Abstract
Background
Clinical benefit from cytotoxic chemotherapy for metastatic papillary thyroid carcinoma (PTC) is disappointing, and effective therapeutic approaches for these patients are urgently needed. Because kinase-activating mutations in the BRAF proto-oncogene commonly occur in advanced PTC, and inhibition of BRAFV600E has shown promising clinical activity in melanoma, BRAF inhibitor therapy may be an effective strategy to treat metastatic PTC.
Methods
The dose escalation portion of a first-in-human, phase I study of vemurafenib, a selective RAF inhibitor, included three patients with metastatic PTC harboring the BRAFV600E mutation. Vemurafenib was initially dosed at 240–360 mg twice a day, later escalated to 720 mg twice a day. Response evaluation was performed every 8 weeks per Response Evaluation Criteria in Solid Tumors (RECIST).
Results
Among the three patients, one had a confirmed partial response with reduction of pulmonary target lesions by 31%, and the duration of response was 7.6 months before the disease progressed in the lungs and the bones. The time to progression was 11.7 months. The other two patients had stable disease, and the time to progression was 13.2 and 11.4 months, respectively.
Conclusions
Vemurafenib appears to have a promising clinical activity in patients with metastatic PTC, and our data suggest that the BRAFV600E mutant kinase is a relevant target for therapy in this patient population. Further investigation of inhibitors of mutated BRAF kinase in patients with PTC in a phase II study is warranted.
Introduction
Thyroid carcinoma is an increasingly common cancer, currently the fifth most frequently diagnosed malignancy in the United States in women (1). Among different variants of thyroid cancer, papillary thyroid carcinoma (PTC) is the most common histologic type, accounting for nearly 80% of all cases. Despite a favorable prognosis in a majority of patients, about 10%–20% of the patients with PTC will develop metastatic disease (2,3), and at least a half of these patients with advanced disease will not respond to conventional therapy such as radioactive iodine and thyrotropin (TSH) suppressive thyroid hormone therapy. Patients with radioactive iodine–refractory disease have a poor overall prognosis with a long-term overall survival rate of approximately 10% (4). Cytotoxic systemic therapy plays a very limited role in durable disease control of PTC (5). Recently, molecularly targeted therapy for PTC, especially small molecules targeting the vascular endothelial growth factor receptor (VEGFR), have been evaluated in clinical trials with great promise and are emerging as standard of care for patients with progressive, radioactive iodine–refractory disease (6–12). However, other kinases that drive the growth, invasion, and survival of PTC cells and facilitate resistance to VEGFR targeting drugs may be appropriate targets for therapy as well.
Among several known genetic aberrations of PTC, kinase-activating mutations in the V-raf murine sarcoma viral oncogene homolog B1 (BRAF) proto-oncogene are the most frequent event, found in nearly half of all PTCs, and the incidence rate of these mutations approaches 80% in recurrent or metastatic PTCs (13–15). A single amino acid substitution, from valine to glutamic acid at codon 600 (V600E), accounts for approximately 90% of all BRAF mutations (13–16). This mutation mimics activated BRAF kinase protein, leading to phosphorylation of the downstream extracellular signal-regulated kinase kinase (MEK) and extracellular signal-regulated kinase (ERK1/2), tumor cell proliferation, and potentially loss of differentiated functions (17–19). In addition, BRAF mutations predict for poorer clinicopathologic outcomes (extrathyroidal extension, capsular invasion, lymph node metastasis), persistence/recurrence of tumor after surgery, and death (13,14,20,21). In vitro treatment of PTC cell lines containing mutant BRAF with a selective BRAF kinase inhibitor dephosphorylates MEK and subsequently ERK1/2, blocks cell cycle progression, and inhibits tumor xenograft growth (22,23). Therefore, it is plausible to hypothesize that inhibition of BRAFV600E kinase would clinically benefit patients with PTC harboring this mutation.
Vemurafenib (also known as PLX4032 or RG7204) is a potent kinase inhibitor of BRAFV600E with an IC50 of 31 nM and V-raf-1 murine leukemia viral oncogene homolog 1 (CRAF) with an IC50 of 48 nM; potency against wild-type BRAF kinase is considerably less (24). In a first-in-human phase I study of vemurafenib, 81% of patients had a significant tumor reduction with a confirmed response rate of 56% among patients with metastatic melanoma harboring the BRAFV600E mutation, who received treatment in a dose-extension cohort at the 960 mg twice daily dose (25). The median progression-free survival duration was at least 7 months. This clinical efficacy among melanoma patients with the relevant mutated BRAF kinase drastically contrasts with a complete absence of clinical response among those lacking the BRAFV600E mutation (25). These results underscore the importance of selecting patients whose tumors bear the appropriate molecular target for the success of molecularly targeted drugs. A recently reported randomized phase III trial demonstrated improved overall survival, progression-free survival, and response rate following treatment with vemurafenib for patients with previously untreated BRAFV600E-mutant melanoma, compared with dacarbazine, and has led to the drug's recent approval (26).
With the demonstrated efficacy of this selective RAF inhibitor in melanoma, interest has arisen in the evaluation of the clinical activity of RAF inhibitors in nonmelanoma malignancies with a BRAFV600E mutation (27). Here we report expanded clinical details and long-term follow-up of three patients with advanced PTC harboring BRAFV600E mutation who were treated with vemurafenib in the phase I clinical trial at The University of Texas MD Anderson Cancer Center, early results of which were previously published (25).
Patients and Methods
Patients
In a multicenter, first-in-human phase I study of vemurafenib, patients with histologically confirmed solid tumors that were refractory to standard therapy or for which standard or curative therapy did not exist, were eligible. They had to be 18 years of age or older and had to have Eastern Cooperative Oncology Group performance status 0 to 1 with adequate hematologic, hepatic, and renal function.
In the dose-escalation phase of the study, patients of any tumor type were eligible regardless of the BRAF mutation status, and in the dose-extension cohort, only patients with melanoma harboring BRAFV600E mutation were eligible because of promising clinical activity to vemurafenib in this subtype during the early phase of the study. Among 55 patients treated with highly bioavailable, microprecipitated bulk powder formulation of vemurafenib during the dose-escalation phase, three patients had metastatic PTC. Archived tumor tissues of these three patients were analyzed for the presence of a BRAF gene mutation by Clinical Laboratory Improvement Amendments–certified, polymerase chain reaction–based pyrosequencing technique performed at the Molecular Diagnostic Laboratory at MD Anderson Cancer Center.
Treatment
In the dose-escalation phase, vemurafenib was administered orally at a range of 160 to 1120 mg twice a day (BID) without interruption, and a cohort of three to six patients were treated at each dose level until the maximum tolerated dose was determined at 960 mg BID (25). One cycle was defined as 4 weeks of treatment. Intrapatient dose escalation to the next dose level was permitted for patients who had not experienced grade 3 or higher toxicity, but only when safety and tolerability had been established in the separate cohort of three to six patients treated for 28 days at that higher dose level.
Study assessments
Safety evaluations, including physical examination, electrocardiogram, laboratory tests comprising of complete blood counts, and clinical chemistry and urinalysis, were performed weekly during the first cycle, and then every 4 weeks afterwards. The Common Terminology Criteria for Adverse Events (version 3.0) were used to assess adverse events.
Clinical response was assessed every two cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (28). Progression‐free survival was defined as the time from the first day of treatment to the date of the first documented disease progression or date of death, whichever occurred first.
The study protocol was approved by the Institutional Review Board; all patients provided written informed consent including disclosure of potential conflicts of interest.
Results
Three patients with metastatic papillary thyroid cancer were initially treated with vemurafenib at 240, 360, and 360 mg BID, respectively. Pyrosequencing analysis of the archived tumor tissues from three patients was performed prior to treatment and identified the BRAFV600E mutations in each. Among these three patients, one had a confirmed partial response, and the other two patients had stable disease as their best initial response to therapy. The times to disease progression among the three patients were 11.4, 11.7, and 13.2 months, and the overall survival durations were 15, 21, and at least 31.7 months. The adverse events were similar to those were reported in a phase I study of patients with metastatic melanoma and reversible upon treatment discontinuation (25). The clinical courses of these patients are described as follows.
Patient 1
Patient 1 was a 57-year-old white man who was diagnosed with PTC in March 1999. He had a total thyroidectomy with central and left neck node dissections, followed by radioactive iodine treatment 2 months later. Between 2000 and 2007, he had multiple invasive recurrences involving bilateral neck, right supraclavicular, and superior mediastinal lymph nodes, for which he underwent extensive lymph node dissections of involved regions; subsequent review at our institution of a pathology specimen from a 2002 resection confirmed classical PTC without poorly differentiated features. In April 2008, a computed tomography (CT) scan revealed an enlarging tumor mass in the thyroid bed encasing the trachea with questionable endoluminal spread along the right anterior subglottis and also approximating the common carotid and vertebral arteries. Left neck and mediastinal lymphadenopathy and bilateral pulmonary nodules were also concerning for metastatic disease. After providing informed consent, he started vemurafenib treatment in May 2008 with 240 mg BID, and the dose was increased to 360 mg BID after six cycles, and then to 720 mg BID after 11 cycles as tolerated. He tolerated therapy well, except for requiring surgical resection of multiple cutaneous keratinocytic eruptions diagnosed as invasive squamous cell carcinomas (SCCs), which appeared beginning 8 months after starting therapy. His best overall response was stable disease by RECIST with 9% reduction of the target lesions. The thyroglobulin level at baseline was 105 ng/mL, subsequently fluctuated between 126 and 567 ng/mL, and at the time of disease progression was 185 ng/mL. Although his pulmonary and mediastinal lesions remained stable, his tumor progressed in the thyroid bed after 14 cycles. He underwent surgical resection of a thyroid bed mass requiring laryngectomy in February 2010 followed by external beam radiation therapy in May 2010. He was lost to follow-up subsequently. The time to progression was 13.2 months and the overall survival duration after initiation of vemurafenib was at least 31.7 months as of the last follow-up in January 2011.
Patient 2
Patient 2 was a 48-year-old white woman who was diagnosed with a 2.5 cm PTC with extrathyroidal extension, and she underwent thyroidectomy in September 2001 followed by adjuvant radioactive iodine treatment. However, in March 2003, she had regional recurrence of PTC, which was managed surgically followed by adjuvant external beam radiation therapy. Histologic review of the recurrent tumor demonstrated regions of squamoid changes with strong expression of EGFR (Fig. 1). With enlarging pulmonary metastases that demonstrated no radioiodine uptake on multiple diagnostic 131I scans, treatment was initiated with sorafenib 400 mg BID in October 2007 with initial disease stabilization. However, disease progression in the lungs bilaterally was noted in May 2008 and treatment was discontinued. After providing informed consent, she started vemurafenib treatment in July 2008 with 360 mg BID, with dose escalation to 720 mg BID after eight cycles. She tolerated therapy well, except for requiring resection of cutaneous keratinocytic eruption diagnosed as invasive SCC from her right lateral chest, which developed after 6 months of therapy. The thyroglobulin level at baseline was 140 ng/mL, which decreased to 102 ng/mL after three cycles. However, thyroglobulin levels subsequently fluctuated between 109 and 96 ng/mL. After four cycles of treatment, she had a partial response in the lungs with approximately 31% reduction of target lesions. But, after 12 cycles of treatment, progression was noted, with marked and irregular enlargement of a small tumor in the right lower lobe lung that had been present at the time of initiation of vemurafenib therapy; further evaluation revealed right hilar adenopathy and new lytic lesions in the left eighth rib and the left distal femur. It was noted that the patient had no history of cigarette smoking. At the time of radiographic disease progression, her serum thyroglobulin level was stable, 84.5 ng/mL. Pathologic examination of a thoracoscopic biopsy specimen of the right lower lung mass showed carcinoma most consistent with squamous cell carcinoma with strong expression of cytokeratin 5/6 (CK5/6) and p63, but absent thyroid transcription factor-1 (TTF-1), thyroglobulin, and mucin expression (Fig. 1). The molecular sequencing of this progressing squamous carcinoma in the lung showed the presence of the BRAFV600E mutation, analogous to her primary thyroid tumor, and suggesting that this lesion represented progression of her underlying thyroid disease since squamous cell carcinoma may be a form of dedifferentiated thyroid carcinoma. She was subsequently treated with radiation therapy to the symptomatic rib and left distal femur lesions, and further chemotherapy was recommended, but she died in October 2009. The duration of response was 7.6 months, time to progression was 11.7 months per RECIST, and the overall survival duration after starting vemurafenib was 15 months.
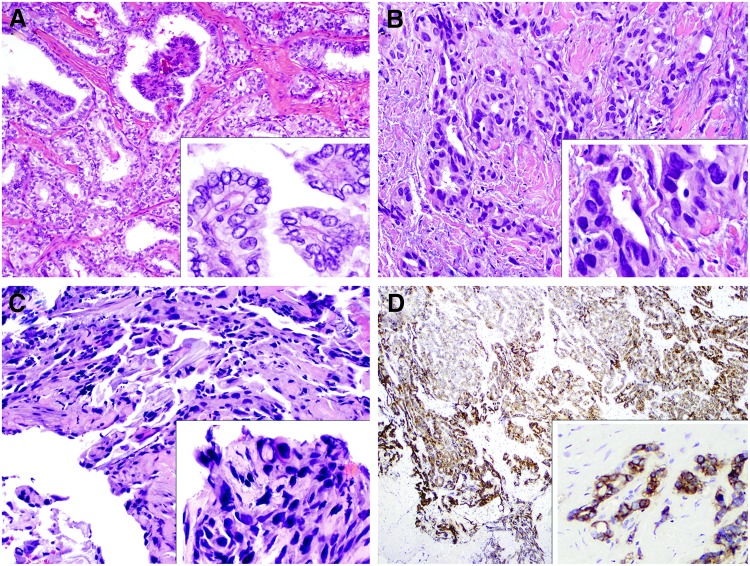
FIG. 1.
Histologic evaluation for patient 2. (A) Hematoxylin and eosin (H&E, 200×) stained section showing classical papillary thyroid carcinoma arising within the thyroid (initial surgery, 2001); higher power showing nuclear clearing, elongation and grooving (inset, 400×) positive for BRAFV600E mutation. (B) Recurrent papillary thyroid carcinoma involving peritracheal soft tissue with squamoid features (2003) (H&E, 200×and inset 400×) and nuclear hyperchromasia. (C) Biopsy of the lung mass in 2009 with squamous features (H&E, 200×) and hyperchromatic nuclei (inset, 200×), also positive for BRAFV600E mutation. (D) Strong, diffuse EGFR expression by immunohistochemical evaluation was present in this recurrent tumor (100×, inset 400×).
Patient 3
Patient 3 was a 63-year-old white man diagnosed with bilateral, multifocal tall cell variant of PTC (without foci of undifferentiated tumor) in July 2005 when he underwent a total thyroidectomy and left modified neck dissection. The left lobe primary tumor was at least 2.2 cm in diameter invading the adjacent skeletal tissue and fat. In September 2005, he received adjuvant radioactive iodine treatment. However, his disease recurred in the left neck and mediastinum, for which he underwent a second surgical resection of the left neck with mediastinal dissection in May 2007 and radioactive iodine treatment 2 months later. He underwent a paratracheal neck dissection for another recurrence in March 2008, but 3 months later, an enlarging, unresectable left superior paratracheal recurrence was identified. After providing informed consent, he started vemurafenib treatment in August 2008 at a dose of 360 mg BID. After two cycles, his serum alanine aminotransferase level increased to 307 IU/L (normal range 7–56 IU/L) from 23 IU/L 4 weeks earlier; therefore, the dose was reduced to 240 mg BID without further elevation in the serum transaminase level. The dose of vemurafenib was again increased to 360 mg BID after the sixth cycle and then to 720 mg BID after the eighth cycle with good tolerance, except for the development of a 5 mm cutaneous keratinocytic eruption, diagnosed as verruca, resected from his left shoulder after 7 months of therapy. His best overall response was stable disease by RECIST with 16% reduction of the target lesions. His left supraclavicular adenopathy, although initially stable, progressed after 12 cycles, and the treatment was discontinued. The thyroglobulin level was, unfortunately, not measured at baseline, and it was 0.4 ng/mL at the end of two cycles. His thyroglobulin level steadily increased to 9.1 ng/mL by the eighth cycle, but decreased to 5.7 ng/mL 4 weeks after dose escalation from 360 to 720 mg BID. However, the thyroglobulin level increased to 21.4 ng/mL by the time of disease progression after 12 cycles. After being taken off protocol, he was subsequently treated with sorafenib for 9 months and then sunitinib for 2 months without disease response. He died in September 2010 due to rapid disease progression with anaplastic transformation involving the lungs, liver, and bones. The time to progression was 11.4 months, and the overall survival duration after starting vemurafenib was 24.9 months.
Discussion
This is the first detailed report to describe clinical response to selective BRAF kinase inhibitor therapy in patients with progressive, metastatic PTC bearing the BRAFV600E mutation. Unfortunately, on-therapy biopsies of metastatic lesions were not performed, and thus the hypothesis that the observed results are due to therapeutic targeting of the BRAFV600E mutant kinase is based only on the agent's known greater selectivity of inhibition. Additionally, the number of patients with PTC treated in this phase I study is quite small. Nonetheless, partial response observed in one patient with distant metastatic disease and the prolonged stabilization of locoregional and distant disease in the other two patients suggests that further study of treatment with vemurafenib in BRAFV600E mutant PTC is warranted.
Response to therapy with other, less selective BRAF inhibitors has been described previously. In published reports from two phase II trials, sorafenib therapy yielded partial response rates of 15% and 23%, respectively, and may have prolonged the time to tumor progression as much as threefold in a retrospective series (29–31). However, this agent has been described as a weak in vivo inhibitor of BRAF kinase, and is more likely to exert its primary effects through inhibition of VEGFR, consistent with the responses seen with multiple other anti-VEGFR tyrosine kinase inhibitors (9). Prolonged stable disease but no objective response has been preliminarily reported in 6 of 12 PTC patients treated in a phase I trial of the pan-BRAF inhibitor XL281 (32). Notably, however, tumor BRAF mutation status was not prospectively and uniformly determined in these studies, was not used to determine eligibility for treatment, and has not clearly correlated with clinical outcomes. Therefore, studies with these less selective agents do not necessarily support the contention that targeting mutant BRAF protein in PTC is an effective strategy.
The development of cutaneous keratinocytic eruptions including SCC and keratoacanthoma (KA) in patients treated with both nonselective and selective BRAF inhibitors has been well described previously and recently reviewed by Robert et al. (33). In larger series, the frequency of such secondary cutaneous neoplasms is 15%–30%. In the recent phase III melanoma trial, cutaneous KA and SCC were reported in 8% and 12%, respectively, of vemurafenib-treated patients, compared with fewer than 1% during dacarbazine therapy (26). Of note, cutaneous keratinocytic eruptions diagnosed as SCC appeared in two of our PTC patients during vemurafenib therapy and a verruca appeared in the third. Examination of larger cohorts of patients with PTC on BRAF inhibitors will be necessary to evaluate if patients with PTC may potentially harbor a higher susceptibility to development of keratinocytic eruptions during treatment when compared to non-PTC patients on similar therapy. In future trials of vemurafenib therapy for BRAFV600E mutant PTC, prospective dermatologic monitoring for cutaneous keratinocytic eruptions is clearly warranted.
The rapidly enlarging lung tumor of squamous histology during vemurafenib therapy in patient 2 is notable. Molecular genotyping identified a BRAFV600E mutation in a biopsy of the lesion, similar to her primary thyroid lesion. The BRAFV600E mutation has been reported to occur with low frequency in adenocarcinoma of the lung and possibly limited to patients with smoking histories (34); in contrast, this mutation was not identified in 104 cases of primary squamous cell cancers of the lung in two recent series (35,36). Although BRAF mutations can be uncommonly observed in metastatic squamous carcinomas arising from the upper aerodigestive tract, imaging studies never demonstrated evidence of an appropriate primary lesion. The clinical course along with mutational and immunohistochemical findings are therefore evidence that the lung lesion was more likely to be a progressive, dedifferentiating metastasis from the original PTC, rather than a secondary lung malignancy. Squamous metaplasia may be encountered in an otherwise well-differentiated thyroid carcinoma, but there is no known association with squamous metaplasia progressing to squamous carcinoma in the thyroid. Nonetheless, patients with PTC showing squamous metaplasia may warrant cautious consideration prior to enrollment in future trials of vemurafenib. Further, the rapid progression of dedifferentiated tumor in two patients, in the form of the lung metastasis of squamous cell carcinoma (considered by many as a form of anaplastic thyroid carcinoma) in one patient during vemurafenib therapy, and clear anaplastic transformation with numerous metastases in another patient after multiple sequential kinase inhibitor therapies, may highlight the limitations of any single drug for treatment in the setting of dedifferentiating disease.
It has been proposed that BRAF kinase inhibitors can stimulate proliferation in cells lacking mutant BRAF by paradoxical activation of signaling of the RAF-MEK-ERK1/2 pathway in the presence of upstream activation of RAS, thus possibly explaining the development of cutaneous SCC (37–39). In contrast, the growth of a squamous metastasis in this patient's lung is not readily explained by this hypothesis of paradoxical activation of signaling in wild-type BRAF cells, given the BRAF mutation found in her tumor. Unfortunately, the available amount of the patient's lung tumor was insufficient for further molecular analyses. It is possible that other mutations were present or subsequently developed that triggered rapid growth and spread, including changes in RAS or in cell surface receptors, such as EGFR or IGFR that signal downstream through RAS, or additional activating mutations (e.g., MEK1 mutations) in the MAPK signaling pathway (40).
In summary, treatment with vemurafenib of three patients with progressive BRAFV600E mutant PTC yielded a partial response in one and prolonged stabilization of disease in the others in this phase I trial. On the basis of these results, a phase II trial of vemurafenib has recently been initiated in patients with progressive metastases from BRAFV600E mutant PTC (ClinicalTrials.gov number NCT01286753).
Acknowledgments
This study was supported by Plexxikon Inc. and Roche Pharmaceuticals and has ClinicalTrials.gov number NCT00215605.
Author Disclosures
A.J.L., M.D.W., D.L.S., J.L.I. have no disclosures relevant to this article. K.K. has received research support and honoraria from Roche and Genentech. M.E.C has received research support from Roche. K.N. is an employee of Plexxikon. R.J.L. is an employee of Roche. S.I.S. provides consulting for Roche, Plexxikon, Bayer, AstraZeneca, Eisai, Exelixis, Pfizer, NovoNordisk, Eli Lilly, and Veracyte and research support to Genzyme and Pfizer.
References
- 1.Siegel R. Naishadham D. Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Shaha AR. Shah JP. Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996;172:692–694. doi: 10.1016/s0002-9610(96)00310-8. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli A. Fallahi P. Ferrari SM. Carpi A. Berti P. Materazzi G. Minuto M. Guastalli M. Miccoli P. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomed Pharmacother. 2008;62:559–563. doi: 10.1016/j.biopha.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 4.Durante C. Haddy N. Baudin E. Leboulleux S. Hartl D. Travagli JP. Caillou B. Ricard M. Lumbroso JD. De Vathaire F. Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22:464–468. doi: 10.1016/j.clon.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Sherman SI. Wirth LJ. Droz JP. Hofmann M. Bastholt L. Martins RG. Licitra L. Eschenberg MJ. Sun YN. Juan T. Stepan DE. Schlumberger MJ. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 7.Cohen EE. Rosen LS. Vokes EE. Kies MS. Forastiere AA. Worden FP. Kane MA. Sherman E. Kim S. Bycott P. Tortorici M. Shalinsky DR. Liau KF. Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta-Abramson V. Troxel AB. Nellore A. Puttaswamy K. Redlinger M. Ransone K. Mandel SJ. Flaherty KT. Loevner LA. O'Dwyer PJ. Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab. 2009;94:1493–1499. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle RM. Ball DW. Byrd D. Dilawari RA. Doherty GM. Duh QY. Ehya H. Farrar WB. Haddad RI. Kandeel F. Kloos RT. Kopp P. Lamonica DM. Loree TR. Lydiatt WM. McCaffrey JC. Olson JA Jr. Parks L. Ridge JA. Shah JP. Sherman SI. Sturgeon C. Waguespack SG. Wang TN. Wirth LJ. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 11.Bible KC. Suman VJ. Molina JR. Smallridge RC. Maples WJ. Menefee ME. Rubin J. Sideras K. Morris JC., 3rd McIver B. Burton JK. Webster KP. Bieber C. Traynor AM. Flynn PJ. Goh BC. Tang H. Ivy SP. Erlichman C. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman SI. Jarzab B. Cabanillas ME. Licitra LF. Pacini F. Martins R. Robinson B. Ball D. McCaffrey J. Shah MH. Bodenner D. Allison R. Newbold K. Elisei R. O'Brien JP. Schlumberger M. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) J Clin Oncol. 2011;29(15 suppl):5503. (Abstract). [Google Scholar]
- 13.Lupi C. Giannini R. Ugolini C. Proietti A. Berti P. Minuto M. Materazzi G. Elisei R. Santoro M. Miccoli P. Basolo F. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–4090. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 14.Elisei R. Ugolini C. Viola D. Lupi C. Biagini A. Giannini R. Romei C. Miccoli P. Pinchera A. Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 15.Henderson YC. Shellenberger TD. Williams MD. El-Naggar AK. Fredrick MJ. Cieply KM. Clayman GL. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–491. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasko V. Hu S. Wu G. Xing JC. Larin A. Savchenko V. Trink B. Xing M. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90:5265–5269. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]
- 17.Davies H. Bignell GR. Cox C. Stephens P. Edkins S. Clegg S. Teague J. Woffendin H. Garnett MJ. Bottomley W. Davis N. Dicks E. Ewing R. Floyd Y. Gray K. Hall S. Hawes R. Hughes J. Kosmidou V. Menzies A. Mould C. Parker A. Stevens C. Watt S. Hooper S. Wilson R. Jayatilake H. Gusterson BA. Cooper C. Shipley J. Hargrave D. Pritchard-Jones K. Maitland N. Chenevix-Trench G. Riggins GJ. Bigner DD. Palmieri G. Cossu A. Flanagan A. Nicholson A. Ho JW. Leung SY. Yuen ST. Weber BL. Seigler HF. Darrow TL. Paterson H. Marais R. Marshall CJ. Wooster R. Stratton MR. Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 18.Mitsutake N. Miyagishi M. Mitsutake S. Akeno N. Mesa C., Jr Knauf JA. Zhang L. Taira K. Fagin JA. BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology. 2006;147:1014–1019. doi: 10.1210/en.2005-0280. [DOI] [PubMed] [Google Scholar]
- 19.Durante C. Puxeddu E. Ferretti E. Morisi R. Moretti S. Bruno R. Barbi F. Avenia N. Scipioni A. Verrienti A. Tosi E. Cavaliere A. Gulino A. Filetti S. Russo D. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–2843. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 20.Xing M. Clark D. Guan H. Ji M. Dackiw A. Carson KA. Kim M. Tufaro A. Ladenson P. Zeiger M. Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2009;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salerno P. De Falco V. Tamburrino A. Nappi TC. Vecchio G. Schweppe RE. Bollag G. Santoro M. Salvatore G. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab. 2010;95:450–455. doi: 10.1210/jc.2009-0373. [DOI] [PubMed] [Google Scholar]
- 23.Xing J. Liu R. Xing M. Trink B. The BRAF(T1799A) mutation confers sensitivity of thyroid cancer cells to the BRAF(V600E) inhibitor PLX4032 (RG7204) Biochem Biophys Res Commun. 2011;404:958–962. doi: 10.1016/j.bbrc.2010.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollag G. Hirth P. Tsai J. Zhang J. Ibrahim PN. Cho H. Spevak W. Zhang C. Zhang Y. Habets G. Burton EA. Wong B. Tsang G. West BL. Powell B. Shellooe R. Marimuthu A. Nguyen H. Zhang KY. Artis DR. Schlessinger J. Su F. Higgins B. Iyer R. D'Andrea K. Koehler A. Stumm M. Lin PS. Lee RJ. Grippo J. Puzanov I. Kim KB. Ribas A. McArthur GA. Sosman JA. Chapman PB. Flaherty KT. Xu X. Nathanson KL. Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaherty KT. Puzanov I. Kim KB. Ribas A. McArthur GA. Sosman JA. O'Dwyer PJ. Lee RJ. Grippo JF. Nolop K. Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman PB. Hauschild A. Robert C. Haanen JB. Ascierto P. Larkin J. Dummer R. Garbe C. Testori A. Maio M. Hogg D. Lorigan P. Lebbe C. Jouary T. Schadendorf D. Ribas A. O'Day SJ. Sosman JA. Kirkwood JM. Eggermont AM. Dreno B. Nolop K. Li J. Nelson B. Hou J. Lee RJ. Flaherty KT. McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopetz S. Desai J. Chan E. Hecht JR. O'Dwyer PJ. Lee RJ. Nolop KB. Saltz L 2010 PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 28(15 suppl):3534. (Abstract). [Google Scholar]
- 28.Therasse P. Arbuck S. Eisenhauer E. Wanders J. Kaplan R. Rubinstein L. Verweij J. Van Glabbeke M. van Oosterom A. Christian M. Gwyther S. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Gupta-Abramson V. Troxel AB. Nellore A. Puttaswamy K. Redlinger M. Ransone K. Mandel SJ. Flaherty KT. Loevner LA. O'Dwyer PJ. Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloos RT. Ringel MD. Knopp MV. Hall NC. King M. Stevens R. Liang J. Wakely PE., Jr Vasko VV. Saji M. Rittenberry J. Wei L. Arbogast D. Collamore M. Wright JJ. Grever M. Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabanillas ME. Waguespack SG. Bronstein Y. Williams MD. Feng L. Hernandez M. Lopez A. Sherman SI. Busaidy NL. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95:2588–2595. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GK. Robertson S. Shen A. Wang E. Pace L. Dials H. Mendelson D. Shannon P. Gordon M. A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (Pts) with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2009;27:3513. [Google Scholar]
- 33.Robert C. Arnault JP. Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23:177–182. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 34.Paik PK. Arcila ME. Fara M. Sima CS. Miller VA. Kris MG. Ladanyi M. Riely GJ. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SY. Kim MJ. Jin G. Yoo SS. Park JY. Choi JE. Jeon HS. Cho S. Lee EB. Cha SI. Park TI. Kim CH. Jung TH. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol. 2010;5:1734–1740. doi: 10.1097/JTO.0b013e3181f0beca. [DOI] [PubMed] [Google Scholar]
- 36.Hoque MO. Brait M. Rosenbaum E. Poeta ML. Pal P. Begum S. Dasgupta S. Carvalho AL. Ahrendt SA. Westra WH. Sidransky D. Genetic and epigenetic analysis of erbB signaling pathway genes in lung cancer. J Thorac Oncol. 2010;5:1887–1893. doi: 10.1097/JTO.0b013e3181f77a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulikakos PI. Zhang C. Bollag G. Shokat KM. Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatzivassiliou G. Song K. Yen I. Brandhuber BJ. Anderson DJ. Alvarado R. Ludlam MJ. Stokoe D. Gloor SL. Vigers G. Morales T. Aliagas I. Liu B. Sideris S. Hoeflich KP. Jaiswal BS. Seshagiri S. Koeppen H. Belvin M. Friedman LS. Malek S. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 39.Lacouture ME. Chapman PB. Ribas A. Sosman JA. McArthur GA. Flaherty KT. Kim KB. Puzanov I. Nolop KB. Joe AK. Spleiss O. Koehler A. Wu W. Robert C. Hauschild A. Schadendorf D. Troy JL. Duvic M. Trunzer K. Presence of frequent underlying RAS mutations in cutaneous squamous cell carcinomas keratoacanthomas (cuSCC/KA) that develop in patients during vemurafenib therapy. J Clin Oncol. 2011;29(15 suppl):8520. (Abstract). [Google Scholar]
- 40.Wagle N. Emery C. Berger MF. Davis MJ. Sawyer A. Pochanard P. Kehoe SM. Johannessen CM. Macconaill LE. Hahn WC. Meyerson M. Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]