Abstract
This study aimed to determine the prevalence and predictors of poor 3 and 12 month quality of life outcomes in a cohort of pediatric patients with isolated mild TBI. We conducted a prospective cohort study of children and adolescents <18 years of age treated for an isolated mild TBI, defined as “no radiographically apparent intracranial injury” or “an isolated skull fracture, and no other clinically significant non-brain injuries.” The main outcome measure was the change in quality of life from baseline at 3 and 12 months following injury, as measured by the Pediatric Quality of Life index (PedsQL). Poor functioning was defined as a decrease in total PedsQL score of >15 points between baseline and follow-up scores (at 3 and 12 months). Of the 329 patients who met inclusion criteria, 11.3% (95% CI 8.3–15.3%) at 3 months and 12.9% (95% CI 9.6–17.2%) at 12 months following injury had relatively poor functioning. Significant predictors of poor functioning included less parental education, Hispanic ethnicity (at 3 months following injury, but not at 12 months); low household income (at 3 and 12 months), and Medicaid insurance (at 12 months only). Children and adolescents sustaining a mild TBI who are socioeconomically disadvantaged may require additional intervention to mitigate the effects of mild TBI on their functioning.
Key words: : epidemiology, outcome measures, prospective study, pediatric brain injury
Introduction
Although traumatic brain injury (TBI) is the leading cause of death in children, ∼75–97% of TBIs have been characterized as “mild.”1–3 While moderate and severe TBIs in children result in more significant long-term functional disability,3,4 there is emerging evidence that, for some patients, mild TBI can lead to prolonged physical and neurocognitive symptoms months to years after injury.5–7 However, it is still unclear how these persistent symptoms translate into everyday quality of life, and how common poor overall functioning is following mild TBI.
Although many prior studies have examined longer-term outcomes following pediatric mild TBI, there is significant heterogeneity in injury severity definition, methodological approach and rigor, outcome measures, and results.8 Less is known about outcomes of mild TBI across a wide age range of children and adolescents, and in whom outcomes are measured in multiple dimensions over time.8 The aim of this study was to determine the prevalence and predictors of poor functioning as defined by large declines from baseline in 3 and 12 month Pediatric Quality of Life Inventory (PedsQL) scores in a cohort of pediatric patients with isolated mild TBI.
Methods
Patient population
As described in previous reports of the Child Health After Injury (CHAI) Study,2,3 we identified all subjects <18 years of age treated for a TBI either in the emergency department (ED) or as in inpatient at nine participating institutions in King County, Washington and one in Philadelphia, Pennsylvania. Study informants were consenting parents or guardians of subjects randomly selected from the list of all eligible subjects treated between March 1, 2007, and September 30, 2008, who were contacted and agreed to be in the study. Adolescent subjects ≥14 years of age at the time of follow-up also completed separate surveys. The human subjects committees of participating institutions approved all study procedures.
Definition of isolated mild traumatic brain injury
We used the definition of TBI described in the 2002 Centers for Disease Control and Prevention (CDC) report:9 an injury to the head that was documented in the medical record, with at least one of the following conditions attributed to brain injury: observed or reported decreased level of consciousness, amnesia, or objective neurological or neuropsychological abnormality, or a diagnosis of intracranial lesion. Mild TBI was based on both the CDC1 and World Health Organization (WHO)10 criteria, and was defined as: any period of transient confusion, disorientation, impaired consciousness, or amnesia lasting <24 h, or signs of other neurological or neuropsychological dysfunction, with the worst Glasgow Coma Scale (GCS) score of 13–15 at initial evaluation, and GCS score of 15 at discharge from the emergency department or at 24 h post-injury if hospitalized. Mild TBI was further subclassified into: mild I, no abnormalities on CT scans, or patients in whom CT scans were not performed who met criteria for mild TBI; mild II, skull fracture without intracranial hemorrhage; and mild III, intracranial hemorrhage, and meeting criteria for mild TBI. Only patients with mild I and mild II were included in this analysis. In order to consider only those with isolated mild TBI, patients were excluded if they had a non-brain injury with an Abbreviated Injury Scale (AIS)11 score of ≥2 (which indicated a clinically significant injury that could have affected outcomes). The AIS is a standard, categorical injury scale from 1 to 6, where 1 is “minor,” and 6 is “maximal, currently untreatable.” Patients were also excluded if the injury was intentional or if they had had a prior TBI by self-report.
Procedures
A baseline telephone survey that gathered information about the subject's pre-injury status was administered as soon as possible after the injury to one parent or guardian, and to adolescent patients ≥14 years of age. Follow-up surveys were conducted 3 and 12 months after the date of the index injury. In addition to the standardized measures described subsequently, data on self-reported race/ethnicity, insurance status, household income, and respondent education were collected.
Measures
The PedsQL is a measure of health-related quality of life that assesses physical, emotional, social, and school functioning of children. The PedsQL has been found to be reliable and valid, including for children with TBI.4,12–14 Total PedsQL scores range from 0 to 100, with higher scores indicating higher quality of life. Poor functioning was defined as a decrease in total PedsQL score of >15 from baseline to 3 and 12 months, respectively. This definition of patients with significantly worse outcomes has been previously shown to correspond to the approximate mean difference on the PedsQL between normal children and those with a moderately severe chronic health condition.15,16 The remainder of the patients in the sample were considered to have “expected” outcomes. The PedsQL was only available for children ≥2 years of age at the time of the assessment.
Other scales measured that were assessed as potentially associated with poor functioning included: a headache scale previously described using the same data set,17 the sleep subcomponent of the PedsQL,18 depressive symptoms from the Patient Health Questionnaire (PHQ-9),19 and the University of California at Los Angeles (UCLA) Reaction Index, a post-traumatic stress disorder (PTSD) checklist.20
Other measures in the baseline survey included child, parental, and family demographic information; the subject's pre-injury medical history; and an assessment of family functioning using the McMaster Family Assessment Device.
Medical record data
Charts were comprehensively abstracted by the principal investigator (Dr. Rivara) or a trained research nurse using an online standardized abstraction form. These abstractors were blinded to the baseline and outcome measures.
Statistical analysis
Demographic and clinical characteristics of subjects with expected versus poor functioning were compared using χ2 or Fisher's exact test for categorical variables, and t test statistics for continuous variables, to assess for significant differences.
Linear mixed models were developed to assess the change of PedsQL score from baseline to 3 months or 12 months in patients with expected outcomes compared with those with poor outcomes. The models for both groups and both time periods were adjusted for age, gender, child race, family income, and parent/guardian education.
Analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC) data analysis software.
Results
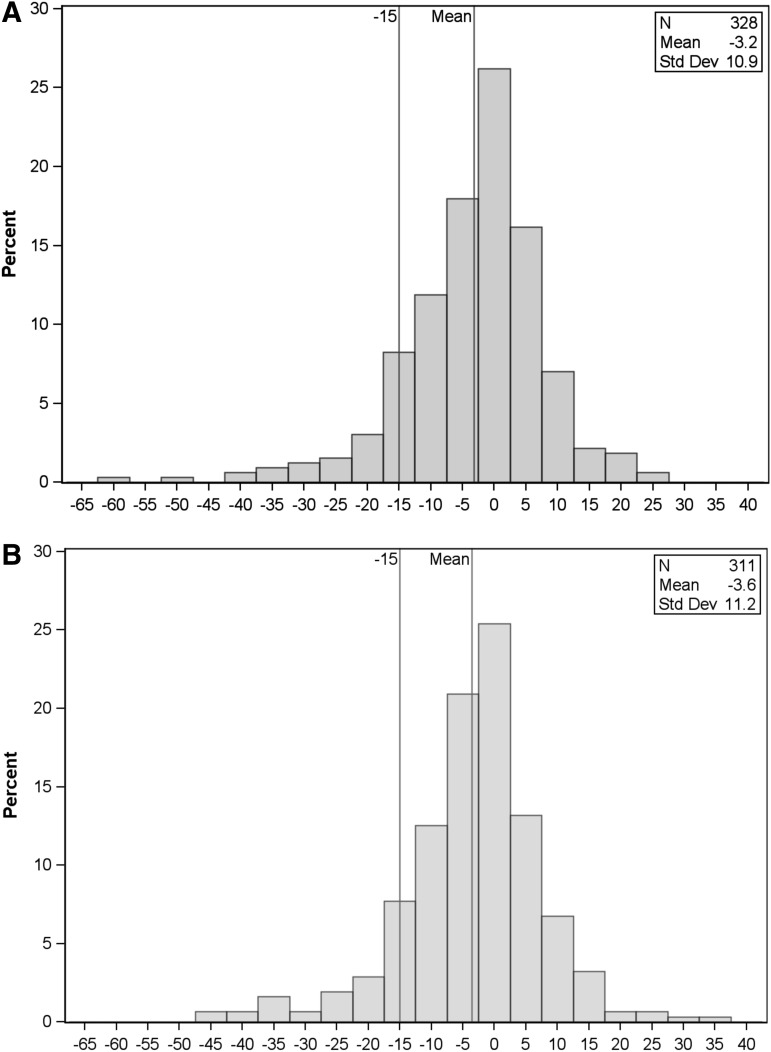
There were 329 patients who met the inclusion criteria, of whom one was missing the 3 month PedsQL score, and 18 were missing 12 month PedsQL scores. The mean PedsQL score for the entire sample was 86.5 (range 23.2–100) at baseline; 83.3 (range 25–100) at 3 months, and 83.0 (range 25.9–100) at 12 months post-injury. Poor functioning, as defined, was seen in 11.3% (95% CI 8.3–15.3%) of the patients at 3 months following injury and 12.9% (95% CI 9.6–17.2%) of the patients at 12 months following injury. Figure 1 shows the distribution of the change in PedsQL scores between baseline and 3 months, and baseline and 12 months.
FIG. 1.
(A) The distribution of the change in Pediatric Quality of Life Index (PedsQL) score between baseline and 3 months following injury. (B) The distribution of the change in PedsQL score between baseline and 12 months following injury.
Table 1 compares the baseline characteristics of the mild TBI patients with expected outcomes and poor outcomes, and Table 2 compares the injury-related characteristics. Poor functioning was significantly associated with: older age (RR[95% CI]=1.006[1.001,1.011] per year of age) at 3 months, Hispanic ethnicity versus white non-Hispanic (RR[95% CI]=3.37[1.47,7.73]) at 3 months, less than high school parental education versus post-college (RR[95% CI]=4.44[1.55,12.76]) at 3 months, some college versus post-college (RR[95% CI]=2.51[1.03,6.10]) at 12 months, and Medicaid insurance versus private insurance (RR[95% CI]=2.22[1.21,4.06]) at 12 months, and low annual household income of <$30,000 versus >$100,000 (RR[95% CI]=2.73[1.28,5.83], 3.10[1.40,6.86]) at 3 and 12 months respectively, and $60,000–$100,000 versus >$100,000 (RR[95% CI]=2.51[1.14,5.53]) at 12 months only.
Table 1.
Characteristics of the Mild TBI Study Population
| 3 month PedsQL | 12 month PedsQL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline total mean PedsQL score | n | Expected n=291 Row% | Poora n=37 Row% | p value | n | Expected n=271 Row% | Poora n=40 Row% | p value | |
| Age at injury | 0.002 | 0.33 | |||||||
| 0–4 years | 91.2 | 62 | 95.2 | 4.8 | 57 | 93.0 | 7.0 | ||
| 5–9 years | 85.6 | 108 | 93.5 | 6.5 | 104 | 87.5 | 12.5 | ||
| 10–14 years | 85.3 | 105 | 79.1 | 20.9 | 99 | 82.8 | 17.2 | ||
| 15–17 years | 85.2 | 53 | 90.6 | 9.4 | 51 | 88.2 | 11.8 | ||
| Gender | 0.09 | 0.92 | |||||||
| Male | 85.9 | 210 | 91.0 | 9.0 | 200 | 87.0 | 13.0 | ||
| Female | 87.6 | 118 | 84.8 | 15.2 | 111 | 87.4 | 12.6 | ||
| Race/ethnicity | 0.11 | 0.65 | |||||||
| White, non-Hispanic | 86.4 | 237 | 90.7 | 9.3 | 230 | 87.8 | 12.2 | ||
| Black, non-Hispanic | 86.0 | 7 | 85.7 | 14.3 | 7 | 71.4 | 28.6 | ||
| Hispanic | 91.4 | 16 | 68.8 | 31.2 | 12 | 83.3 | 16.7 | ||
| Asian | 85.1 | 5 | 100 | 0 | 5 | 100 | 0 | ||
| Other or multiple | 86.0 | 61 | 85.3 | 14.7 | 55 | 85.5 | 14.5 | ||
| Unknown | 83.6 | 2 | 100 | 0 | 2 | 100 | 0 | ||
| Health insurance | 0.12 | 0.07 | |||||||
| None | 88.5 | 8 | 75.0 | 25.0 | 8 | 87.5 | 12.5 | ||
| Medicaid | 83.8 | 76 | 82.9 | 17.1 | 67 | 77.6 | 22.4 | ||
| Private | 87.3 | 216 | 90.7 | 9.3 | 208 | 89.9 | 10.1 | ||
| Unknown | 86.8 | 28 | 92.9 | 7.1 | 28 | 89.3 | 10.7 | ||
| Household income | 0.02 | 0.04 | |||||||
| <$30,000 | 81.8 | 56 | 78.6 | 21.4 | 49 | 77.6 | 22.4 | ||
| $30,000–$60,000 | 84.8 | 47 | 83.0 | 17.0 | 44 | 86.4 | 13.6 | ||
| >$60,000–$100,000 | 86.5 | 71 | 91.6 | 8.4 | 66 | 81.8 | 18.2 | ||
| >$100,000 | 88.7 | 140 | 92.1 | 7.9 | 138 | 92.8 | 7.2 | ||
| Unknown | 89.5 | 14 | 100 | 0 | 14 | 92.9 | 7.1 | ||
| Respondent education | 0.02 | 0.25 | |||||||
| Less than high school | 84.2 | 15 | 66.7 | 33.3 | 11 | 90.9 | 9.1 | ||
| High school, GED | 83.7 | 34 | 91.2 | 8.8 | 32 | 90.6 | 9.4 | ||
| Some college | 85.2 | 90 | 84.4 | 15.6 | 83 | 80.7 | 19.3 | ||
| College graduate | 88.5 | 109 | 91.7 | 8.3 | 107 | 86.9 | 13.1 | ||
| Post-college | 86.8 | 80 | 92.5 | 7.5 | 78 | 92.3 | 7.7 | ||
| Family Assessment Device baseline, mean score (SD) | 86.5 | 328 | 17.8 (5.1) | 17.9 (4.2) | 0.94 | 311 | 17.8 (4.9) | 17.6 (6.1) | 0.89 |
| Pre-injury comorbiditiesb | 0.33 | 0.24 | |||||||
| None | 91.7 | 102 | 91.2 | 8.8 | 95 | 91.6 | 8.4 | ||
| 1 | 88.6 | 90 | 90.0 | 10.0 | 88 | 88.6 | 11.4 | ||
| 2 | 87.7 | 60 | 90.0 | 10.0 | 58 | 84.5 | 15.5 | ||
| ≥3 | 76.3 | 76 | 82.9 | 17.1 | 70 | 81.4 | 18.6 | ||
Defined by a decrease in total PedsQL score of >15 points from baseline to follow-up.
Pre-injury comorbidities assessed included developmental delay, seizures, hemiplegia or paraplegia, lung disease, diabetes, attention-deficit/hyperactivity disorder, depression, other mental health or behavioral problems, learning problems, previous fractures, and previous surgery.
TBI, traumatic brain injury; PedsQL, Pediatric Quality of Life Index.
Table 2.
Injury Characteristics from Medical Record Abstraction
| 3 month PedsQL | 12 month PedsQL | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Expected n=291 Row% | Poora n=37Row% | p value | n | Expected n=271Row% | Poora n=40Row% | p value | |
| Mild TBI | 0.74 | 0.49 | ||||||
| 1 | 306 | 88.6 | 11.4 | 290 | 86.6 | 13.4 | ||
| 2 | 22 | 90.9 | 9.1 | 21 | 95.2 | 4.8 | ||
| Mechanism of injury | 0.84 | 0.26 | ||||||
| Motor vehicle (occupant) | 11 | 81.8 | 18.2 | 11 | 72.7 | 27.3 | ||
| Pedestrian or bicycle | 25 | 92.0 | 8.0 | 23 | 78.3 | 21.7 | ||
| Fall | 189 | 88.9 | 11.1 | 178 | 88.2 | 11.8 | ||
| Stuck by/against | 102 | 88.2 | 11.8 | 98 | 88.8 | 11.2 | ||
| Emergency medical services level | 0.30 | 0.56 | ||||||
| Advanced life support | 21 | 81.0 | 19.0 | 20 | 80.0 | 20.0 | ||
| Basic life support | 40 | 85.0 | 15.0 | 37 | 89.2 | 10.8 | ||
| Not transported by emergency medical services | 259 | 89.6 | 10.4 | 246 | 87.4 | 12.6 | ||
| Isolated TBI | 221 | 89.1 | 10.9 | 0.73 | 208 | 87.5 | 12.5 | 0.79 |
| Head maximum AIS | 0.51 | 0.21 | ||||||
| 1 | 230 | 90.0 | 10.0 | 219 | 89.0 | 11.0 | ||
| 2 | 86 | 86.1 | 13.9 | 81 | 81.5 | 18.5 | ||
| 3 | 12 | 83.3 | 16.7 | 11 | 90.9 | 9.1 | ||
Defined by a decrease in total PedsQL score of >15 points from baseline to follow-up.
TBI, traumatic brain injury; PedsQL, Pediatric Quality of Life Index; AIS, Abbreviated Injury Scale score.
Table 3 shows the mean PedsQL scores at baseline, 3 months, and 12 months, as well as the change and adjusted change in score from baseline to 3 months and baseline to 12 months. Subjects with poor outcomes had ∼14–24 points lower in the adjusted change in PedsQL scores than did those with expected outcomes.
Table 3.
Scores on the Pediatric Quality of Life (PedsQL) Scale Total Score for Children and Adolescents with Mild TBI 1 or Mild TBI 2, and with a Non-Head Maximum AIS of <2
| Mean at month | Change 0→3 months | Change 0→12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 12 | Δb | 95% CI | Diffb | 95% CI | Δb | 95% CI | Diffb | 95% CI | |
| n | 328 | 328 | 310 | ||||||||
| Expected outcomes at 3 months | 86.3 | 85.8 | 84.7 | −0.5 | −1.4,0.4 | Ref | −1.7 | −2.8, −0.7 | Ref | ||
| Poor outcomesa at 3 months | 88.4 | 63.8 | 70.0 | −24.6 | −27.8, −21.3 | −24.1 | −27.4, −20.7 | −18.1 | −23.2, −13.0 | −16.4 | −21.6, −11.1 |
| n | 311 | 310 | 311 | ||||||||
| Expected outcomes at 12 months | 86.1 | 84.7 | 85.6 | −1.4 | −2.5, −0.4 | Ref | −0.5 | −1.5,0.4 | Ref | ||
| Poor outcomesb at 12 months | 90.0 | 75.1 | 66.1 | −15.0 | −19.4, −10.6 | −13.5 | −18.1, −9.0 | −23.9 | −26.7, −21.2 | −23.4 | −26.3, −20.5 |
Defined by a decrease in total PedsQL score of >15 points from baseline to follow-up.
Adjusted for age, gender, child race, family income, and parent education.
TBI, traumatic brain injury; AIS, Abbreviated Injury Scale score.
There were no significant differences at pre-injury baseline between the two groups on trouble sleeping, headache, or depressive symptoms. Subjects who had trouble sleeping “sometimes, often, or almost always” at 3 months and “often or almost always” at 12 months, were more likely to have poor outcomes at 3 and 12 months, respectively (p<0.0001 for both comparisons). Similarly, subjects who had headache or other pain symptoms at 3 months and 12 months were also more likely to have poor outcomes at 3 and 12 months, respectively (p<0.05 for both comparisons). Depressive symptoms and PTSD symptoms were not associated with poor outcomes at either the 3 or 12 month time period.
Patients with poor outcomes were more likely to have lower mean absolute PedsQL scores across all four subdomains (physical, social, school, cognitive) at the 3 and 12 month time period compared than were those with expected outcomes (p<0.001 for all comparisons).
Discussion
This large study of the prevalence and predictors of low PedsQL scores in a cohort of patients with isolated, mild TBI found that ∼12% of subjects with mild TBI had poor quality of life at 3 and 12 months following their injury. Patients who were socioeconomically disadvantaged were more likely to have poor outcomes at 3 and/or 12 months following injury, across all domains of the PedsQL. Our prior work using the full cohort found that subjects with mild TBI overall generally had good outcomes over time as measured by average PedsQL scores, particularly when compared with those with moderate and severe TBI. However, averages obscure the full range of PedsQL scores among those with mild TBI.3 Therefore, those with poor functioning were “hidden” in the larger group.
The mean absolute total PedsQL scores for subjects with expected outcomes at 3 and 12 months were 85.8 and 85.6, respectively, whereas those for subjects with poor outcomes were 63.8 and 66.1, respectively. This is comparable to prior pediatric TBI research in which parents' subjective measurement of their child's cognition being “somewhat worse” at 3 and 12 months following injury corresponded to a mean total PedsQL score of 66.4.21 Interestingly, the clinical definition of poor outcomes (decrease in total PedsQL score of >15 from baseline to follow-up) based on prior literature15 approximated a change in PedsQL of >1 standard deviation (SD) below the mean difference (Fig. 1). Other work has shown poor quality of life at 1 SD below the absolute mean PedsQL score.16
According to the AIS scoring system, an AIS 1 injury is “minor,”11 with a very low risk of fatality. In the most recent version of the AIS manual (AIS 2005, Update 2008), concussion without loss of consciousness is an AIS 1 injury. Although AIS was originally designed as a “threat to life scale,” the morbidity associated with nonfatal injuries is also routinely measured with the instrument. Given the persistent disability of those in this study with seemingly “mild” injuries, use of the AIS system to accurately categorize severity of injury for certain injuries may need to be reconsidered.22
Of the 40 patients with poor outcomes at 12 months, 20 (50%) did not have poor outcomes at 3 months. There are a few possible explanations. First, subjects could have sustained an additional injury during the follow-up period, which was not captured in our data collection. Another possibility is that symptoms actually do increase over time, leading to poorer quality of life measures. This was observed in a recent study of children and adolescents 8–15 years old with mild TBI who had increases in post-concussive symptoms and impaired functionality up to 1 year following injury.7 There is a growing body of literature demonstrating that providers who care for children with mild TBI, including concussion, may not be consistently recognizing symptoms or providing evidence-based treatment.23
Our work is consistent with prior studies showing disparities in mortality and long-term functionality following more severe TBI, with disadvantaged youth and patients with inflicted brain injury having poorer outcomes.24–27 These studies emphasize the need to further identify causes of such outcomes, such as disparities in care, variable compliance with recovery plans, and the lack of social support in these populations.
There are some limitations of this study worth noting. Self-reported measures of functionality may not consistently be accurate, although the PedsQL has proven valid and reliable for a variety of disease states and severities.12 Although not all subjects in this mild TBI group had a head CT scan completed immediately following their injury, the rate of CT use in the poor outcome group was similar to that in the expected outcome group. Another potential limitation is that follow-up surveys did not inquire about re-injury (TBI or otherwise), which could contribute to lower PedsQL scores at follow-up. However, it is unlikely that re-injury could account for the entire proportion of patients with poor outcomes. Finally, the definition of poor functioning using a decrease of >15 total PedsQL points may not have captured all of the subjects with clinically relevant poor quality of life, and, therefore, likely results in a conservative estimate of the prevalence of poor functioning following mild TBI.
In addition to maximizing acute care, health care providers should ensure that multidimensional support and resources are available, particularly for patients who are at risk for poorer outcomes. Finally, primary prevention should always be considered as a means of minimizing the burden of mild TBI. Regular anticipatory guidance surrounding child passenger safety in motor vehicles, injury prevention in the home, and recreational safety is paramount. Attention should be focused on children and adolescents who are socioeconomically disadvantaged, and who have premorbid conditions that may predispose them to TBI or prolong their recovery.
Future work should focus on identifying additional risk factors for poor outcomes following mild TBI, and improved ascertainment about re-injury in longitudinal TBI studies, as well as improved diagnostics for patients with “mild” TBI, such as biomarkers or advanced non-radiating diagnostic imaging. In addition, providers who care for patients with mild TBI should consider adapting standardized protocols for the evaluation of and treatment of mild TBI,28 and testing the efficacy of these treatments with trials.
Acknowledgments
This project was supported by the National Center for Injury Prevention and Control, CDC grant R49 CE 001021, and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH) grant 1K08HD073241-02. The CDC and the NIH had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention-National Center for Injury Prevention and Control Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. 2003. Available at: http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf Accessed August31, 2012
- 2.Koepsell T.D., Rivara F.P., Vavilala M.S., Wang J., Temkin N., Jaffe K.M., and Durbin D.R. (2011). Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics 128, 946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivara F.P., Koepsell T.D., Wang J., Temkin N., Dorsch A., Vavilala M.S., Durbin D., and Jaffe K.M. (2011). Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics 128, e1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy M.L., MacKenzie E.J., Durbin D.R., Aitken M.E., Jaffe K.M., Paidas C.N., Slomine B.S., Dorsch A.M., Christensen J.R., and Ding R. (2006). Health-related quality of life during the first year after traumatic brain injury. Arch. Pediatr. Adolesc. Med. 160, 252–260 [DOI] [PubMed] [Google Scholar]
- 5.Sroufe N.S., Fuller D.S., West B.T., Singal B.M., Warschausky S.A., and Maio R.F. (2010). Postconcussive symptoms and neurocognitive function after mild traumatic brain injury in children. Pediatrics 125, e1331–e1339 [DOI] [PubMed] [Google Scholar]
- 6.Babikian T., Satz P., Zaucha K., Light R., Lewis R.S., and Asarnow R.F. (2011). The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 17, 886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeates K.O., Kaizar E., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., and Taylor H.G. (2012). Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch. Pediatr. Adolesc. Med. 166, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinlay A. (2010). Controversies and outcomes associated with mild traumatic brain injury in childhood and adolescences. Child Care Health Dev. 36, 3–21 [DOI] [PubMed] [Google Scholar]
- 9.Marr A., and Coronado V.G. (2004). Central Nervous System Injury Surveillance Data Submission Standards–2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control [Google Scholar]
- 10.Carroll L.J., Cassidy J.D., Holm L., Kraus J., and Coronado V.G. (2004). Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, 113–125 [DOI] [PubMed] [Google Scholar]
- 11.Association for the Advancement of Automotive Medicine (2008). The Abbreviated Injury Scale 2005, Update 2008. Association for the Advancement of Automotive Medicine: Barrington, IL [Google Scholar]
- 12.Varni J.W., Seid M., and Kurtin P.S. (2001). PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 generic core scales in healthy and patient populations. Med. Care 39, 800–812 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy M.L., MacKenzie E.J., Durbin D.R., Aitken M.E., Jaffe K.M., Paidas C.N., Slomine B.S., Dorsch A.M., Berk R.A., Christensen J.R., and Ding R. (2005). The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch. Phys. Med. Rehabil. 86, 1901–1909 [DOI] [PubMed] [Google Scholar]
- 14.McCarthy M.L. (2007). Measuring children's health-related quality of life after trauma. J. Trauma 63, S122–S129 [DOI] [PubMed] [Google Scholar]
- 15.Huang I.C., Thompson L.A., Chi Y.Y., Knapp C.A., Revicki D.A., Seid M., and Shenkman E.A. (2009). The linkage between pediatric quality of life and health conditions: establishing clinically meaningful cutoff scores for the PedsQL. Value Health 12, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varni J.W., Burwinkle T.M., Seid M., and Skarr D. (2003). The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul. Pediatr. 3, 329–341 [DOI] [PubMed] [Google Scholar]
- 17.Blume H.K., Vavilala M.S., Jaffe K.M., Koepsell T.D., Wang J., Temkin N., Durbin D., Dorsch A., and Rivara F.P. (2012). Headache after pediatric traumatic brain injury: a cohort study. Pediatrics 129, e31–e39 [DOI] [PubMed] [Google Scholar]
- 18.Tham S.W., Palermo T.M., Vavilala M.S., Wang J., Jaffe K.M., Koepsell T.D., Dorsch A., Temkin N., Durbin D., and Rivara F.P. (2012). The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. J. Neurotrauma 29, 154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K., Spitzer R.L., and Williams J.B.W. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg A.M., Brymer M.J., Decker K.B., and Pynoos R.S. (2004). The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. Curr. Psychiatry Rep. 6, 96–100 [DOI] [PubMed] [Google Scholar]
- 21.McCarthy M.L., MacKenzie E.J., Durbin D.R., Aitken M.E., Jaffe K.M., Paidas C.N., Slomine B.S., Dorsch A.M., Berk R.A., Christensen J.R., and Ding R. (2005). The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch. Phys. Med. Rehabil. 86, 1901–1909 [DOI] [PubMed] [Google Scholar]
- 22.Carroll C.P., Cochran J.A., Price J.P., Guse C.E., and Wang M.C. (2010). The AIS-2005 revision in severe traumatic brain injury: mission accomplished or problems for future research? Ann. Adv. Automot. Med. 54, 233–238 [PMC free article] [PubMed] [Google Scholar]
- 23.Zonfrillo M.R., Master C.L., Grady M.F., Winston F.K., Callahan J.M., and Arbogast K.B. (2012). Pediatric providers' self-reported knowledge, practices, and attitudes about concussion. Pediatrics. 52, 397–402 [DOI] [PubMed] [Google Scholar]
- 24.Haider A.H., Efron D.T., Haut E.R., DiRusso S.M., Sullivan T., and Cornwell E.E. (2007). Black children experience worse clinical and functional outcomes after traumatic brain injury: An analysis of the National Pediatric Trauma Registry. J. Trauma 62, 1259–1262 [DOI] [PubMed] [Google Scholar]
- 25.Hakmeh W., Barker J., Szpunar S.M., Fox J.M., and Irvin C.B. (2010). Effect of race and insurance on outcome of pediatric trauma. Acad. Emerg. Med. 17, 809–812 [DOI] [PubMed] [Google Scholar]
- 26.Keenan H.T., Runyan D.K., and Nocera M. (2006). Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 117, 1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan H.T., Runyan D.K., and Nocera M. (2006). Child outcomes and family characteristics 1 year after severe inflicted or noninflicted traumatic brain injury. Pediatrics 117, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention, National Centers for Injury Prevention and Control Concussion and Mild TBI. Available at http://www.cdc.gov/concussion/index.html Accessed February3, 2012