Abstract
Evidence has accumulated that both murine and human adult tissues contain early-development stem cells with a broader differentiation potential than other adult monopotent stem cells. These cells, being pluripotent or multipotent, exist at different levels of specification and most likely represent overlapping populations of cells that, depending on the isolation strategy, ex vivo expansion protocol, and markers employed for their identification, have been given different names. In this review, we will discuss a population of very small embryonic-like stem cells (VSELs) in the context of other stem cells that express pluripotent/multipotent markers isolated from adult tissues as well as review the most current, validated working criteria on how to properly identify and isolate these very rare cells. VSELs have been successfully purified in several laboratories; however, a few have failed to isolate them, which has raised some unnecessary controversy in the field. Therefore, in this short review, we will address the most important reasons that some investigators have experienced problems in isolating these very rare cells and discuss some still unresolved challenges which should be overcome before these cells can be widely employed in the clinic.
Introduction
The name “very small embryonic-like stem cells” (VSELs) has been assigned to a rare population of small cells that were initially isolated from murine bone marrow (BM) [1–3]. Subsequently, the presence of cells that phenotypically correspond to VSELs has been reported in multiple murine organs [4]. Finally, small cells that, similar to murine VSELs, are lineage negative (lin−), do not express CD45 antigen (CD45−), and display a primitive morphology (high nuclear/cytoplasm ratio and the presence of euchromatin in nuclei) have been reported in human (i) umbilical cord blood (UCB) [5–8], (ii) mobilized peripheral blood (mPB) [9–13], and (iii) gonads [14,15]. Human VSELs isolated from UCB and mPB are highly enriched in a population of CD133+ cells [8,16,17], and those isolated from gonads were sorted as SSEA-4+ cells [18].
Since a VSEL-specific marker has not yet been identified, these developmentally early cells are currently isolated by a multiparameter sorting strategy employing a cocktail of antibodies and proper gating [17,19–22]. Both murine and human purified VSELs express several early-development markers, including Oct-4, Nanog, SSEA-1 (mouse), and SSEA-4 (human) [1,8,23] and are highly quiescent [24,25]. Despite their small size, similar morphological features, and phenotype, VSELs are, to some degree, heterogenous. In particular, cDNA libraries created from purified, double-sorted VSELs (20 cells/library) revealed that these cells, while having a characteristic morphology, small size, and overlapping molecular signature, still differ slightly in the expression of some genes [26]. The best method for assessing the quality of sorted VSELs is the combination of ImageStream analysis, which enables the identification of real, nucleated cell events and excludes those from cell debris, with 7AAD staining, which excludes cells that become damaged during the sorting procedure [19,27].
The best-characterized VSELs at the molecular level using microarray analysis are murine BM-derived VSELs [23,26,28] and small SSEA-4+ cells corresponding to murine VSELs isolated from human gonads [14,29,30]. Therefore, more work is needed to characterize molecular signature of VSELs isolated from other murine organs (eg, brain, heart, and skeletal muscles) and, in particular, the phenotypically corresponding populations of human VSELs in BM, UCB, and mPB. The crucial question to ask is whether VSELs are precommitted to monopotent tissue committed stem cells (TCSCs) in the tissues of their residence.
In this short editorial review, we will address the current validated working criteria for how to properly isolate these rare small cells. This is an important issue, because, as was recently observed, by changing the well-established isolation protocols, an incorrect population of cells was isolated and misidentified as VSELs [31–33]. We will also discuss the relationship of VSELs to other potential pluripotent stem cells (PSCs) and multipotent stem cells (MPSCs), which have been isolated from adult tissues by several other investigators. We noticed that very often cells which correspond to VSELs are given different names [5,6,14,34–38]. Finally, we will also try to justify why we named these cells “embryonic-like,” despite the fact that they are isolated from adult tissues.
Multiparameter Sorting of VSELs–How to Avoid Sorting the Wrong Cell Populations
The current validated strategy for the isolation of cells enriched for VSELs from BM, UCB, or mPB is based on multiparameter sorting of viable small cellular events. Murine VSELs can be isolated as small Sca-1+Lin−CD45− cells, and this approach has been successfully employed not only by us but also by other independent groups [9,39–41] who followed our detailed sorting protocols [17,19,20,22]. As reported, the highly quiescent populations of VSELs sorted from murine BM in appropriate experimental settings may give rise to hematopoietic stem cells (HSCs) [42], mesenchymal stem cells (MSCs) [40], lung alveolar type II pneumocytes [39], cardiomyocytes [43,44], and gametes and VSELs isolated from rat BM have been shown to give rise to cardiomyocytes and endothelial cells [45]. Moreover, murine VSELs may support the development of stroma in growing tumors [46]. In parallel, human VSELs have been already shown to become specified into HSCs [47] and MSCs [41].
The detailed protocol on how to sort VSELs by FACS has been described in detail in a chapter in Current Protocols in Cytometry [19]. Unfortunately, a few other groups significantly modified this protocol, which, instead of VSELs, resulted in an incorrect population of sorted cells [31–33]. This has already been the subject of an extensive analysis that we recently published [21]. However, here, we will address some of the most important issues on how to avoid such mistakes in the future.
The major concern with the work of some groups which reported negative data [31–33] is that, despite their claims, they did not follow our published protocol for isolating VSELs. However, we have to admit that these are not trivial sorting strategies, and failure to isolate these rare cells occasionally happens even in our experience in VSELs sorting hands.
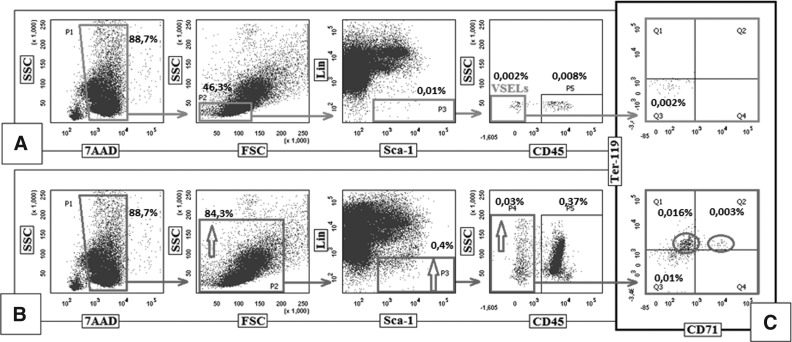
The most critical step in VSEL sorting is proper setting of the gate to exclude contamination by cell debris and small erythroblasts. Enrichment in undesired objects may have a critical impact on subsequent genetic and in vitro functional characterization of these cells. Of great importance, despite the presence of anti-glycophorin antibodies (anti-Ter119) employed in the lineage cocktail to deplete murine lineage-positive cells [19], if the wrong gating strategy is employed, it may result in unwanted enrichment of the sorted cells for erythroblasts [32]. In brief, one of the groups [32] failed to isolate murine VSELs because of (i) setting up an enlarged input gate on the FSC versus SSC plot that included granulocytes and apoptotic cells resulted in enrichment of sorted fractions with artifacts, which were reflected in their further analyses (eg, Annexin V binding) and excluded some of the critical very small objects from further sorting; (ii) additional loss of very small objects by their exclusion of VSELs by gating for “singlets”; (iii) employing some selection markers that are unproved as VSEL markers (eg, c-kit); and (iv) focusing on some populations and discarding other fractions (potentially containing VSELs) based on results such as Annexin V binding. For instance, the entire fraction of CD45−/Lin−/Sca-1+/c-kit−/KDR− cells was excluded by these authors from further sorting [32], because it was deemed “apoptotic,” while it most likely contained not only real Annexin V+ FSClow SSCdim/hi apoptotic objects, but, in addition, VSELs. Importantly, we have reported [48] that healthy normal cells (including VSELs and HSCs) may bind Annexin V after lysis of RBCs due to microvesicle/microparticle release and posphatydylserine transfer to the membranes of the normal cells. Thus, not all Annexin V+ objects should be interpreted as “apoptotic,” as these may represent normal, functional cells [48]. Figure 1 shows a simulation of sorting strategy employed by one of the groups that failed to sort VSELs [32] to demonstrate how important it is to set a proper gate during sorting of murine BM-residing VSELs because of the possibility of contamination by small CD45-negative erythroblasts.
FIG. 1.
Comparison of a correct and incorrect gating strategy for murine BM very small embryonic-like stem cells (VSELs). (A) The correct gating strategy to analyze or sort murine BM-derived VSELs by FACS. Cells were fixed and stained with 7AAD to show nucleated events in gate P1. Gate P2 includes small, agranular cells. Gate P3 includes Sca-1+ Lin− cells, which are visualized on the next dot plot as CD45-negative (VSELs) and CD45-positive cells (HSCs). Expanding this gate into lineagedim population will result in contamination of VSELs by erythroblasts. Percentage shows the average content of each cellular subpopulation among total BM nucleated cells after fixation procedure and staining with 7ADD to gate for nucleated cells only. (B) An incorrect gating strategy (we followed the sorting procedure employed by Szade et al. [32]). As shown, this group employed extended regions P2, P3, and P4, (indicated by arrows) which resulted in enrichment of erythroblasts. (C) Expression of erythroblastic markers: CD71 and Ter-119 (markers of early-stage and more differentiated erythroblasts) in cells sorted by employing correct (A) and incorrect (B) sorting strategies. Cells enclosed by circles in the lower dot plot indicate an erythroblast population that contaminates the VSELs, which is not observed in cells sorted by the correct strategy (upper dot plot).
Unfortunately, the second group [33] also did not avoid several major technical mistakes that could result both in VSEL loss/exclusion and dilution with extraneous objects. The reason for this was (i) expanding the gating for VSEL isolation to include extraneous objects (including Lindim and CD45dim cells), resulting in VSEL dilution and enrichment in erythroblasts and (ii) relying on inexact Syto-16 staining as the main indicator of “VSEL candidates,” which, in fact, are an incorrect, non-VSEL fraction [33]. Together, these approaches led to both VSEL dilution and loss and had a critical effect on the subsequent gene expression and functional analysis of these cells.
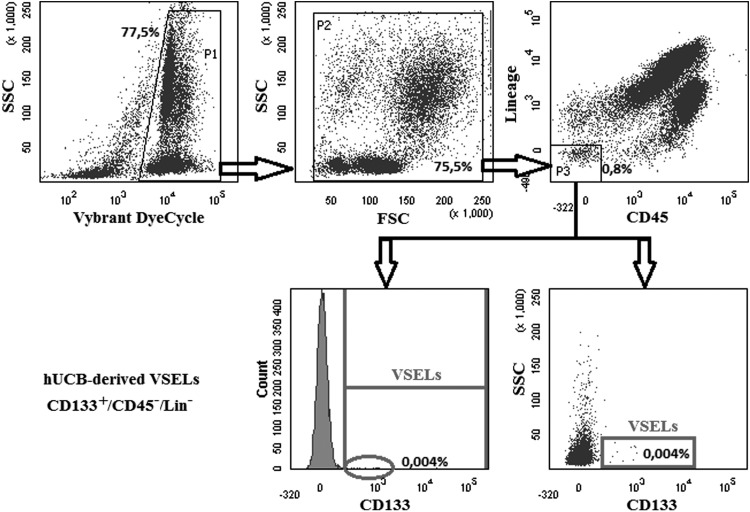
In contrast to murine VSELs, phenotypically corresponding human VSELs are isolated as a population of small CD133+Lin−CD45− cells. We reported that expression of CD133 antigen is, so far, the most important positive marker of human early-development VSELs [49]. However, human VSELs may also co-express CXCR4, CD34, and SSEA-4, the rarest population of CD133+Lin−CD45− cells is highly enriched for small Oct-4+ VSELs [8]. If a sorted population of human cells does not express CD133, it cannot be considered to be enriched for VSELs [31]. This can be observed in one report where [31] an FACS-sorted population of CXCR4+Lin−CD45− cells that lacked expression of CD133 was mistakenly identified as UCB-derived VSELs [31]. Furthermore, this group not only replaced a reliable anti-CD133 antibody clone with one that is less effective for CD133 detection, but, in addition, “proved” the absence of a CD133+Lin−CD45− population of VSELs in UCB by employing histograms in their paper instead of dot plots [31]. It is well known that since VSELs are very rare, dot plot analysis is required to show the presence of these cells. Figure 2 demonstrates an example of the analysis of UCB-derived CD133+lin−CD45− cells by histogram as was performed by this group [31] versus a dot plot, as they should be presented. Thus, based on histogram data, this group [31] isolated an incorrect population of cells for further analysis, and what is even more interesting, failed to realize that, in fact, on the original histogram curves in their paper, small “teeth” corresponding to rare VSELs are visible, and not displaying their data in a dot plot [31].
FIG. 2.
FACS analysis of umbilical cord blood (UCB) CD133+Lin−CD45− VSELs. UCB VSELs are very rare cells, and their content varies, sometimes significantly, between UCB units. Upper panel–P1–shows DNA positive events shown in P2 as SSC versus FSC dot plot. P3–includes Lin− CD45− cells. These human UCB Lin−CD45− cells were subsequently evaluated by FACS for the expression of CD133 antigen. The lower left panel illustrates an analysis for the presence of CD133+ cells in cells from P1/P2/P3 by employing a histogram, and the right panel is a visualization of these rare cells by dot plot. Danova-Alt et al. [31], in their recent studies, concluded that CD133+CD45−Lin− cells as well as CD34+CD45−Lin− cells do not exist in UCB. In fact, one of the reasons that these rare stem cell populations were overlooked by Danova-Alt and colleagues is they employed histograms instead of dot plot cytograms [31].
VSELs Express Several PSCs Markers and Exhibit the In Vitro Criteria of PSCs But Do Not Complete Blastocyst Development and Do Not Grow Teratomas
Several stringent in vitro and in vivo criteria for defining stem cells as PSCs have been proposed by embryologists who are working with embryonic stem cells (ESCs) isolated from embryos or induced PSCs (iPSCs), and we reviewed these criteria in our recent publications [50,51].
In brief, recent experimental data showed that murine BM VSELs fulfill all the in vitro criteria expected for PSCs. Specifically, they possess the primitive morphology of early-development cells (high nuclear/cytoplasmic ration, the presence of euchromatin in nuclei, and a few mitochondria) and express markers typical of PSCs (eg, Oct-4, Nanog, and Rex-1). More importantly, we recently demonstrated the presence of an open-type chromatin in the Oct-4 promoter in murine BM VSELs. Specifically, molecular analysis revealed its hypomethylation and association with transcription-permissive histones (indicated by a high H3Ac/H3K9me2 ratio) [24]. Moreover, the promoter of another core transcription factor, Nanog, despite having a higher level of methylation in VSELs (∼50%) than the Oct-4 promoter, is also transcriptionally active and has a high H3Ac/H3K9me2 ratio that favors transcription [24]. Based on these results, we conclude that murine VSELs truly express both Oct-4 and Nanog [24]. With regard to the other in vitro criteria of pluripotency, murine VSELs also possess bivalent domains in promoters that encode developmentally important homeobox-containing transcription factors, such as Sox21, Nkx2.2, Dlx1, Lbx1h, Hlxb9, Pax5, and HoxA3 [26]. Furthermore, VSELs derived from female mice reactivate the X-chromosome [51]. Finally, we and other groups have succeeded in differentiating VSELs in vitro into cells of all three germ layers [4,36,39,40,42,52].
Nevertheless, in contrast to pluripotent ESCs and iPSCs, murine VSELs do not complete blastocyst development and do not grow teratomas in immunodeficient mice [24,50]. This discrepancy between in vitro and in vivo pluripotency criteria can be explained by epigenetic changes in the expression of some paternally imprinted genes [24] that govern quiescence of these rare cells. Specifically, VSELs, similar to primordial germ cells (PGCs), erase imprinting in regulatory regions for paternally imprinted genes at the Igf2-H19 and RasGRF1 loci and increase imprinting at some regulatory regions for maternally imprinted genes such as Igf2R and KCNQ1. Thus, murine BM VSELs, by epigenetic modulation of imprinted genes (Igf2-H19, RasGRF1, and IGF2R) that play an important a role in IIS, remain resistant to stimulation by insulin, IGF-1, and IGF-2 and to additional modulation of expression of the KCNQ1 locus, which regulates expression of the cell cycle inhibitor p57Kip2. As a result of this resistance, VSELs remain in adult tissues as a population of highly quiescent cells [24], and, therefore, these epigenetic changes explain their lack of ex vivo expansion.
Considering the in vivo criteria of stem cell pluripotentiality, one has to consider that while all these in vivo criteria apply very well to ESCs and iPSCs [53,54], they are not always applicable for other pluripotent stem cells such as epiblast stem cells (EpiSCs) [55–58] or PGCs [59–61]. In particular, PGCs, by changes in expression of imprinted genes, remain quiescent, do not proliferate, do not complement blastocyst development, and do not form teratomas [62–65]. However, they may be converted by appropriate manipulation to embryonic germ cells (EGCs), but this requires appropriate changes in the expression of the imprinted genes mentioned earlier [24,66]. This well-known fact has also important practical implications. As previously reported, since murine BM VSELs express several markers of migrating PGCs and undergo similar (but not identical) epigenetic changes as PGCs in the expression of imprinted genes [24], it might be possible by manipulating the expression of imprinted genes to reprogram them to a proliferation-permissive state and expand them ex vivo for therapeutic purposes. This is one of the current challenges in our laboratory.
In Table 1, we have summarized the most important characteristics of murine and human VSELs.
Table 1.
The Most Important Identification Criteria That Are Attributable to BM-Purified Murine VSELs and Human UCB-Isolated VSELs
| Source of VSELs | Characteristics |
|---|---|
| Murine VSELsa | Enriched in population of cells that are slightly smaller than red blood cells. |
| Primitive morphology, large nuclei contain euchromatin, high nuclear/cytoplasmic ratio. | |
| Quiescent cells due to epigenetic changes in expression of some genes regulated by paternal imprinting (eg, Igf2-H19, RasGrf1, IGF2R, and p57Kip2). It also explains that they neither form teratomas nor complement blastocyst development. | |
| Purified VSELs are enriched for cells that express some markers characteristic for pluripotent stem cells (Oct-4, Nanog, SSEA-1, and presence of bivalent domains), epiblast and germ line cell markers. | |
| Number of VSELs decreases with the age and correlates with life span in experimental animals. | |
| In appropriate experimental models murine VSELs differentiate into cells from all three germ layers, including germ line. | |
| Highly resistant to stress, irradiation and cytostatics. | |
| Human VSELsb | Enriched in a population of cells that are slightly smaller than red blood cells. |
| Primitive morphology, large nuclei contain euchromatin, high nuclear/cytoplasmic ratio. | |
| Purified human UCB-VSELs are enriched for cells that express some markers characteristic for pluripotent stem cells (eg, Oct-4, Nanog, and SSEA-4) and some epiblast and primordial germ cell markers. | |
| Do not form teratomas in experimental animals | |
| Quiescent cells due to epigenetic changes in expression of some genes regulated by paternal imprinting (eg, Igf2-H19). | |
| In appropriate experimental models, human VSELs differentiate into hematopoietic cells, mesenchymal stem cells, and neural cells. Moreover, VSELs isolated from human gonads also differentiate into germ line cells. |
The best characterized so far at molecular level are murine VSELs purified from BM. More work is needed to compare VSELs isolated from other tissues with BM-purified ones.
Recent data reported on VSELs-like cells isolated from human ovaries indicate their molecular similarity to murine BM-purified VSELs [14,15,18,68,117,118].
VSEL, very small embryonic-like stem cells; UCB, umbilical cord blood; BM, bone marrow.
Why “Small Embryonic-Like” Stem Cells?
The name “embryonic” has been proposed historically, based initially on the morphology of these cells and transmission electron microscope images that revealed a similar chromatin structure as the chromatin in ESCs [3,67]. Furthermore, molecular analysis of gene expression performed later revealed that VSELs express not only Oct-4, Nanog, and Rex-1 but, in addition, also several markers characteristic of EpiSCs and epiblast-derived migratory PGCs [23,28]. We are aware that the name “embryonic-like” may create some confusion, in particular, when size and morphology of VSELs are compared with that of established immortalized ESC lines. However, one should take into consideration that stem cells in the preimplantation blastocyst or epiblast are very small and, in addition, we have to consider that quiescent cells residing in adult tissues have reduced cytoplasm and a high nuclear/cytoplasmic ratio.
In fact, several investigators have described populations of very small stem cells in adult murine [15,34–37,45,68], and most likely, some of these cell populations that possess broader differentiation potential across germ layers could be related to the VSELs (Table 2).
Table 2.
Examples of Selected Reports from Other Independent Groups on Small Stem Cells That Are Attributable to VSELs
| Cells name as originally described in the literature | References |
|---|---|
| ELH cells—Very small cells ∼5 μm in diameter isolated by elutriation and FACS sorting or by elutriation (E), lineage depletion (L), and recovered after homing (H) to BM. Give rise to long-term reconstituting hematopoietic cells (LT-HSCs) and epithelial cells. | [34,35,119] |
| Small nonhematopoietic Sca-1+ Lin−CD45− cells—Isolated by FACS from murine BM give rise to type II pneumocytes, producing surfactant in lung alveolar epithelium. Recently, these cells have been confirmed to be VSELs. | [39,52] |
| Pluripotent CD45-Sca-1+c-kit- cells—Isolated by FACS from murine BM, muscles, and intestinal epithelium that are able to differentiate into cells from all three germ layers. | [36] |
| Spore-like stem-cells—Very small cells, ∼5 μm in diameter, isolated from various murine tissues, resistant to freeze/thawing, expressing Oct-4, and showing broad differentiation. The isolation procedure of these small cells not revealed in original paper. | [37] |
| Rat embryonic-like stem cells (ELSCs)—Very small cells, ∼5 μm in diameter, isolated by FACS from rat bone marrow as SSEA-1+ Lin−CD45− population. These cells as reported express Oct-4 and are endowed with in vitro and in vivo cardiomyogenic and endothelial potential. | [45] |
| Ovarian and testicular VSELs—Small Oct-4+ SSEA+ cells isolated by FACS from ovarian surface epithelium (OSE) from mice (SSEA-1+) and humans (SSEA-4+)–precursors of female gametes. Human OSE-derived VSELs were characterized extensively by gene array for mRNA expression. Small Oct-4+ SSEA+ cells were also identified in murine and human testes as precursors of male gametes. | [14,15,18,68,117] |
| Embryonic-like stem-cells from UCB—Small CD45−, CD33−, CD7−, CD235a− pluripotent stem-cells (∼3 μm in diameter) co-expressing embryonic stem-cell markers, including Oct-4 and Sox2, and able to differentiate into neuronal cells. | [5,6] |
| Human UCB-derived VSELs—Small Oct-4+, SSEA-4+, Nanog+, Sox2+, Rex-1+, and Tert+ cells. | [7,20] |
| Human PB-derived VSELs—Oct-4+ very small cells isolated by FACS. In one of the reports SSEA-4+ CD133+ CXCR4+ Lin− and CD45− VSELs from human PB formed in immunodeficient mice vascularized bone fragments. | [9,41] |
| Omnicytes—Small Oct-4+ stem-cells identified in UCB, able to establish fetal-maternal chimerism. | [38] |
| UCB-derived nonhematopoietic CD34− Oct-3/4+, Sox2+, Rex-1+ cells—that are able to differentiate into neural lineage (neurons, astrocytes, oligodendrocytes). | [120] |
The Biological Function of VSELs in BM and Other Adult Tissues
We and others postulate that in BM, VSELs are a dormant population of stem cells which serve as precursors for long-term repopulating HSCs (LT-HSCs) [50,69,70] and MSCs [40,41]. By contrast, VSELs residing in the gonads give rise to gametes [15,68,71]. However, more studies are required to see whether VSELs in other organs play a similar role as precursors for monopotent TCSCs. Evidence has accumulated that VSELs are activated and mobilized into PB during tissue or organ injuries in murine and human models of heart infarct [10,44,72], stroke [11], skin burns [13], colitis ulcerosa [12], and tumor expansion [46] and in several of these pathologic situations, the number of VSELs circulating in peripheral blood may be of some prognostic value.
The number of VSELs in adult murine tissues reportedly decreases with age [73–75], and the number of these cells also correlates with lifespan in mice. Mice that live longer (eg, Laron and Ames dwarfs) maintain higher numbers of these cells in BM [73]. Preliminary not published data also indicate a positive effect of physical activity and calorie restriction in maintaining a high number of VSELs in adult murine tissues.
Based on this evidence, VSELs could be a back-up population of dormant stem cells in adult tissues that plays, on the one hand, an important role in organ regeneration during tissue injuries, and on the other hand, is involved in rejuvenation of the tissues. Since the number of these cells decreases with the age [73–75], this could help explain both the impaired regeneration and tissue rejuvenation observed in older individuals.
What Is the Rationale for Why PSCs/MPSCs Reside in Adult Tissues?
A decade ago, the concept of stem cell plasticity was proposed, based on the assumption that adult monopotent TCSCs (eg, HSCs) may trans-dedifferentiate into cells from other germ layers (eg, neural cells) [76–80]. This concept is currently rejected by a majority of the scientific community, and alternative explanations for the “phenomenon of stem cell plasticity” have been proposed, such as cell fusion [81–83] or the presence of heterogeneous populations of stem cells, for example, in BM, UCB, or mPB, including some rare stem cells endowed with broader differentiation potential [1,5,6,36,47,84,85]. Moreover, in parallel, cumulative evidence from several laboratories shows that in the adult tissues may reside cells which express some early development embryonic markers [5,6,30,86–95], and some of these cells may even possess germ line potential [96–103]. Some of these intriguing cells are listed in Table 3. It is important to emphasize that murine BM-purified VSELs are enriched as mentioned earlier in cells which express several markers characteristic for migrating PGCs [23].
Table 3.
Selected Reports from Other Groups on Stem Cells in Adult Nongonadal Tissues That Possess Germ Line Potential and/or Express Embryonic Stem Cell Markers (eg, Oct-4, SSEA, and MvH)
| Cells name as originally described in the literature | References |
|---|---|
| Stem cells with germ line potential from newborn mouse skin—Oct-4+ cells isolated by FACS from Oct-4-GFP mice that are able to give rise in vitro and in vivo to early oocytes. | [96] |
| Porcine multipotent stem/stromal cells—Oct-3/4+, Nanog+, Sox2+ cells isolated from porcine skin and adipose tissue able to differentiate into oocyte like cells. | [97] |
| SSEA-1+ murine BM cells—Isolated from murine BM by anti-SSEA-1 immunomagnetic beads. In the presence of BMP4 (bone morphogenic factor-4), they differentiate into Oct-4+Stella+Mvh+ and into gamete precursors. | [98] |
| BM-derived putative germ cells—Oct-4+Mvh+Dazl+Stella+ cells present in BM that may affect recurrence of oogenesis in mice sterilized by chemotherapy. | [99,100] |
| BM-derived male germ cells—Oct-4+, Mvh+, Stella+ cells isolated as Stra8-GFP cells from BM from Stra8-GFP transgenic mice. These cells express several molecular markers of spermatogonial stem cells and spermatogonia. | [101] |
| BM-derived precursors of male germ cells—GFP+ transgenic chicken Oct-4+SSEA-1/3/4+ BM cells after injection into testes give rise to functional sperm. | [102] |
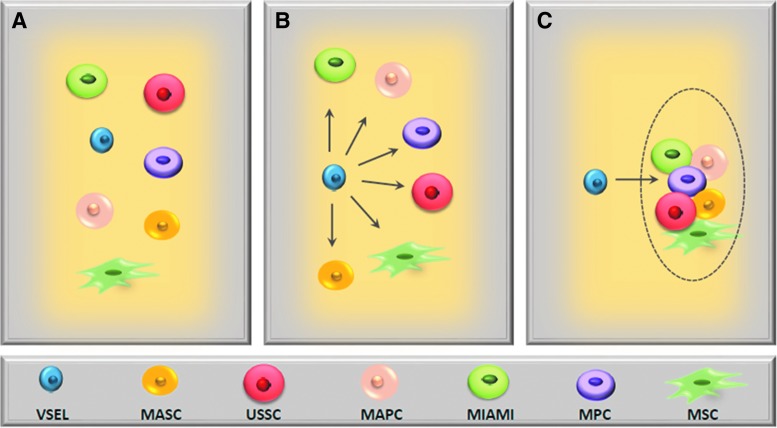
In support of the presence of early development stem cells in postnatal life, several types of putative PSCs or MPSCs have been described and isolated, primarily from hematopoietic tissues and that are able to give rise to cells from more than one germ layer [36,87,104–109]. These cells were isolated by employing various strategies, such as ex vivo expansion of partially purified immunomagnetic- or FACS-based sorted cells [35,36,39,45,87,104,107,108]. Nevertheless, in most of the expansion cultures, those rare cells that were able to initiate expansions and cross germ-layer commitment were not characterized at the single-cell level [78,87,105,106,108], and in most of these cases, the phenotype of the putative stem cell with PSC/MPSC properties was described “post factum,” after phenotyping clones of already differentiated, in vitro-expanded cells [87,104,108,110]. Nevertheless, many of the investigators would agree that if early-development stem cells endowed with broader differentiation potential reside in adult tissues, they are probably closely related and exist at different levels of tissue specification. Most likely, they represent overlapping populations of early-development stem cells that, depending on isolation strategy, ex vivo expansion protocol, and markers employed for their identification, have been given different names [87,104,105,107,108,110–112]. Figure 3 shows a hypothetical relationship to other populations of multi/pluripotent stem cells described in adult BM, PB, and UCB such as, for example, Multipotent Adult Stem Cells (MASC) [104], Multilineage-differentiating stress-enduring cells (Muse) [110,111], MSCs [112], Multipotent Adult Progenitor Cells [106], Unrestricted Somatic Stem Cells [107], Marrow-Isolated Adult Multilineage-Inducible Cells [108], and Multipotent Progenitor Cells [87,104].
FIG. 3.
Hypothetical relation of VSELs to other multi/pluripotent stem cells identified in adult bone marrow (BM), peripheral blood (PB), and UCB. (A) VSELs and other multi/pluripotent stem cells identified in hematopoietic tissues are independent populations of stem cells. (B) VSELs are the most primitive small dormant stem cells that on proper activation give rise to expanding other larger multi/pluripotent stem cells. (C) VSELs are the most primitive small dormant stem cells that on proper activation give rise to other larger multi/pluripotent overlapping stem cell populations. We hypothesize that it is the most likely scenario.
Overall, the presence of PSCs/MPSCs in adult tissues can be explained by the possibility that early, during embryogenesis, not all of the earliest-development stem cells disappear from the embryo after giving rise to TCSCs, but some may survive in developing organs as “a dormant back-up population of more primitive stem cells” [113]. These cells could give rise to monopotent TCSCs and, thus, be involved in tissue/organ rejuvenation and in organ regeneration after organ injury. In support of this notion, evidence has accumulated that adult murine tissues, in fact, contain, in addition to rapidly proliferating stem cells, a back-up population of more primitive dormant stem cells [3,4]. We have proposed as mentioned earlier that these most-primitive dormant stem cells are kept quiescent in adult tissues by changes in the expression of imprinted genes which mostly regulate insulin/insulin like growth factor-1 and -2 signaling (IIS) [24,66]. Interestingly, our proposal that the most-primitive stem cells in adult tissues which give rise to BM LT-HSCs [42,47] follow this mechanism has been recently somehow confirmed by another group [114]. Thus, we envision that VSELs, or stem cells very closely related to them, could fulfill the criteria for such dormant stem cells in adult tissues [113]. This, however, requires further experimental evidence, in particular for the most-primitive stem cells residing in other extra-hematopoietic tissues.
Future Directions
Despite significant progress in the field, there are still many problems with VSELs that should be solved. First, most of the data has been generated so far in murine BM-derived VSELs. We do not know whether phenotypically similar VSELs residing in other murine organs are regulated in the same way. Second, we are aware that we may be purifying a collection of small cells which are at different levels of tissue specification and development. Third, we also do not know whether human VSELs have a same molecular signature as their murine counterparts. Finally, as mentioned earlier, expansion in vitro is still a problem for both murine and human VSELs. We believe that the most important reason for these obvious obstacles is epigenetic modification of some imprinted genes in these cells [24] and unfortunately, our in vitro models which we applied so far did not provide optimal signals and microenvironment to reverse this phenomenon. Therefore, we need to explore the possibility that modification of imprinted genes in VSELs, as reported for PGCs [62–64], could help expand these cells. Another strategy would be to find a proper scaffold or supportive microenvironment that will force expansion and differentiation of VSELs, similarly as has been recently reported for other types of stem cells [115,116].
We expect that the next few years will bring answers about the developmental origin and biological role of this distinct and intriguing population of stem cells residing in adult tissues. We should also seriously consider a new hierarchy for the stem cell compartment not only in adult BM but also in other tissues and try to investigate the mutual relationship between VSELs and PSCs and MPSCs described by different investigators in adult organs [5,15,35,36,41,85,88,109].
Finally, we ask the scientific community to follow our well-described isolation protocols [19] and directly contact our group if there are problems with gating and sorting of these very rare cells. This will avoid confusion in the field and situations in which cells are identified as VSELs but lack a true VSEL phenotype [31–33]. While this paper was prepared for print two recent reports confirmed presence of VSELs in adult human and murine tissues [121, 122].
Acknowledgments
This study was supported by UE structural funds, the Innovative Economy Operational Program POIG.01.01.02-00-109/09 grant, NIH grants 2R01 DK074720 and R01HL112788, and the Stella and Henry Endowment to MZR.
Author Disclosure Statement
The University of Louisville is the owner of patents on VSELs and some areas of VSEL technology are licensed to Neostem, Inc., New York. None of the authors have any stock in Neostem or any other biotechnological stem cell company.
References
- 1.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J. and Ratajczak MZ. (2006). A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 20:857–869 [DOI] [PubMed] [Google Scholar]
- 2.Kucia M, Wu W. and Ratajczak MZ. (2007). Bone marrow-derived very small embryonic-like stem cells: their developmental origin and biological significance. Dev Dyn 236:3309–3320 [DOI] [PubMed] [Google Scholar]
- 3.Kucia M, Wysoczynski M, Ratajczak J. and Ratajczak MZ. (2008). Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res 331:125–134 [DOI] [PubMed] [Google Scholar]
- 4.Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr., Ratajczak J. and Ratajczak MZ. (2008). Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A 73A:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R. and Denner L. (2005). Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif 38:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuckin C, Jurga M, Ali H, Strbad M. and Forraz N. (2008). Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc 3:1046–1055 [DOI] [PubMed] [Google Scholar]
- 7.Bhartiya D, Shaikh A, Nagvenkar P, Kasiviswanathan S, Pethe P, Pawani H, Mohanty S, Rao SG, Zaveri K. and Hinduja I. (2012). Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev 21:1–6 [DOI] [PubMed] [Google Scholar]
- 8.Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B. and Ratajczak MZ. (2007). Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia 21:297–303 [DOI] [PubMed] [Google Scholar]
- 9.Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V. and Henon P. (2011). Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/Lin(-)CD45(-) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol 39:495–505 [DOI] [PubMed] [Google Scholar]
- 10.Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Paczkowska E, Ciosek J, Halasa M, Krol M, Kazmierski M, et al. (2009). Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol 53:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ. and Machalinski B. (2009). Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke 40:1237–1244 [DOI] [PubMed] [Google Scholar]
- 12.Marlicz W, Zuba-Surma E, Kucia M, Blogowski W, Starzynska T. and Ratajczak MZ. (2012). Various types of stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients with Crohn's disease. Inflamm Bowel Dis 18:1711–1722 [DOI] [PubMed] [Google Scholar]
- 13.Drukala J, Paczkowska E, Kucia M, Mlynska E, Krajewski A, Machalinski B, Madeja Z. and Ratajczak MZ. (2012). Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev 8:184–194 [DOI] [PubMed] [Google Scholar]
- 14.Virant-Klun I, Zech N, Rozman P, Vogler A, Cvjeticanin B, Klemenc P, Malicev E. and Meden-Vrtovec H. (2008). Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 76:843–856 [DOI] [PubMed] [Google Scholar]
- 15.Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K. and Hinduja I. (2011). Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev 20:1451–1464 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, Greco NJ, Tendera M. and Ratajczak MZ. (2013). Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells—implications for stem cell therapies in regenerative medicine. Stem Cells Dev 22:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuba-Surma EK, Klich I, Greco N, Laughlin MJ, Ratajczak J. and Ratajczak MZ. (2010). Optimization of isolation and further characterization of umbilical cord blood-derived very small embryonic/epiblast-like stem cells (VSELs). Eur J Haematol 84:34–46 [DOI] [PubMed] [Google Scholar]
- 18.Virant-Klun I, Skutella T, Hren M, Gruden K, Cvjeticanin B, Vogler A. and Sinkovec J. (2013). Isolation of small SSEA-4-positive putative stem cells from the ovarian surface epithelium of adult human ovaries by two different methods. Biomed Res Int 2013:690415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuba-Surma EK. and Ratajczak MZ. (2010). Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Curr Protoc Cytom Chapter 9:Unit9 29. [DOI] [PubMed] [Google Scholar]
- 20.Halasa M, Baskiewicz-Masiuk M, Dabkowska E. and Machalinski B. (2008). An efficient two-step method to purify very small embryonic-like (VSEL) stem cells from umbilical cord blood (UCB). Folia Histochem Cytobiol 46:239–243 [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak MZ, Zuba-Surma E, Wojakowski W, Suszynska M, Mierzejewska K, Liu R, Ratajczak J, Myung-Shin D. and Kucia M. (2013). Very Small Embryonic Like Stem Cells (VSELs) represent a real challenge in stem cell biology. Recent pros and cons in the midst of a lively debate. Leukemia [Epub ahead of print]; DOI: 10.1038/leu.2013.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratajczak MZ, Kucia M, Ratajczak J. and Zuba-Surma EK. (2009). A multi-instrumental approach to identify and purify very small embryonic like stem cells (VSELs) from adult tissues. Micron 40:386–393 [DOI] [PubMed] [Google Scholar]
- 23.Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M. and Ratajczak MZ. (2010). Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia 24:1450–1461 [DOI] [PubMed] [Google Scholar]
- 24.Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ. and Kucia M. (2009). Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia 23:2042–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mierzejewska K, Heo J, Kang JW, Kang H, Ratajczak J, Ratajczak MZ, Kucia M. and Shin DM. (2013). Genome-wide analysis of murine bone marrowderived very small embryonic-like stem cells reveals that mitogenic growth factor signaling pathways play a crucial role in the quiescence and ageing of these cells. Int J Mol Med 32:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ. and Kucia M. (2012). Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev 21:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuba-Surma EK. and Ratajczak MZ. (2011). Analytical capabilities of the ImageStream cytometer. Methods Cell Biol 102:207–230 [DOI] [PubMed] [Google Scholar]
- 28.Shin DM, Liu R, Klich I, Ratajczak J, Kucia M. and Ratajczak MZ. (2010). Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs). Mol Cells 29:533–538 [DOI] [PubMed] [Google Scholar]
- 29.Virant-Klun I, Skutella T, Kubista M, Vogler A, Sinkovec J. and Meden-Vrtovec H. (2013). Expression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid. Biomed Res Int 2013:861460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virant-Klun I, Stimpfel M, Cvjeticanin B, Vrtacnik-Bokal E. and Skutella T. (2013). Small SSEA-4-positive cells from human ovarian cell cultures: related to embryonic stem cells and germinal lineage? J Ovarian Res 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danova-Alt R, Heider A, Egger D, Cross M. and Alt R. (2012). Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS One 7:e34899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szade K, Bukowska-Strakova K, Nowak WN, Szade A, Kachamakova-Trojanowska N, Zukowska M, Jozkowicz A. and Dulak J. (2013). Murine bone marrow Lin(-)Sca(-)1(+)CD45(-) very small embryonic-like (VSEL) cells are heterogeneous population lacking Oct-4A expression. PLoS One 8:e63329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CK, Nakauchi H. and Weissman IL. (2013). Do pluripotent stem cells exist in adult mice as very small embryonic stem cells? Stem Cell Rep 1:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S. and Sharkis SJ. (2001). Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105:369–377 [DOI] [PubMed] [Google Scholar]
- 35.Jones RJ, Collector MI, Barber JP, Vala MS, Fackler MJ, May WS, Griffin CA, Hawkins AL, Zehnbauer BA, et al. (1996). Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood 88:487–491 [PubMed] [Google Scholar]
- 36.Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC. and Srour EF. (2003). Pluripotent stem cells identified in multiple murine tissues. Ann NY Acad Sci 996:158–173 [DOI] [PubMed] [Google Scholar]
- 37.Vacanti MP, Roy A, Cortiella J, Bonassar L. and Vacanti CA. (2001). Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem 80:455–460 [PubMed] [Google Scholar]
- 38.Mikhail MA, M'Hamdi H, Welsh J, Levicar N, Marley SB, Nicholls JP, Habib NA, Louis LS, Fisk NM. and Gordon MY. (2008). High frequency of fetal cells within a primitive stem cell population in maternal blood. Hum Reprod 23:928–933 [DOI] [PubMed] [Google Scholar]
- 39.Kassmer SH, Jin H, Zhang PX, Bruscia EM, Heydari K, Lee JH, Kim CF. and Krause DS. (2013). Very small embryonic-like stem cells from the murine bone marrow differentiate into epithelial cells of the lung. Stem Cells. [Epub ahead of print]; DOI: 10.1002/stem.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taichman RS, Wang Z, Shiozawa Y, Jung Y, Song J, Balduino A, Wang J, Patel LR, Havens AM, et al. (2010). Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev 19:1557–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havens AM, Shiozawa Y, Jung Y, Sun H, Wang J, McGee S, Mishra A, Taichman LS, Danciu T, et al. (2013). Human very small embryonic-like cells generate skeletal structures, in vivo. Stem Cells Dev 22:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC. and Ratajczak MZ. (2011). Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol 39:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, et al. (2008). Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells 26:1646–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, et al. (2011). Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med 15:1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu JH, Wang HJ, Tan YZ. and Li ZH. (2012). Characterization of rat very small embryonic-like stem cells and cardiac repair after cell transplantation for myocardial infarction. Stem Cells Dev 21:1367–1379 [DOI] [PubMed] [Google Scholar]
- 46.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, et al. (2013). Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratajczak J, Zuba-Surma E, Klich I, Liu R, Wysoczynski M, Greco N, Kucia M, Laughlin MJ. and Ratajczak MZ. (2011). Hematopoietic differentiation of umbilical cord blood-derived very small embryonic/epiblast-like stem cells. Leukemia 25:1278–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu R, Klich I, Ratajczak J, Ratajczak MZ. and Zuba-Surma EK. (2009). Erythrocyte-derived microvesicles may transfer phosphatidylserine to the surface of nucleated cells and falsely “mark”. them as apoptotic. Eur J Haematol 83:220–229 [DOI] [PubMed] [Google Scholar]
- 49.Ratajczak MZ, Mierzejewska K, Ratajczak J. and Kucia M. (2013). CD133 Expression strongly correlates with the phenotype of very small embryonic-/epiblast-like stem cells. Adv Exp Med Biol 777:125–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratajczak MZ, Shin DM, Liu R, Mierzejewska K, Ratajczak J, Kucia M. and Zuba-Surma EK. (2012). Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation—an update and comparison to other primitive small stem cells isolated from adult tissues. Aging (Albany NY) 4:235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratajczak MZ, Liu R, Ratajczak J, Kucia M. and Shin DM. (2011). The role of pluripotent embryonic-like stem cells residing in adult tissues in regeneration and longevity. Differentiation 81:153–161 [DOI] [PubMed] [Google Scholar]
- 52.Kassmer SH, Bruscia EM, Zhang PX. and Krause DS. (2012). Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells 30:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller FJ, Goldmann J, Loser P. and Loring JF. (2010). A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell 6:412–414 [DOI] [PubMed] [Google Scholar]
- 54.Smith KP, Luong MX. and Stein GS. (2009). Pluripotency: toward a gold standard for human ES and iPS cells. J Cell Physiol 220:21–29 [DOI] [PubMed] [Google Scholar]
- 55.Xue K, Ng JH. and Ng HH. (2011). Mapping the networks for pluripotency. Philos Trans R Soc Lond B Biol Sci 366:2238–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernemann C, Greber B, Ko K, Sterneckert J, Han DW, Arauzo-Bravo MJ. and Scholer HR. (2011). Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells 29:1496–1503 [DOI] [PubMed] [Google Scholar]
- 57.Hayashi K. and Surani MA. (2009). Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development 136:3549–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillich A, Bao S, Grabole N, Hayashi K, Trotter MW, Pasque V, Magnusdottir E. and Surani MA. (2012). Epiblast stem cell-based system reveals reprogramming synergy of germline factors. Cell Stem Cell 10:425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leitch HG, Nichols J, Humphreys P, Mulas C, Martello G, Lee C, Jones K, Surani MA. and Smith A. (2013). Rebuilding pluripotency from primordial germ cells. Stem Cell Rep 1:66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginsburg M, Snow MH. and McLaren A. (1990). Primordial germ cells in the mouse embryo during gastrulation. Development 110:521–528 [DOI] [PubMed] [Google Scholar]
- 61.Eguizabal C, Shovlin TC, Durcova-Hills G, Surani A. and McLaren A. (2009). Generation of primordial germ cells from pluripotent stem cells. Differentiation 78:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reik W. and Walter J. (2001). Genomic imprinting: parental influence on the genome. Nat Rev Genet 2:21–32 [DOI] [PubMed] [Google Scholar]
- 63.Hayashi K. and Surani MA. (2009). Resetting the epigenome beyond pluripotency in the germline. Cell Stem Cell 4:493–498 [DOI] [PubMed] [Google Scholar]
- 64.Wylie C. (1999). Germ cells. Cell 96:165–174 [DOI] [PubMed] [Google Scholar]
- 65.Hayashi K, de Sousa Lopes SM. and Surani MA. (2007). Germ cell specification in mice. Science 316:394–396 [DOI] [PubMed] [Google Scholar]
- 66.Ratajczak MZ, Shin DM, Schneider G, Ratajczak J. and Kucia M. (2013). Parental imprinting regulates insulin-like growth factor signaling: a Rosetta Stone for understanding the biology of pluripotent stem cells, aging and cancerogenesis. Leukemia 27:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melcer S. and Meshorer E. (2010). Chromatin plasticity in pluripotent cells. Essays Biochem 20:245–262 [DOI] [PubMed] [Google Scholar]
- 68.Bhartiya D, Kasiviswananthan S. and Shaikh A. (2012). Cellular origin of testis-derived pluripotent stem cells: a case for very small embryonic-like stem cells. Stem Cells Dev 21:670–674 [DOI] [PubMed] [Google Scholar]
- 69.Ratajczak MZ, Suszynska M, Pedziwiatr D, Mierzejewska K. and Greco NJ. (2012). Umbilical cord blood-derived very small embryonic like stem cells (VSELs) as a source of pluripotent stem cells for regenerative medicine. Pediatr Endocrinol Rev 9:639–643 [PubMed] [Google Scholar]
- 70.Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J. and Kucia M. (2008). Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev 4:89–99 [DOI] [PubMed] [Google Scholar]
- 71.Sandhya A, Bhartiya D, Sriraman K, Patel H, Manjramkar D, Bakshi G, Dhamankar V. and Kurkure P. (2013). Quiescent very small embryonic-like stem cells resist oncotherapy and can restore spermatogenesis in germ cell depleted mammalian testis. Stem Cells Dev [Epub ahead of print]; DOI: 10.1089/scd.2013.0059 [DOI] [PubMed] [Google Scholar]
- 72.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R. and Ratajczak MZ. (2004). Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res 95:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A. and Ratajczak MZ. (2011). Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice—novel view on Igf-1, stem cells and aging. Leukemia 25:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kucia M, Shin DM, Liu R, Ratajczak J, Bryndza E, Masternak MM, Bartke A. and Ratajczak MZ. (2011). Reduced number of VSELs in the bone marrow of growth hormone transgenic mice indicates that chronically elevated Igf1 level accelerates age-dependent exhaustion of pluripotent stem cell pool: a novel view on aging. Leukemia 25:1370–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kucia M, Masternak M, Liu R, Shin DM, Ratajczak J, Mierzejewska K, Spong A, Kopchick JJ, Bartke A. and Ratajczak MZ. (2013). The negative effect of prolonged somatotrophic/insulin signaling on an adult bone marrow-residing population of pluripotent very small embryonic-like stem cells (VSELs). Age (Dordr) 35:315–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A. and Anversa P. (2003). Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant 7 (Suppl. 3):86–88 [DOI] [PubMed] [Google Scholar]
- 77.Mezey E. and Chandross KJ. (2000). Bone marrow: a possible alternative source of cells in the adult nervous system. Eur J Pharmacol 405:297–302 [DOI] [PubMed] [Google Scholar]
- 78.Prockop DJ. (2003). Further proof of the plasticity of adult stem cells and their role in tissue repair. J Cell Biol 160:807–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wagers AJ. and Weissman IL. (2004). Plasticity of adult stem cells. Cell 116:639–648 [DOI] [PubMed] [Google Scholar]
- 80.Mezey E, Chandross KJ, Harta G, Maki RA. and McKercher SR. (2000). Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290:1779–1782 [DOI] [PubMed] [Google Scholar]
- 81.Vassilopoulos G. and Russell DW. (2003). Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev 13:480–485 [DOI] [PubMed] [Google Scholar]
- 82.Scott EW. (2004). Stem cell plasticity or fusion: two approaches to targeted cell therapy. Blood Cells Mol Dis 32:65–67 [DOI] [PubMed] [Google Scholar]
- 83.Eisenberg LM. and Eisenberg CA. (2003). Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Res C Embryo Today 69:209–218 [DOI] [PubMed] [Google Scholar]
- 84.Ratajczak MZ, Zuba-Surma EK, Wojakowski W, Ratajczak J. and Kucia M. (2008). Bone marrow—home of versatile stem cells. Transfus Med Hemother 35:248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kassmer SH. and Krause DS. (2013). Very small embryonic-like cells: Biology and function of these potential endogenous pluripotent stem cells in adult tissues. Mol Reprod Dev 80:677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anjos-Afonso F. and Bonnet D. (2007). Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood 109:1298–1306 [DOI] [PubMed] [Google Scholar]
- 87.Cesselli D, Beltrami AP, Rigo S, Bergamin N, D'Aurizio F, Verardo R, Piazza S, Klaric E, Fanin R, et al. (2009). Multipotent progenitor cells are present in human peripheral blood. Circ Res 104:1225–1234 [DOI] [PubMed] [Google Scholar]
- 88.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, et al. (2011). Evidence for human lung stem cells. N Engl J Med 364:1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Atari M, Barajas M, Hernandez-Alfaro F, Gil C, Fabregat M, Ferres Padro E, Giner L. and Casals N. (2011). Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol 26:1057–1070 [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, et al. (2011). Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell 145:1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.d'Aquino R, Tirino V, Desiderio V, Studer M, De Angelis GC, Laino L, De Rosa A, Di Nucci D, Martino S, et al. (2011). Human neural crest-derived postnatal cells exhibit remarkable embryonic attributes either in vitro or in vivo. Eur Cell Mater 21:304–316 [DOI] [PubMed] [Google Scholar]
- 92.Andreadis D, Bakopoulou A, Leyhausen G, Epivatianos A, Volk J, Markopoulos A. and Geurtsen W. (2013). Minor salivary glands of the lips: a novel, easily accessible source of potential stem/progenitor cells. Clin Oral Investig [Epub ahead of print]; DOI: 10.1007/s00784-013-1056-6 [DOI] [PubMed] [Google Scholar]
- 93.Roy S, Gascard P, Dumont N, Zhao J, Pan D, Petrie S, Margeta M. and Tlsty TD. (2013). Rare somatic cells from human breast tissue exhibit extensive lineage plasticity. Proc Natl Acad Sci USA 110:4598–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murrell W, Palmero E, Bianco J, Stangeland B, Joel M, Paulson L, Thiede B, Grieg Z, Ramsnes I, et al. (2013). Expansion of multipotent stem cells from the adult human brain. PLoS One 8:e71334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stimpfel M, Skutella T, Cvjeticanin B, Meznaric M, Dovc P, Novakovic S, Cerkovnik P, Vrtacnik-Bokal E. and Virant-Klun I. (2013). Isolation, characterization and differentiation of cells expressing pluripotent/multipotent markers from adult human ovaries. Cell Tissue Res 354:593–607 [DOI] [PubMed] [Google Scholar]
- 96.Dyce PW, Liu J, Tayade C, Kidder GM, Betts DH. and Li J. (2011). In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PLoS One 6:e20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song SH, Kumar BM, Kang EJ, Lee YM, Kim TH, Ock SA, Lee SL, Jeon BG. and Rho GJ. (2011). Characterization of porcine multipotent stem/stromal cells derived from skin, adipose, and ovarian tissues and their differentiation in vitro into putative oocyte-like cells. Stem Cells Dev 20:1359–1370 [DOI] [PubMed] [Google Scholar]
- 98.Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K. and Ragerdi Kashani I. (2012). BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int 36:1185–1193 [DOI] [PubMed] [Google Scholar]
- 99.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122:303–315 [DOI] [PubMed] [Google Scholar]
- 100.Selesniemi K, Lee HJ, Niikura T. and Tilly JL. (2009). Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY) 1:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J. and Engel W. (2006). Derivation of male germ cells from bone marrow stem cells. Lab Invest 86:654–663 [DOI] [PubMed] [Google Scholar]
- 102.Heo YT, Lee SH, Yang JH, Kim T. and Lee HT. (2011). Bone marrow cell-mediated production of transgenic chickens. Lab Invest 91:1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hua J, Yu H, Dong W, Yang C, Gao Z, Lei A, Sun Y, Pan S, Wu Y. and Dou Z. (2009). Characterization of mesenchymal stem cells (MSCs) from human fetal lung: potential differentiation of germ cells. Tissue Cell 41:448–455 [DOI] [PubMed] [Google Scholar]
- 104.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, et al. (2007). Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood 110:3438–3446 [DOI] [PubMed] [Google Scholar]
- 105.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49 [DOI] [PubMed] [Google Scholar]
- 106.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M. and Verfaillie CM. (2002). Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol 30:896–904 [DOI] [PubMed] [Google Scholar]
- 107.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, et al. (2004). A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA. and Schiller PC. (2004). Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 117:2971–2981 [DOI] [PubMed] [Google Scholar]
- 109.Ratajczak MZ, Zuba-Surma E, Kucia M, Poniewierska A, Suszynska M. and Ratajczak J. (2012). Pluripotent and multipotent stem cells in adult tissues. Adv Med Sci 57:1–17 [DOI] [PubMed] [Google Scholar]
- 110.Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M. and Dezawa M. (2013). Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8:1391–1415 [DOI] [PubMed] [Google Scholar]
- 111.Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, et al. (2011). Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA 108:9875–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le Blanc K. and Pittenger M. (2005). Mesenchymal stem cells: progress toward promise. Cytotherapy 7:36–45 [DOI] [PubMed] [Google Scholar]
- 113.Li L. and Clevers H. (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327:542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Venkatraman A, He XC, Thorvaldsen JL, Sugimura R, Perry JM, Tao F, Zhao M, Christenson MK, Sanchez R, et al. (2013). Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature 500:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nadri S, Kazemi B, Eslaminejad MB, Yazdani S. and Soleimani M. (2013). High yield of cells committed to the photoreceptor-like cells from conjunctiva mesenchymal stem cells on nanofibrous scaffolds. Mol Biol Rep 40:3883–3890 [DOI] [PubMed] [Google Scholar]
- 116.Nadri S, Yazdani S, Arefian E, Gohari Z, Eslaminejad MB, Kazemi B. and Soleimani M. (2013). Mesenchymal stem cells from trabecular meshwork become photoreceptor-like cells on amniotic membrane. Neurosci Lett 541:43–48 [DOI] [PubMed] [Google Scholar]
- 117.Bhartiya D, Unni S, Parte S. and Anand S. (2013). Very small embryonic-like stem cells: implications in reproductive biology. Biomed Res Int 2013:682326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anand S, Bhartiya D, Sriraman K, Patel H, Manjramkar D, Bakshi G, Dhamankar V. and Kurkure P. (2013). Quiescent very small embryonic-like stem cells resist oncotherapy and can restore spermatogenesis in germ cell depleted mammalian testis. Stem Cells Dev [Epub ahead of print]; DOI: 10.1089/scd.2013.0059 [DOI] [PubMed] [Google Scholar]
- 119.Jones RJ, Wagner JE, Celano P, Zicha MS. and Sharkis SJ. (1990). Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature 347:188–189 [DOI] [PubMed] [Google Scholar]
- 120.Habich A, Jurga M, Markiewicz I, Lukomska B, Bany-Laszewicz U. and Domanska-Janik K. (2006). Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp Hematol 34:914–925 [DOI] [PubMed] [Google Scholar]
- 121.Chang YJ, Tien KF, Wen CH, Hsieh TB, and Hwang SM. (2013). Recovery of CD45-/Lin-/SSEA-4+ very small embryonic-like stem cells by cord blood bank standard operating procedures. Cytotherapy [Epub ahead of print]; DOI: 10.1016/j.jcyt.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 122.Havens A, Sun H, Shiozawa Y, Jung Y, Wang J, Mishra A, Jiang Y, O'Neill DW, Krebsbach PH, et al. (2013). Human and murine very small embryonic-like (VSEL) cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev [Epub ahead of print]; DOI: 10.1089/scd.2013.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]