Abstract
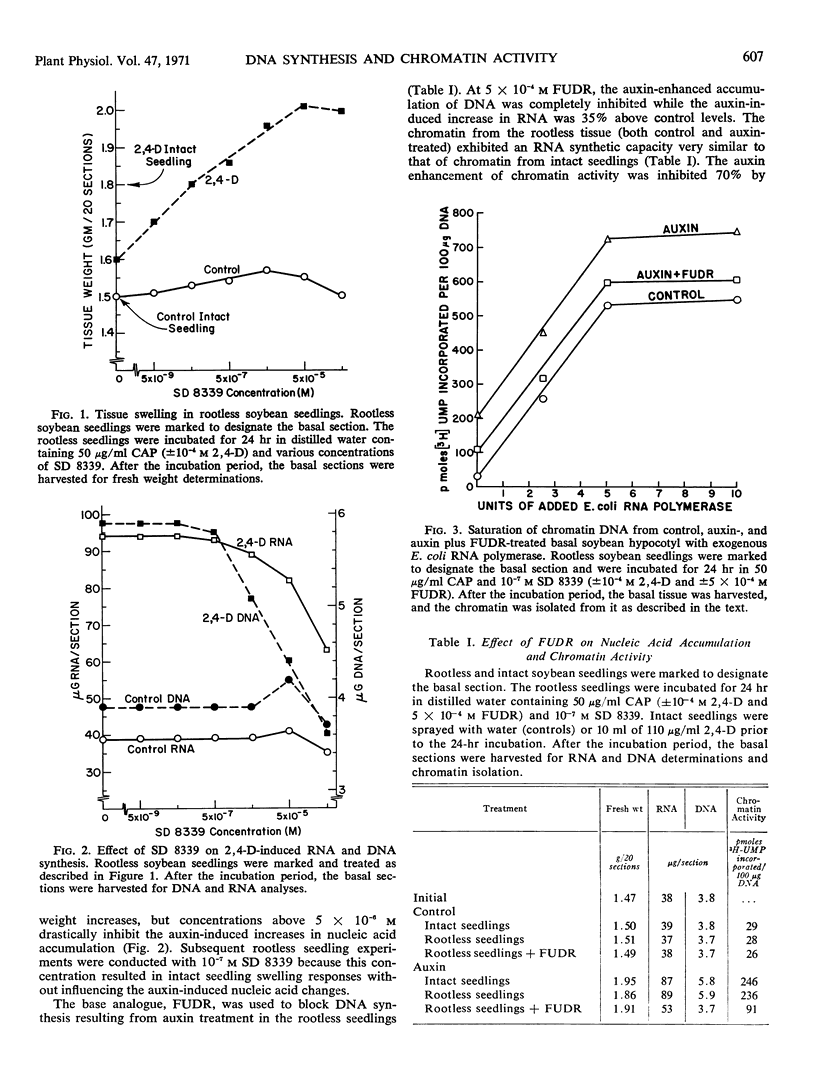
Rootless soybean (Glycine max) seedlings were used as a test system to examine the action of auxin on chromatin-directed RNA synthesis. Chromatin from the basal tissue of rootless seedlings (both control and auxin-treated) had RNA synthetic capacity similar to that of chromatin from comparably treated intact seedlings. When DNA synthesis normally induced in the basal tissue by auxin was blocked in the rootless seedlings by 5-fluorodeoxyuridine, the auxin enhancement of chromatin activity was inhibited 70%. This level was still three times the control level, indicating that auxin influenced the synthetic activity of existing DNA template. Experiments with Escherichia coli RNA polymerase revealed that chromatin from both auxin- and auxin plus 5-fluorodeoxyuridine-treated tissue saturated at higher levels than chromatin from control tissue.
Full text
PDF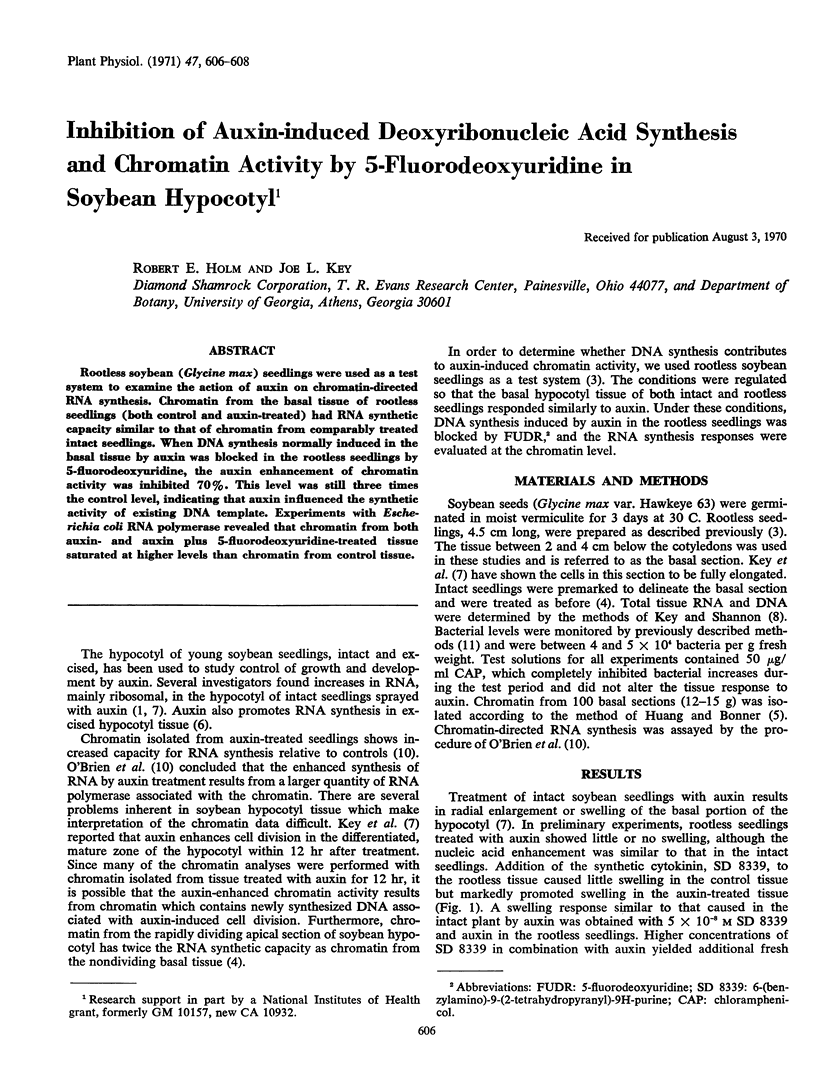

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. E., Key J. L. Hormonal regulation of cell elongation in the hypocotyl of rootless soybean: an evaluation of the role of DNA synthesis. Plant Physiol. 1969 Sep;44(9):1295–1302. doi: 10.1104/pp.44.9.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. E., O'brien T. J., Key J. L., Cherry J. H. The Influence of Auxin and Ethylene on Chromatin-directed Ribonucleic Acid Synthesis in Soybean Hypocotyl. Plant Physiol. 1970 Jan;45(1):41–45. doi: 10.1104/pp.45.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Ribonucleic Acid and Protein Synthesis as Essential Processes for Cell Elongation. Plant Physiol. 1964 May;39(3):365–370. doi: 10.1104/pp.39.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Phillips C. A protein intermediary in the interaction of a hormone with the genome. Proc Natl Acad Sci U S A. 1969 Jul;63(3):897–903. doi: 10.1073/pnas.63.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Sobota A. E., Leaver C. J., Key J. L. A detailed evaluation of the possible contribution of bacteria to radioactive precursor incorporation into nucleic acids of plant tissues. Plant Physiol. 1968 Jun;43(6):907–913. doi: 10.1104/pp.43.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]



