Abstract
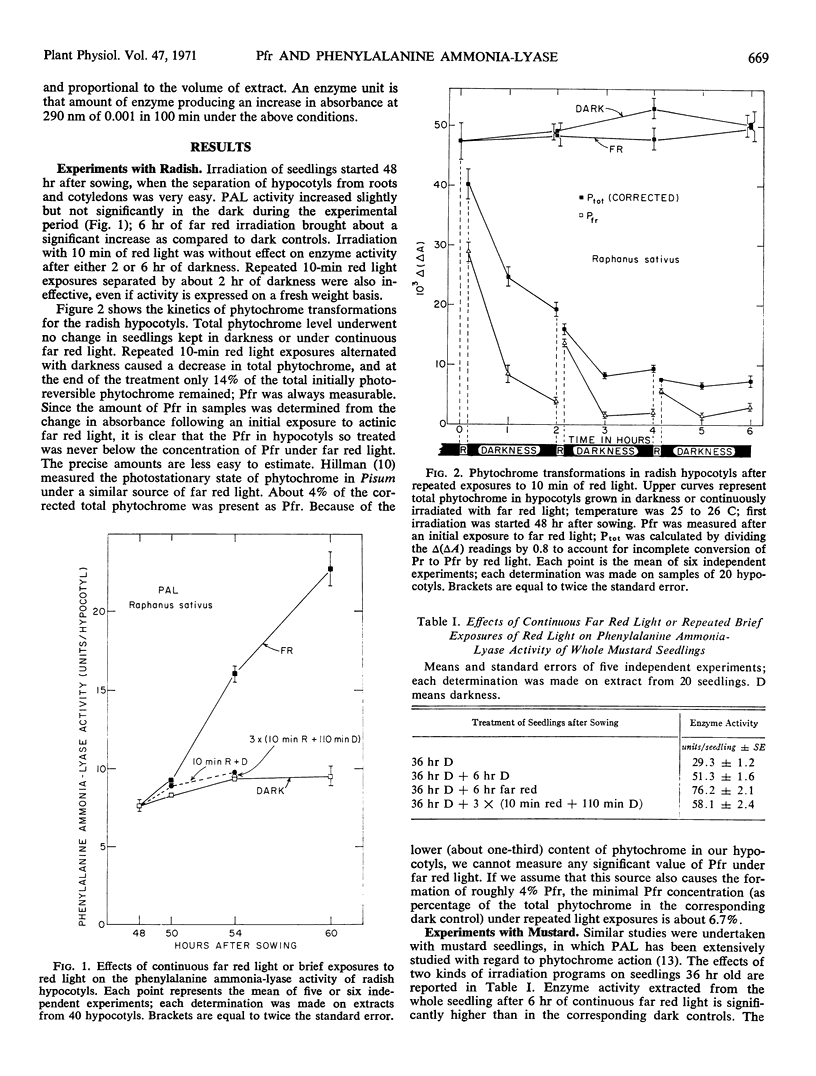
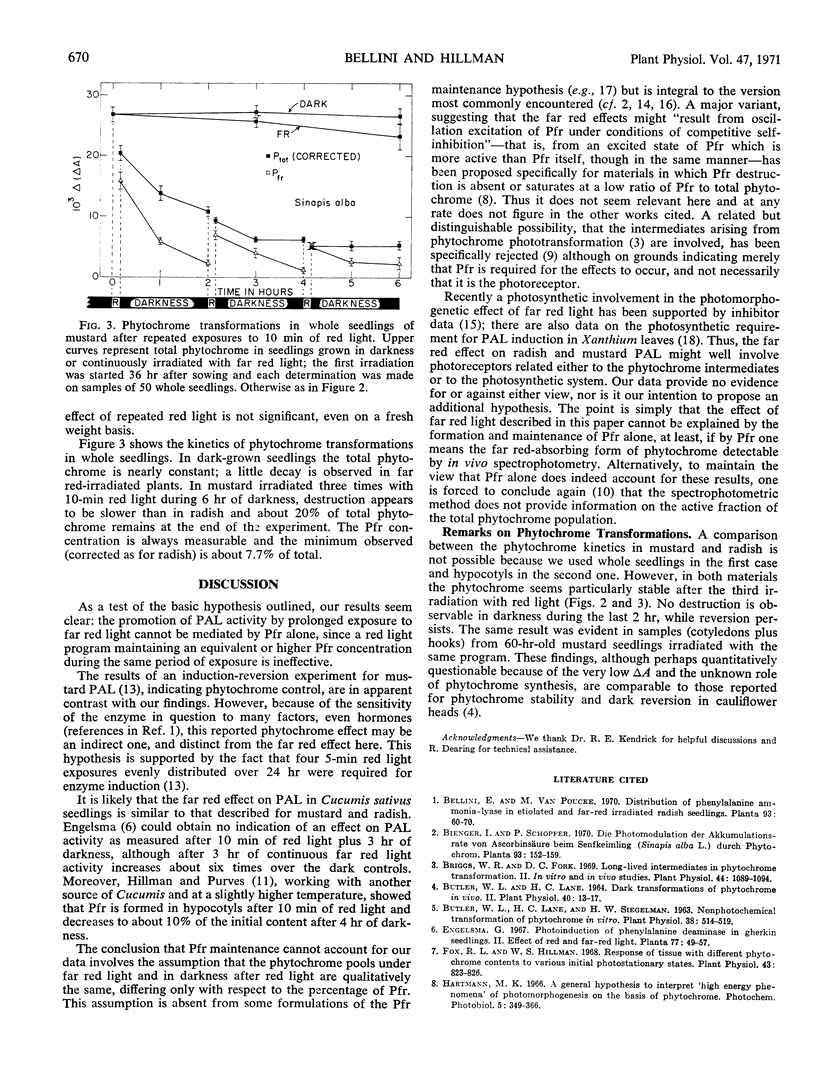
In seedlings of Raphanus sativus (radish) and Sinapis alba (mustard), irradiation for 6 hours with far red light significantly increases the extractable activity of phenylalanine ammonia-lyase by the end of the light period. A schedule of 10 minutes red light-110 minutes darkness-10 minutes red-110 minutes darkness-10 minutes red-110 minutes darkness has no effect as compared to dark controls. However, the red light program maintains a level of far red-absorbing phytochrome always measurable by in vivo spectrophotometry during the 6-hour experimental period. We conclude that the far red effect on this enzyme and for this specific material cannot be explained solely by formation and maintenance of far red-absorbing phytochrome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation II: In Vitro and In Vivo Studies. Plant Physiol. 1969 Aug;44(8):1089–1094. doi: 10.1104/pp.44.8.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. R., Hillman W. S. Response of tissue with different phytochrome contents to various initial photostationary States. Plant Physiol. 1968 May;43(5):823–826. doi: 10.1104/pp.43.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. M. Phytochrome 730 (Pfr), the effector of the "high energy photomorphogenic reaction" in the far-red region. Naturwissenschaften. 1967 Oct;54(20):544–544. doi: 10.1007/BF00627231. [DOI] [PubMed] [Google Scholar]
- Klein W. H., Edwards J. L., Shropshire W. Spectrophotometric Measurements of Phytochrome in vivo and Their Correlation with Photomorphogenic Responses of Phaseolus. Plant Physiol. 1967 Feb;42(2):264–270. doi: 10.1104/pp.42.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze-Karow H., Schopfer P., Mohr H. Phytochrome-mediated repression of enzyme synthesis (lipoxygenase): a threshold phenomenon. Proc Natl Acad Sci U S A. 1970 Jan;65(1):51–57. doi: 10.1073/pnas.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Phytochrome and photomorphogenesis in plants. Nature. 1970 Aug 15;227(5259):665–668. doi: 10.1038/227665a0. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]



