Abstract
Heterogeneity within brain injury presents a challenge to the development of informative molecular diagnostics. Recent studies show progress, particularly in cerebrospinal fluid, with biomarker assays targeting one or a few structural proteins. Protein-based assays in peripheral fluids, however, have been more challenging to develop, in part because of restricted and intermittent barrier access. Further, a greater number of molecular variables may be required to inform on patient status given the multi-factorial nature of brain injury. Presented is an alternative approach profiling peripheral fluid for a class of small metabolic by-products rendered by ongoing brain pathobiology. Urine specimens were collected for head trauma subjects upon admission to acute brain injury rehabilitation and non-traumatized matched controls. An innovative data-independent mass spectrometry approach was employed for reproducible molecular quantification across osmolarity-normalized samples. The postacute human traumatic brain injury urinary signature encompassed 2476 discriminant variables reproducibly measured in specimens for subject classification. Multiple subprofiles were then discerned in correlation with injury severity per the Glasgow Comma Scale and behavioral and neurocognitive function per the Patient Competency Rating Scale and Frontal Systems Behavioral Scale. Identified peptide constituents were enriched for outgrowth and guidance, extracellular matrix, and post-synaptic density proteins, which were reflective of ongoing post-acute neuroplastic processes demonstrating pathobiological relevance. Taken together, these findings support further development of diagnostics based on brain injury urinary signatures using either combinatorial quantitative models or pattern-recognition methods. Particularly, these findings espouse assay development to address unmet diagnostic and theragnostic needs in brain injury rehabilitative medicine.
Key words: : biomarker; brain injury, mass spectrometry; metabolomics; rehabilitation; urine
Introduction
Providers cite brain injury variability as a primary challenge to accurate characterization of symptoms and progress of their patients.1–4 Brain injury heterogeneity has also complicated the development of informative diagnostics, which must be sensitive and selective to an individualized trajectory, diversified by a varied set of factors, including mechanism, severity, and localization of injury, demographics, individualized pathobiological response, and comorbidity with other trauma.5–7 Effective diagnostics must then reflect injury metrics, target acute or chronic pathology, and employ appropriate models that are robust to the degree of variance present within brain injury.
To this end, the field of brain injury diagnostics may benefit from new approaches. To date, brain injury research has produced several candidate molecular biomarkers based on quantifying one or a few target proteins in cerebrospinal fluid or blood (see recent reviews).8–12 Though these groundbreaking assays are promising, the target-protein approach presents several diagnostic limitations: underpowered pathobiological factor specificity from too few variables; quantitative variability resulting from underfitting individualized aspects of disease response; and restricted, intermittent access to peripheral fluids, particularly unfavorable in the post-acute period when brain barrier stability is restored.8,10,13,14 Alternatively, molecular efflux into peripheral fluid (e.g., blood and urine) is enhanced for smaller, ionic by-products rendered by ongoing neurobiological processes.15–17 Encompassing an abundant class of pathobiologically informative molecules portends use of pattern detection methods to develop more-robust brain injury diagnostics.8,13,18,19
Post-acute brain injury rehabilitative care would particularly benefit from assays based on molecules with more-consistent brain barrier efflux that are accessible in easily attained peripheral fluids. Clear advances have been made over the last two decades in rehabilitation of traumatic brain injury (TBI) patients.2,20 Rehabilitative therapy improves cognition, quality of life, and perceived competence.2,3,21 Further, therapeutic intensity is predictive of improved function.22 However, therapeutic needs vary widely among brain injury patients, demanding individualized therapeutic strategies and assessments to provide maximal rehabilitative benefit.20,23 Rehabilitation practitioners are challenged to quickly, yet precisely, characterize their patient's cognitive and behavioral performance to facilitate effective treatment planning and long-term recommendations. Patient readiness for acute rehabilitation is particularly difficult to assess with conventional neuropsychological testing that often does not capture the capacity to participate meaningfully in therapies.20,24,25 Rehabilitation readiness and therapeutic responsiveness diagnostics that are independent of human verbal or written responses would advance our ability to identify and improve individualized care for persons with brain injury.
Thus, there is a growing call for molecular diagnostics in brain injury rehabilitative medicine.20,21 Four particular goals have been set: 1) aid admitting and stratifying patients for customized therapy; 2) monitor therapeutic progress and guide treatment course; 3) reflect underlying pathobiology in evaluating new treatments; and 4) predict outcome. Structural protein biomarkers under study for brain injury reflect on acute degenerative pathobiology and thus may be less informative on post-acute regeneration.21,26,27 Alternative diagnostics and theragnostics would preferentially reflect neuroplastic processes ongoing weeks to months after injury to target and optimize neurological and functional recovery.23,28,29 Confronting all of the above, this study reports on proof of principle for a new diagnostic approach assessing small metabolic brain injury by-products released into patient urine in the early rehabilitative phase of recovery. Complexity of the urinary TBI metabolomic signature was assessed along with the capacity to classify subjects and stratify based on clinical metrics of injury and function. Discriminant variables were probed for relevance to regenerative pathobiology substantiating a basis for further development into brain injury rehabilitation diagnostics.
Methods
A controlled demographic of young adult Caucasian male subjects was recruited with informed consent and approval by the Virginia Commonwealth University Institutional Review Board (Richmond, VA). TBI subjects were enrolled upon admission to inpatient rehabilitation at a mean 17 days postinjury (n=5; 26±6 years old; 5±3 initial Glasgow Coma Scale [GCS] score assessed acutely after injury). Non-traumatized matched control subjects were then recruited (n=5; 26±5 years old). Criteria excluded subjects with noncranial bone fractures, renal dysfunction at time of rehabilitation admission, and a positive history for past brain injury or neurological disease. Admission to the Brain Injury Rehabilitation Unit was based on standards of care for demonstrating readiness, with required medical stability and capacity to progress in an acute rehabilitation program. Consent was obtained within 48 h of admission to the unit. Beginning at 72 h on unit, three mid-stream urine specimens were acquired within a 48-h window. Urine specimens were placed at 4°C after collection and centrifuged at 1500g and 4°C for 15 min. Aliquots were then stored at −80°C.
Specimens (three per subject) were load standardized to an osmolarity of 130 mOsm/kg with Nanopure water. Balanced specimens (100 μL) were filtered with 0.1-μm pore Ultrafree-MC units (Millipore, Billerica, MA), with the supernatants transferred to vials for direct injection (8 μL on column) in a group-interspersed order. Reversed-phase separation was performed with a nanoAcquity chromatography system, using a Symmetry C18 trapping column (2 cm×180 μm i.d.) and an HSS T3 nanoAcquity (15 cm×75 μm i.d.) capillary column (Waters, Milford, MA). Components were gradient separated using 0.1% formic-acid–modified acetonitrile and water. Eluting peptides were electrosprayed into a Synapt G2 hybrid ion mobility/mass spectrometer (Waters) operated in a data-independent analysis mode, as described previously.30 All analytical work was performed within a climate-controlled clean room.
Data were processed using PLGS software v.2.5.2 (Waters). Precursor and product ion measures exceeding 150 and 20 counts, respectively, were extracted, deisotoped, and charge state collapsed. Accurate mass and retention time (AMRT) tables for triplicate specimens were merged to generate a single composite molecular profile per subject that accounted for intradaily variance. All subject profiles were then aligned by AMRT values (±7 ppm mass accuracy;±0.5 min retention time) using Expressions software (v.2.5).31 Non-reproducing AMRT measures (<3/group) were removed. Values from a simulated Gaussian distribution randomized about the limit of quantification were imputed for left-censored data denoting a non-random, group-specific level below the detection limit.30 Intersubject normalization (median intensity, 1000 most intense ions) and log(2) transformation procedures were performed.
Statistical analysis
Aligned composite molecular profiles (one per subject) were statistically tested using the MultiExperimentViewer (v.4.8.1) informatics package for array data.32 Principle component analysis and Welch's t-test methods were applied with alpha corrected for multiple measures using a q value false-discovery rate (FDR) method.33 Pearson's analysis tested for correlation between TBI-responsive molecular variables and Pavlidis templates of subject GCS, Galveston Orientation and Amnesia Test (GOAT), Disability Rating Scale (DRS), Frontal Systems Behavioral Scale (FrSBe), Neurobehavioral Rating Scale (NRS), and Patient Competency Rating Scale (PCRS) total scores. A one-sample t-test method assessed for chance correlation relative to a set of random templates; the significance level was adjusted with Bonferroni's correction method. Correlation between subject clinical scores was assessed by Pearson's analysis using SPSS (v.20; IBM, Armonk, NY).
Sequence search analysis was performed on TBI-positive responding variables (PLGS v.2.5.2) against a Human UniProtKB fasta database (2012_10 release). Parameters selected for no restrictive enzyme, variable methionine oxidation, neutral loss of ammonia or water, mass spectrometry (MS) tolerance of 5 ppm, and tandem MS tolerance of 15 ppm. Results (raw peptide score) were controlled to a 10% false sequence identification rate using a reversed decoy database method. Identified peptide products were matched to their parent protein or protein family. Enrichment analysis was performed with corresponding protein symbols against Gene Ontology (GO) annotation terms (molecular function, biochemical process, and cellular component), biochemical pathways, and protein-protein interaction clusters using a Fisher's inverse chi-square method with a Bonferroni correction of alpha (ToppGene v.9.56.45).34 TBI-responsive results were further analyzed using protein-protein interaction network analysis (STRING v.9.0, action view),35 with a minimum interaction confidence score of 0.6 and 10 added interactor nodes.
Results
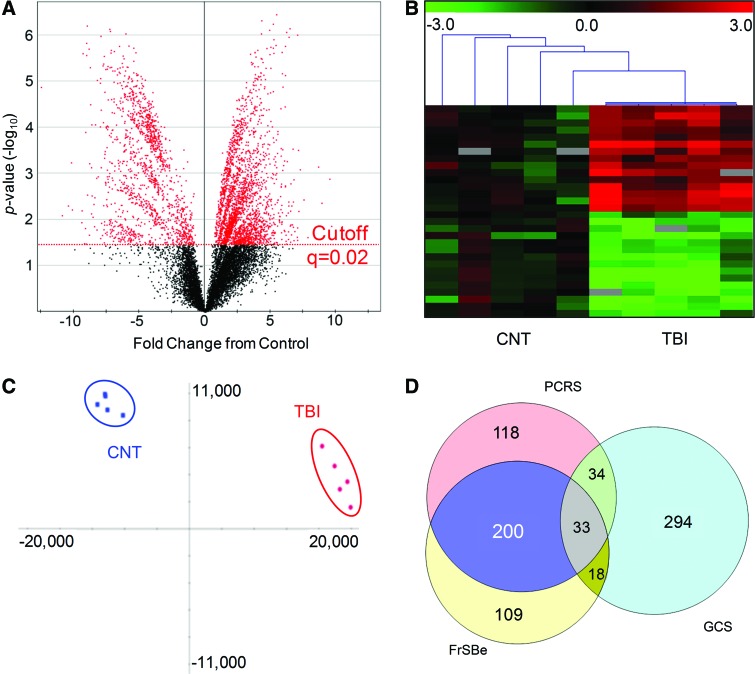
Using innovative data-independent mass spectrometry (MS) analysis, 10,929 distinct molecular measures were reproducibly quantified across group subjects. This urinary metabolome was comprised of small molecules with a median mass of 1274 Da, with only 5% exceeding 5 kDa. Supervised statistical testing revealed 3897 TBI-responsive measures (Fig. 1A) with an FDR of 2% (76 false detections). Measures reproducibly discriminated TBI from control subjects (Fig. 1B). A 64% majority of the measures (2476) were significantly increased in TBI urine (TBI urinary signature). A nonsupervised orthogonal transformation into principal components unambiguously classified TBI subjects from matched controls (Fig. 1C), supporting utility in diagnostic model building. Principal component 1 with a dominant eigenvalue of 17.5 represented over 66.4% of total variance and effectively bisected the subjects into two distinct clusters (Fig. 1C, x-axis). Secondary, intragroup variability was accounted for in principal components 2 (Fig. 1C, y-axis) through 4, where a definitive Scree plot breakpoint was identified. Eigenvalues for these components were appreciably smaller (3.1–1.2), accounting for an additional 21.7% of total variance.
FIG. 1.
TBI urinary signature discriminates postacute traumatic brain injury (TBI) subjects from controls. (A) Volcano projection of fold-change, relative to control (CNT), plotted against statistical probability (p) values for 10,929 reproducing molecular measures in human urine specimens. Adjusting the significance level to a false-discovery rate FDR of 2% (q=0.02), 3897 measures (red) were found statistically responsive to TBI. (B) Heatmap plot of 30 representative molecular measures detected across CNT and TBI subjects (n=5/group). Measures are plotted as fold-change from control, scaled between −3.0 and 3.0, with gray fields denoting absent values. K-means hierarchical clustering results are illustrated by leader lines at top, with TBI data clustered together apart from CNT data. (C) Multi-variate presentation of TBI (red) and CNT (blue) subjects by factor scores across principal components 1 (x-axis) and 2 (y-axis), with ellipsoids demarking eigenvector covariance. The maximum proportion of variance (PC1) comprised of discriminate molecular variables effectively resolved TBI subjects from CNT. (D) Venn diagram presentation of confluence between subprofiles found in correlation with subject GCS (379), PCRS (385), and FrSBe (360) scores. GCS, Glasgow Comma Scale; PCRS, Patient Competency Rating Scale; FrSBe, Frontal Systems Behavioral Scale. Color image is available online at www.liebertpub.com/neu
To further evaluate diagnostic potential, the TBI urinary signature was assessed for correlation to templates of scalar clinical metrics of interest to rehabilitation practitioners. The number of correlative molecular variables (Pearson's R, >0.95) was not significantly different from random template matching for GOAT, DRS, and NRS total scores once corrected for repeated measures (p>0.004). However, three significant subprofile factors were found in correlation with the following clinical metrics (Table 1): GCS, with a subset of 379 molecules; (p=1.91e–8); PCRS, with a subset of 385 molecules (p=2.23e–7); and FrSBe, with a subset of 360 molecules (p<9.07e–9). Relationships between the three subprofiles were summarized using a Venn diagram (Fig. 1D). Total scores for PCRS and FrSBe were found to be correlative (Pearson's R, 0.91; p=0.034). Thus, it fit that there was a two-thirds overlap in PCRS and FrSBe correlative molecular variables. In contrast, the GCS factor was largely distinct (294, or 77% unique) from the other two subprofiles. In agreement, subject GCS scores were not predictive of either rehabilitation assessments (GCS to PCRS, Pearson's R=0.63, p=0.251; GCS to FrSBe, Pearson's R=0.62, p=0.261; see Table 1). Findings suggest that the TBI urinary signature comprises multiple clinically informative factors.
Table 1.
Details of Correlation Analyses with TBI Clinical Metrics
| TBI clinical assessments | ||||
|---|---|---|---|---|
| GCS | PCRS | FrSBe | ||
| Subject metrics | ||||
| 1 | 5 | 128 | 67 | |
| 2 | 3 | 141 | 119 | |
| 3 | 3 | 124 | 74 | |
| 4 | 6 | 137 | 85 | |
| 5 | 8 | 149 | 188 | |
| Correlated subprofile results | ||||
| Pearson's R | >0.95 | >0.95 | >0.95 | |
| No. of correlation variables | 379 | 385 | 360 | |
| t score | 7.787 | 8.114 | 6.752 | |
| p value | 1.91E-08 | 9.07E-09 | 2.23E-07 | |
| Correlation between clinical metrics | ||||
| GCS | R | 1 | 0.634 | 0.624 |
| p | 0.251 | 0.261 | ||
| PCRS | R | 0.634 | 1 | 0.906 |
| p | 0.251 | 0.034 | ||
| FrSBe | R | 0.624 | 0.906 | 1 |
| p | 0.261 | 0.034 | ||
TBI, traumatic brain injury; GCS, Glasgow Comma Scale; PCRS, Patient Competency Rating Scale; FrSBe, Frontal Systems Behavioral Scale.
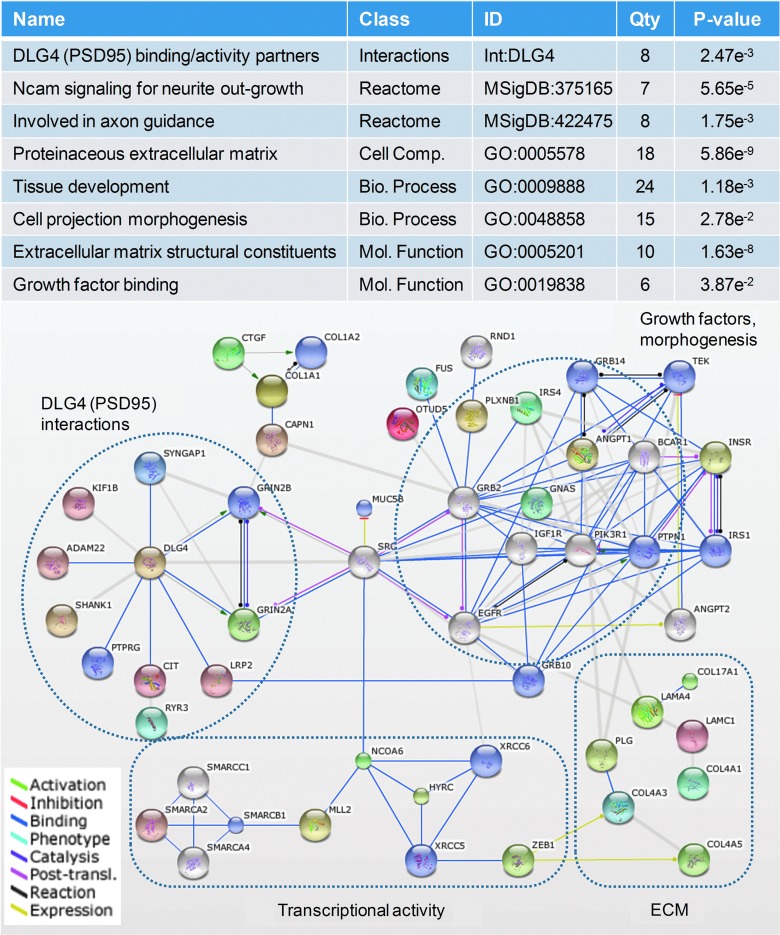
To assess a pathobiological connection, tandem mass spectra from the TBI urinary signature were matched with peptide sequences, which were then examined for their biomolecular relevance. Using accurate mass measurement and selective fragmentation spectra, 238 sequences were identified at a 10% FDR. These endogenous peptides had an average mass of 2223±755 Da (ranging from 812 to 3948 Da) and were of sufficient sequence length to discriminate 144 source proteins (or protein families). A 57% majority of proteins had only one corresponding endogenous peptide, with only 16% having three or more matched peptides. These findings suggested that most peptides were select metabolic products excreted from circulation, rather than from non-specific protein catabolism, in urine.
Of the 144 source proteins, 119 were annotated in molecular ontology databases and were mined for enriched biomolecular associations (Fig. 2). Identified peptides were largely fragments of extracellular matrix (ECM) and membranous proteins that are known to, or may likely, shed peptides during synaptic reorganization.36,37 Four distinct activity networks of interest were related to outgrowth, guidance and morphogenesis, the ECM, transcriptional activity, and postsynaptic density interaction. This later network included peptides from several major components of the excitatory glutamate receptor complex, including glutamate receptor 2A and B (Grin2A/B), synaptic Ras GTPase activating protein 1(Syngap1), and SH3 ankyrin repeat domain 1 (Shank1). Taken together, these results provide a mechanistic link with neuroplastic dynamics.
FIG. 2.
Traumatic brain injury (TBI) urinary signature reflects on an ongoing neuroplastic response to TBI during the post-acute rehabilitative phase of care. Identified peptide fragments found increased within TBI urine were metabolized products of proteins associated with the postsynaptic density complex (DLG4 interactions), neurite outgrowth, guidance cues, and projection morphogenesis factors (growth factors, morphogenesis), extracellular matrix components (ECM), and transcriptional activity. (Top) Biological classes significantly enriched among represented proteins with selective relevance to a neuroplastic response after TBI. (Bottom) Protein-protein interaction network of proteins metabolized to form by-product peptide detected within the TBI urinary signature (nodes, with protein symbols). MSigDB, Molecular Signature Database; GO, Gene Ontology. Color image is available online at www.liebertpub.com/neu
Discussion
Presented are results supporting an alternative approach for brain injury molecular diagnostics espoused particularly to address needs in post-acute care. Results unveil the discriminant capacity, pathobiological relevance, and diagnostic potential of a TBI urinary signature. Findings suggest applicability for multi-variate analyses of small molecular by-products released into peripheral fluids to assess brain injury pathobiological and functional status.
Results newly reveal that human urine specimens contain several thousand TBI discriminant measures (Fig. 1A). Urine has largely been overlooked as a brain injury biomarker source, with proteins of interest generally excluded through normal renal function.9 However, urine is a noninvasive, readily attainable, and stable biofluid—preferable attributes for longitudinal monitoring in rehabilitative care (including outpatient) or with sensitive populations, such as pediatric TBI.38 The extensive molecular diversity (10,929 reproducible measurements) uncovered in urine is an advantageous finding. The present approach capitalizes on renal barrier function in excreting small metabolites from circulation, providing filtration enrichment for by-products of interest here. In particular, barrier-permeable small ionic metabolites, such as proteolytically shed peptides, are also amenable to MS analysis. Figure 1C provides a reduced multi-variate projection of the extensive factor space objectively classifying TBI from control subjects (component 1, x-axis). The factor space included 834 molecular variables that were over 3-fold more abundant in TBI specimens, relative to non-traumatized controls. These measures could provide for sensitive, robust multi-variate diagnostic models to address multi-factor heterogeneity in TBI.
Further study assessed whether the TBI urinary signature consisted of clinically informative subset factors (Fig. 1D). Pearson's analysis revealed molecular factors correlated with subject PCRS and FrSBe rehabilitation scores (Table 1). The two subprofiles were, however, confluent with a two-thirds overlap in variables. In explanation, PCRS and FrSBe instruments reflected upon similar aspects of behavioral and neurocognitive competencies at the same postacute period, with significant correlation between their total scores. GCS, conversely, was found in correlation with an independent subprofile. A measure of the conscious state, GCS was assessed acutely after TBI as used to gauge injury severity, thus a different metric and period in the pathobiology. It rationally followed that GCS related to a distinct subset of biochemical products. Taken together, these results denote multiple distinct molecular factors within the TBI urinary signature in correlation with different clinical metrics.
Sample collection was standardized with admission to rehabilitation assessed with standard-of-care examination. This, in part, accounted for variable recovery rates among individuals. However, more precise diagnostic indicators of maximal readiness may be possible using longitudinal assessment of measures associated with regenerative pathobiology. Readiness may be linked with priming of neuroplastic processes underlying regeneration and influenced by rehabilitative activities.28,29 Timing is all too critical because early intervention may negatively affect regeneration and functional recovery.39,40 Post-acute neuroplastic reorganization involves changes to brain matrix and release of extracellular signaling factors (e.g., peptides). The TBI urinary signature demonstrated a significant overrepresentation of peptide by-products of proteins involved in neuroplastic processes (Fig. 2). Included were peptide growth factors and matrix components connected with Ncam signaling for neurite outgrowth and axon guidance as well as an enrichment of post-synaptic density interacting components. These findings underscore the neuroplastic relevance of the TBI urinary signature, supporting potential utility in brain injury rehabilitative medicine. Subsequent studies are needed, however, to examine temporal biokinetics of the TBI urinary signature in association with rehabilitative care and outcome.7
Several limitations of the present study also need to be addressed in future studies. Foremost, the present findings provide support for larger cohort studies. Enrollment criteria for these initial studies aimed to minimize confounders by restricting subject demographics, injury severity, and exclusion of other major organ trauma.7,41 However, follow-up studies are needed to evaluate the effect of these relevant factors on the TBI urinary signature. Individualized factors associated with patient symptomology and associated pharmacology and therapeutic care no doubt add further variability across subsets of molecular variables. Though our study design focused on factors reproducibly responsive across all subjects, there are likely other molecules that remain to be explored within the TBI urinary signature that reflect such individualized aspects. Acknowledging these shortcomings, these findings provide proof of principle to support further research. Innovation was enabled by data-independent quantitative MS, providing for reproducible measurement of endogenous biofluid constituents across subjects (Fig. 1B). Conventional data-dependent methods lack a sufficient duty cycle to provide consistent observation and precise quantification as necessary for these studies without employing molecular labeling methods, which are not suited to metabolomic analysis. The present approach rapidly sampled, accurate precursor and product ion mass measurements to assess a large array of molecules with femtomolar detection and a general dynamic range on par with singular target enzyme-linked immunosorbent assay kits. Given the stability of urine specimens, daily MS assessment of the post-acute TBI urinary signature is feasible through properly equipped regional clinical service laboratories.
Conclusion
Findings reveal a diverse class of molecular products present within human urine that effectively classify TBI subjects from matched non-traumatized controls. The encompassed TBI urinary signature provided a reproducible pattern across a controlled cohort of severe TBI study subjects. Measures are directly linked with neuroplastic processes with relevance to brain injury pathobiology during the post-acute rehabilitative phase. Further, the TBI urinary signature comprises multiple subsets found to correlate with clinical metrics of acute injury severity and postacute behavioral and neurocognitive function. These results support further development of pattern-based urinary metabolite diagnostics and theragnostics to assess rehabilitation readiness and efficacy of intervention applicable broadly to brain injuries from traumatic, ischemic, and hemorrhagic insults.
Acknowledgments
This research was supported in part by the Virginia Commonwealth University School of Medicine and the National Institutes of Health National Center for Medical Rehabilitation Research/National Institute of Child Health and Human Development (grant HD052922) and the National Center for Advancing Translational Sciences (grant TR000058). The authors thank William Korzun for assistance with osmolarity analysis. Many thanks to the nursing staff of the Brain Injury Rehabilitation Unit of Virginia Commonwealth University Health Systems for their crucial assistance on the unit. The authors are grateful to the participants and their families for their voluntary contribution to this research.
Authors Disclosure Statement
No competing financial interests exist.
References
- 1.Salazar A.M., Warden D.L., Schwab K., Spector J., Braverman S., Walter J., Cole R., Rosner M. M., Martin E.M., Ecklund J., and Ellenbogen R.G. (2000). Cognitive rehabilitation for traumatic brain injury: a randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. JAMA 283, 3075–3081 [DOI] [PubMed] [Google Scholar]
- 2.Cicerone K.D., Mott T., Azulay J., Sharlow-Galella M.A., Ellmo W.J., Paradise S., and Friel J.C. (2008). A randomized controlled trial of holistic neuropsychologic rehabilitation after traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 2239–2249 [DOI] [PubMed] [Google Scholar]
- 3.Vanderploeg R.D., Schwab K., Walker W.C., Fraser J.A., Sigford B.J., Date E.S., Scott S.G., Curtiss G., Salazar A.M., Warden , and D. L. ; Defense and Veterans Brain Injury Center Study Group (2008). Rehabilitation of traumatic brain injury in active duty military personnel and veterans: Defense and Veterans Brain Injury Center randomized controlled trial of two rehabilitation approaches. Arch. Phys. Med. Rehabil. 89, 2227–2238 [DOI] [PubMed] [Google Scholar]
- 4.Niemeier J.P., Kreutzer J.S., Marwitz J.H., Gary K.W., and Ketchum J.M. (2011). Efficacy of a brief acute neurobehavioural intervention following traumatic brain injury: a preliminary investigation. Brain Inj. 25, 680–690 [DOI] [PubMed] [Google Scholar]
- 5.Adamczak S., Dale G., de Rivero Vaccari J.P., Bullock M.R., Dietrich W.D., and Keane R.W. (2012). Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J. Neurosurg. 117, 1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roozenbeek B., Chiu Y.L., Lingsma H.F., Gerber L.M., Steyerberg E.W., Ghajar J., and Maas A. I. (2012). Predicting 14-day mortality after severe traumatic brain injury: application of the IMPACT models in the brain trauma foundation TBI-trac(R) New York State database. J. Neurotrauma 29, 1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niyonkuru C., Wagner A.K., Ozawa H., Amin K., Goyal A., and Fabio A. (2013). Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: a practical example. J. Neurotrauma 30, 938–945 [DOI] [PubMed] [Google Scholar]
- 8.Jeter C.B., Hergenroeder G.W., Hylin M.J., Redell J.B., Moore A.N., and Dash P.K. (2013). Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma 30, 657–670 [DOI] [PubMed] [Google Scholar]
- 9.Papa L., Ramia M.M., Kelly J.M., Burks S.S., Pawlowicz A., and Berger R.P. (2013). Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J. Neurotrauma 30, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zetterberg H., Smith D.H., and Blennow K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochanek P.M., Berger R.P., Fink E.L., Au A.K., Bayir H., Bell M.J., Dixon C.E., and Clark R.S. (2013). The potential for bio-mediators and biomarkers in pediatric traumatic brain injury and neurocritical care. Front. Neurol. 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokobori S., Hosein K., Burks S., Sharma I., Gajavelli S., and Bullock R. (2013). Biomarkers for the clinical differential diagnosis in traumatic brain—a systematic review. CNS Neurosci. Ther. 19, 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siman R., Giovannone N., Toraskar N., Frangos S., Stein S.C., Levine J.M., and Kumar M.A. (2011). Evidence that a panel of neurodegeneration biomarkers predicts vasospasm, infarction, and outcome in aneurysmal subarachnoid hemorrhage. PLoS One 6, e28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walder B., Robin X., Rebetez M.M., Copin J.C., Gasche Y., Sanchez J.C., and Turck N. (2013). The prognostic significance of the serum biomarker H-FABP in comparison with S100b in severe traumatic brain injury. J. Neurotrauma 30, 1631–1637 [DOI] [PubMed] [Google Scholar]
- 15.Gabathuler R. (2010). Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 37, 48–57 [DOI] [PubMed] [Google Scholar]
- 16.Witt K.A., Gillespie T.J., Huber J.D., Egleton R.D., and Davis T.P. (2001). Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides 22, 2329–2343 [DOI] [PubMed] [Google Scholar]
- 17.Kusuhara H., and Sugiyama Y. (2005). Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2, 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robin X., Turck N., Hainard A., Lisacek F., Sanchez J.C., and Muller M. (2009). Bioinformatics for protein biomarker panel classification: what is needed to bring biomarker panels into in vitro diagnostics? Expert Rev. Proteomics 6, 675–689 [DOI] [PubMed] [Google Scholar]
- 19.Rajalahti T., and Kvalheim O.M. (2011). Multivariate data analysis in pharmaceutics: a tutorial review. Int. J. Pharm. 417, 280–290 [DOI] [PubMed] [Google Scholar]
- 20.Gordon W.A., Zafonte R., Cicerone K., Cantor J., Brown M., Lombard L., Goldsmith R., and Chandna T. (2006). Traumatic brain injury rehabilitation: state of the science. Am. J. Phys. Med. Rehabil. 85, 343–382 [DOI] [PubMed] [Google Scholar]
- 21.Wagner A.K. (2010). TBI translational rehabilitation research in the 21st century: exploring a rehabilomics research model. Eur. J. Phys. Rehabil. Med. 46, 549–556 [PubMed] [Google Scholar]
- 22.Cifu D.X., Kreutzer J.S., Kolakowsky-Hayner S.A., Marwitz J.H., and Englander J. (2003). The relationship between therapy intensity and rehabilitative outcomes after traumatic brain injury: a multicenter analysis. Arch. Phys. Med. Rehabil. 84, 1441–1448 [DOI] [PubMed] [Google Scholar]
- 23.Zitnay G.A., Zitnay K.M., Povlishock J.T., Hall E.D., Marion D.W., Trudel T., Zafonte R.D., Zasler N., Nidiffer F.D., DaVanzo J., and Barth J.T. (2008). Traumatic brain injury research priorities: the Conemaugh International Brain Injury Symposium. J. Neurotrauma 25, 1135–1152 [DOI] [PubMed] [Google Scholar]
- 24.Ragnarsson K.T. (2006). Traumatic brain injury research since the 1998 NIH Consensus Conference: accomplishments and unmet goals. J Head Trauma Rehabil 21, 379–387 [DOI] [PubMed] [Google Scholar]
- 25.Ruttan L., Martin K., Liu A., Colella B., and Green R.E. (2008). Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch. Phys. Med. Rehabil. 89, S69–S76 [DOI] [PubMed] [Google Scholar]
- 26.Berger R.P. (2006). The use of serum biomarkers to predict outcome after traumatic brain injury in adults and children. J. Head Trauma Rehabil. 21, 315–333 [DOI] [PubMed] [Google Scholar]
- 27.Kobeissy F.H., Guingab-Cagmat J.D., Razafsha M., O'Steen L., Zhang Z., Hayes R.L., Chiu W.T., and Wang K.K. (2011). Leveraging biomarker platforms and systems biology for rehabilomics and biologics effectiveness research. PM R 3, S139–S147 [DOI] [PubMed] [Google Scholar]
- 28.Nudo R.J. (2011). Neural bases of recovery after brain injury. J. Commun. Disord. 44, 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishibe M., Barbay S., Guggenmos D., and Nudo R.J. (2010). Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J. Neurotrauma 27, 2221–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes D.F., Landis M.K., and Ottens A.K. (2012). High-capacity peptide-centric platform to decode the proteomic response to brain injury. Electrophoresis 33, 3712–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva J.C., Denny R., Dorschel C.A., Gorenstein M., Kass I.J., Li G.Z., McKenna T., Nold M.J., Richardson K., Young P., and Geromanos S. (2005). Quantitative proteomic analysis by accurate mass retention time pairs. Anal. Chem. 77, 2187–2200 [DOI] [PubMed] [Google Scholar]
- 32.Saeed A.I., Bhagabati N.K., Braisted J.C., Liang W., Sharov V., Howe E.A., Li J., Thiagarajan M., White J.A., and Quackenbush J. (2006). TM4 microarray software suite. Methods Enzymol. 411, 134–193 [DOI] [PubMed] [Google Scholar]
- 33.Kall L., Storey J.D., MacCoss M.J., and Noble W.S. (2008). Posterior error probabilities and false discovery rates: two sides of the same coin. J. Proteome Res. 7, 40–44 [DOI] [PubMed] [Google Scholar]
- 34.Chen J., Bardes E.E., Aronow B.J., and Jegga A.G. (2009). ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P., Jensen L.J., and von Mering C. (2011). The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren K.M., Reeves T.M., and Phillips L.L. (2012). MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J. Neurotrauma 29, 1922–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajor M., and Kaczmarek L. (2013). Proteolytic remodeling of the synaptic cell adhesion molecules (CAMs) by metzincins in synaptic plasticity. Neurochem. Res. 38, 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger R.P., and Kochanek P.M. (2006). Urinary S100B concentrations are increased after brain injury in children: a preliminary study. Pediatr. Crit. Care Med. 7, 557–561 [DOI] [PubMed] [Google Scholar]
- 39.Matter A.M., Folweiler K.A., Curatolo L.M., and Kline A.E. (2011). Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 25, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griesbach G.S., Hovda D.A., Molteni R., Wu A., and Gomez-Pinilla F. (2004). Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125, 129–139 [DOI] [PubMed] [Google Scholar]
- 41.Pelinka L.E., Hertz H., Mauritz W., Harada N., Jafarmadar M., Albrecht M., Redl H., and Bahrami S. (2005). Nonspecific increase of systemic neuron-specific enolase after trauma: clinical and experimental findings. Shock 24, 119–123 [DOI] [PubMed] [Google Scholar]