |
| Drug×time NS | ||||||||||
| ODDb | CBT+DCS | 11.1 | 8.4 | 7.9 | 7.5 | 0.4 | 8.4 | 8.7 | 0.3 | Drug p<0.0001 |
| CBT+P | 10.6 | 8.4 | 5.5 | 6.2 | 0.6 | 6.6 | 6.5 | 0.5 | Time p<0.0001 | |
| Drug×time NS | ||||||||||
| Inattentionb | CBT+DCS | 17.4 | 11.0 | 10.8 | 10.2 | 0.6 | 10.8 | 10.1 | 0.6 | Drug p<0.0001 |
| CBT+P | 15.4 | 8.0 | 8.5 | 7.1 | 0.9 | 9.8 | 7.3 | 0.7 | Time p<0.0001 | |
| Drug p<0.05 | ||||||||||
| Hyperactivityb | CBT+DCS | 8.3 | 6.6 | 6.4 | 7.5 | 0.3 | 6.4 | 7.6 | 0.3 | Drug p<0.0001 |
| CBT+P | 7.7 | 7.2 | 4.7 | 6.4 | 0.4 | 4.8 | 5.7 | 0.5 | Time p<0.0001 | |
| Drug×time NS | ||||||||||
Either/or joint ratings from youth and parents.
Parent ratings.
CBT, cognitive behavioral therapy; DCS, D-cycloserine; CPSS, Child Posttraumatic Stress Disorder (PTSD) Symptom Scale; P, placebo; NS, not significant; CDI, Child Depression Inventory; SCARED, Screen for Child Anxiety Related Disorders; ODD, oppositional defiant disorder.
The test of interest, the time-by-drug interaction, was not significant (p=0.96). Both groups improved markedly with treatment; the effect sizes for the CBT and DCS group (1.5) and the CBT and placebo group (1.9) were both large (Table 2). The results were unchanged when using child or parent ratings alone as opposed to joint ratings. Because the groups differed at baseline (i.e., randomization produced unequal groups so we supplemented the main analyses with matched groups analyses [Kazdin 2003]). For these supplemental analyses, we matched the groups at baseline by deleting the four subjects with the highest joint CPSS pretreatment ratings in the CBT and DCS group and the four subjects with the lowest ratings pretreatment in the CBT and placebo group. With these subjects removed, the groups no longer differed on pretreatment joint CPSS ratings. The time-by-drug interaction term was still not significant (p=0.77). Entering the baseline joint SCARED or CDI ratings to control for initial differences between groups also did not alter the results.
Depression, anxiety, ODD, and ADHD
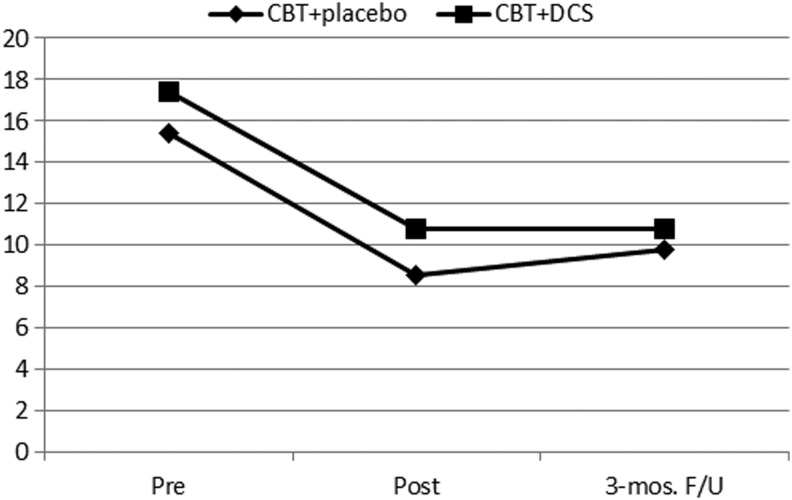
Using joint ratings for depression and anxiety, and parent ratings for hyperactivity and ODD, there were significant effects of drug and time, but nonsignificant drug-by-time interactions. For inattention, however, the drug-by-time effect was significant (F=3.8, df=2, p<0.05). The CBT and DCS group significantly reduced inattention problems at posttreatment and 3 month follow up compared with pretreatment, and remained stable from posttreatment to 3 month follow-up. Whereas the CBT and placebo inattention ratings were reduced at posttreatment and 3 months follow up compared with pretreatment, the ratings increased significantly from posttreatment (mean=8.5) to 3 month follow-up (mean=9.8) (Fig. 2). Using the subsample with the groups matched on pre-treatment CPSS ratings (i.e., eight subjects removed as described previously), the results were not altered.
FIG. 2.
Inattention ratings for cognitive behavioral therapy (CBT) and D-cycloserine (DCS) group versus CBT and placebo group.
Covariates
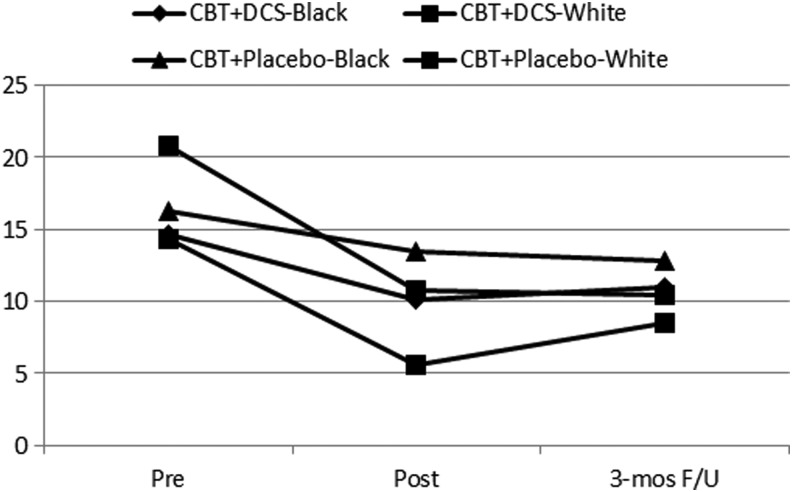
When age, sex, race, or type of trauma were entered as covariates, there were no changes in the results, with one exception. With race as a covariate (using only the subset of African American/black vs. white), the group-by-time effect on inattention was less powerful than before, and was only marginally significant (F=2.5, df=2, p=0.09). Figure 3 illustrates that the worsening of inattention in the CBT and placebo group between posttreatment and 3 month follow-up was largely the result of white participants. Black CBT and placebo participants did not show worsening of inattention (Fig. 3). Formal tests of inattention by race separately, however, included nonsignificant drug-by-time interaction effects for both black (p=0.19, n=23) and white (p=0.18, n=24) participants.
FIG. 3.
Inattention ratings for cognitive behavioral therapy (CBT) and D-cycloserine (DCS) group versus CBT and placebo group by black and white races.
Completer analysis
With treatment response determined as ≥50% reduction in joint CPSS scores, 52% of participants in the CBT and DCS group were treatment responders compared with 75% in the CBT and placebo group, but this was not statistically significant (χ2=2.7, df=1, p=0.10). The results were not substantially different when using only child reports or only parent reports.
Hypothesis 2: Rapidity of effectiveness
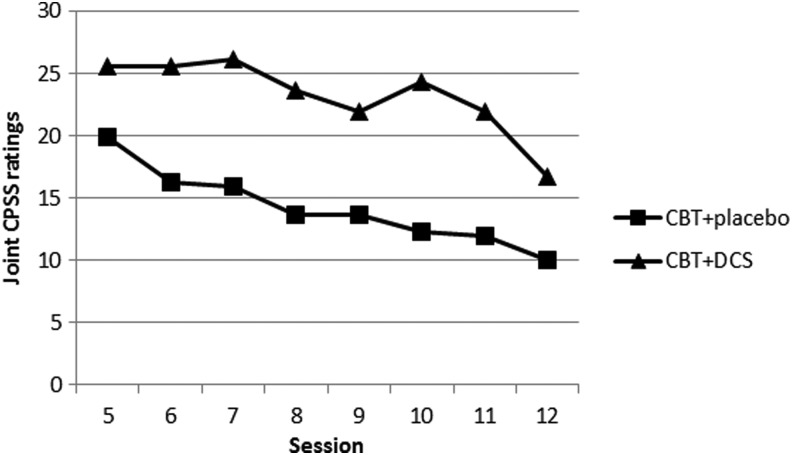
Using weekly joint CPSS ratings for sessions 5–12, the quadratic term (time by time by drug) was not statistically significant at the traditional 0.05 level, but was very close (t=1.92, df=275, p=0.056). Using an alternative test of ratings from only sessions 5–8, a simple test of linear slopes between drug groups, the drug-by-time interaction, was F=2.3, df=4, p=0.07. The results were identical when using the subsample matched on pretreatment CPSS ratings (i.e., eight subjects removed as described previously).
Because the results from both tests were nearly significant, and the size of effects suggested trends, we conducted post-hoc explorations. Figure 4 indicates that the nearly significant quadratic effect appears to have been caused by a relatively sharp decrease in the CBT and DCS ratings after sessions 7 and 8, then an increase after session 9, and then decreases again after sessions 10 and 11; whereas the CBT and placebo group showed a more steady decline.
FIG. 4.
Weekly joint Child Posttraumatic Stress Disorder (PTSD) Symptom Scale (CPSS) ratings during administration of D-cycloserine (DCS) or placebo pills.
Adverse effects
Twenty-three percent of those who received DCS reported an adverse effect: Drowsiness (6%), irritability (6%), dizziness (3%), headache (3%), and dry mouth (3%). Twenty-six percent of those who received placebo reported an adverse effect: Drowsiness (13%), dizziness (10%), lips burning (3%), increased appetite (3%), stomach pain (3%), and feeling as if “things going too fast” (3%); the sum of percentages exceeds 100% because three participants reported two adverse effects.
Discussion
These data represent the first randomized trial on the adjunctive use of DCS paired with CBT to treat PTSD in children and adolescents. It is also the largest pediatric DCS treatment study to date. Per expectations, CBT was associated with significant reductions in symptoms; however, findings provided little support for relatively improved effectiveness of CBT and DCS. This is consistent with recent findings in two studies with adults showing no significant effect for DCS in treating PTSD (de Kleine et al. 2012; Litz et al. 2012). In one of these studies, Litz et al. actually found significantly less symptom reduction in the CBT and DCS group than in the CBT and placebo group. Whereas DCS adjunctive treatment with CBT appears more effective than CBT alone for some other anxiety disorders (Norberg et al. 2008), PTSD appears to be an exception. Litz and colleagues (2012) speculated that DCS may have inadvertently enhanced reconsolidation of the trauma memory rather than a more positive and adaptive memory. We speculate further that the substantial differences in the sources of anxiety (memories of actual, life-threatening events in PTSD vs. imagined dreadful outcomes in the anxiety disorders) may also have contributed to the differences in outcomes with different syndromes.
These results are also consistent with the only other pediatric DCS studies, which found preliminary support for DCS for treating obsessive-compulsive disorder (OCD) even though the main tests of greater effectiveness were not significant. Those studies were limited by small sample sizes, with n=30 8–17-year-old youth (Storch et al. 2010) and n=17 8–18-year-old youth (Farrell et al. 2013). It could be considered that the negative outcomes in these two studies and the present study were in part the result of developmental differences. For the augmentation to work, clients need to cooperate with exposure exercises to make themselves anxious. Pediatric clients, who are generally brought to therapy by their parents, may be less inclined to cooperate. In our study however, the patients cooperated sufficiently, according to our fidelity checks, and we found no effect for age. Different physiology or metabolism in children and adolescents compared with those of adults could account for different findings. DCS is primarily excreted unchanged in the urine, however, so developmental differences in hepatic metabolism could play only a minor part.
This study did reveal a potentially positive effect for DCS on attention. The CBT and DCS group showed stability of the pre- to posttreatment gains at the 3 month follow-up, whereas the CBT and placebo group showed some significant relapse of inattention ratings, even though they did not approach the pretreatment levels. Given the established effect of DCS on learning and memory, a preferential effect on inattention ratings would not be surprising. There are few data from prior pediatric PTSD treatment studies to know if stability or relapse of inattention gains is the norm. One prior study in 3–6-year-old children treated with CBT showed no improvement in ADHD severity; however, the ADHD rating was not broken down by inattention and hyperactivity/impulsivity type (Scheeringa et al. 2011). The data suggested that the inattention effect may have been limited to white subjects, but small sample sizes limited the power to detect such an effect.
The tests of hypothesis 2 were nearly significant with effects suggesting a trend toward the CBT and DCS group improving more rapidly in some stages of the exposure sessions (although they did not improve more overall). Future research needs to examine the characteristics of the subset who improved rapidly to help identify better ways to take advantage of the pharmacological properties of DCS.
Limitations
Limitations include that this sample was largely minority and of low socioeconomic status in contrast to samples in prior DCS studies, and this limits generalizations across studies. This may also be viewed as a strength of this study in terms of extending DCS research into different sociodemographic populations. This sample may have been less motivated for treatment as shown by the substantial dropout rate, which may be a reflection of the sociodemographics of this sample. Research suggests that certain groups, such as ethnic minorities, may perceive less potential benefit from traditional treatment options for anxiety-related problems (Chavira et al. 2003). Relatively higher dropout rates, however, are typical of PTSD studies because of the inherent avoidance in PTSD phenomenology (de Kleine et al. 2012). An additional limitation could be the heterogeneous types of traumatic experiences in this sample. It is not known if youth with certain types of trauma would respond better to DCS, but this may be a worthwhile variable to explore in future studies.
Conclusions
Straightforward extensions of the DCS adjunctive treatment model in PTSD and in youth have met with limited success to date. Future work with DCS may need to exploit effects on attention by capitalizing on, for example, recent successes in attention training toward positive stimuli in anxious children (Waters et al. 2013). It may also be more productive to focus on consolidation of adaptive memories with systematically limited exposure to traumatic memories.
Clinical Significance
Because partial response and attrition remain considerable obstacles in the treatment of many individuals with PTSD, augmentation with DCS may be a promising and safe alternative for enhancing CBT outcomes that is worth exploring, but additional work remains. These findings may point to new avenues for exploration of attentional capacities, and the types of memories or cognitions that can be constructively augmented with DCS.
Disclosures
No competing financial interests exist.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Birmaher B, Khetarpal S, Cully M, Brent DA, McKenzie S: Screen for Child Anxiety Related Disorders (SCARED)–Parent form and child form (8 years and older). In: Innovations in Clinical Practice: Focus on Children & Adolescents, edited by VandeCreek L. and Jackson T.L. Sarasota, FL: Professional Resource Press/Professional Resource Exchange, 99–104, 2003 [Google Scholar]
- Carle AC, Millsap RE, Cole DA: Measurement bias across gender on the Children's Depression Inventory. Educ Psychol Meas 68, 281–303, 2008 [Google Scholar]
- Chavira DA, Stein MB, Bailey K, and Stein MT: Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr 24, 315–322, 2003 [DOI] [PubMed] [Google Scholar]
- Cohen J, Mannarino A: A treatment outcome study for sexually abused preschool children: Initial findings. J Am Acad Child Adolesc Psychiatry 35, 42–50, 1996 [DOI] [PubMed] [Google Scholar]
- Cohen JA, Deblinger E, Mannarino AP, Steer RA: A multisite, randomized controlled trial for children with sexual abuse-related PTSD symptoms. J Am Acad Child Adolesc Psychiatry 43, 393–402, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP: Interventions for sexually abused children: Initial treatment outcome findings. Child Maltreat 3, 17–26, 1998 [Google Scholar]
- Crane GE: The psychotropic effects of cycloserine: A new use for an antibiotic. Compr Psychiatry 2, 51–59, 1961 [Google Scholar]
- de Kleine RA, Hendriks G, Kusters WJC, Broekman TG, van Minnen A: A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry 71, 962–968, 2012 [DOI] [PubMed] [Google Scholar]
- Deblinger E, Stauffer L, Steer R: Comparative efficacies of supportive and cognitive behavioral group therapies for young children who have been sexually abused and their nonoffending mothers. Child Maltreat 6, 332–343, 2001 [DOI] [PubMed] [Google Scholar]
- Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, Collings N, Zimmer-Gembeck M, Shelton D, Ollendick TH, Testa C, Storch EA: Difficult-to-treat pediatric obsessive-compulsive disorder: Feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress Anxiety 30, 721–731, 2013 [DOI] [PubMed] [Google Scholar]
- Foa EB, Johnson KM, Feeny NC, Treadwell KR: The Child PTSD Symptom Scale: A preliminary examination of its psychometric properties. J Clin Child Psychol 30, 376–384, 2001 [DOI] [PubMed] [Google Scholar]
- Gau S, Shur-Fen Shang C, Liu S, Lin CH, Swanson JM, Liu YC, Tu CL: Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale–parent form. Int J Methods Psychiatr Res 17, 35–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa–Schechtman E, Foa EB, Shafran N, Aderka IM, Powers MB, Rachamim L, Rosenbach L, Yadin E, Apter A.: Prolonged exposure versus dynamic therapy for adolescent PTSD: A pilot randomized controlled trial. J Am Acad Child Adolesc Psychiatry 49, 1034–1042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco–Levy U, Kremer I, Javitt DC, Goichman R, Reshef A, Blanaru M, Cohen T: Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol 5, 301–307, 2002 [DOI] [PubMed] [Google Scholar]
- Jaycox LH, Cohen JA, Mannarino AP, Walker DW, Langley AK, Gegenheimer KL, Scott M, Schonlau M: Children's mental health care following Hurricane Katrina: A field trial of trauma-focused psychotherapies. J Trauma Stress 23, 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka SH, Stein BD, Jaycox LH, Wong M, Escudero P, Tu W, Zaragoza C, Fink A: A school-based mental health program for traumatized Latino immigrant children. J Am Acad Child Adolesc Psychiatry 42, 311–318, 2003 [DOI] [PubMed] [Google Scholar]
- Kazdin AE: Research Design in Clinical Psychology, 4th ed. Needham Heights, MA: Allyn & Bacon, 2003 [Google Scholar]
- King N, Tonge B, Mullen P: Treating sexually abused children with posttraumatic stress symptoms: A randomized clinical trial. J Am Acad Child Adolesc Psychiatry 39, 1347–1355, 2000 [DOI] [PubMed] [Google Scholar]
- Kovacs M: The Children's Depression Inventory Manual. Toronto: Multi-Health Systems,1992 [Google Scholar]
- Lenth RV. (2006–9) Java Applets for Power and Sample Size (computer software) [Google Scholar]
- Litz BT, Salters–Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG: A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res 46, 1194–1190, 2012 [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF: A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63, 1118–1126, 2008 [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Weems CF, Cohen JA, Amaya-Jackson L, Guthrie D: Trauma-focused cognitive-behavioral therapy for posttraumatic stress disorder in three through six year-old children: A randomized clinical trial. J Child Psychol Psychiatry 52, 853–860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa MS, Wright MJ, Hunt JP, Zeanah CH: Factors affecting the diagnosis and prediction of PTSD symptomatology in children and adolescents. Am J Psychiatry 163, 644–651, 2006 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39, 28–38, 2000 [DOI] [PubMed] [Google Scholar]
- Silverman WK, Ortiz CD, Viswesvaran C, Burns BJ, Kolko DJ, Putnam FW, Amaya-Jackson L: Evidence-based psychosocial treatments for children and adolescents exposed to traumatic events. J Clin Child Adolesc Psychol 37, 156–183, 2008 [DOI] [PubMed] [Google Scholar]
- Smith P, Yule W, Perrin S, Tranah T, Dalgleish T, Clark DM: Cognitive-behavioral therapy for PTSD in children and adolescents: A preliminary randomized controlled trial. J Am Acad Child Adolesc Psychiatry 46, 1051–1061, 2007 [DOI] [PubMed] [Google Scholar]
- Stein B, Jaycox L, Kataoka S: A mental health intervention for schoolchildren exposed to violence: A randomized controlled trial. JAMA 290, 603–611, 2003 [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, Micco JA, Sprich S, Wilhelm S, Bengtson M, Geller DA: A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry 68, 1073–1076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Lipsch C, Timp S, Gispen-de Wied C, Wynne H, van Ree JM, Kahn RS: Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology 16, 317–324, 1997 [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M: Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 22, 2343–2351, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Pittaway M, Mogg K, Bradley BP, Pine DS: Attention training towards positive stimuli in clinically anxious children. Dev Cogn Neurosci 4, 77–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]