Abstract
We present a case of an elderly man with coronary artery disease who was diagnosed with non-Hodgkin lymphoma. Soon after the administration of chemotherapy, which included rituximab and vincristine, he developed acute myocardial infarction with cardiogenic shock. The condition was managed successfully with primary percutaneous coronary intervention. We briefly discuss the possible pathogenic mechanisms of chemotherapy-induced ischemic syndrome and the management of chemotherapy in patients with high cardiovascular risk.
Key words: Acute coronary syndrome/chemically induced; acute myocardial infarction/chemically induced; antineoplastic agents/toxicity; cardiotoxins; endothelium, vascular/drug effects; lymphoma, non-Hodgkin; percutaneous coronary intervention; rituximab/adverse effects; tubulin/metabolism; vincristine/adverse effects
Chemotherapy-induced cardiovascular complications include myocardial dysfunction, conduction abnormalities (arrhythmias), hypertension, venous thrombosis, and ischemic syndromes.1 Chemotherapy-induced left ventricular (LV) dysfunction is often encountered in clinical practice, but chemotherapy-mediated ischemic syndromes are rarely seen. The incidence of acute myocardial infarction (AMI) after chemotherapy varies from 1% to 5%.1 We describe the case of an elderly man with non-Hodgkin lymphoma (NHL) who developed AMI after the administration of chemotherapeutic agents.
Case Report
A 68-year-old man with coronary artery disease (CAD) and severe single-vessel disease of the left anterior descending coronary artery (LAD) had undergone coronary angioplasty with a 3 × 18-mm bare-metal stent. Four years later, while under evaluation for neck and abdominal pain, he was diagnosed with NHL (diffuse large β-cell lymphoma). His LV function, as evaluated with 2-dimensional transthoracic echocardiography (TTE) before chemotherapy, was normal, and he was free of angina. He received chemotherapy that included rituximab, cyclophosphamide, vincristine, and prednisolone (R-CVP).
Forty-eight hours later, he had sudden-onset heaviness of the chest and difficulty in breathing. On examination, his heart rate was 150 beats/min, his blood pressure was 80/60 mmHg, and crepitations could be heard bilaterally over his entire chest. He was immediately intubated and started on intravenous diuretic agents and inotropic support. An electrocardiogram was indicative of ST-elevation anterior-wall myocardial infarction, and TTE showed severely hypokinetic myocardium (involving the LAD territory), with an estimated LV ejection fraction of 0.30. He was transferred for diagnostic angiography and primary percutaneous coronary intervention. Before the procedure, the patient received a loading dose of aspirin and clopidogrel; then an intra-aortic balloon pump was inserted. A left coronary angiogram revealed a patent stent in the mid-LAD with a discrete, thrombotic, tight stenosis proximal to the stent; there was slow flow in the LAD and substantial ostio-proximal disease of the left circumflex coronary artery (LCx) and first obtuse marginal branch (Fig. 1). A right coronary angiogram showed diffuse but insignificant disease. After cannulating the left coronary system with a Judkin left 3.5 catheter, we crossed the LAD lesion with a floppy wire and stented it with a 3 × 13-mm bare-metal stent, without predilation. In view of his hemodynamic instability, we also performed stenting (without predilation) of the LCx lesion with a bare-metal stent (Fig. 2). A week after the procedure, the patient was discharged from the hospital in a stable condition.
Fig. 1.
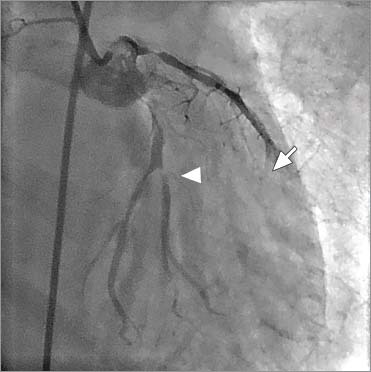
Left coronary angiogram (right anterior caudal projection) shows a patent left anterior descending coronary artery (LAD) stent with a tight, thrombotic stenosis proximal to it, with slow flow in the LAD (arrow) and significant thrombotic disease involving the proximal left circumflex coronary artery and the first obtuse marginal branch (arrowhead).
Supplemental motion image is available for Figure 1 (1.4MB, m1v) .
Fig. 2.

Left coronary angiogram (right anterior caudal projection) after primary percutaneous coronary intervention shows good flow in the left anterior descending and left circumflex coronary arteries
Supplemental motion image is available for Figure 2 (1.6MB, m1v) .
Discussion
This case highlights the importance of severe ischemic complications in a patient with a history of CAD who is placed on a multidrug chemotherapeutic regimen. Some of the chemotherapeutic agents that are known to cause AMI include taxanes, vinca alkaloids, 5-fluorouracil, cisplatin, carboplatin, bevacizumab, sorafenib, and erlotinib.1 In our patient, an R-CVP regimen had been used. Rituximab is one of the agents commonly used in treating CD20+ leukemias. Although it has been implicated as a cause of cardiac arrhythmias in 8% of patients treated for lymphoma,2 less than 0.1% of rituximab infusions have been associated with acute coronary syndrome (ACS), including AMI. The mechanism of ACS after rituximab therapy is not well understood. However, rituximab infusion is known to be associated with the release of cytokines, such as interleukin-6 and tumor necrosis factor-α.2 Such cytokine storms are postulated to lead to vasoconstriction, platelet activation, and rupture of atherosclerotic plaque,3 thus leading to acute ischemic syndromes. Armitage and colleagues4 described the cases of 3 patients with lymphoproliferative disorders who experienced ACS associated with an initial infusion of rituximab. Arunprasath and associates5 have reported on one such patient who was managed conservatively after an infusion with rituximab-precipitated AMI.
Similarly, vincristine is associated with AMI.2,6,7 A study by Mikaelian and colleagues8 showed that tubulin-binding drugs (including vincristine and vinblastine) can induce cell-cycle arrest of the rapidly proliferating cardiac endothelial cells and cause myocardial infarction. Vincristine is more often reported to cause ischemic syndromes than is rituximab, but a catastrophic synergism of vincristine and rituximab certainly cannot be ruled out.
Our patient experienced an AMI that probably was secondary to chemotherapeutic infusions of rituximab and vincristine. Both of these drugs have been reported to cause ACS. We propose that the synergistic effect of such drugs in causing ACS should be considered before their administration, especially in a high-risk patient with existing CAD. Whether such patients should be considered for an alternative chemotherapeutic regimen is a matter of conjecture.
References
- 1.Force T, Chen MH. The cancer patient and cardiovascular disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's heart disease. Philadelphia: Saunders; 2011. pp. 1896–902. [Google Scholar]
- 2.Foran JM, Rohatiner AZ, Cunningham D, Popescu RA, Solal-Celigny P, Ghielmini M. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma [published erratum appears in J Clin Oncol. J Clin Oncol. 2000;2000;1818(9)(2):2006. 317–24. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 3.Garypidou V, Perifanis V, Tziomalos K, Theodoridou S. Cardiac toxicity during rituximab administration. Leukolymphoma. 2004;45(1):203–4. doi: 10.1080/10428190310001607160. [DOI] [PubMed] [Google Scholar]
- 4.Armitage JD, Montero C, Benner A, Armitage JO, Bociek G. Acute coronary syndromes complicating the first infusion of rituximab. Clin Lymphoma Myeloma. 2008;8(4):253–5. doi: 10.3816/CLM.2008.n.035. [DOI] [PubMed] [Google Scholar]
- 5.Arunprasath P, Gobu P, Dubashi B, Satheesh S, Balachander J. Rituximab induced myocardial infarction: a fatal drug reaction. J Cancer Res Ther. 2011;7(3):346–8. doi: 10.4103/0973-1482.87003. [DOI] [PubMed] [Google Scholar]
- 6.Mandel EM, Lewinski U, Djaldetti M. Vincristine-induced myocardial infarction. Cancer. 1975;36(6):1979–82. doi: 10.1002/cncr.2820360908. [DOI] [PubMed] [Google Scholar]
- 7.Barra M, Brignole M, Sartore B, Bertulla A. Myocardial infarction induced by vincristine in patients with Hodgkin's lymphoma. description of 2 cases and review of the literature [in Italian] G Ital Cardiol. 1985;15(1):107–11. [PubMed] [Google Scholar]
- 8.Mikaelian I, Buness A, de Vera-Mudry MC, Kanwal C, Coluccio D, Rasmussen E. Primary endothelial damage is the mechanism of cardiotoxicity of tubulin-binding drugs. Toxicol Sci. 2010;117(1):144–51. doi: 10.1093/toxsci/kfq189. [DOI] [PubMed] [Google Scholar]


