Abstract
Aims: We have recently shown that fenretinide preferentially targets CD34+ cells of acute myeloid leukemia (AML), and here, we test whether this agent exerts the effect on CD34+ cells of chronic myeloid leukemia (CML), which are refractory to imatinib. Results: As tested by colony-forming cell assays using clinical specimens, both number and size of total colonies derived from CD34+ CML cells were significantly reduced by fenretinide, and by combining fenretinide with imatinib. In particular, colonies derived from erythroid progenitors and more primitive pluripotent/multipotent progenitors were highly sensitive to fenretinide/fenretinide plus imatinib. Accordantly, fenretinide appeared to induce apoptosis in CD34+ CML cells, particularly with regard to the cells in the subpopulation of CD34+CD38−. Through cell quiescent assays, including Ki-67 negativity test, we added evidence that nonproliferative CD34+ CML cells were largely eliminated by fenretinide. Transcriptome and molecular data further showed that mechanisms underlying the apoptosis in CD34+ CML cells were highly complex, involving multiple events of oxidative stress responses. Innovation and Conclusion: As compared with CD34+ AML cells, the apoptotic effects of fenretinide on CD34+ CML cells were more prominent whereas less varied among the samples of different patients, and also various stress-responsive events appeared to be more robust in fenretinide-treated CD34+ CML cells. Thus, the combination of fenretinide with imatinib may represent a more sophisticated strategy for CML treatment, in which imatinib mainly targets leukemic blast cells through the intrinsic pathway of apopotosis, whereas fenretinide primarily targets CML stem/progenitor cells through the oxidative/endoplasmic reticulum stress-mediated pathway. Antioxid. Redox Signal. 20, 1866–1880.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder characterized by a t(9;22) reciprocal translocation that gives rise to the Philadelphia chromosome, eventually producing the constitutionally active BCR-ABL tyrosine kinase (46, 51). Imatinib mesylate (STI571; Gleevec) selectively blocks the tyrosine kinase activity and, consequently, induces apoptosis in CML cells (11). Treatment with imatinib for CML patients in chronic phase (CP) largely achieves a complete hematologic response (CHR), and reaches a complete cytogenetic response (CCR) (10). However, imatinib fails to provide a cure to CML patients. Minimal residual disease (MRD) appears to be common in CML patients treated with imatinib (22, 27). Clinical trials prove that a relapse could rapidly occur (2–7 months) after discontinuation of imatinib therapy even in those CML patients who have achieved genetic and molecular remission (8, 45, 55). Moreover, patients in accelerated phase (AP) and blast crisis (BC), characterized by the block of hematopoietic differentiation and thus the sharp accumulation of immature blasts, are mostly resistant to imatinib therapy (47, 53). Although other more potent BCR-ABL inhibitors, such as dasatinib (BMS-354825) and nilotinib (Amn107), may provide a possibility to improve the CHR and CCR rates in CML, it is now debatable whether the therapeutic strategy only targeting the BCR-ABL kinase is sufficient to prevent imatinib resistance or relapse in CML (5, 9).
Innovation.
Impairment of redox homeostasis plays a critical role in the genesis of cancer stem/initiation cells, and, thus, targeting redox homeostasis in these cells represents a new approach in cancer therapy. In this setting, we provide evidence demonstrating that fenretinide, a well-known oxidative stress-inducing agent in cancer cells, can effectively induce apoptosis in chronic myeloid leukemia (CML) stem/progenitor cells which are escapable from imatinib therapy. Thus, a combination of fenretinide with imatinib may represent a more sophisticated strategy for the treatment of CML, in which fenretinide targets imatinib-resistant CML stem/progenitor cells whereas imatinib targets leukemic blasts.
Numerous lines of evidence suggest that imatinib resistance and relapse can be largely attributed to CML stem/progenitor cells that are escapable from imatinib therapy (3, 17, 19, 60). These cells are present in small percentages in the leukemic cell mass of CML patients, whereas they are significantly enriched in the primitive CD34+ cells (3), and are indeed able to regenerate CML cell populations in immunodeficient mice (21). In vitro studies demonstrate that these primitive CML cells are insensitive to imatinib (17), dasatinib (5), and nilotinib (26). Accordingly, much attention has been focused on the development of agents and strategies for targeting CML stem/progenitor cells and for potentiating the efficacy of imatinib.
The redox signaling cascade may represent a new target in cancer stem/progenitor cells (37, 39). Mechanistically, PI3K/AKT pathways are abnormally activated by BCR-ABL, which may consequently impair downstream FoxOs, a key regulator in the maintenance of redox homeostasis in hematopoietic stem/progenitor cells (4, 54). It is, therefore, interesting to test whether agents that are able to perturb the redox homeostasis in tumor cells can be effective in targeting CML stem/progenitor cells. In this regard, fenretinide N-4-hydroxyphenylretinamide (4HPR), a well-known cancer chemo-preventive agent that effectively induces oxidative stress in many types of cancer cells, may represent a promising candidate. We have in parallel investigated potential effects of fenretinide on CD34+ cells of acute myeloid leukemia (AML) specimens, and found that this agent preferentially targets CD34+ cells in many of the tested specimens, while having limited cytotoxicity to normal CD34+ cells (58). Since CML is typically considered a hematopoietic stem cell disorder and the maintenance of redox homeostasis in its stem/progenitor cells is potentially impaired by the BCR-ABL signaling, it would be of great interest to investigate whether fenretinide is able to eradiate CML stem/progenitor cells that are refractory to imatinib.
Results
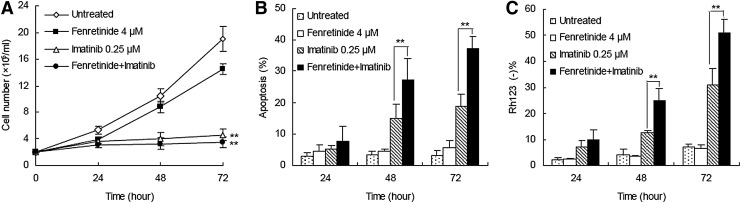
Fenretinide enhances the ability of imatinib for growth inhibition and apoptosis induction in CML-derived K562 cells
In an attempt to evaluate whether fenretinide was able to potentiate the ability of imatinib for growth inhibition and apoptosis induction in CML, a low dose (0.25 μM) of imatinib (12) and a physiologically achievable concentration (4 μM) of fenretinide (14) were utilized to treat CML-derived K562 cells. Fenretinide alone appeared to exert a minor effect (23.9%±4.4%) of inhibition on cell growth and a minimal effect on apoptosis induction (Fig. 1A–C). In contrast, the combination of fenretinide with imatinib induced significantly more apoptosis than imatinib alone, as evaluated by either Annexin V (37.2%±3.9% vs. 18.8%±4.0%) or Rh123 staining (51.0%±5.3% vs. 31.0%±6.5%). These results indicate that fenretinide is able to potentiate the efficacy of imatinib for apoptosis induction in CML-derived K562 cells.
FIG. 1.
Effects of fenretinide and imatinib on proliferation and apoptosis in K562 cells. (A) Growth curve of K562 cells under the indicated drug exposure as assessed by total viable cell counts. The combination of fenretinide with imatinib induced significantly growth inhibition (p<0.01) than untreated and fenretinide-alone treated group. (B) Induction of apoptosis in K562 cells under the indicated treatments, as evaluated through Annexin V-specific antibody and PI staining, and followed by flow cytometry analysis. The combination of fenretinide with imatinib significantly induced apoptosis than imatinib alone, as evaluated by Annexin V (p<0.01). (C) Loss of ΔΨm in K562 cells under the indicated treatments as determined by rhodamine 123 and PI double staining, and followed by flow cytometry analysis. Data represent the mean of three independent experiments. The combination of fenretinide with imatinib significantly induced more apoptosis than imatinib alone, as evaluated by Rh123 staining (p<0.01). **p<0.01 denotes the statistically significant level of difference among fenretinide, imatinib, fenretinide+imatinib, and untreated groups. ΔΨm, mitochondrial transmembrane potential; PI, propidium iodide.
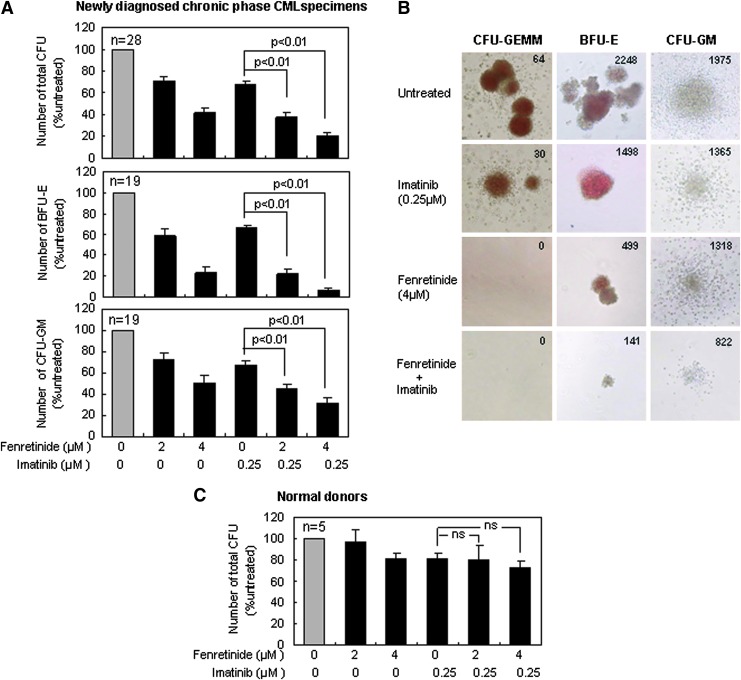
Fenrentinide potentiates the efficacy of imatinib for growth inhibition of colonies derived from CD34+ CML cells
CD34+ cells isolated from bone marrow or leukapheresis products of newly diagnosed CP CML patients (Table 1) were applied to colony-forming cell (CFC) assays on semi-solid methycellulose medium for 14 days with the presence of imatinib (0.25 μM), fenretinide (2 and 4 μM), and the combination of the two, respectively. The median frequency of total colony-forming units (CFUs) from untreated CP specimens was 161 per 1000 cells plated, ranging from 68 to 440. In contrast, the frequency of CFUs on the medium with fenretinide was reduced to 69%±4% (2 μM) and 39%±5% (4 μM) of that from the untreated specimens (the top panel of Fig. 2A). Imatinib was reported to exert a moderate effect on the suppression of primitive CML cells (20). Consistent with this report, the frequency of CFUs on the medium with imatinib was reduced to the level (68%±4%) equivalent to that resulting from fenretinide. With the addition of 2 and 4 μM fenretinide present in the medium, however, frequencies of CFUs dropped to 35%±3% (p<0.01) and 18%±3% (p<0.01), respectively, implicating that fenretinide significantly increases the ability of imatinib to suppress primitive CML cells.
Table 1.
Clinical Profile of the Patients with Chronic Myeloid Leukemia Involved in the Study
| Patient no. | Age/Sex | Phase | WBC (×109/L) | Hb (g/L) | PLT (×109/L) | Mutation | BCR-ABL variant | Previous therapy | Cell type |
|---|---|---|---|---|---|---|---|---|---|
| CML1 | 25/F | CP | 90 | 116 | 311 | ND | b3a2 | None | BM |
| CML2 | 42/F | CP | 202 | 72 | 720 | – | b3a2 | None | BM |
| CML3 | 37/M | CP | 201 | 118 | 496 | – | b3a2 | None | BM |
| CML4 | 26/M | CP | 216 | 97 | 360 | – | b3a2 | None | BM |
| CML5 | 35/F | CP | 376 | 78 | 350 | ND | ND | None | LP |
| CML6 | 71/M | CP | 196 | 106 | 267 | – | b3a2 | None | BM |
| CML7 | 36/M | CP | 219 | 112 | 153 | – | b2a2 | None | BM |
| CML8 | 35/M | CP | 387 | 100 | 602 | – | b3a2 | None | LP |
| CML9 | 19/M | CP | 403 | 113 | 308 | – | b2a2 | None | BM |
| CML10 | 51/M | CP | 113 | 116 | 662 | ND | b3a2 | None | BM |
| CML11 | 30/M | CP | 90 | 136 | 303 | ND | b2a2 | None | BM |
| CML12 | 55/M | CP | 177 | 90 | 852 | ND | ND | None | BM |
| CML13 | 55/M | CP | 207 | 72 | 75 | – | b2a2 | None | BM |
| CML14 | 23/F | CP | 61 | 102 | 724 | – | b3a2 | None | BM |
| CML15 | 40/M | CP | 74 | 147 | 240 | – | b2a2 | None | BM |
| CML16 | 32/F | CP | 106 | 111 | 216 | – | b3a2 | None | BM |
| CML17 | 30/F | CP | 82 | 94 | 419 | – | b3a2 | None | BM |
| CML18 | 42/M | CP | 61 | 131 | 490 | – | b3a2 | None | BM |
| CML19 | 28/M | CP | 28 | 124 | 392 | – | b3a2 | None | BM |
| CML20 | 44/M | CP | 127 | 131 | 269 | – | b2a2 | None | BM |
| CML21 | 41/M | CP | 324 | 16 | 324 | ND | b2a2 | None | BM |
| CML22 | 43/F | CP | 107 | 125 | 728 | – | b2a2 | None | BM |
| CML23 | 40/F | CP | 130 | 114 | 399 | – | ND | None | BM |
| CML24 | 55/F | CP | 251 | 97 | 702 | ND | b3a2 | None | BM |
| CML25 | 51/M | CP | 39 | 142 | 212 | – | b2a2 | None | BM |
| CML26 | 29/M | CP | 130 | 136 | 341 | ND | b3a2 | None | BM |
| CML27 | 56/F | CP | 14.3 | 154 | 273 | – | b3a2 | None | BM |
| CML28 | 35/F | CP | 312 | 86 | 644 | – | b3a2 | None | BM |
| CML29 | 53/M | CP | 142 | 110 | 380 | – | b2a2 | None | BM |
| CML30 | 46/M | CP | 291 | 13 | 469 | – | b2a2 | None | BM |
| CML31 | 58/F | CP | 114 | 95 | 44 | ND | ND | None | BM |
| CML32 | 47/M | CP | 154 | 106 | 310 | – | b2a2 | None | LP |
| CML33 | 68/F | CP | 221 | 93 | 445 | ND | b3a2 | None | LP |
| CML34 | 31/F | CP | 49 | 120 | 437 | – | b2a2 | None | BM |
| CML35 | 55/M | CP | 311 | 121 | 370 | – | b2a2 | None | LP |
| CML36 | 46/M | CP | 137 | 100 | 196 | – | ND | None | BM |
| CML37 | 36/M | CP | 166 | 10 | 392 | – | b3a2 | None | BM |
CP, chronic phrase; M, male; F, female; ND, not determined; IM, imatinib; BM, bone marrow; LP, leukapheresis product; –, no mutation found; CML, chronic myeloid leukemia; CP, chronic phase.
FIG. 2.
Effects of fenretinide and imatinib on colony formation of CD34+ cells derived from CML patients or normal donors. (A) Effects of fenretinide combined with imatinib on colony formation of CD34+ CML cells obtained from newly diagnosed CP specimens. Compared with imatinib alone, the addition of fenretinide to imatinib significantly reduced the number of total CFUs, CFUs-GM, and BFUs-E. CD34+ cells were planted into the semi-solid methylcellulose-based media under the indicated drug exposure for 14 days. (B) The morphology of CFUs-GEMM, BFUs-E, and CFUs-GM in newly diagnosed CP specimens under the indicated drug exposure. Total counts of each type of colonies are shown on the upper right corner of each figure. (C) Effects of fenretinide and imatinib on the number of total CFUs derived from normal donor CD34+ cells. No significant difference between the co-treatment and imatinib alone was observed. Error bar represents SEM. CFUs, colony-forming units; CFUs-GM, colony-forming units-granulocyte/macrophage; CFUs-GEMM, granulocyte, erythrocyte and monocyte, megakaryocyte-colony-forming units; BFUs-E, burst-forming units-erythroid; CML, chronic myeloid leukemia; CP, chronic phase.
CFUs are mainly composed of burst-forming units-erythroid (BFUs-E) and colony-forming units-granulocyte/macrophage (CFUs-GM), respectively derived from erythroid progenitors and myeloid progenitors. As illustrated in the middle panel of Figure 2A, BFUs-E appeared to be highly sensitive to fenretinide or fenretinide plus imatinib. The relative number of BFUs-E was reduced from 59%±7% to 23%±6% (second and third columns), when the concentration of fenretinide was increased from 2 to 4 μM. With the addition of imatinib, such numbers were further reduced to 19%±5% and 6%±3% (fifth and sixth columns), respectively. CFUs-GM appeared to be less sensitive to fenretinide or fenretinide plus imatinib (the bottom panel of Fig. 2A), as compared with BFUs-E. However, significantly (p<0.01) reduced CFUs-GM, as resulting from imatinib combined with 2 μM of fenretinide or 4 μM of fenretinide, suggests that the efficacy of imatinib is potentiated. In addition to the reduction in colony number, a marked reduction in colony size of CFUs-GM appeared to be particularly evident in the medium with imatinib plus fenretinide (Fig. 2B).
Granulocyte, erythrocyte, and monocyte, megakaryocyte-colony-forming units (CFUs-GEMM) are a distinct category of colonies that are derived from more primitive hematopoietic progenitor cells. Although present in a small percentage, they may give rise to a variety of myeloid lineages under certain circumstances. As demonstrated in Figure 2B, a total of 64 such colonies were detected with untreated CML specimens and 30 were observed with the specimens treated by imatinib. Remarkably, no such colonies were detectable with the specimens treated with either fenretinide or fenretinide plus imatinib, indicating that fenretinide and its combinations with imatinib are particularly effective for the growth inhibition of CFUs-GEMM in CML patients.
In addition, we included CD34+ cells from five healthy donors in CFC assays to evaluate the potential cytotoxicity of the agents and their combinations with the indicated concentrations to normal cells. As shown in Figure 2C, the cytotoxicity of the agents and their combinations on these cells appeared to be minor or minimal. Based on CFC assays of thousands of colonies derived from several dozens of CML specimens, we have shown that fenretinide is able to inhibit growth of colonies from multi-lineages of CD34+ CML progenitor cells, particularly with regard to erythroid progenitor and pluripotent/multipotent progenitor cells. In addition, we have shown that this agent increases the ability of imatinib for growth inhibition of various progenitor-derived colonies in CML.
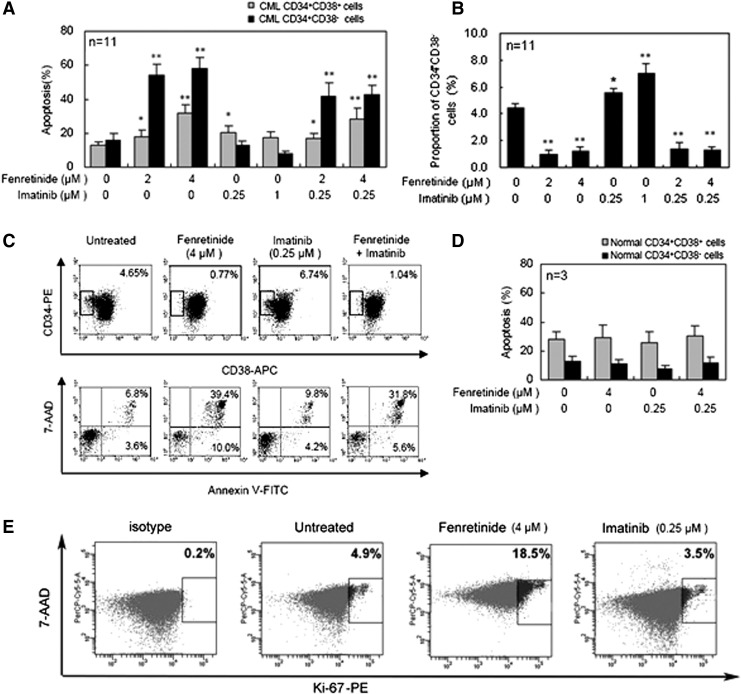
Fenretinide induces apoptosis in CD34+ CML cells that are refractory to imatinib
Next, we conducted apoptosis assays on CD34+ CML cells cultured in serum-free medium (SFM) under physiological growth factor conditions (20) and, respectively, with the presence of fenretinide, imatinib, or combinations of the two for 48 h. CD34+ cells are known hierarchical, and categorized into the minor portion of CD34+CD38− cells and the major portion of CD34+CD38+ cells. CD34+CD38− cells are composed of more primitive progenitors, including hematopoietic stem cells, whereas CD34+CD38+ cells are composed of more mature progenitor cells. Previous reports have implicated that imatinib is ineffective to eradicate CD34+ CML progenitor cells, particularly with regard to those which are more primitive (7, 17). Probably consistent with these reports, apoptotic effects exerted by imatinib on CD34+CD38+ cell were significant, whereas those on CD34+CD38− cells were minor/minimal (Fig. 3A). Interestingly, a four-fold increase in the concentration of imatinib (i.e., from 0.25 to 1 μM) was unable to increase the apoptosis level in either CD34+CD38+ or CD34+CD38− cells. In contrast, a higher concentration of fenretinide appeared to induce more apoptosis in both categories of the cells, in which CD34+CD38− cells were particularly sensitive to fenretinide-induced apoptosis. In the combined treatments, although the apoptosis levels were lower than or equivalent to those in the fenretinide-alone treatments, it was evident that apoptosis rates revealed by the combined treatments were significantly higher than those revealed by imatinib-alone treatments, particularly with apoptosis rates revealed in CD34+CD38− CML cells. In a reversed manner, we examined proportional changes of CD34+CD38− cells under the indicated treatment conditions (Fig. 3B, C). Indeed, percentages of CD34+CD38− cells in the samples treated with fenretinide or fenretinide plus imatinib were reduced significantly (p<0.01), as highlighted by a roughly three- to fourfold reduction. It is reported that cells in the CD34+CD38− subpopulation are particularly refractory to imatinib (17, 20). However, recent in vitro data show that imatinib reduces the number of CD34+CD38− cells to some extent (7). Probably consistent with these data, our in vitro experiments revealed a slight effect of apoptosis on CD34+CD38− CML cells by imatinib, though statistically not significant (Fig. 3A, C). Relatively, CD34+CD38+ CML cells are more sensitive to imatinib (Fig. 3A). Since the absolute number of CD34+CD38+ CML cells or CD34+CD38− CML cells are reduced rather than increased, a proportional increase in CD34+CD38− CML cells on imatinib treatment (Fig. 3B) suggests a proportional decrease in CD34+CD38+ CML cells of the treated sample. Nevertheless, our data add strong evidence that CD34+CD38− CML cells which are largely refractory to imatinib are highly sensitive to fenrentinide. Of note, the results cited earlier may also implicate that the inhibitory effects of fenretinide, as observed in CFC assay (Fig. 2A, B), are likely due to apoptotic mechanisms triggered by the agent. Although imatinib appears to exert some inhibitory effects on the formation of CFUs, CFUs-GM, and BFUs-E as well, mechanisms underlying the effects are possibly different, as apoptotic induction by imatinib is not obvious in CD34+ CML cells, particularly with regard to the cells of the CD34+CD38− subpopulation. As additional controls, CD34+ cells from three healthy donors were included in the assays (Fig. 3D), providing evidence that the apoptotic impacts of fenretinide or fenretinide plus imatinib with the indicated concentrations on normal CD34+CD38−/CD34+CD38+ cells were minimal.
FIG. 3.
Targeting CD34+CD38− CML cells while sparing normal counterparts by fenretinide. (A) Apoptosis rate of CD34+CD38− and CD34+CD38+ CML cells under the indicated drug exposure for 48 h in SFM. Compared with the untreated control, fenretinide alone and the co-treatment markedly induced apoptosis of CD34+CD38− and CD34+CD38+ CML cells, especially the CD34+CD38− cells. CD34+ cells were stained with CD34-PE, CD38-APC, AnnexinV-FITC, and 7-AAD, and then tested by flow cytometry analysis. (B) The proportion of CD34+CD38− cells under the indicated drug exposure. Compared with untreated control, fenretinide alone and the co-treatment markedly decreased the proportion of CD34+CD38− CML cells. (C) Representative flow cytometry dot plots showing the effects of fenretinide and imatinib on CD34+CD38− CML cells. The bottom panels show the apoptosis rate of CD34+CD38− CML cells linked to the top panels. Numbers on the top panels indicate the proportions of CD34+CD38− CML cells after 48 h of drug exposure, and numbers on the bottom panels represent the apoptosis rates. (D) Apoptosis rate of normal CD34+CD38− and CD34+CD38+ cells induced by the indicated drug treatments. The CD34+ cells were derived from three normal donors. No significant difference was observed for each pair-wise comparison. (E) Flow cytometry dot plots showing Ki-67 expression of CD34+ cells population. Proportions of quiescent cells under the indicated drug exposure are lacking expression of Ki-67, as normalized by isotype. Compared with untreated control, fenretinide greatly increased the proportion of Ki-67+ cells. Error bar represents SEM. *p<0.05, **p<0.01, respectively denote the statistically significant level of difference between the drug treatments and the untreated control. 7-AAD, 7-aminoactinomycin; SFM, serum-free medium.
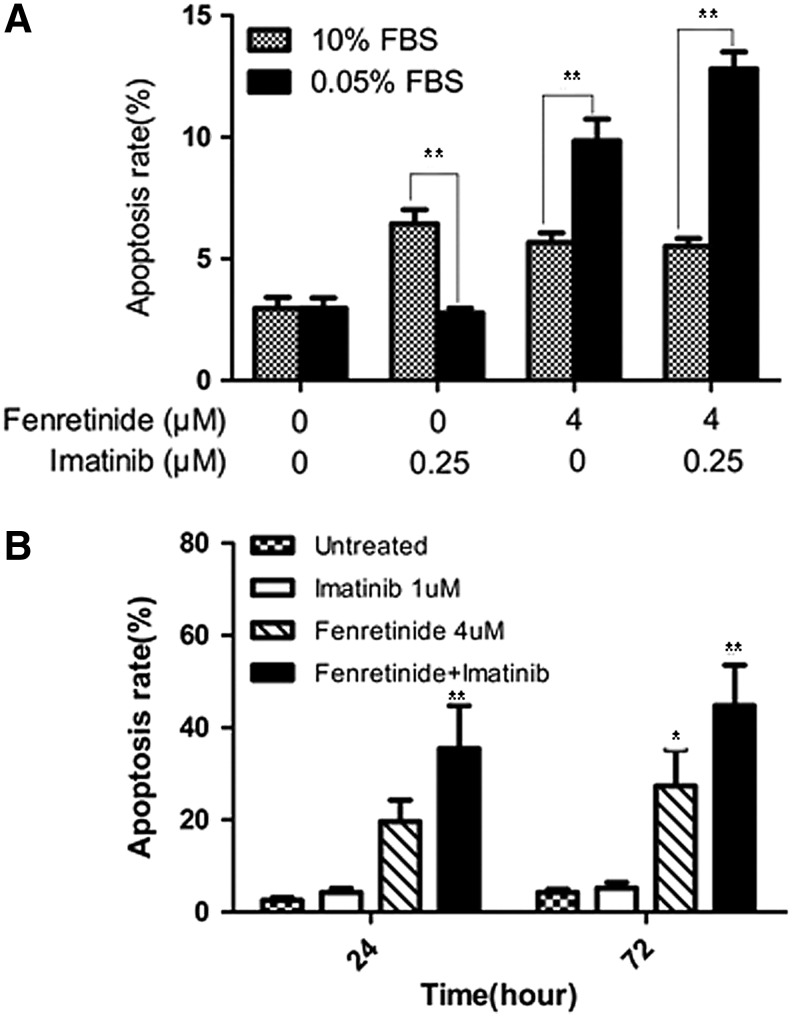
To test whether fenretinide exerts an effect on nonproliferative CML cells in the CD34+ population, we first conducted a Ki-67 expression assay (7) to monitor potential changes of proliferative/nonproliferative cells before and after treatment by fenretinide, as compared with imatinib. Ki-67 is a nuclear protein expressed in proliferating cells. It is expressed in all phases of the cell cycle except G0. The Ki-67 protein is exclusively expressed in proliferative cells and whose negativity is considered quiescence in CML (7). As illustrated in Figure 3E, 4.9% of the untreated CD34+ cells were positive with Ki-67 and a similar percentage (3.5%) of the imatinib-treated cells were positive with Ki-67. In sharp contrast, 18.5% of the fenretinide-treated cells were positive with Ki-67, implicating that the proportion of the nonproliferative cells which were quiescent in fenretinide-induced apoptosis cells were higher. Since the absolute number of CD34+ CML cells are reduced rather than increased after fenrentinide treatment for 48 h (∼30%; Fig. 3A), a sharp increase in the percentage of proliferating cells indicates a considerable decrease of nonproliferative cells in fenretinide-treated samples. In addition, we conducted a serum-starvation assay in K562 cells, which may result in the cell cycle arrest at the G0 phase and, thus, cell quiescence. As illustrated in Figure 4A, serum-starved K562 cells become sensitive to fenretinide even at the 12-h time point. With prolonged exposure to fenrentinide or fenretinide plus imatinib, the apoptosis rate of the serum-starved cells is much faster than that of the cells in the presence of 10% serum. In addition, imatinib-resistant BCR-ABL-T315I Ba/F3 cells were separately treated with imatinib (1 μM), fenretinide (4 μM), or the combination of the two for 24 and 72 h. The combination of fenretinide with imatinib induced more apoptosis than imatinib or fenretinide alone, as evaluated by Annexin V kit analysis (Fig. 4B). Thus, the inclusion of fenretinide for the treatment of CML may represent a strategy that potentiates the efficacy of imatinib in CML patients.
FIG. 4.
Effects of fenretinide and imatinib on apoptosis in serum-starved K562 cells and imatinib-resistant BCR-ABL-T315I Ba/F3 cells. (A) Fenretinide induces apoptosis in K562 cells under culture of serum-starvation. K562 cells were maintained in medium consisting of RPMI 1640 supplemented with 0.05% FBS and 10% FBS, respectively. Induction of apoptosis in K562 cells under the indicated treatments for 12 h, as evaluated through Annexin V-specific antibody and PI staining, and followed by flow cytometry analysis. Data represent the mean of three independent experiments. Error bar represents SD. **p<0.01 denotes the statistically significant level of difference between 10% FBS culture treatment and 0.05% FBS culture treatment. (B) Effects of fenretinide and imatinib on apoptosis in BCR-ABL-T315I Ba/F3 cells. BCR-ABL-T315I Ba/F3 cells were separately treated with imatinib (1 μM), fenretinide (4 μM), or the combination of the two for 24 and 72 h. Apoptosis assays were performed using Annexin V-FITC apoptosis detection kit. Data represent the mean of three independent experiments. Error bar represents SD. *p<0.05, **p<0.01, respectively denote the statistically significant level of difference between the drug treatments and the untreated control. FBS, fetal bovine serum.
Involvement of oxidative stress responses in apoptosis induced by fenretinide in CD34+ CML cells
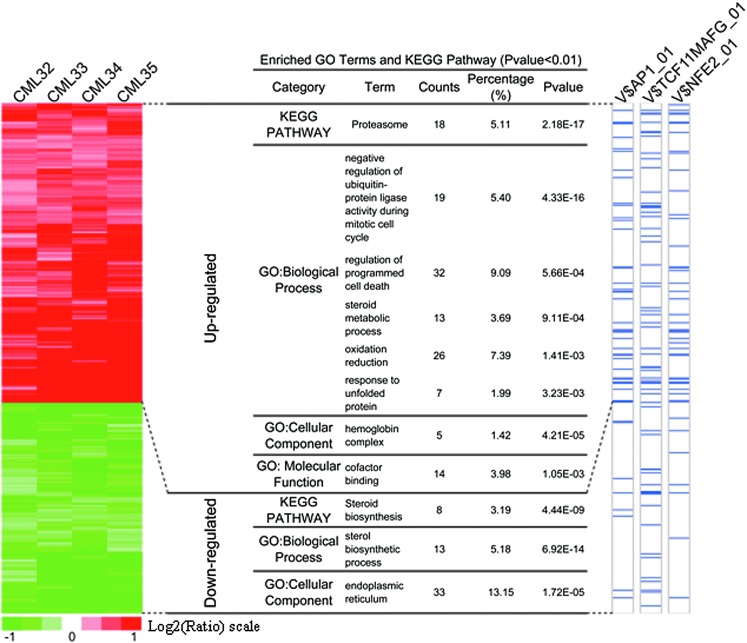
Although the CD34+ cell population is hierarchical, possible changes in genes or proteins associated with the apoptosis might be detectable through global approaches. Accordingly, CD34+ CML cells with and without fenretinide treatment were profiled using whole genome expression arrays (Affymetrix HG-U133 Plus 2.0 platform) (patients CML32, CML33, CML34, and CML 35; Table 1). To minimize potential data biases, both treated and untreated cell samples were maintained in culture for 48 h before hybridizations were performed. After data acquisition, the linear models for microarray data (LIMMA) method (15) was used to identify differentially expressed genes (based on the adjusted p-values<0.1; see Materials and Methods section) in a pair-wise manner. Based on four sample pairs, a total of 610 genes (813 probe sets) were identified as commonly regulated genes, of which 357 (478 probe sets) were up-regulated and 253 (335 probe sets) were down-regulated (the left panel of Fig. 5 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ars). As illustrated in the left panel of Figure 5, genes with similar expression patterns were displayed adjacently, suggesting that they may share common features. The expression level of genes in the upper column (the left panel of Fig. 5) gradually increased, whereas that of genes in the lower column tended to be gradually repressed in all the paired samples.
FIG. 5.
Gene expression signature underlying fenretinide-induced apoptosis in CML CD34+ cells from four patients. Left panel illustrates hierarchical visualization of the gene expression signature groups identified, an induced group (478 probe sets, representing 357 unique Entrezgenes) and a repressed group (335 probe sets, representing 253 unique Entrezgenes). Functional and regulatory relevance to each group is also displayed. Middle panel summarizes GO functional enrichments and KEGG pathway (p-value<0.01), and lists genes annotated in specific GO terms. The enrichment of the stress-responsive transcription factors AP-1 (FDR<10−3), TCF11/MafG (FDR<10−3) and NF-E2 (FDR<10−4) was integrated on the right panel of display, in which genes harboring the indicated TFBSs are marked by blue in the bars. GO, gene ontology; TFBS, transcription factor binding site.
To recognize prominent features inherited to the data, we, respectively, employed UCSC conserved transcription factor binding site (TFBS) (35) and gene ontology (GO) (1, 34) for the enrichment analysis (13). As illustrated in the middle panel of Figure 5, a number of significant GO terms/KEGG pathways were revealed in either the up-regulated or down-regulated gene category. Interestingly, the terms or pathways enriched in the up-regulated category strongly implicate the occurrence of oxidative stress-mediated apoptosis in fenretinide-treated cells, as typically highlighted by genes involved in programmed cell death, redox reactions, and unfolded protein response (UPR). In contrast, the most significant GO terms in down-regulated genes were those associated with endoplasmic reticulum (ER) functions such as steroid biosynthesis. Through hierarchical clustering followed by integration of genomic TFBS information (the right panel of Fig. 5), significantly enriched TFBSs in promoter regions of the up-regulated genes were represented by those of AP-1, TCF11/MafG, and NF-E2 (p-value: p=2.72×10−6, p=6.33×10−6, and p=2.25×10−7, respectively), suggesting that these transcription factors were potentially important for transcriptional changes in response to fenretinide treatment in CD34+ CML cells. AP-1 represents a dimeric complex consisting of various members of the Jun, Fos, and activation transcription factor sub-families, and it, thus, regulates a variety of biological events, including stress response and cell apoptosis (23, 49). TCF11, also known as NRF1, regulates a battery of cytoprotective genes through antioxidant response elements (AREs) in their promoter regions under stress stimuli (41). TCF11 also mediates proteasome homeostasis by inducing proteasome gene transcription, a crucial event in UPR (43). Heterodimerization with the small Maf protein MafG increases the DNA-binding affinity of TCF11, mediating gene transcription that is important for redox signaling and UPR (25). NF-E2 is a key transcription factor in controlling pathways of heme and globin synthesis involved in erythroid cell differentiation (2), thus regulating gene transcription of erythroid and megakaryocytic cells (29, 42). It was known as a negative regulator of erythroid proliferation in erythroleukemias (30), and its regulatory role was largely attributed to the control of its homeostatic concentration, which was mediated by P-JNK (29).
Fenretinide is known as an agent with a primary mode of action to increase cellular levels of reactive oxygen species (ROS) (24, 52, 57). In accordance, prominent features commonly implicated by transcriptome responses of CD34+ cells from the four patients appeared to be indeed relevant to various cellular stress responses, which were probably triggered by ROS. For instance, up-regulation of a number of oxidative responsive genes (e.g., HMOX1, SRXN1, NQO1, NQO2, DDIT3, and GCLM) suggested that anti-oxidative activities occurred in CD34+ cells after fenretinide treatment (Supplementary Table S2). Genes involved in these processes are represented by those encoding heat shock proteins (e.g., HSPA1A, HSPAIB, HSPA6, DNAJB1, DNAJB4, and BAG3) and components of proteasome (e.g., PSMB4, PSMB7, PSMC2, PSMC6, and PSMD14) suggested the occurrence of ER stress and, thus, UPR in the cells. Enhanced steroid metabolic process and reduced steroid biosynthesis, as strongly suggested by the data (Fig. 5), might be relevant to ER stress and UPR as well (28). The markedly induced hemoglobin genes were probably correlated with NF-E2-mediated erythroid differentiation. Taken together, transcriptome changes induced by fenretinide in CD34+ cells of CML suggest the occurrence of a series of stress-responsive events during the apoptosis induced by fenretinide.
Molecular evidence for the involvement of stress-mediated apoptosis in CD34+ CML cells on fenretinide treatment
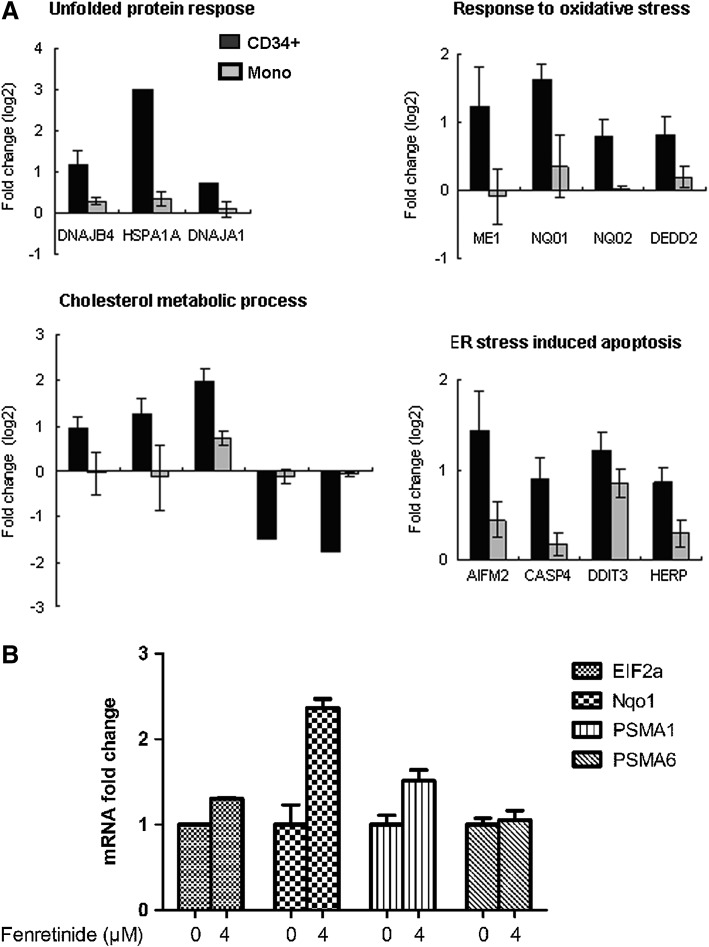
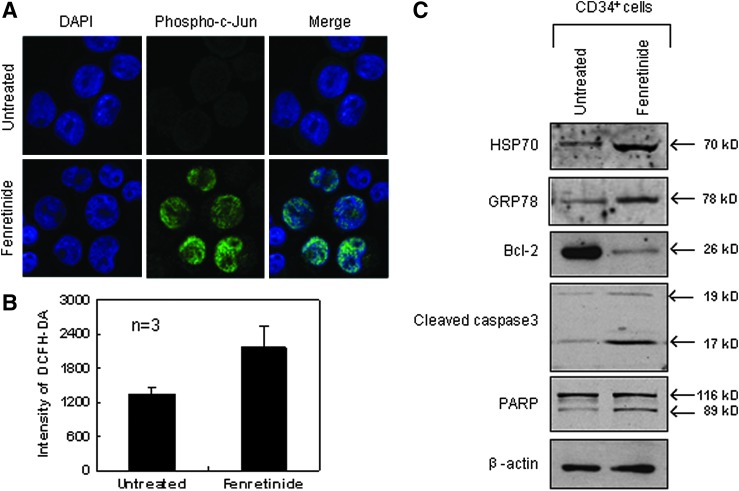
Next, we randomly selected genes, and compared their expression levels between the CD34+ cells and the monocytes after fenretinide treatment of six patients through reverse transcription-polymerase chain reaction (RT-PCR). As shown in Figure 6A, genes involved in oxidative stress, UPR, and ER stress-mediated apoptosis were significantly up-regulated in the CD34+ cells, as compared with the monocytes. In addition, aberrant ER function of steroid metabolic processes appeared to be evident. We also checked expression levels of four ARE cis-element containing genes in the CD34+ cells before and after fenrentinide treatment (Fig. 6B). As illustrated, three out of four revealed obvious changes, adding further evidence that redox signaling is involved in transcriptional regulation of this setting. Since activation of c-Jun, a potent member of AP-1 complex, was considered a pro-apoptotic signal under various stress conditions (50), we performed immunofluorescence assays using specific antibodies for phosphorylated c-Jun to test whether this transcription factor was activated in CD34+ CML cells after fenretinide treatment. As shown in Figure 7A, c-Jun was significantly phosphorylated and translocated into the nucleus, suggesting the activation of AP-1 in the fenretinide-induced apoptosis. To test whether the level of ROS was elevated in the fenretinide-treated cells, flow cytometry analysis by DCFH-DA staining was applied (Fig. 7B), showing that the ROS level was roughly increased by twofold, as compared with the untreated cells (p<0.05). Elevated levels of HSP70 and GRP78 are often used as landmarks for ER stress and UPR. As shown in Figure 7C, both HSP70 and GRP78 were up-regulated in CD34+ CML cells after fenretinide treatment. Bcl-2 is a distinguished protein of anti-apoptosis. The marked reduction of this protein, as illustrated in Figure 7C, indicated the occurrence of apoptosis in CD34+ CML cells treated with fenretinide. Caspase-3 is a downstream executor, and PARP is a common target of caspases. Accordingly, increased amounts of cleaved forms of both the proteins, as detected by the indicated antibodies (Fig. 7C), added additional layers of evidence that apoptosis occurred in cells that were fenretinide treated. Collectively, cellular and molecular data appear to be consistent with transcriptome data that fenretinide induces oxidative stress in CD34+ CML cells, which trigger a series of stress-responsive activities, including anti-oxidative activities, ER stress, and UPR, and, consequently, activate apoptotic caspases.
FIG. 6.
Results of real-time PCR revealed fenretinide specifically inducing the expression of ER stress-related genes in CML CD34+ cells. (A) Real-time PCR examine the expression level of genes related to UPR to oxidative stress, cholesterol metobolic process, and ER stress-induced apoptosis, which were functional enriched in microarray data, in CML CD34+ cells and mononuclear cells exposed to fenretinide for 48 h (n=6). (B) Endogenous mRNA levels of representative gene products induced by TCF11 and AP1 by real-time PCR in CML CD34+ cells exposed to fenretinide for 48 h (n=6). ER, endoplasmic reticulum; UPR, unfolded protein response; PCR, polymerase chain reaction.
FIG. 7.
Stress-responsive signatures underlying fenretinide-induced apoptosis in CD34+ CML cells from patients. (A) Representative confocal images of oxidative stress involved in fenretinide-induced apoptosis in CD34+ CML cells. Nuclear translocation of phosphorylated c-Jun induced by fenretinide in CML CD34+ cells after 48 h of drug exposure. (B) Elevation of intracellular ROS level in fenretinide-treated CML CD34+ cells, as determined by flow cytometry analysis using DCFH-DA staining after 24 h of drug exposure (p<0.05). (C) Western blot analysis of the selected proteins involved in oxidative stress and apoptotic cascades. Fenretinide induced expression of Hsp70 and Grp78 proteins, which responded to oxidative stress and UPR, and repressed expression of anti-apoptotic protein Bcl-2, followed by the cleavages of caspase-3 and PAPR. Error bar represents SEM. ROS, reactive oxygen species.
Discussion
Using imatinib to treat CML represents the first generation of targeted therapy in human malignancy, yielding a remarkable clinical outcome in terms of 5-year disease-free survival. However, late relapse is almost inevitable in imatinib therapy, which is largely attributed to BCR-ABL positive stem/progenitor cells, as these cells are refractory to imatinib and they are able to re-establish CML cell populations. Accordingly, much attention has been now attracted to the development of more sophisticated strategies by combining additional agent(s), which may, on the one hand, target more matured CML cells and, on the other hand, eradicate CML stem/progenitor cells. In this regard, fenretinide may represent a promising candidate. With decades of history in both laboratory and clinical studies, this vitamin A derivative has revealed distinct advantages compared with many other agents for treating tumor and leukemia (24, 36, 40). In addition to its efficacy against a wide range of tumor types, side effects exhibited by fenretinide are minimal, such as impaired night vision adaptation and dry skin, which readily disappear after treatment cessation (32). This agent also appears to be able to synergize with many other anti-cancer agents for apoptosis induction (31, 33, 48). In particular, fenretinide is known as a chemo-preventive agent and is able to reduce the risk of second breast cancer in premenopausal women (56), which has led us to speculate whether this agent possesses the ability to kill cancer cells at early stages. In this setting, we have shown data that fenretinide significantly enhances the efficacy of imatinib, thus offering a promising potential to overcome/reduce drug resistant and probably disease relapse as well in CML patients. Fenretinide markedly improves the ability of imatinib to induce apoptosis in K562 cells (Fig. 1) and exerts similar effects on primary CML cells (Figs. 2 and 3). Through CFC assays, we have shown that colonies derived from CD34+ primitive CML cells are suppressed by fenretinide or fenretinide plus imatinib, particularly with regard to those colonies derived from erythroid progenitors and pluripotent/multipotent stem/precursor cells of CML. In accordance with CFC results cited earlier, apoptosis assessments show that fenretinide induces cell death in both subpopulations of CD34+CD38+ and CD34+CD38− in a dose-dependent manner, whereas the apoptotic induction in the later is more efficient than that in the former, indicating that the stem-cell-enriched CD34+CD38− subpopulation is highly sensitive to fenretinide. Obviously, much remains to be learned regarding the potential of fenretinide for targeting CML stem cells. However, data of this setting have shown that fenretinide may enhance the efficacy of imatinib for the treatment of CML, as primitive CD34+ CML cells are refractory to imatinib. Even in imatinib-resistant cells, fenretinide appears to potentiate the efficacy of imatinib (Fig. 4).
Numerous studies have shown that fenretinide can induce apoptosis in cancer cells via oxidative stress (6, 18). However, mechanisms underlying the apoptosis can be complex and versatile. In this report, our data suggest that mechanisms underlying the apoptosis induced in CD34+ CML cells can be multifaceted, possibly attributed to the heterogeneity of the CD34+ cells. Nevertheless, downstream events of redox signaling that triggers the apoptosis appear to be more definitive, as highlighted by the occurrence of typical ER stress and UPR. In contrast, oxidative stress responses in our recent study of fenretinide on CD34+ AML cells (58) were not as prominent as those in fenretinide-treated CD34+ CML. Possible explanations would include that CML stem/progenitor cells were more close to hematopoietic stem/progenitor cells than AML stem/progenitor cells whereas less oncogenic. Based on TFBS analysis of the prominently regulated genes in this setting, transcription factors, including AP-1, TCF11/MafG (NRF1) and NF-E2, are likely to play important roles in the regulation of genes involved in the stress-responsive JNK pathway. However, transcription factors responding to fenretinide treatment in cancer cells can be versatile and cell type dependent. For instance, fenretinide-induced apoptosis in leukemic NB4 cells appears to be a typical process of oxidative stress-mediated apoptosis, involving a number of stress-responsive events such as ER stress and UPR, and transcriptionally orchestrated by NRF2 and HSF1 (57). In addition, stress-activated signaling pathways in fenrentinide-treated cancer cells can be different to some extent. For example, ceramide signaling is important to oxidative stress-mediated apoptosis in many type of cancer cells; whereas in three fenretinide-treated leukemic cell lines (i.e., NB4, U937, and HL-60), this signaling is only critical to the apoptosis in one of them (i.e., HL-60) (24). It should be noted that mechanistic studies of this setting are based on hierarchical CD34+ CML cells rather than on isolated CML progenitor or stem cells, and, thus, additional efforts are needed to elucidate the detailed mechanisms involved. On the other hand, intracellular redox homeostasis plays a crucial role in various biological processes under both physiological and pathological conditions (59). As byproducts of numerous cellular events, ROS may serve in turn as signaling molecules to regulate cellular events such as differentiation and apoptosis. In hematopoiesis, erythroid lineages and hematopoietic stem cells are highly sensitive to ROS signaling (16, 37). Erythroid precursors synthesize and accumulate hemoglobin, and circulating erythrocytes carry oxygen bound to hemoglobin and, as such, they are prone to oxidative damage. In erythroid cells of healthy individuals, over-produced ROS can be scavenged by antioxidants, protecting the cells from oxidative damage. In erythroid cells of CML, however, the ability to maintain redox homeostasis is probably impaired, which may explicate why erythroid progenitor cells are sensitive to fenretinide treatment in this setting. Hematopoietic stem cells are known to have low levels of metabolic activities, thus reducing the risk of the damage from metabolic products such as ROS. However, increased ROS may cause DNA damage and loss of quiescence, leading to accelerated aging of hematopoietic stem cells or formation of hematopoietic malignancies (16, 44). Since PI3K/AKT pathways are abnormally activated by BCR-ABL in CML cells, which may consequently impair downstream FoxOs, a key regulator in the maintenance of redox homeostasis in hematopoietic stem/progenitor cells (54). Interestingly, although ROS levels of CD34+CD38− CML cells appear to be equivalent to those of CD34+CD38+ CML cells, higher levels of ROS are observed in quiescent CML cells, as compared with proliferative CML cells (38). Such observations may, therefore, explicate why quiescent CML cells are highly sensitive to fenretinide in this setting. Since much of our knowledge about redox perturbation by fenretinide comes from various cancer cell lines, it remains to be elucidated whether this agent induces equivalent changes in ROS in CML stem/progenitor cells in order to trigger efficient apoptosis. However, the nature of fenretinide for inducing apoptosis in these stem/progenitor cells may allow us to address such a complex question in near future.
Materials and Methods
Cell culture, viability, and apoptosis assay
CML-derived cell line K562 cells and serum-starved K562 cells were maintained in RPMI 1640 supplemented with 10% and 0.05% fetal bovine serum (FBS) (PAA), respectively. Imatinib-resistant BCR-ABL-T315I Ba/F3 cells were kindly provided by Dr. Wenli Feng of the Department of Clinical Hematology, Key Laboratory of Laboratory Medical Diagnostics Designated by the Ministry of Education, Chongqing Medical University. BCR-ABL-T315I Ba/F3 cells were separately treated with imatinib (1 μM), fenretinide (4 μM), or the combination of the two for 24 and 72 h. Imatinib was kindly provided by Novartis Pharma, and fenretinide was purchased from Sigma. K562 cells were, respectively, treated with imatinib (0.25 μM), fenretinide (4 μM), and a combination of the two for 24, 48, and 72 h. Serum-starved K562 cells were also treated with imatinib (0.25 μM), fenretinide (4 μM), and a combination of the two for 12 h. Viable cells were counted by trypan blue exclusion. Apoptosis assays were performed using Annexin V-FITC apoptosis detection kit (BD Biosciences PharMingen), and mitochondrial transmembrane potential (ΔΨm) was evaluated using rhodamine 123 (Sigma) and propidium iodide staining, followed by flow cytometry.
CML specimens, CD34+ cell isolation, and culture
Fresh bone marrow cells or leukapheresis products were obtained from 37 CML patients in the newly diagnosed CP and five normal donors with informed consent and approval of Institutional Review Board at the School of Medicine in Shanghai Jiao Tong University (Table 1). Mononuclear cells were isolated by Ficoll density gradient centrifugation. CD34+ cells were enriched using EasySep® Human CD34 Positive Selection kit (Stem Cell Technologies) according to the manufacturer's instructions. The purity of CD34+ cells ranged between 92% and 98% in all samples determined by flow cytometry. CD34+ cells were cultured in a STEMPRO-34® SFM Complete Medium (Invitrogen) supplemented with growth factors (PeproTech) (20). The growth factors consisted of GM-CSF (200 pg/ml), G-CSF (1 ng/ml), SCF (200 pg/ml), LIF (50 pg/ml), MIP-1α (200 pg/ml), and IL-6 (1 ng/ml).
CFC assay
CD34+ cells were, respectively, mixed with imatinib (0.25 μM), fenretinide (2 or 4 μM), and the combinations of the two agents, and a quantity of 1000 CD34+ cells were plated in Methocult H4434 (Stem Cell Technologies). After incubation at 37°C for 14 days, CFUs-GEMM, CFUs-GM, and BFUs-E were, respectively, counted, and all the colony assays were performed in triplicate.
Apoptosis assessment of primitive CD34+ CML cells
CD34+ cells were, respectively, treated with imatinib (0.25 or 1 μM), fenretinide (2 or 4 μM), and the combinations of the two for 48 h in SFM. Then, CD34+ cells were first stained with antibodies against the surface markers CD34-PE and CD38-APC (Beckman Coulter) and incubated at room temperature for 15 min. Cells were then washed with cold phosphate-buffered saline (PBS) and resuspended in Annexin V 1×binding buffer with AnnexinV–FITC (BD PharMingen) and 7-aminoactinomycin (7-AAD) (Molecular Probes). Subsequently, the samples were incubated at room temperature for 15 min and analyzed by flow cytometry using FACSCalibur (BD Biosciences).
Ki-67 analysis
CD34+ cells were, respectively, treated with imatinib (0.25 μM) or fenretinide (4 μM) for 48 h in SFM. Then, the treated CD34+ CML cells were washed twice with wash buffer (PBS with 1% FBS, 0.09% NaN3 pH7.2) and stained for Ki-67 antibody according to the manufacturer's instructions (Beckman Coulter) and 7-AAD staining solution (Molecular Probes), and then tested by flow cytometry analysis using FACS Calibur (BD Biosciences).
Microarray hybridization and data mining
Total RNAs of CD34+ cells from four CML patients with or without 4 μM fenretinide treatment for 48 h were amplified and labeled with biotin according to the standard Affymetrix® protocol. The fragmented and biotinylated cDNA was then subjected to hybridization with the GeneChip® Human Genome-U133 Plus 2.0 array (Affymetrix). Raw expression data were normalized using robust multi-array averaging (RMA) with quantile normalization. The normalized expression data were subsequently imported into LIMMA bioconductor library (15) for the detection of differentially expressed probe sets using paired t-test for the fenretinide versus control comparison across four CML patients. The criteria for identifying the top significant probe sets was based on adjusted p-values (<0.1) corrected using Benjamini and Hochberg procedure. Hypergeometric distribution-based enrichment analyses of biological themes were performed to explore the underlying themes of those differentially expressed genes (p-values<0.1) in terms of biological relevance, for example, functional relevance as revealed by GO (1, 34) enrichment analysis and regulatory relevance as revealed by UCSC conserved TFBS enrichment analysis (13, 35). p-Value was applied to account for multiple hypothesis testing, thus to assess the significance of the biological theme enrichments.
Confocal microscopy
CD34+ CML cells were treated with 4 μM fenretinide for 48 h. Then, cells were fixed, rinsed, and incubated with phospho-c-Jun antibody (Cell Signaling Technology) at 4°C overnight. Cells were stained with goat anti-rabbit Alexa 488 secondary antibodies and Hoechst 33342 (Invitrogen) for nuclear staining. Fluorescence was observed using the×100 oil immersion objective (NA 1.4), further magnified by a×3 zoom, on a Leica SP5 confocal microscope (Leica Microsystems). Images were acquired using Leica confocal software v. 1.8.2 Build 1465.
ROS detection
CD34+ CML cells were treated with 4 μM fenretinide for 24 h. After washing with SFM, cells were incubated in SFM containing 10 μM 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma) for 30 min. The elevations of intracellular ROS induced by fenretinide were detected using flow cytometry.
Western blot analysis
Cells were harvested and lysed in 100 μl of ice-cold Triton lysis buffer (0.1% v/v Tx-100). Lysates were cleared by centrifugation, and a sample of the supernatant was removed for protein determination. Equivalent amounts of protein lysate had sodium dodecylsulfate sample buffer added and were boiled for 5 min. Samples were resolved by sodium dodecylsulfate–polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride filters. Immunoblotting was performed with specific antibodies for GRP78 (Santa Cruz Biotechnology), HSP70 and Bcl-2 (BD PharMingen), PARP, cleaved caspase-3, and β-actin (Cell Signaling Technology). For detection, blots were incubated with secondary HRP-linked antibodies and visualized using the ECL Plus system (GE Healthcare).
Statistical analysis
Statistical analysis was performed using the Student's t-test and one-way analysis of variance (ANOVA). A p-value less than or equal to 0.05 was chosen to be a statistically significant difference, unless specified otherwise.
Publicly deposited data
The transcriptome profilings of fenretinide-treated and untreated CD34+ cells from CML patients are available at GEO accession GSE17480. The following link is provided for review of record GSE17480 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=xxofhymccieuwdc&acc=GSE17480).
Supplementary Material
Abbreviations Used
- ΔΨm
mitochondrial transmembrane potential
- 7-AAD
7-aminoactinomycin
- AML
acute myeloid leukemia
- ANOVA
one-way analysis of variance
- AP
accelerated phase
- AREs
antioxidant response elements
- BC
blast crisis
- BFUs-E
burst-forming units-erythroid
- CCR
complete cytogenetic response
- CFC
colony forming cell
- CFUs
colony-forming units
- CFUs-GEMM
granulocyte, erythrocyte and monocyte, megakaryocyte-colony-forming units
- CFUs-GM
colony-forming units-granulocyte/macrophage
- CHR
complete hematologic response
- CML
chronic myeloid leukemia
- CP
chronic phase
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GO
gene ontology
- LIMMA
linear models for microarray data
- PBS
phosphate-buffered saline
- PI
propidium iodide
- RMA
robust multi-array averaging
- ROS
reactive oxygen species
- SFM
serum-free medium
- TFBS
transcription factor binding site
- UPR
unfolded protein response
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation (81170503 and 90919059), Chinese Academy of Sciences (KSCX2-EW-Q-1-08), Ministry of Science and Technology of China (2012AA02A505 and 2013CB966802), the Shanghai Commission of Science and Technology (11431922402), and SA-SIBS Scholarship Program (Y.D.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gene Ontology Consortium The Gene Ontology project in 2008. Nucleic Acids Res 36: D440–D444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews NC. The NF-E2 transcription factor. Int J Biochem Cell Biol 30: 429–432, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, and Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 101: 4701–4707, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, and Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, Barow M, Mountford JC, and Holyoake TL. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 107: 4532–4539, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Corazzari M, Lovat PE, Armstrong JL, Fimia GM, Hill DS, Birch-Machin M, Redfern CP, and Piacentini M. Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: the role of stress proteins ERdj5 and ERp57. Br J Cancer 96: 1062–1071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, and Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 121: 396–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes J, O'Brien S, and Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood 104: 2204–2205, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Donato NJ, Wu JY, Stapley J, Lin H, Arlinghaus R, Aggarwal BB, Shishodia S, Albitar M, Hayes K, Kantarjian H, and Talpaz M. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res 64: 672–677, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, and Larson RA. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355: 2408–2417, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, and Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2: 561–566, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Du Y, Wang K, Fang H, Li J, Xiao D, Zheng P, Chen Y, Fan H, Pan X, Zhao C, Zhang Q, Imbeaud S, Graudens E, Eveno E, Auffray C, Chen S, Chen Z, and Zhang J. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood 107: 1582–1590, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fang H, Yang Y, Li C, Fu S, Yang Z, Jin G, Wang K, Zhang J, and Jin Y. Transcriptome analysis of early organogenesis in human embryos. Dev Cell 19: 174–184, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, De Bernardi B, Bellani FF, and Formelli F. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res 9: 2032–2039, 2003 [PubMed] [Google Scholar]
- 15.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, and Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10: 1923–1940, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, and Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99: 319–325, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Hail N, Jr., Kim HJ, and Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis 11: 1677–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Helgason GV, Young GA, and Holyoake TL. Targeting chronic myeloid leukemia stem cells. Curr Hematol Malig Rep 5: 81–87, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, and Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood 99: 3792–3800, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Holyoake T, Jiang X, Eaves C, and Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 94: 2056–2064, 1999 [PubMed] [Google Scholar]
- 22.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, and Radich JP. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 349: 1423–1432, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal AK. Jun and Fos regulation of NAD(P)H: quinone oxidoreductase gene expression. Pharmacogenetics 4: 1–10, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Pan X, Chen Y, Wang K, Du Y, and Zhang J. Preferential involvement of both ROS and ceramide in fenretinide-induced apoptosis of HL60 rather than NB4 and U937 cells. Biochem Biophys Res Commun 405: 314–318, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Johnsen O, Skammelsrud N, Luna L, Nishizawa M, Prydz H, and Kolsto AB. Small Maf proteins interact with the human transcription factor TCF11/Nrf1/LCR-F1. Nucleic Acids Res 24: 4289–4297, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, and Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood 109: 4016–4019, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Talpaz M, O'Brien S, Garcia-Manero G, Verstovsek S, Giles F, Rios MB, Shan J, Letvak L, Thomas D, Faderl S, Ferrajoli A, and Cortes J. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood 103: 2873–2878, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kedi X, Ming Y, Yongping W, Yi Y, and Xiaoxiang Z. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis 207: 123–130, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lee TL, Shyu YC, Hsu PH, Chang CW, Wen SC, Hsiao WY, Tsai MD, and Shen CK. JNK-mediated turnover and stabilization of the transcription factor p45/NF-E2 during differentiation of murine erythroleukemia cells. Proc Natl Acad Sci USA 107: 52–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YJ, Higgins RR, Pak BJ, Shivdasani RA, Ney PA, Archer M, and Ben-David Y. p45(NFE2) is a negative regulator of erythroid proliferation which contributes to the progression of Friend virus-induced erythroleukemias. Mol Cell Biol 21: 73–80, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovat PE, Ranalli M, Bernassola F, Tilby M, Malcolm AJ, Pearson AD, Piacentini M, Melino G, and Redfern CP. Distinct properties of fenretinide and CD437 lead to synergistic responses with chemotherapeutic reagents. Med Pediatr Oncol 35: 663–668, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Malone W, Perloff M, Crowell J, Sigman C, and Higley H. Fenretinide: a prototype cancer prevention drug. Expert Opin Investig Drugs 12: 1829–1842, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Maurer BJ, Melton L, Billups C, Cabot MC, and Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst 92: 1897–1909, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Mi H, Guo N, Kejariwal A, and Thomas PD. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res 35: D247–D252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller W, Rosenbloom K, Hardison RC, Hou M, Taylor J, Raney B, Burhans R, King DC, Baertsch R, Blankenberg D, Kosakovsky Pond SL, Nekrutenko A, Giardine B, Harris RS, Tyekucheva S, Diekhans M, Pringle TH, Murphy WJ, Lesk A, Weinstock GM, Lindblad-Toh K, Gibbs RA, Lander ES, Siepel A, Haussler D, and Kent WJ. 28-way vertebrate alignment and conservation track in the UCSC Genome Browser. Genome Res 17: 1797–1808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon RC, McCormick DL, Becci PJ, Shealy YF, Frickel F, Paust J, and Sporn MB. Influence of 15 retinoic acid amides on urinary bladder carcinogenesis in the mouse. Carcinogenesis 3: 1469–1472, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Naka K, Muraguchi T, Hoshii T, and Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10: 1883–1894, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nieborowska-Skorska M, Kopinski PK, Ray R, Hoser G, Ngaba D, Flis S, Cramer K, Reddy MM, Koptyra M, Penserga T, Glodkowska-Mrowka E, Bolton E, Holyoake TL, Eaves CJ, Cerny-Reiterer S, Valent P, Hochhaus A, Hughes TP, van der Kuip H, Sattler M, Wiktor-Jedrzejczak W, Richardson C, Dorrance A, Stoklosa T, Williams DA, and Skorski T. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood 119: 4253–4263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogasawara MA. and Zhang H. Redox regulation and its emerging roles in stem cells and stem-like cancer cells. Antioxid Redox Signal 11: 1107–1122, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Ohshima M, Ward JM, and Wenk ML. Preventive and enhancing effects of retinoids on the development of naturally occurring tumors of skin, prostate gland, and endocrine pancreas in aged male ACI/segHapBR rats. J Natl Cancer Inst 74: 517–524, 1985 [PubMed] [Google Scholar]
- 41.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, and Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem 283: 33554–33562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono Y, Wang Y, Suzuki H, Okamoto S, Ikeda Y, Murata M, Poncz M, and Matsubara Y. Induction of functional platelets from mouse and human fibroblasts by p45NF-E2/Maf. Blood 120: 3812–3821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, and Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell 38: 17–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues MS, Reddy MM, and Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal 10: 1813–1848, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J, Gardembas M, and Mahon FX. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 109: 58–60, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243: 290–293, 1973 [DOI] [PubMed] [Google Scholar]
- 47.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, Fischer T, O'Brien SG, Stone RM, Gambacorti-Passerini CB, Russell NH, Reiffers JJ, Shea TC, Chapuis B, Coutre S, Tura S, Morra E, Larson RA, Saven A, Peschel C, Gratwohl A, Mandelli F, Ben-Am M, Gathmann I, Capdeville R, Paquette RL, and Druker BJ. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99: 3530–3539, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Shan D, Gopal AK, and Press OW. Synergistic effects of the fenretinide (4-HPR) and anti-CD20 monoclonal antibodies on apoptosis induction of malignant human B cells. Clin Cancer Res 7: 2490–2495, 2001 [PubMed] [Google Scholar]
- 49.Shaulian E. and Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Shen HM. and Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 40: 928–939, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Shtivelman E, Lifshitz B, Gale RP, and Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 315: 550–554, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Sun SY, Li W, Yue P, Lippman SM, Hong WK, and Lotan R. Mediation of N-(4-hydoxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res 59: 2493–2498, 1999 [PubMed] [Google Scholar]
- 53.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, Fischer T, Deininger MW, Lennard AL, Hochhaus A, Ottmann OG, Gratwohl A, Baccarani M, Stone R, Tura S, Mahon FX, Fernandes-Reese S, Gathmann I, Capdeville R, Kantarjian HM, and Sawyers CL. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99: 1928–1937, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, and Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Usuki K, Iijima K, Iki S, and Urabe A. CML cytogenetic relapse after cessation of imatinib therapy. Leuk Res 29: 237–238, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L, Decensi A, Camerini T, Del Turco MR, Di Mauro MG, Muraca MG, Del Vecchio M, Pinto C, D'Aiuto G, Boni C, Campa T, Magni A, Miceli R, Perloff M, Malone WF, and Sporn MB. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst 91: 1847–1856, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wang K, Fang H, Xiao D, Zhu X, He M, Pan X, Shi J, Zhang H, Jia X, Du Y, and Zhang J. Converting redox signaling to apoptotic activities by stress-responsive regulators HSF1 and NRF2 in fenretinide treated cancer cells. PLoS One 4: e7538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Mi JQ, Fang H, Wang Z, Wang C, Wu L, Zhang B, Minden M, Yang WT, Wang HW, Li JM, Xi XD, Chen SJ, Zhang J, Chen Z, and Wang KK. Preferential eradication of acute myelogenous leukemia stem cells by fenretinide. Proc Natl Acad Sci USA 110: 5606–5611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Du Y, Le W, Wang K, Kieffer N, and Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal 15: 2867–2908, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Zong Y, Zhou S, and Sorrentino BP. Loss of P-glycoprotein expression in hematopoietic stem cells does not improve responses to imatinib in a murine model of chronic myelogenous leukemia. Leukemia 19: 1590–1596, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.