Abstract
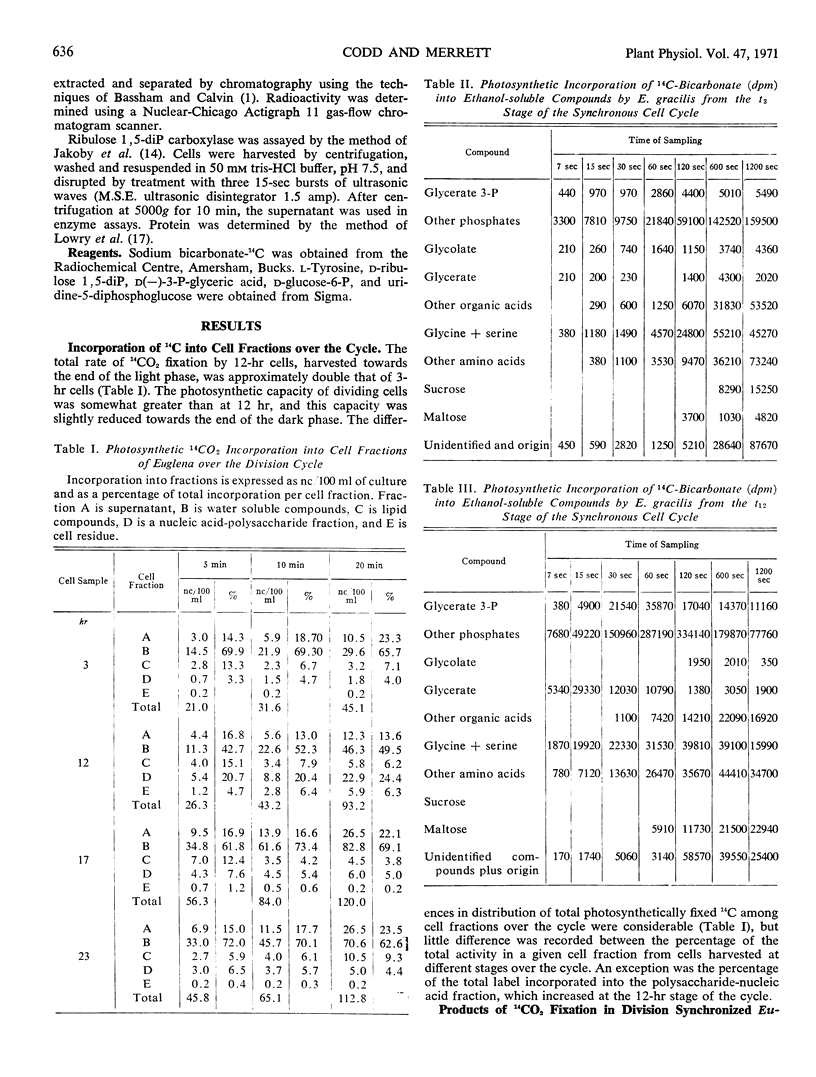
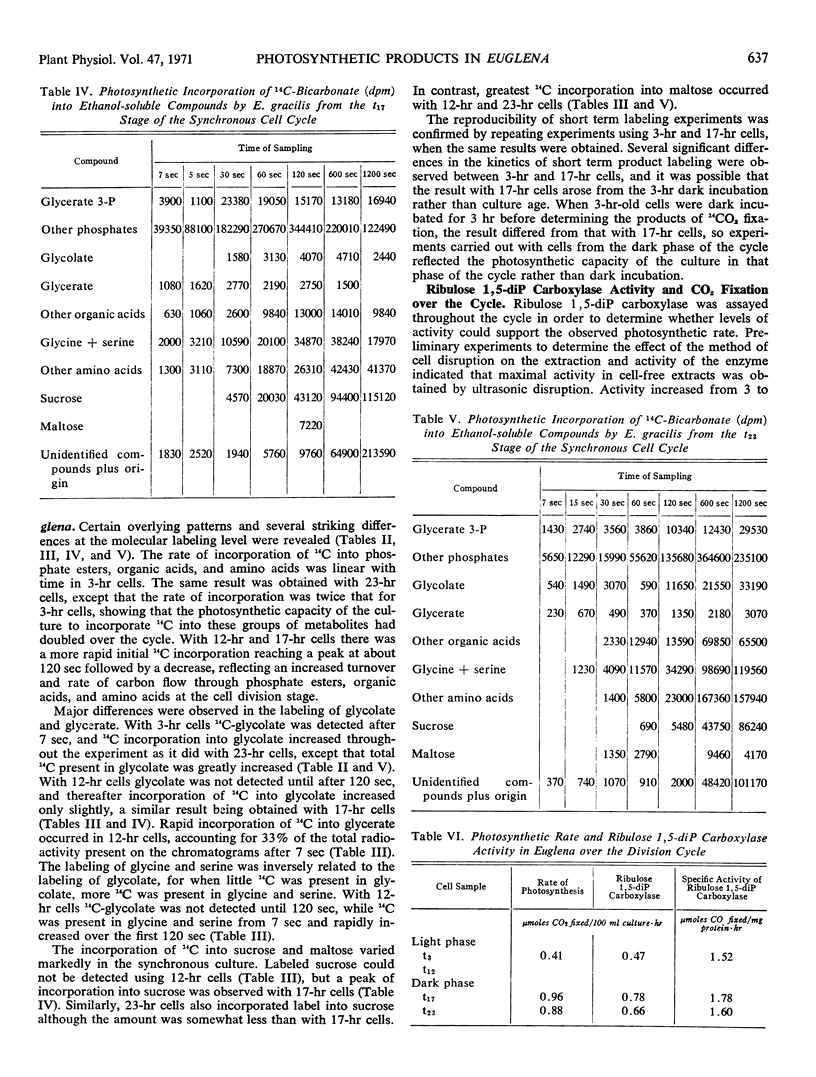
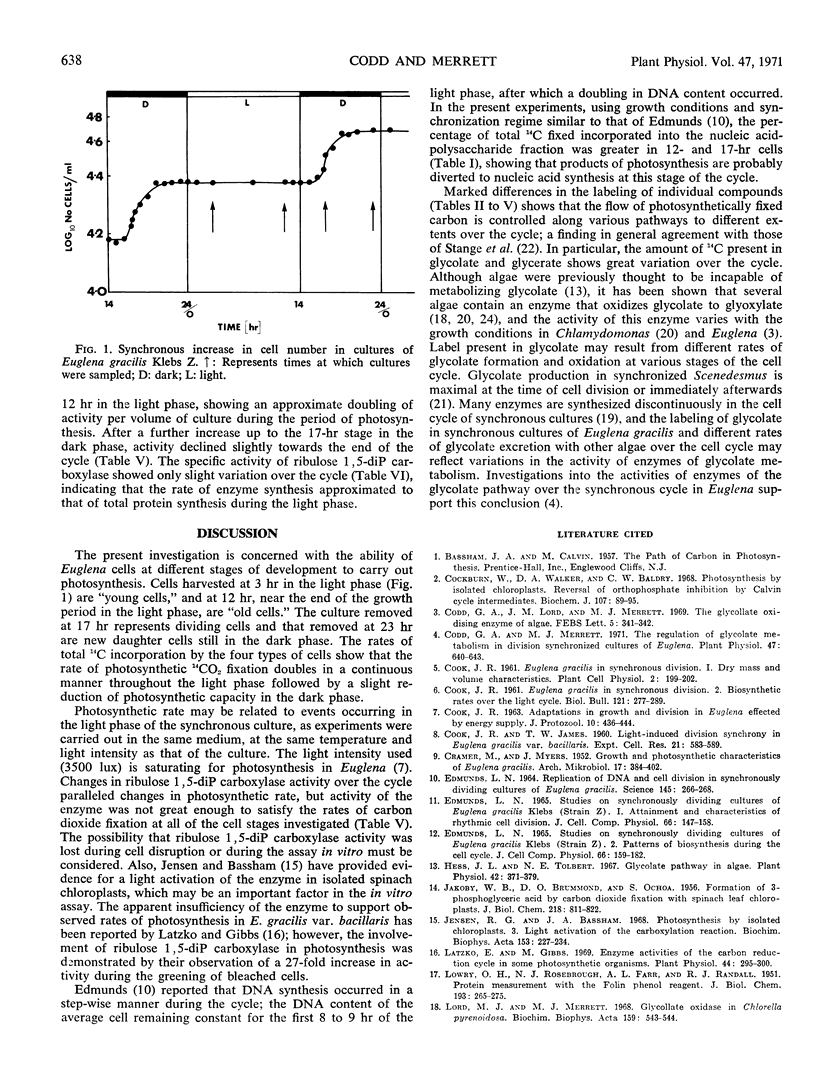
Rates and products of photosynthetic 14CO2 fixation by division synchronized cultures of Euglena gracilis strain Z were determined over the cycle. Rate of 14CO2 fixation doubled in a continuous manner throughout the light phase followed by a slight reduction of photosynthetic capacity in the dark phase. Greater 14C incorporation into the nucleic acid-polysaccharide fraction occurred with mature cells. Products of 14CO2 fixation varied markedly over the cycle: although with mature cells 14C-labeled sucrose was not detected, with dividing cells this was the main sugar labeled; in young cells 14C maltose was formed. Cells removed at end of dark phase accumulated 14C in glycolate, whereas at other stages over the cycle less 14C was present in glycolate, and this was accompanied by a rapid incorporation of 14C into glycine and serine. Glycerate was an early and major product of photosynthesis with cells at the mature stage of the cycle.
Changes in ribulose 1,5-diphosphate carboxylase activity paralleled changes in photosynthetic rate, but activity was not great enough to account for the observed rates of CO2 fixation at most stages of the division cycle investigated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOK J. R. ADAPTATIONS IN GROWTH AND DIVISION IN EUGLENA EFFECTED BY ENERGY SUPPLY. J Protozool. 1963 Nov;10:436–444. doi: 10.1111/j.1550-7408.1963.tb01703.x. [DOI] [PubMed] [Google Scholar]
- COOK J. R., JAMES T. W. Light-induced division synchrony in Euglena gracills var. bacillaris. Exp Cell Res. 1960 Dec;21:583–589. doi: 10.1016/0014-4827(60)90292-5. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. The regulation of glycolate metabolism in division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):640–643. doi: 10.1104/pp.47.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds L. N., Jr Studies on synchronously dividing cultures of Euglena gracilis Klebs (strain Z). I. Attainment and characterization of rhythmic cell division. J Cell Physiol. 1965 Oct;66(2):147–158. doi: 10.1002/jcp.1030660204. [DOI] [PubMed] [Google Scholar]
- Edmunds L. N., Jr Studies on synchronously dividing cultures of Euglena gracilis Klebs (strain Z). II. Patterns of biosynthesis during the cell cycle. J Cell Physiol. 1965 Oct;66(2):159–181. doi: 10.1002/jcp.1030660205. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B., BRUMMOND D. O., OCHOA S. Formation of 3-phosphoglyceric acid by carbon dioxide fixation with spinach leaf enzymes. J Biol Chem. 1956 Feb;218(2):811–822. [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. 3. Light activation of the carboxylation reaction. Biochim Biophys Acta. 1968 Jan 15;153(1):227–234. doi: 10.1016/0005-2728(68)90164-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M. J., Merrett M. J. Glycollate oxidase in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jul 9;159(3):543–544. doi: 10.1016/0005-2744(68)90140-x. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E., Hess J. L. Glycolate stimulation of oxygen evolution during photosynthesis. Plant Physiol. 1969 Jan;44(1):55–59. doi: 10.1104/pp.44.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- STANGE L., BENNETT E. L., CALVIN M. Short-time radiocarbon-labelled carbon dioxide incorporation experiments with synchronously growing Chlorella cells. Biochim Biophys Acta. 1960 Jan 1;37:92–100. doi: 10.1016/0006-3002(60)90082-2. [DOI] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Glycolate oxidase activity in algae. Plant Physiol. 1968 Feb;43(2):289–291. doi: 10.1104/pp.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]



