Abstract
Background: Zinc supplementation prevents incident pneumonia in children; however, the effect for pneumonia treatment remains unclear.
Methods: A randomized, double-blind, placebo-controlled trial of zinc supplements (daily 25 mg) adjunct to antibiotic treatment of radiology-confirmed acute pneumonia was conducted among hospitalized children (6–36 months) in Dar es Salaam, Tanzania.
Results: The trial was stopped early due to low enrollment, primarily owing to exclusion of children outside the age range and >3 days of prior illness. Among children enrolled (n = 94), zinc supplementation indicated no beneficial effect on the duration of hospitalization (IRR: 0.69; 95% CI 0.45–1.06; p = 0.09) or the proportion of children who were hospitalized for <3 days (RR: 0.85; 95% CI: 0.57–1.25; p = 0.40) or <5 days (RR: 1.01; 95% CI: 0.83–1.23; p = 0.92) (IRRs and RRs >1.0 favor zinc).
Conclusions: Although underpowered, this randomized trial provided no evidence for a beneficial effect of zinc supplementation adjunct to antibiotics for hospitalized children.
Keywords: zinc, pneumonia, micronutrients, child, respiratory tract infections
Introduction
Pneumonia is a leading cause of mortality among children <5 years worldwide and has been estimated to account for 18% of all child deaths in sub-Saharan Africa [1]. Accordingly, interventions to reduce the incidence or to improve treatment of pneumonia are likely critical for achievement of Millennium Development Goal 4 in sub-Saharan Africa.
Zinc deficiency is common worldwide, but children in sub-Saharan Africa and South Asia are at notably high risk due to inadequate intake of zinc-containing foods [2]. The prevalence of zinc deficiency in Tanzania has been found to be as high as 70% among children aged 6 months to5 years [3]. Children deficient in zinc are at increased risk for developing pneumonia, diarrhea and growth retardation [4]. A recent meta-analysis determined that zinc supplementation was associated with a 19% reduction in the incidence of pneumonia [5]. Nevertheless, the effect of zinc adjunct to antibiotic treatment of pneumonia among hospitalized children (<5 years) is not clear. Zinc supplementation was found to reduce the duration of severe pneumonia among children in Bangladesh [6], reduce duration of very ill status and fever among a sub-analysis of Indian boys [7], reduce mortality due to severe pneumonia in Uganda [8] and reduce time to recovery among a subset of children with very severe pneumonia in India [9] and a subset of children with radiographic pneumonia in Nepal [10]. Nevertheless, no beneficial effect was found in trials conducted in Nepal [11], India [12, 13] and Australia [14]. There is also concern that some of these trials may have included children with bronchiolitis, for whom zinc supplementation may differently affect the duration of hospitalization and resolution of illness as compared with children with pneumonia [15]. The effect of zinc supplementation may also be modified by the severity of pneumonia at hospital admission [9] or by the pathogen causing pneumonia [16].
Here, we present a trial of zinc supplementation as an adjunct therapy to standard antibiotic treatment of radiologically confirmed acute pneumonia among Tanzanian children aged 6–36 months. The primary study outcome was duration of hospitalization. We secondarily assessed the effect of zinc supplementation on the resolution of clinical signs and symptoms of pneumonia and the need to switch to second-line antibiotics.
Methods
Study design and setting
The study was a randomized, double-blind, placebo-controlled trial designed to assess the efficacy of zinc supplementation as an adjunct therapy to standard antibiotic treatment of pneumonia for Tanzanian children aged 6–36 months (clinicaltrials.gov identifier NCT00133432). The trial was conducted between September 2005 and October 2007 at the general pediatric wards of the Muhimbili National Hospital (MNH) in Dar es Salaam, Tanzania. MNH is the national referral hospital and the primary teaching hospital for Muhimbili University of Health and Allied Sciences.
Participant eligibility
Children aged 6–36 months admitted to MNH were assessed by study physicians and were considered eligible for enrollment in the trial if they received a primary clinical diagnosis of acute pneumonia with confirmation by radiological findings from chest X-ray. Primary clinical diagnosis of acute pneumonia was based on the World Health Organization (WHO) Integrated Management of Childhood Illness algorithm, and consisted of a child presenting with cough or difficulty breathing and all of the following features: (i) tachypnea defined by a respiratory rate ≥50 breaths/min for children aged 6–12 months and ≥40 breaths/min for children older than 12 months, (ii) a fever of ≥37.5°C as measured by axillary thermometer and (iii) any one of the following signs: nasal flaring, visible indrawing of the lower chest wall muscles on inspiration, central cyanosis, inability to feed, lethargy or crepitations [17]. In addition, all clinical diagnoses of pneumonia also had to be confirmed by chest X-ray using WHO criteria with abnormalities consistent of an inflammatory process, such as a distinctly confined dense abnormality or large pleural effusion, to meet study eligibility criteria. The study pediatrician and radiologist interpreted the chest X-ray, and in case of discordant findings a third physician reviewed and served as the tiebreaker.
Children were excluded if they required immediate nutritional intervention, which included micronutrient supplements containing zinc, according to a Wellcome Classification of marasmus, marasmic-kwashiorkor or kwashiorkor [18]. Children were also excluded if they needed immediate life-saving measures at presentation (i.e. resuscitation), had a prior known or current diagnosis of clinical AIDS as per the WHO clinical case definition [19], were diagnosed with active pulmonary tuberculosis or measles, presented with signs of systemic illness (e.g. sepsis, acute meningitis), reported diarrhea (≥3 loose or watery stools in past 24 h), reported illness for >3 days prior to admission, had a known intolerance or allergy to zinc or were currently receiving supplements containing zinc. Infants whose mother or guardian refused HIV testing for the child were also excluded. Written informed consent was obtained from parents or guardians for all children.
In addition to the clinical data needed to assess study eligibility at the time of enrollment, study physicians performed a complete physical examination and assessed oxygen saturation using a pulse oximeter. Study nurses collected data on demographics and obtained anthropometric measurements using calibrated instruments. Length-for-age and weight-for-length z-scores were calculated using the 2006 WHO Child Growth Standards [20]. Blood samples were drawn at enrollment for HIV testing, complete blood count and plasma zinc quantification. Hemoglobin concentrations were measured using the AcT5 Diff AL hematology analyzer (Beckman Coulter, Jersey City, NJ, USA). Children under 15 months of age were tested for HIV infection by PCR using the Amplicor HIV-1 DNA assay version 1.5 (Roche Molecular Systems, Inc., Branchburg, NJ, USA), while children aged 15 months or older were tested for HIV infection by two sequential HIV ELISAs, the Murex HIV antigen/antibody (Abbott Murex, Kent, UK) followed by the Enzygnost anti-HIV-1/2 Plus (Dade Behring, Marburg, Germany), with discordant results resolved by a Western blot test (Bio-Rad Laboratories, Herfordshire, UK).
Randomization, blinding and treatment allocation
Children were randomly assigned to receive adjunctive zinc therapy or placebo. A randomization list from 1 to 600 was prepared by the study biostatistician in Boston using permuted blocks of 20, which was then sent to the study pharmacists at MNH. Study pharmacists stored the randomization list in a locked file cabinet and concealed allocation by covering the numeric regimen code on each bottle with a sticker. Children enrolled in the study were provided the next consecutive regimen number in the series. The manufacturer of zinc and placebo supplements labeled all bottles with a regimen code and kept the treatment identification codes until completion of follow-up for all participants. As a result, all study participants and research personnel (including the principal investigator, physicians and nurses) were unaware of treatment groups.
Children randomized to adjunctive zinc therapy received an effervescent tablet that contained 12.5 mg of elemental zinc in the form of zinc sulfate monohydrate (Hermes Arzneimittel GmbH, Munich, Germany) dissolved in water twice daily (daily dose of 25 mg) from enrollment until hospital discharge. The zinc tablets also contained 1700 mg of citric acid to produce a strong lemon–lime flavor to mask the metallic taste of zinc. Children randomized to the placebo group received a zinc-free effervescent tablet (produced by the same manufacturer), which was taken twice daily dissolved in water from enrollment until discharge. The placebo tablets contained 1700 mg of citric acid that produced an identically strong lemon–lime flavor as the zinc preparation to blind the participants and research personnel to the randomized treatment group. Tablets for both treatment groups were also identical in packaging and appearance. Children who vomited within 15 min of treatment administration were provided with a repeat dose.
Provision of standard of care
All participants were treated according to standard of care at MNH, which is based on WHO standard case management guidelines [21]. Participants received 25 mg/kg chloramphenicol intravenously every 8 h for the initial period of 72 h. Second-line antibiotics were provided for children who failed to improve at 48 h, defined by persistence of respiratory distress and lack of improvement in oxygen saturation, or for children who had a deterioration of clinical status characterized by a new danger sign or hypoxia. The second-line antibiotic regimen included ampicillin (50 mg/kg intravenously every 6 h), cloxacillin (50 mg/kg intravenously every 6 h) and gentamicin (7.5 mg/kg intravenously once a day). Oxygen therapy was provided to maintain an oxygen saturation >92%.
Follow-up and outcome definitions
The clinical condition of participants was recorded every 6 h by study nurses and once every 24 h by a study clinician. Nurses and clinicians recorded the child’s pulse rate, respiratory rate, oxygen saturation and auxiliary temperature. Clinicians also performed a complete clinical examination.
The primary outcome of the study was duration of hospitalization. Children were discharged from the hospital when the signs and symptoms of pneumonia resolved and all of the following criteria were met for a 24-h period: afebrile (<37.5°C), a normal respiratory rate (<50 breaths/min for children under 12 months and <40 breaths/min for children aged 12–36 months) and oxygen saturation >96%. The time of discharge was recorded as the end of the 8-h shift during which these criteria were met.
Secondary outcomes included resolution of clinical indicators of pneumonia, switching to second-line antibiotics and vomiting within 15 min of treatment administration. Resolution of fever and tachypnea were defined as the beginning of a consecutive 24-h period of nurse and physician assessments with auxiliary temperatures <37.5°C and a respiratory rate <50 breaths/min for children aged 6–12 months and <40 breaths/min for children older than 12 months. Resolution of nasal flaring and chest indrawing was defined as the beginning of a 24-h period of physician assessments without these signs.
Measurement of plasma zinc
Blood samples for plasma zinc concentration were obtained at enrollment and hospital discharge. Special precautions were taken to ensure that for each child the blood samples were collected using zinc-free gloves and syringes and transferred to a zinc-free polypropylene tube (Becton Dickinson, Franklin Lakes, NJ, USA). The blood was centrifuged at 3000 g for 15 min and plasma was separated and stored in a zinc-free polypropylene tube below −70°C. Plasma zinc quantification was undertaken at Boston Children’s Hospital, Boston, using an atomic absorption spectrophotometer method with deuterium background correction and a magnesium nitrate modifier (Model AA 800, Perkin Elmer, Boston, MA, USA). The assay had a day-to-day coefficient of variation of <5.0% over a range of concentrations.
Sample size
The target enrollment size for the trial was 300 in each treatment arm, totaling 600 children. This sample size was selected to detect a 20% reduction in the duration of hospital stay. We assumed a mean length of hospital stay of 5 days with a standard deviation of 3.7 days based on a trial of vitamin A for the treatment of childhood pneumonia conducted in the same setting [22]. Calculations also assumed 80% power, an α error rate of 5 and 4% loss to follow-up. The trial was stopped early due to low enrollment (95 children) in October 2007 by the recommendation of the Data and Safety Monitoring Board. Slow recruitment of study participants was primarily due to the exclusion of infants who were not within the age range of 6–36 months and those reported illness >3 days prior to hospital admission.
Statistical analysis
The intention-to-treat principle was used for statistical analyses. One child in the placebo group who later had consent withdrawn had missing baseline data and was excluded from baseline comparisons. Three children (including the child with missing baseline data) had consent withdrawn and were excluded from follow-up analyses. Kaplan–Meier survival curves were generated for the duration of hospitalization by randomized treatment group. Cox proportional hazard models were used to produce incidence rate ratios (IRRs) for the effect of treatment group on duration of hospitalization and clinical indicators of pneumonia. Only individuals with nasal flaring or chest indrawing at baseline were included in analyses for resolution of these clinical indicators. IRRs and relative risks (RRs) >1.0 for duration of hospitalization and clinical indicator indicate a beneficial effect of zinc. Chi-square tests were used to compare the observed proportion of children who were hospitalized for <3 and <5 days, as well as the proportion of children who were switched to second-line antibiotics, by treatment group. The association of randomized treatment group with vomiting within 15 min of receiving the regimen was analyzed using generalized estimating equations with the log link and exchangeable working covariance to produce RRs [23]. RRs for vomiting <1.0 show a beneficial effect of zinc supplementation. Change in plasma zinc per 12 h of hospitalization was compared between treatment groups using the Wilcoxon rank-sum test to account for time on regimen. All p-values were 2-sided with p < 0.05 considered statistically significant. We did not perform adjustments for multiple comparisons because we only considered a single primary outcome. All secondary endpoints and analyses should therefore be considered exploratory. Statistical analyses were performed using the SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Ethics
Institutional approval was granted by the Harvard School of Public Health Human Subjects Committee (protocol 11419), the Muhimbili University of Health and Allied Sciences Committee of Research and Publications, the Tanzanian National Institute of Medical Research and theTanzanian Food and Drugs Authority.
Results
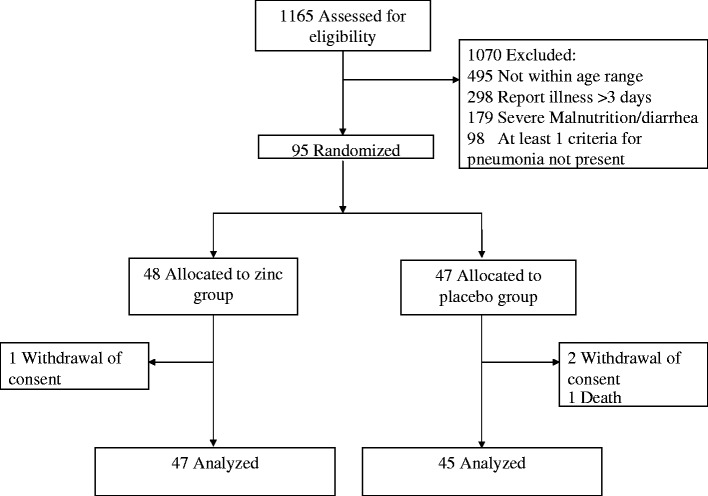
A total of 1165 children were screened for enrollment in the trial between September 2005 and October 2007. The flow of participants through the trial is shown in Fig. 1. Of these screened children, 1070 were excluded for not being within 6–36 months of age (n = 495), reporting illness >3 days (n = 298), being severely malnourished and/or reporting diarrhea (n = 179) or not meeting all criteria for the case definition of radiologically confirmed pneumonia (n = 98). The trial was stopped early in October 2007 per recommendations of the DSMB, primarily due to low enrollment.
Fig. 1.
Profile of a randomized trial of zinc supplementation as an adjunct therapy to standard antibiotic treatment of acute pneumonia for Tanzanian children aged 6–36 months.
Baseline characteristics of the 94 randomized children (excluding child with missing data) by treatment regimen are presented in Table 1. Treatment groups appeared to be relatively comparable with respect to sex, age, HIV status, nutritional status, clinical characteristics, most indicators of pneumonia severity and plasma zinc concentration. There was some indication that individuals randomized to the zinc group were slightly older, had increased respiratory rates among those <1 year and had reduced prevalence of chest indrawing at baseline as compared with the placebo group. The proportion of children with plasma zinc concentrations <70 µg/dl was 50% (n = 24) and 46% (n = 21) for the zinc and placebo groups, respectively. One HIV-infected child in the placebo treatment group died during the trial.
Table 1.
Baseline characteristics of zinc and placebo treatment groups
| Zinc group (n = 48) | Placebo group (n = 46) | |
|---|---|---|
| Characteristic | Frequency (%) or Mean ± SD | Frequency (%) or Mean ± SD |
| Male | 26 (54.2) | 30 (53.6) |
| Age | ||
| 6–11 months | 19 (39.6) | 26 (56.5) |
| 12–23 months | 24 (50.0) | 18 (39.1) |
| 24–36 months | 5 (10.4) | 2 (4.4) |
| HIV-positive | 4 (8.7) | 3 (6.5) |
| Weight-for-height z score ≤2 | 10 (20.8) | 8 (17.4) |
| Height-for-age z score ≤2 | 12 (25.0) | 14 (30.4) |
| Mean hemoglobin (g/dl) | 9.3 ± 1.2 | 9.3 ± 1.8 |
| Mean pulse (beats/min) | 153 ± 18 | 141 ± 46 |
| Mean auxiliary temperature (°C) | 38.1 ± 0.7 | 38.1 ± 0.8 |
| Mean respiratory rate (breaths/min) | ||
| 6–11 months | 60.6 ± 8.4 | 56.2 ± 8.5 |
| 12–36 months | 52.8 ± 11.0 | 51.6 ± 9.5 |
| Mean arterial O2 saturation (%) | 96.0 ± 2.8 | 96.8 ± 2.2 |
| Hypoxia (arterial O2 saturation <93%) | 1 (2.1) | 1 (2.2) |
| Nasal flaring | 26 (54.2) | 34 (73.9) |
| Chest indrawing | 32 (66.7) | 39 (84.8) |
| Cyanosis | 0 (0.0) | 0 (0.0) |
| Inability to feed | 1 (2.1) | 1 (2.2) |
| Convulsions | 0 (0.0) | 0 (0.0) |
| Plasma zinc (µg/dl) | 72.1 ± 16.1 | 68.0 ± 31.1 |
| Plasma zinc <70 µg/dl | 24 (50.0) | 21 (45.7) |
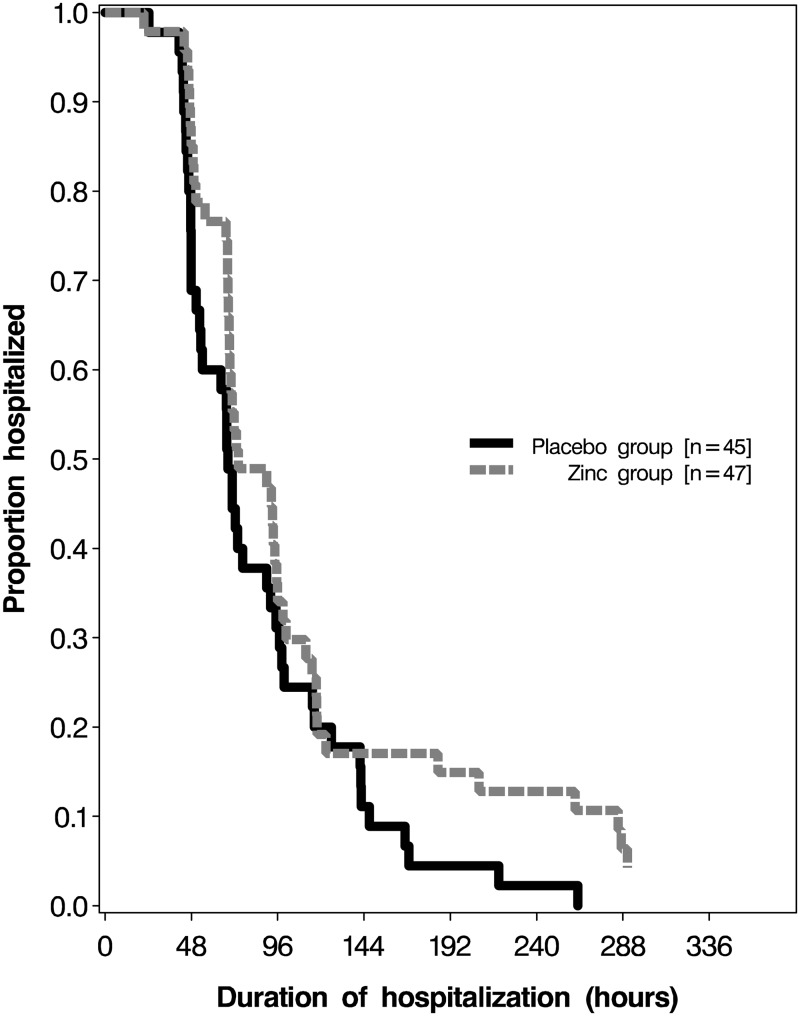
The effects of treatment on duration of hospitalization and clinical indicators of pneumonia are presented in Table 2. There was no significant difference in the duration of hospitalization between the zinc and placebo groups (IRR for discharge: 0.69; 95% CI: 0.45–1.06; p = 0.09) (IRR > 1.0 favors zinc). There was also no significant effect of zinc supplementation on hospitalization duration among individuals with baseline plasma zinc <70 µg/dl (IRR for discharge: 0.54; 95% CI: 0.28–1.02; p = 0.06) or after adjustment for potential imbalances in baseline age, respiratory rate, pulse, nasal flaring and plasma zinc between treatment groups (IRR for discharge: 0.70; 95% CI: 0.44–1.14; p = 0.15). We found no significant difference in the proportion of children who were hospitalized for <3 days (RR: 0.85; 95% CI: 0.57–1.25; p = 0.40) and <5 days (RR: 1.01; 95% CI: 0.83–1.23; p = 0.92) between treatment groups (RR > 1.0 favors zinc). A Kaplan-Meir curve also suggested no difference in duration of hospitalization by treatment arm (Fig. 2).
Table 2.
Effect of randomized treatment on duration hospitalization and clinical indicators (IRR>1.0 favors zinc)
| Zinc group (n = 48) | Placebo group (n = 46) | IRR for recovery (95% CI) | p-value | |
|---|---|---|---|---|
| Outcome | Median duration in h (IQR) | Median duration in h (IQR) | ||
| Hospitalization | 74.2 (67.3–117.5) | 68.5 (47.8–99.5) | 0.69 (0.45–1.06) | 0.089 |
| Fever (>37.5°C) | 30.2 (16.6–57.1) | 26.9 (12.4–41.2) | 0.78 (0.52–1.18) | 0.237 |
| Tachypnea* | 47.3 (28.7–86.9) | 32.5 (23.7–56.3) | 0.68 (0.44–1.03) | 0.071 |
| Nasal flaring | 39.1 (22.7–69.3) | 42.8 (23.9–72.4) | 1.00 (0.62–1.62) | 0.985 |
| Chest indrawing | 48.7 (24.6–74.3) | 46.5 (26.9–68.5) | 0.75 (0.49–1.14) | 0.176 |
*Respiratory rate ≥50 breaths/min for children aged 6–12 months and ≥40 breaths/min for children aged 12–36 months.
Fig. 2.
Kaplan–Meier survival curves for hospital discharge in the zinc-supplemented group (dashed line) and placebo group (solid line).
There was also no significant difference between treatment groups in the duration of fever, tachypnea, nasal flaring and chest indrawing (Table 2). No significant difference in the proportion of children who were switched to second-line antibiotics for zinc (n = 13; 28%) versus placebo group (n = 13; 27%) was observed (p = 0.90). Only 10 vomiting events (six for zinc and four for placebo) were recorded within 15 min of treatment administration, and there was no significant difference in occurrence between treatment groups (RR: 1.02; 95% CI: 0.29–3.65; p = 0.97).
There was no significant difference in mean change in plasma zinc concentrations per 12 h of hospitalization between the zinc group (mean change: +1.98 µg/dl) and the placebo group (mean change: +0.88 µg/dl) (p = 0.29).
Discussion
This trial of zinc as an adjunct therapy to standard antibiotic treatment of acute pneumonia showed no indication of a benefit of supplementation on hospital discharge time for Tanzanian children aged 6–36 months. There also appeared to be no benefit of zinc supplementation on the duration of fever, tachypnea, nasal flaring, chest indrawing and switching to second-line antibiotics. Nevertheless, the trial was stopped early and the sample size was likely too small to detect a modest effect.
Our trial results are in line with previous clinical trials conducted in Southeast Asia [11–13] and Australia [14] finding no significant beneficial effect of zinc supplementation on duration of hospitalization for children with pneumonia, but in contrast to trials in Bangladesh [6] and India [7, 9] that found some beneficial effects. Only one trial of zinc for treatment of pneumonia has been conducted in sub-Saharan Africa [8]. That trial, conducted in Uganda, found no effect of zinc supplementation on normalization of respiratory rate, temperature and oxygen saturation, which is consistent with our findings; however, there was a significantly decreased case-fatality rate among children in the zinc group. Furthermore, the benefits of zinc on pneumonia mortality in the Uganda zinc treatment trial appeared to be greater for HIV-infected children as compared with HIV-uninfected children [8]. Because of the small sample size and low prevalence of HIV infection (∼4%) in our trial, we were unable to assess the effect of zinc on mortality or among the subgroup of HIV-infected children. Future studies of zinc supplementation on mortality and among HIV-infected children in sub-Saharan Africa appear to be warranted. There were also few cases of severe pneumonia in our trial as assessed by hypoxia, and children with very severe pneumonia at hospital admission may benefit from zinc supplements [9].
A strength of our study is that all randomized children had radiologically confirmed pneumonia, thereby excluding children with bronchiolitis or other etiologies of lower respiratory tract infection who may experience a different effect of zinc on resolution of illness. Previous trials have used WHO criteria to define severe pneumonia, which has a high specificity for lower respiratory tract infections but can include a wide range of etiologies [24]. The proportion of children with wheezing has varied in previous trials. Trials conducted in Nepal [10], India [12] and Bangladesh [6] had wheezing prevalence rates of 82, 63 and 37%, respectively. The Bangladeshi trial, which had the lowest prevalence of wheezing, found a significant beneficial effect of zinc [6]. Nevertheless, the Ugandan trial, which found no effect of zinc on duration of pneumonia, excluded children presenting with wheezing [8].
A major limitation of our trial is the small sample size because the study was stopped early due to low enrollment. As a result, the study did not have the statistical power to detect a modest effect. Nevertheless, a generalized synthesis of results from our study with other clinical trials suggests there may be limited to no effect of zinc as an adjunct therapy to antibiotics on duration of pneumonia hospitalization, but there is some evidence that zinc may reduce pneumonia case fatality rates, particularly for HIV-infected children. Given these circumstances, it is difficult to come to a definitive conclusion whether zinc supplements should be provided as an adjunct to standard treatment of pneumonia in sub-Saharan Africa. Trials powered to detect differences in mortality and specific to HIV-infected children are needed.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases [R01 AI056235-02]. CR Sudfeld was supported by the National Institute of Allergy and Infectious Diseases [award number T32AI007358]. The authors report no conflicts of interest.
Acknowledgements
The authors thank the mothers, children and fileld teams, including physicians, nurses, pharmacists, laboratory staff and the administrative staff, who made this study possible; and Muhimbili University of Health and Allied Sciences for their institutional support. M.R. Fataki, R.R. Kisenge, S. Aboud and W.W. Fawzi: designed the research; M.R. Fataki, R.R. Kisenge, S. Aboud, S. Mehta and W.W. Fawzi: conducted the research; C.R. Sudfeld, J. Okuma and D. Spiegelman: analyzed the data; M.R. Fataki, C.R. Sudfeld and W.W. Fawzi: drafted the initial manuscript. All authors contributed to and approved the final manuscript.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2. International Zinc Nutrition Consultative Group, Brown KH, Rivera JA, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25:S99–203. [PubMed]
- 3.Veenemans J, Milligan P, Prentice AM, et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8:e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119:1120–30. doi: 10.1542/peds.2006-3481. [DOI] [PubMed] [Google Scholar]
- 5.Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011;11(Suppl. 3):S23. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363:1683–8. doi: 10.1016/S0140-6736(04)16252-1. [DOI] [PubMed] [Google Scholar]
- 7.Mahalanabis D, Lahiri M, Paul D, et al. Ransomized, double-blind, placebo controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004;79:430–6. doi: 10.1093/ajcn/79.3.430. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan MG, Ndeezi G, Mboijana CK, et al. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: a randomized double blind placebo-controlled trial. BMC Med. 2012;10:14. doi: 10.1186/1741-7015-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhwa N, Chandran A, Aneja S, et al. Efficacy of zinc given as an adjunct in the treatment of severe and very severe pneumonia in hospitalized children 2-24 mo of age: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2013;97:1387–94. doi: 10.3945/ajcn.112.052951. [DOI] [PubMed] [Google Scholar]
- 10.Basnet S, Shrestha PS, Sharma A, et al. A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics. 2012;129:701–8. doi: 10.1542/peds.2010-3091. [DOI] [PubMed] [Google Scholar]
- 11.Shah GS, Dutta AK, Shah D, Mishra OP. Role of zinc in severe pneumonia: a randomized double bind placebo controlled study. Ital J Pediatr. 2012;38:36. doi: 10.1186/1824-7288-38-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose A, Coles CL, Gunavathi, et al. Efficacy of zinc in the treatment of severe pneumonia in hospitalized children <2 y old. Am J Clin Nutr. 2006;83:1089–96; quiz 1207. doi: 10.1093/ajcn/83.5.1089. [DOI] [PubMed] [Google Scholar]
- 13.Bansal A, Parmar VR, Basu S, et al. Zinc supplementation in severe acute lower respiratory tract infection in children: a triple-blind randomized placebo controlled trial. Indian J Pediatr. 2011;78:33–7. doi: 10.1007/s12098-010-0244-5. [DOI] [PubMed] [Google Scholar]
- 14.Chang AB, Torzillo PJ, Boyce NC, et al. Zinc and vitamin A supplementation in Indigenous Australian children hospitalised with lower respiratory tract infection: a randomised controlled trial. Med J Aust. 2006;184:107–12. doi: 10.5694/j.1326-5377.2006.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 15.Howie S, Zaman SM, Omoruyi O, et al. Severe pneumonia research and the problem of case definition: the example of zinc trials. Am J Clin Nutr. 2007;85:242–3. doi: 10.1093/ajcn/85.1.242. author reply 243. [DOI] [PubMed] [Google Scholar]
- 16.Coles CL, Bose A, Moses PD, et al. Infectious etiology modifies the treatment effect of zinc in severe pneumonia. Am J Clin Nutr. 2007;86:397–403. doi: 10.1093/ajcn/86.2.397. [DOI] [PubMed] [Google Scholar]
- 17.WHO/UNICEF. Handbook on Integrated Management of Childhood Illness. Geneva: WHO; 2000. [Google Scholar]
- 18.Wellcome Trust Working Party. Classification of infantile malnutrition. Lancet. 1970;2:302–3. [PubMed] [Google Scholar]
- 19.WHO. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: WHO; 2007. [Google Scholar]
- 20.The WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/ (19 November 2012, date last accessed) [Google Scholar]
- 21.WHO. Management of the Child with a Serious Infection or Severe Malnutrition. Guidelines for Care at the First-Referral Level in Developing Countries. Geneva: WHO; 2000. [Google Scholar]
- 22.Fawzi WW, Mbise RL, Fataki MR, et al. Vitamin A supplementation and severity of pneumonia in children admitted to the hospital in Dar es Salaam, Tanzania. Am J Clin Nutr. 1998;68:187–192. doi: 10.1093/ajcn/68.1.187. [DOI] [PubMed] [Google Scholar]
- 23.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 24.Puumalainen T, Quiambao B, Abucejo-Ladesma E, et al. Clinical case review: a method to improve identification of true clinical and radiographic pneumonia in children meeting the World Health Organization definition for pneumonia. BMC Infect Dis. 2008;8:95. doi: 10.1186/1471-2334-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]