Abstract
Pluripotent stem cells, both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the ability to differentiate into several cell types that can be used in drug testing and also in the study and treatment of diseases. These cells can be differentiated by in vitro systems, which may serve as models for human diseases and for cell transplantation. In this review, we address the pluripotent cell types, how to obtain and characterize these cells, and differentiation assays. We also focus on the potential of these cells in clinical trials, and we describe the clinical trials that are underway.
Introduction
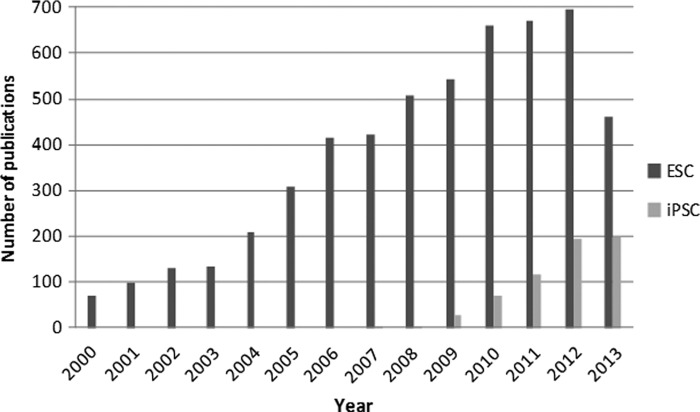
Pluripotent stem cells are a unique cells that are able to self-renew and differentiate into any adult tissue (epithelial, connective, muscle, neural, and others). This great differentiation capacity makes pluripotent stem cells very attractive to research studies with the hope of their being used in cell therapies in the future. We can divide pluripotent cells basically into two types. The first type, embryonic stem cells (ESCs), is physiological and is present in the blastocyst stage of embryonic development. These cells can be isolated from the inner cell mass (ICM) of the blastocyst (Bongso et al., 1994) during the stage of embryonic development when implantation occurs. The second type is an artificial or “induced” cell, called induced pluripotent stem cells (iPSCs); these cells were obtained for the first time in 2006 by the introduction of four genes able to reprogram somatic mouse cells into pluripotent stem cells (Takahashi and Yamanaka, 2006). One year later, it was demonstrated that human fibroblast cells also be reprogrammed (Takahashi et al., 2007). This new source of pluripotent cells has accelerated the number of studies in the pluripotent area. Figure 1 shows the evolution of publications in the field of ESCs and iPSCs since 2000 using data from PubMed.
FIG. 1.
Articles on pluripotent stem cells published from 2000–2014. (Data from Pubmed www.ncbi.nlm.nih.gov/pubmed; accessed 10/12/2013.)
The main objective of research with pluripotent stem cells is that these cells can be used in clinical trials. However, to use these cells in clinical applications, their efficiency and safety need to be proven scientifically. At the moment, there are still more questions than answers: What are the characteristics of a pluripotent cell? What is the best way to obtain and manipulate them? Are the differentiated cell lines derived from them really functional? Are iPSCs and ESCs equivalent? These questions still do not have answers. What we have is the hope that stem cells may one day provide therapies for human diseases, a hope that seems more likely with the advancement of scientific research. In this review, we will discuss the types of pluripotent cells and their characterization, pluripotent pathways, differentiation process, and the clinical trials using pluripotent stem cells.
Pluripotent Cell Types
There are two types of pluripotent cells that occur in nature: (1) ESCs and (2) embryonic germ cells (EGCs). ESCs can be isolated from the ICM of the blastocyst 4–5 days postfertilization. Human (h) ESCs are isolated from frozen embryos that were not used in in vitro fertilization procedures. ESCs are isolated and cultured in specific culture media and expanded in vitro. These cells are fed daily and can be enzymatically or mechanically separated for expansion to be used in experiments (Thomson et al., 1998).
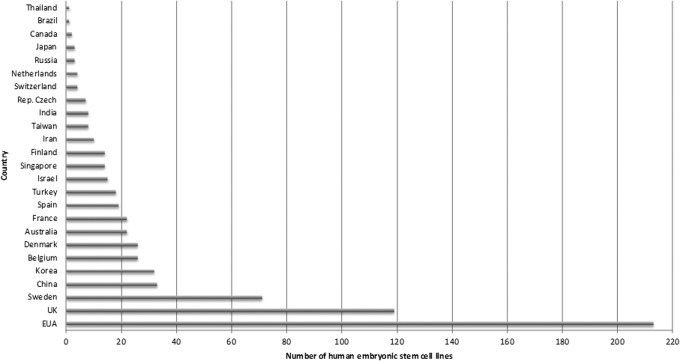
ESCs hold out the promise of being used in different cell therapies, and the demand continues for more hESC lines that can be used in clinical trials. Twenty-five countries are investing in the development of hESC lines (Fig. 2). There are a total of 695 validated hESC human cell lines (data from European Human Embryonic Stem Cell Registry, www.hescreg.eu/), of which 213 are in the United States and 119 in the United Kingdom.
FIG. 2.
Numbers of hESC lines per country deposited in European Human Embryonic Stem Cell Registry. (Data fromwww.hescreg.eu/; accessed 10/12/2013.)
The second type of pluripotent stem cells, EGCs, can be isolated from fetal gonads over 6–8 weeks postconception (Liu et al., 2004). In general, germ cells originate near the gut of an embryo and migrate to the developing gonads. EGCs can also be isolated from teratocarcinomas; these cells are usually aneuploid and cannot be used in therapeutic approaches.
Human EGCs cells express the transcription factor Oct4, a marker of pluripotency, at a high and steady level. These cells also can be differentiated in vitro into embryoid bodies (EBs) (Liu et al., 2004). Despite several similarities with ESCs, EGCs display some differences, such as transient self-renewal capability and distinct lineage-specific characteristics. In fact, under normal conditions, EGCs are believed to differentiate into germ cells only—oogonia/oocytes in the female and prospermatogonia in the male—that will produce eggs and sperm, respectively (De Felici et al., 2009).
In addition to these two natural types of pluripotent stem cells, there is another type, the artificial or “induced” cells, or iPSCs. This type of pluripotent stem cell is artificially derived from a nonpluripotent cell—typically an adult somatic cell—by inducing a “forced” expression of specific genes. The first human iPSCs were derived in 2007 from human fibroblasts in a series of experiments by Shinya Yamanaka's team at Kyoto University, Japan, and by James Thomson's team at the University of Wisconsin–Madison (Takahashi et al., 2007). Yamanaka had transformed human fibroblasts into pluripotent stem cells using four transcription factors—OCT3/4, SOX2, KLF4, and c-MYC—cloned in retroviral vectors, whereas Thomson and colleagues used OCT4, SOX2, NANOG, and LIN28 using a lentiviral system (Yu et al., 2007).
iPSCs emerged as a potential cell type to be used in cell therapy approaches. They represented a source of autologous cells that can avoid immune rejection frequently associated with allogeneic source such as ESCs or donated cells (Nishikawa et al., 2008; Yamanaka, 2008; Zhao and Daley, 2008). Only recently has the possibility that these cells have some immunogenic potential been discussed (Fairchild, 2010; Kadereit and Trounson, 2011). This idea was shown more clearly by Zhao et al. (2011), who demonstrated that there was rejection of syngeneic undifferentiated iPSCs when they were transplanted into mice. The authors showed that iPSCs were frequently rejected and showed T cell infiltration in the teratomas that originated from these cells. The same results were not observed in syngeneic ESC transplantation.
In 2013, four important studies tried to solve this issue (Araki et al., 2013; Guha et al., 2013; Morizane et al., 2013; Thanasegaran et al., 2013). Araki and Zhao's groups demonstrated that syngeneic integration-free iPSCs were rejected when injected into syngeneic mice (Araki et al., 2013; Zhao et al., 2011). However, unlike the results by Zhao's group, syngenic ESCs also showed a similar frequency of rejection. These data suggest that rejection must be connected to the expression of pluripotency genes and not to the specific features of iPSCs. Araki and colleagues also tested the rejection of differentiated cells. When ESCs or iPSCs were differentiated in vivo (matured in chimera mice), they were rarely rejected in syngeneic mice. However, when iPSCs were differentiated in vitro, rejection occurs frequently and infiltration of T cells was observed in the transplant. Guha and colleagues showed that autologous iPSCs could be used for cell replacement therapy without eliciting immune rejection. They tested viral iPSCs, episomal-generated iPSCs, and ESCs and observed no evidence of increased T cell proliferation in vitro, rejection of syngeneic cells after transplantation, or an antigen-specific secondary immune response. They concluded that undifferentiated or differentiated iPSCs were not rejected after transplantation. Likewise, Thanasegaran and colleagues analyzed the immunogenicity of iPSC clones derived from different ages of mice and, similarly to Guha, they did not find immunogenicity of undifferentiated iPSCs in syngeneic host mice (Thanasegaran et al., 2013; Guha et al., 2013). A similar result was founded by Morizane's group, which compared autologous and allogeneic transplantation of iPSCs differentiated into neural cells (Morizane et al., 2013). Pluripotent cells were differentiated in vitro in dopaminergic neurons and transplanted into the primate brain. They observed that the autologous transplantation of iPSC-derived neurons elicited only a minimal immune response in the brain, in contrast to allogeneic transplants that showed a great number of T cells infiltrated in the transplant.
The factors that lead to immune response when iPSCs are injected into syngeneic animals can be many. There can be many changes in gene expression and epigenetic pattern during the reprogramming process; injected cells are not differentiated into mature cells or are not identical to adult cells. Chemical reagents can be present in the culture medium, nonhuman sialic acid Neu5Gc can be secreted by mice feeder cells (Martin et al., 2005), and gene correction of a protein that is not expressed can occur (for example, iPSCs derived from hemophilia A and B patients) (Wang et al., 2008). All of these factors can potentially trigger an immune response that results in the rejection of the transplanted cells. It is not possible to conclude that iPSCs are more immunogenic than ESCs. The main difference among these studies is the site of injection of the cells (subcapsular renal space, brain, or subcutaneous), which certainly influences the rejection of the implanted cells. Despite the efforts that are being made in this area, studies need to continue to try to clarify whether iPSCs can trigger immune reactions before so they can be used in clinical trials.
Many studies have been showing that iPSCs are very similar or equivalent to ESCs in many aspects, including the expression of certain stem cell genes and proteins, chromatin methylation patterns, doubling time, EB formation, teratoma formation, viable chimera formation, and potency and differentiation potential. However, the full extent of their relation to natural pluripotent stem cells is still being assessed. There is a hypothesis that this variation results from residual transgene expression (Sommer et al., 2012). However, preliminary data suggest that even transgene-free iPSCs are epigenetically distinct from ESCs. Thus, the most acceptable hypothesis is that iPSCs contain a residual epigenetic signature of the origin tissue (“epigenetic memory”), and that the reprogramming process confers unique molecular features on iPSCs (Kim et al., 2010).
Pluripotent Pathways: Mechanisms That Induce and Support Pluripotency
Pluripotency and self-renewal are two important characteristics of pluripotent cells. These features enable these cells to be maintained indefinitely in culture and to differentiate into several cell types. Some signaling pathways regulate pluripotency and self-renewal. In the case of hESCs, the process of self-renewal is regulated mainly by transforming growth factor-β (TGF-β). The TGF-β pathway is activated by the signal transducer Smad2/3, and the fibroblast growth factor receptor (FGFR) activates mitogen-activated protein kinase (MAPK) and Akt (James et al., 2005; Ichida et al., 2009; Maherali and Hochedlinger 2009). The Wnt pathway also is involved in maintaining pluripotency in ESCs by a mechanism involving the inactivation of TCF3 (Sokol, 2011). These pathways result in the expression and activation of three main factors—Oct-4, Sox2, and Nanog—that will activate other genes related to pluripotency.
In the case of iPSCs, after the classic combination used by Takahashi and Yamanaka (2006), other combinations of transcription factors have been used successfully to derive the pluripotent state from different human somatic cell sources (for review, see Stadtfeld and Hochedlinger, 2010). These discoveries indicate that distinct gene combinations can also result in iPSC generation. Other genes, such as Esrrb (Feng et al., 2009), Nr5a2 (Heng et al., 2010), UTF1 (Zhao et al., 2008), TCL-1 (Picanço-Castro et al., 2011), Glis 1 (Maekawa et al., 2011), E-cadherin (Samavarchi-Tehrani et al., 2010), and selected microRNAs (mir 34, Choi et al., 2011), can also improve the reprogramming efficiency.
In addition to different combinations of transcription factors tested, other efforts have been made to improve the efficiency of iPSC generation and also the quality of these cells. This includes different types of vectors, such as polycistronic lentiviruses, adenoviruses, PiggyBac transposons, Sendai viruses, episomal DNA, mRNA transfections of the pluripotent genes, plasmid DNA, and also proteins of the pluripotent factors (Carey et al., 2009; Fusaki et al., 2009; Kim et al., 2009; Okita et al., 2008; Sommer et al., 2009; Stadtfeld et al., 2008; Warren et al., 2010; Woltjen et al., 2009; Yu et al., 2009; Yusa et al., 2009; Zhou and Freed 2009). Nonintegration approaches generate iPSCs free from exogenous genetic material, representing in the future, a source of cells that can be used in cell therapy trials.
Pluripotency Characterization
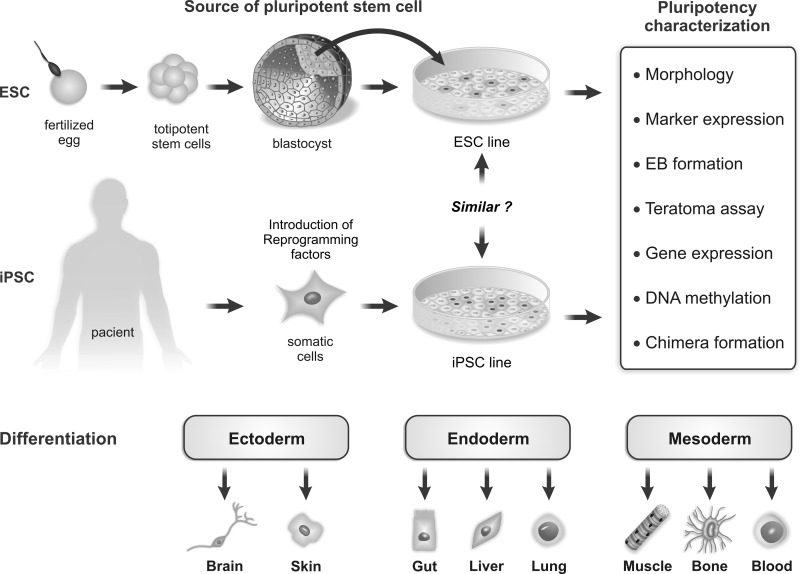
The pluripotency of ESCs and iPSCs can be assessed mainly by morphologic characteristics, marker and gene expression, and in vitro and in vivo differentiation. Figure 3 shows the generation of these cells and the main laboratory methods to prove the pluripotency.
FIG. 3.
Isolation, characterization, and differentiation potential of pluripotent stem cells. ESCs and iPSCs are obtained from different sources: hESCs are isolated from ICM cells of the blastocyst postfertilization. iPSCs can be generated from adult somatic cells and reprogrammed into pluripotent stem cells by the “forced” expression of specific genes. In culture, both pluripotent stem cells grow colonies, but the question is: Are ESCs and iPSCs equivalents? These cells can be characterized as “pluripotent” using different methodologies and the ability to differentiate into cells derived from ectoderm, mesoderm, and endoderm.
Morphology
The pluripotent stem cells cultured on inactivated mouse embryonic feeder cells (MEFs) monolayer or extracellular matrix (Matrigel) grow in colonies formed by small round cells. During reprogramming, iPSCs change their fibroblastoid morphology and begin to grow in colonies, similarly to ESCs.
Marker expression
There are several surfaces or intracellular antigens used to identify pluripotent stem cells, such as SOX2, Nanog, Oct3/4, TRA-1-60, and SSEA-1 in hESCs. These markers are widely used for characterization of hESCs and iPSCs.
Gene expression
The gene expression profile of pluripotent stem cells can be usually evaluated by real-time PCR and microarrays assay. These methodologies are frequently used for identifying genes that are differentially expressed in ESCs and iPSCs.
In vitro and in vivo differentiation
In culture (in vitro assay), pluripotent stem cells spontaneously differentiate into structures called EBs. These structures are composed of cells of three germ layers (ectoderm, mesoderm, and endoderm). To verify the pluripotency of ESCs and iPSCs in vivo, these cells are injected into immune-compromised mice, and their capacity to form teratomas, benign tumors composed of cell types from three germ layers, is evaluated.
The Differentiation Process of Pluripotent Stem Cells
Pluripotent stem cells have the ability to differentiate into all 216 cell types found in an adult organism. The first embryonic stem cell was isolated from mice in 1981 by Martin and colleagues (Martin, 1981). Since the late 1990s, when the first human line of ESCs was established in culture by Thomson and colleagues (Thomson et al., 1998), several cell types could be obtained from these cells, such as neurons, cardiomyocytes, smooth muscle cells, osteocytes, hepatocytes, keratinocytes, insulin-producing cells, hematopoietic cells, and endothelial cells (Kaufman et al., 2001; Kehat et al., 2001; Levenberg et al., 2002; Rambhatla et al., 2003; Reubinoff et al., 2001; Segev et al., 2004; Zhang et al., 2001). Moreover, the in vitro differentiation system allows the study of mechanisms involved in lineage specification of pluripotent stem cells to distinct cell types.
Currently, two methods are widely used to differentiate ESCs and iPSCs: (1) By EB formation; and (2) by co-culture of pluripotent stem cells with a mesenchymal stromal cell (MSC) monolayer. In the first method, pluripotent cells differentiate spontaneously and then form three-dimensional (3D) aggregates called EBs. This method has been used to obtain several cellular lineages in vitro. When sectioned, these structures contain cells of endodermal, mesodermal, and ectodermal tissues. The second method uses mainly mouse stromal cell lines. Pluripotent stem cells are cultivated with these stromal cells, which secrete growth factors and cytokines that induce differentiation. The choice of the differentiation method depends on the cellular type desired, with variations in the efficiency of differentiation.
The major cell types used from the endoderm include hepatic cells (Agarwal et al., 2008; Lavon and Benvenisty, 2005), lung epithelium (Van Vranken et al., 2005; Wang et al., 2007), and insulin-producing cells (Assady et al., 2001; Brolen et al., 2005). Studies have demonstrated that ESCs can be differentiated into functional hepatocytes both in vitro and in vivo (Agarwal et al., 2008; Duan et al., 2010; Yi et al., 2012). More recently, it was demonstrated that human iPSCs could also be differentiated into functional hepatocytes (Chen et al., 2012).
The mesodermal progenitors obtained from ESCs and iPSCs includes cardiomyocytes, endothelial cells, and hematopoietic cells. These cell types could be used for treatment of ischemic heart disease, repair of ischemic tissue, and to obtain all types of blood cells, respectively. However, the generation of these cell types is still inefficient, and thus the establishment of a refined protocol applicable for regenerative medicine is necessary.
The cells differentiated into the ectoderm lineage include cells of the epidermis, external sense organs, and central and peripheral nervous system (Gilbert, 2006). The generation of functional neurons has been shown by several groups employing the EB method (Reubinoff et al., 2000; Reubinoff et al., 2001) or mouse stromal cell line (Kawasaki et al., 2000; Zeng et al., 2004), and these cells can be used for the treatment of neurodegenerative diseases, such as acute spinal cord injury.
The induction of ESC and iPSC differentiation to produce different cell types requires complex differentiation steps with specific culture medium and growth factors, addition of cytokines, and supplements, representing a challenge in differentiation control. The high differentiation potential of ESCs into specific cell lineages through in vitro systems, EB formation, or co-culture with stromal cells represents a source of cells that can be used for testing new drugs and also for cell therapy clinical trials. For this purpose, it is essential that the protocols for maintenance or differentiation of these cells are carried out in the absence of animal components.
Clinical Trials
The most frequent question asked today is whether hESCs can be used in clinical trials. In 2009, the company Geron received by the Food and Drug Administration (FDA) approval to begin a Phase I clinical trial using hESCs. This clinical trial aimed at treating patients with acute spinal cord injury using hESCs differentiated into oligodendrocytes. Initial studies had shown significant restoration of mobility in animals with spinal injuries that received these cells (Keirstead et al., 2005). However, this study was discontinued in November, 2011, and in July, 2012, Geron abandoned its studies using stem cells due to financial constraints (Walsh, 2012).
In 2010, two new clinical trials were approved. Advanced Cell Technology (ACT), located in Marlborough, Massachusetts, leads studies to improve the vision of patients with Stargardt's macular dystrophy (SMD) and dry age-related macular degeneration (dry AMD), studies 4, 9, and 10. Originally, 12 patients participated in this clinical trial in three hospitals in the United States. In this clinical trial, patients were injected with retinal pigment epithelial cells (RPE) derived from hESCs (Vergano, 2010). In January, 2012, an article with preliminary results was published and showed that the hESC-derived RPE cells had no signs of hyperproliferation, tumorigenicity, ectopic tissue formation, or apparent rejection after 4 months (Schwartz et al., 2012). This study is still ongoing. In February, 2013, ACT received approval to initiate a Phase I/II clinical trial with RPE cells derived from hESCs for the treatment of severe myopia. CHA Bio & Diostech has two clinical trials very similar to the ACT studies (Table 1).
Table 1.
Clinical Studies Registry in Clinicaltrials.gov Website Using Search Term “Embryonic Stem Cells”
| Rank | Study | Cell type | Disease | Sponsor | Status |
|---|---|---|---|---|---|
| 1 | The Derivation of Human Embryonic Stem Cell Lines From PGD Embryos | Human embryonic stem cell lines from PGD embryos | Not applied | Hadassah Medical Organization | Recruiting |
| 2 | Derivation of New Human Embryonic Stem Cell Lines Lines for Clinical Use | Human embryonic stem cell | Not applied | Hadassah Medical Organization | Recruiting |
| 3 | A Study Of Implantation Of Human Embryonic Stem Cell Derived Retinal Pigment Epithelium In Subjects With Acute Wet Age Related Macular Degeneration And Recent Rapid Vision Decline | Human embryonic stem cell–derived retinal pigment epithelium (RPE) | Age-related macular degeneration | Pfizer | Not yet recruiting |
| 4 | Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cells Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt's Macular Dystrophy (SMD) | Human embryonic stem cell–derived retinal pigmented epithelial (hESC-RPE) cells | Stargardt's macular dystrophy (SMD) | Advanced Cell Technology | Recruiting |
| 5 | A Phase I/IIa, Open-Label, Single-Center, Prospective Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (MA09-hRPE) Cells in Patients With Advanced Dry Age-related Macular Degeneration(AMD) | Human embryonic stem cell–derived retinal pigmented epithelial (MA09-hRPE) cells | Age-related macular degeneration | CHA Bio & Diostech | Recruiting |
| 6 | Derivation of New Human Embryonic Stem Cell Lines: Identification of Instructive Factors for Germ Cells Development | Human embryonic stem cells | Not applied | Soroka University Medical Center | Unknown |
| 7 | The Role of TBX3 in Human ES Cell Differentiation | Human embryonic stem cells | Not applied | University of California, Irvine | Unknown |
| 8 | Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cells Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt's Macular Dystrophy (SMD) | Human embryonic stem cell–derived retinal pigmented epithelial (MA09-hRPE) cells | Stargardt's macular dystrophy (SMD) | CHA Bio & Diostech | Recruiting |
| 9 | Sub-retinal Transplantation of hESC Derived RPE (MA09-hRPE) Cells in Patients With Stargardt's Macular Dystrophy | Human embryonic stem cell–derived retinal pigmented epithelial (MA09-hRPE) cells | Stargardt's macular dystrophy (SMD) | Advanced Cell Technology | Recruiting |
| 10 | Safety and Tolerability of Sub-retinal Transplantation of hESC Derived RPE (MA09-hRPE) Cells in Patients With Advanced Dry Age Related Macular Degeneration (Dry AMD) | Human embryonic stem cell–derived retinal pigmented epithelial (MA09-hRPE) cells | Advanced dry age- related macular degeneration (dry AMD) | Advanced Cell Technology | Recruiting |
| 11 | Isolation and Characterization of Mammary Stem Cells | Tumor stem cells | Not applied | National Cancer Institute (NCI) | Completed |
| 12 | Patient Specific Induced Pluripotency Stem Cells (PSiPS) | Pluripotent stem cells from skin | Not applied | Royan Institute | Completed |
| 13 | Skin and Blood Research Samples From Healthy Volunteers and Patients With Hematologic Diseases | Not applied | Hematologic diseases | Washington University School of Medicine | Terminated |
| 14 | Development of iPS From Donated Somatic Cells of Patients With Neurological Diseases | iPSCs from donated somatic cells of patients with neurological diseases | Neurological disease | Hadassah Medical Organization | Active, not recruiting |
| 15 | Evaluation of Circulating Levels of Adult Stem Cells in the Peripheral Blood of Patients With Acute Decompensated Heart Failure and Following Stabilization, in Comparison With Healthy Volunteers (CIRCSTEM-HF) | Examine the levels of a number of different types of stem cells in patients with heart failure | Acute decompensated heart failure | Monash University | Active, not recruiting |
| 16 | The Transendocardial Autologous Cells (hMSC or hBMC) in Ischemic Heart Failure Trial (TAC-HFT) | Bone marrow–derived cells | Ischemic heart failure trial (TAC-HFT) | University of Miami | Completed |
| 17 | The Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis Pilot Study (The POSEIDON-Pilot Study) | Bone marrow–derived cells | Not applied | University of Miami | Completed |
| 18 | Percutaneous Stem Cell Injection Delivery Effects On Neomyogenesis in Dilated CardioMyopathy (The POSEIDON-DCM Study) (PoseidonDCM) | Bone marrow–derived cells | Dilated cardiomyopathy | University of Miami | Recruiting |
Data are from www.clinicaltrials.gov; accessed on October 24, 2013. The search was for studies: “embryonic stem cells.”
There are currently 18 registered clinical trials (www.clinicaltrials.gov) related to ESCs. However, three studies (1, 2, and 6 in Table 1) are related to the generation of ESC lines and not related to their use in clinical trials, and other studies (16, 17, and 18) use adult stem cells, such as mesenchymal stem cells. Moreover, there are two studies with iPSCs (12 and 14). Studies 7, 11, 13, and 15 are related to differentiation of hESCs, isolation and characterization of mammary stem cells, hematologic disease, and heart failure. In summary, only six studies (3, 4, 5, 8, 9, and 10) actually use ESCs in clinical trials, all of them to treat SMD or dry AMD.
There is no doubt that many advances have been made since the isolation of the first human embryonic lineage in 1994 (Bongso et al., 1994), and the possibilities for stem cell therapy seem limitless. However, there is still a great lack of knowledge about the processes of cell differentiation and how to control cell differentiation. The use of differentiated cells requires the development of well-controlled and safe differentiation protocols. One major issue is the risk of teratoma formation. Studies aimed at understanding the basic biology of these cells will determine whether these cells can be used effectively in cellular therapies. In relation to iPSCs, several studies on the derivation of these cells from adult cells belonging to affected tissues have been enrolled, but there is no study based on differentiation of these cells and their use in patients.
Conclusions
There are currently six clinical trials using hESCs to treat diseases. However, many basic questions about the efficiency and safety of the use of these cells still remain unanswered. In recent years, great progress has been made in the characterization of pluripotent cells. Differentiation protocols have been optimized; however, it is still necessary to ensure full differentiation, so that any remaining pluripotent cells are capable of generating teratomas. In the field of iPSCs, great advancement has been made in the generation of these cells from a variety of cell types and different combinations of transcription factors, and a huge variety of vectors have been tested. However, iPSCs still have large genetic and epigenetic variability in comparison with ESCs and do not represent a suitable source for clinical use. If the clinical application is the main goal of stem cell research, there are still many molecular mechanisms that need to be understood before these cells can be efficiently used in clinical trials in the future.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Agarwal S., Holton K.L., and Lanza R. (2008). Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 26, 1117–1127 [DOI] [PubMed] [Google Scholar]
- Araki R., Uda M., Hoki Y., Sunayama M., Nakamura M., Ando S., Sugiura M., Ideno H., Shimada A., Nifuji A., and Abe M. (2013). Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494,100–104 [DOI] [PubMed] [Google Scholar]
- Assady S., Maor G., Amit M., Itskovitz-Eldor J., Skorecki K.L., and Tzukerman M. (2001). Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697 [DOI] [PubMed] [Google Scholar]
- Bongso A., Fong C.Y., Ng S.C., and Ratnam S. (1994). Isolation and culture of inner cell mass cells from human blastocysts. Hum. Reprod. 9, 2110–2117 [DOI] [PubMed] [Google Scholar]
- Brolen G.K., Heins N., Edsbagge J., and Semb H. (2005). Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes 54, 2867–2874 [DOI] [PubMed] [Google Scholar]
- Carey B.W., Markoulaki S., Hanna J., Saha K., Gao Q., Mitalipova M., and Jaenisch R. (2009). Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl. Acad. Sci. USA 106, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.F., Tseng C.Y., Wang H.W., Kuo H.C., Yang V.W., and Lee O.K. (2012). Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 55, 1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Lin C.P., Ho J.J., He X., Okada N., Bu P., Zhong Y., Kim S.Y., Bennett M.J., Chen C., Ozturk A., Hicks G.G., Hannon G.J., and He L. (2011). miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 13, 1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M., Farini D., and Dolci S. (2009). In or out stemness: Comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells. Curr. Stem Cell Res. Ther. 4, 87–97 [DOI] [PubMed] [Google Scholar]
- Duan Y., Ma X., Zou W., Wang C., Bahbahan I.S., Ahuja T.P., Tolstikov V., and Zern M.A. (2010). Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 28, 674–686 [DOI] [PubMed] [Google Scholar]
- Fairchild P.J. (2010). The challenge of immunogenicity in the quest for induced pluripotency. Nat. Rev. Immunol. 10, 868–875 [DOI] [PubMed] [Google Scholar]
- Feng B., Jiang J., Kraus P., Ng J.H., Heng J.C., Chan Y.S., Yaw L.P., Zhang W., Loh Y.H., Han J., Vega V.B., Cacheux-Rataboul V., Lim B., Lufkin T., and Ng H.H. (2009). Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat. Cell Biol. 11, 197–203 [DOI] [PubMed] [Google Scholar]
- Fusaki N., Ban H., Nishiyama A., Saeki K., and Hasegawa M. (2009). Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85, 348–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.F. (2006). Developmental Biology. 8. Sunderland, MA: Sinauers Associates, Inc., Publishers [Google Scholar]
- Guha P., Morgan J.W., Mostoslavsky G., Rodrigues N.P., and Boyd A.S. (2013). Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 12, 407–412 [DOI] [PubMed] [Google Scholar]
- Heng J.C., Feng B., Han J., Jiang J., Kraus P., Ng J.H., Orlov Y.L., Huss M., Yang L., Lufkin T., Lim B., and Ng H.H. (2010). The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 6, 167–174 [DOI] [PubMed] [Google Scholar]
- Ichida J.K., Blanchard J., Lam K., Son E.Y., Chung J.E., Egli D., Loh K.M., Carter A.C., Di Giorgio F.P., Koszka K., Huangfu D., Akutsu H., Liu D.R., Rubin L.L., and Eggan K. (2009). A small-molecule inhibitor of TGF-beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell 5, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D., Levine A.J., Besser D., and Hemmati-Brivanlou A. (2005). TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132, 1273–1282 [DOI] [PubMed] [Google Scholar]
- Kadereit S., and Trounson A. (2011). In vitro immunogenicity of undifferentiated pluripotent stem cells (PSC) and derived lineages. Semin. Immunopathol. 33, 551–562 [DOI] [PubMed] [Google Scholar]
- Kaufman D.S., Hanson E.T., Lewis R.L., Auerbach R., and Thomson J.A. (2001). Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 98, 10716–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., Nishikawa S.I., and Sasai Y. (2000). Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40 [DOI] [PubMed] [Google Scholar]
- Kehat I., Kenyagin-Karsenti D., Snir M., Segev H., Amit M., Gepstein A., Livne E., Binah O., Itskovitz-Eldor J., and Gepstein L. (2001). Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 108, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead H.S., Nistor G., Bernal G., Totoiu M., Cloutier F., Sharp K., and Steward O. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B., Greber B., Arauzo-Bravo M.J., Meyer J., Park K.I., Zaehres H., and Scholer H.R. (2009). Direct reprogramming of human neural stem cells by OCT4. Nature 461, 649–653 [DOI] [PubMed] [Google Scholar]
- Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M.J., Ji H., Ehrlich L.I., Yabuchi A., Takeuchi A., Cunniff K.C., Hongguang H., McKinney-Freeman S., Naveiras O., Yoon T.J., Irizarry R.A., Jung N, Seita J., Hanna J., Murakamai P., Jaenisch R., Weissleder R.,.Orkin S.H., Weissman I.L., Feinberg A.P., and Daley G.Q. (2010). Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavon N., and Benvenisty N. (2005). Study of hepatocyte differentiation using embryonic stem cells. J. Cell. Biochem. 96, 1193–1202 [DOI] [PubMed] [Google Scholar]
- Levenberg S., Golub J.S., Amit M., Itskovitz-Eldor J., and Langer R. (2002). Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 99, 4391–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu H., Pan Y., Tang S., Xiong J., Hui N., Wang S., Qi Z., and Li L. (2004). Human embryonic germ cells isolation from early stages of post-implantation embryos. Cell Tiss. Res. 318, 525–531 [DOI] [PubMed] [Google Scholar]
- Maekawa M., Yamaguchi K., Nakamura T., Shibukawa R., Kodanaka I., Ichisaka T., Kawamura Y., Mochizuki H., Goshima N., and Yamanaka S. (2011). Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 474, 225–229 [DOI] [PubMed] [Google Scholar]
- Maherali N., and Hochedlinger K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 19, 1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M.J., Muotri A., Gage F., and Varki A. (2005). Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 11, 228–232 [DOI] [PubMed] [Google Scholar]
- Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T., Hayashi T., Onoe H., Shiina T., Yamanaka S., and Takahashi J. (2013). Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Rep. 1, 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Goldstein R.A., and Nierras C.R. (2008). The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 9, 725–729 [DOI] [PubMed] [Google Scholar]
- Okita K., Nakagawa M., Hyenjong H., Ichisaka T., and Yamanaka S. (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science 322, 949–953 [DOI] [PubMed] [Google Scholar]
- Picanço-Castro V., Russo-Carbolante E., Reis L.C., Fraga A.M., de Magalhaes D.A., Orellana M.D., Panepucci R.A., Pereira L.V., and Covas D.T. (2011). Pluripotent reprogramming of fibroblasts by lentiviral mediated insertion of SOX2, C-MYC, and TCL-1A. Stem Cells Dev. 20, 169–180 [DOI] [PubMed] [Google Scholar]
- Rambhatla L., Chiu C.P., Kundu P., Peng Y., and Carpenter M.K. (2003). Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 12, 1–11 [DOI] [PubMed] [Google Scholar]
- Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., and Bongso A. (2000). Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404 [DOI] [PubMed] [Google Scholar]
- Reubinoff B.E., Itsykson P., Turetsky T., Pera M.F., Reinhartz E., Itzik A., and Ben-Hur T. (2001). Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 19, 1134–1140 [DOI] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P., Golipour A., David L., Sung H.K., Beyer T.A., Datti A., Woltjen K., Nagy A., and Wrana J.L. (2010). Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 [DOI] [PubMed] [Google Scholar]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., and Lanza R. (2012). Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 [DOI] [PubMed] [Google Scholar]
- Segev H., Fishman B., Ziskind A., Shulman M., and Itskovitz-Eldor J. (2004). Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells 22, 265–274 [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (2011). Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138, 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C.A., Stadtfeld M., Murphy G.J., Hochedlinger K., Kotton D.N., and Mostoslavsky G. (2009). Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C.A., Christodoulou C., Gianotti-Sommer A., Shen S.S., Sailaja B.S., Hezroni H., Spira A., Meshorer E., Kotton D.N., and Mostoslavsky G. (2012). Residual expression of reprogramming factors affects the transcriptional program and epigenetic signatures of induced pluripotent stem cells. PLoS One 7, e51711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M., and Hochedlinger K. (2010). Induced pluripotency: History, mechanisms, and applications. Genes Dev. 24, 2239–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M., Nagaya M., Utikal J., Weir G., and Hochedlinger K. (2008). Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- Thanasegaran S., Cheng Z., Ito S., Nishio N., and Isobe K. (2013). No immunogenicity of IPS cells in syngeneic host studied by in vivo injection and 3D scaffold experiments. Biomed. Res. Int. 2013, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., and Jones J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- Van Vranken B.E., Romanska H.M., Polak J.M., Rippon H.J., Shannon J.M., and Bishop A.E. (2005). Coculture of embryonic stem cells with pulmonary mesenchyme: A microenvironment that promotes differentiation of pulmonary epithelium. Tiss. Eng. 11, 1177–1187 [DOI] [PubMed] [Google Scholar]
- Vergano D. (2010). Second human embryonic stem cell clinical trial to start. Retrieved Feb. 1, 2011, from USAToday website: http://content.usatoday.com/communities/sciencefair/post/2010/11/second-human-embryonic-stem-cell-clinical-trial-to-start/1
- Walsh F. (2012). “BBC News.” Daily Events. BBC News. Retrieved July24, 2012, from website: http://www.bbc.co.uk/news/health-15740133
- Wang D., Haviland D.L., Burns A.R., Zsigmond E., and Wetsel R.A. (2007). A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 4449–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lozier J., Johnson G., Kirshner S., Verthelyi D., Pariser A., Shores E., Rosenberg A. (2008). Neutralizing antibodies to therapeutic enzymes: Considerations for testing, prevention and treatment. Nat Biotechnol. 26, 901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A., Daley G.Q., Brack A.S., Collins J.J., Cowan C, Schlaeger T.M., and Rossi D.J. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hamalainen R., Cowling R., Wang W., Liu P., Gertsenstein M., Kaji K., Sung H.-K., Nagy A. (2009). piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. (2008). Pluripotency and nuclear reprogramming. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2079–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F., Qu J., Li M., Suzuki K., Kim N.Y., Liu G.H., and Belmonte J.C. (2012). Establishment of hepatic and neural differentiation platforms of Wilson's disease specific induced pluripotent stem cells. Protein Cell 3, 855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., and Thomson J.A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin II, and Thomson J.A. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K., Rad R., Takeda J., and Bradley A. (2009). Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat. Methods 6, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Cai J., Chen J., Luo Y., You Z.B., Fotter E., Wang Y., Harvey B., Miura T., Backman C., Chen G.J., Rao M.S., and Freed W.J. (2004). Dopaminergic differentiation of human embryonic stem cells. Stem Cells 22, 925–940 [DOI] [PubMed] [Google Scholar]
- Zhang S.C., Wernig M., Duncan I.D., Brustle O., and Thomson J.A. (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 [DOI] [PubMed] [Google Scholar]
- Zhao R., and Daley G.Q. (2008). From fibroblasts to iPS cells: Induced pluripotency by defined factors. J. Cell. Biochem. 105, 949–955 [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.N., Rong Z., and Xu Y. (2011). Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yin X., Qin H., Zhu F., Liu H., Yang W., Zhang Q., Xiang C., Hou P., Song Z., Li Y., Yong J., Zhang P., Cai J., Liu M., Li H., Li Y., Qu X., Cui K., Zhang W., Xiang T., Wu Y., Zhao Y., Liu C., Yu C., Yuan K., Lou J., Ding M., and Deng H. (2008). Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 3, 475–479 [DOI] [PubMed] [Google Scholar]
- Zhou W., and Freed C.R. (2009). Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 27, 2667–2674 [DOI] [PubMed] [Google Scholar]