Abstract
Mercury (Hg) is neurotoxic and children may be particularly susceptible to this effect. A current major challenge is identification of children who may be uniquely susceptible to Hg toxicity because of genetic disposition. This study examined the hypothesis that genetic variants of catechol-O-methyltransferase (COMT) that are reported to alter neurobehavioral functions that are also affected by Hg in adults might modify the adverse neurobehavioral effects of Hg exposure in children. Five hundred and seven children, 8–12 yr of age at baseline, participated in a clinical trial to evaluate the neurobehavioral effects of Hg from dental amalgam tooth fillings. Subjects were evaluated at baseline and at seven subsequent annual intervals for neurobehavioral performance and urinary Hg levels. Following the clinical trial, genotyping assays were performed for single-nucleotide polymorphisms (SNPs) of COMT rs4680, rs4633, rs4818, and rs6269 on biological samples provided by 330 of the trial participants. Regression-modeling strategies were employed to evaluate associations between allelic status, Hg exposure, and neurobehavioral test outcomes. Similar analysis was performed using haplotypes of COMT SNPs. Among girls, few interactions for Hg exposure and COMT variants were found. In contrast, among boys, numerous gene–Hg interactions were observed between individual COMT SNPs, as well as with a common COMT haplotype affecting multiple domains of neurobehavioral function. These findings suggest increased susceptibility to the adverse neurobehavioral effects of Hg among children with common genetic variants of COMT, and may have important implications for strategies aimed at protecting children from the potential health risks associated with Hg exposure.
Children are recognized as having heightened susceptibility to the adverse effects of environmental chemicals, compared to adults with similar exposures (Faustman et al., 2000; Landrigan and Goldman, 2011; Makri et al., 2004). Of particular concern in this respect are possible neurologic deficits associated with mercury (Hg) exposure, including impairment of the developing central nervous system (CNS) along with attendant personality, cognitive function, and behavioral disorders (Counter and Buchanan, 2004). A current major challenge is the identification of those children who may be uniquely susceptible to Hg-mediated neurological deficits because of genetic predisposition.
Among the biological variants likely to be associated with altered susceptibility to Hg toxicity are enzymes affecting the processing of neurotransmitters that mediate behavioral functions known to be compromised by Hg exposure. Notable among these is catechol-O-methyltransferase (COMT, EC 2.1.1.6), a ubiquitously expressed enzyme that maintains basic neurologic functions by deactivating catechol substrates (epinephrine, norephinephrine, and dopamine) and catecholestrogens (Weinshilboum et al., 1999). The human COMT gene is located on chromosome 22q11, where it encodes two distinct proteins, soluble COMT (S-COMT) and membrane-bound COMT (MB-COMT), via alternative promoters and translation initiation sites (Tenhunen et al., 1993). S-COMT is found mostly in peripheral tissues, whereas MB-COMT is predominantly expressed in the CNS (Tenhunen et al., 1994). The specific cell and tissue distribution of COMT isoforms conforms largely to that of its catechol substrates. In the CNS, the prefrontal cortex, a region that is primarily involved in integration of cognitive functions in humans (Malhotra et al., 2002), is particularly dependent on COMT to terminate the action of synaptic dopamine (DA). Notably, COMT methylation accounts for approximately 60% of total DA turnover in the prefrontal cortex, compared with approximately 15% in other brain regions (Garris and Wrightman, 1994). Genetic variants or other factors that modify COMT activity may, therefore, exert a substantial effect on DA-dependent neurobehavioral processes.
Studies in humans demonstrated that polymorphisms that modify the catalytic activity of COMT are associated with a number of neuropsychiatric disorders (Nackley et al., 2009), as well as with deficits in process-specific psychological functions such as cognition and brain metabolic activities accompanying neurobehavioral tasks, particularly in children (Barnett et al., 2007, 2009; Diamond et al., 2004). Many of these association studies focused specifically on a common functional missense mutation (G > A) in exon 4 of the MB-COMT gene (COMT rs4680, minor allelic frequency [MAF] = 0.3892), producing a transcriptional substitution of methionine for valine at codon 158. This val158 met polymorphism decreases COMT enzymatic activity by more than fourfold (Lachman et al. 1996), allowing DA to remain active in and around synaptic clefts substantially longer. Studies demonstrated that cognitive function, particularly on more complex tasks, improved in proportion to the increased availability of frontal DA associated with the met158 allele (Bilder et al., 2002). Conversely, higher synaptic DA levels associated with met158 may heighten affective responses to negative stimuli and reduce coping ability (Heyer et al., 2009). Recent studies report that more inclusive haplotypes involving other common single nucleotide polymorphisms (SNPs) in the MB-COMT gene acting in concert with rs4680 may more definitively characterize susceptibility to COMT-dependent neurologic processes, including those affecting myogenous temporomandibular (TMD) pain sensitivity (Diatchenko et al., 2005; Nackley et al., 2006) and cognitive function in children (Barnett et al. 2009). SNPs of particular interest in this respect include rs6269 (MAF = 0.3718), an A > G transition in the P1 promoter region; rs4633 (MAF = 0.3892), a C > T transition encoding a his62 his in exon 3; and rs4818 (MAF = 0.3228), a C > G transition encoding a leu136 leu in exon 4, which together affect changes in these phenotypes by encoding mRNAs with alternative secondary structures that display differential levels of COMT protein expression (Nackley et al., 2006).
The aim of the present study was to examine the hypothesis that the genetic variants of COMT (rs4680, rs4633, rs4818, and rs6269) that, individually or in combination, have been reported to alter neurobehavioral functions that are also affected by Hg would modify the adverse neurobehavioral effects of Hg exposure in children. In addition to evaluating these specific COMT variants, the hypothesis that COMT haplotype classification was significantly better than COMT val158 met genotype classification in terms of predicting increased sensitivity of children to Hg neurotoxicity was also investigated. Because previous studies (Woods et al., 2007, 2012, 2013) suggested possible gender-related differences in Hg toxicokinetics and susceptibility to Hg toxicity, these assessments were made independently in boys and girls.
MATERIALS AND METHODS
The Study Population
The current study included 330 subjects who participated as children in the Casa Pia Dental Amalgam Clinical Trial (DeRouen et al., 2002; 2006) conducted between 1996 and 2006. Participants in the clinical trial included 279 boys and 228 girls, aged 8–12 years at baseline, who were students of the Casa Pia school system in Lisbon, Portugal. Children were initially randomized to Hg amalgam (treatment) or composite resin (control) dental treatment groups. Subjects were evaluated at baseline and at seven subsequent annual intervals following initial dental treatment using an extensive battery of neurobehavioral assessments (Martins et al., 2005). Follow-up data were obtained on a similar number of subjects in each treatment group. Baseline urinary Hg concentrations were 1.5 ± 1.2 (0.1–7.7) and 1.4 ± 1.1 (0.0–8.6) µg/L for amalgam and composite groups, respectively. Mean urinary Hg concentrations by treatment group and by gender for each year of the clinical trial were previously described (Woods et al., 2007). A detailed description of the study design and methods, including factors measured over the course of the study and how these factors were considered in constructing analytical models, has been published (DeRouen et al., 2002).
Neurobehavioral Tests
A comprehensive neurobehavioral test battery was used in this analysis, including measures from the Rey Auditory Verbal Learning Test (RAVLT), subtests from the Wide Range Assessment of Visual Motor Abilities (WRAVMA), the Wechsler Adult Intelligence Scale-III (WAIS-III), the Wechsler Memory Scale for Adults-III (WMS-III), Standard Reaction Time, Finger Tapping, Trailmaking A and B, and the Stroop word, color, and word-color tests. The validity and rationale underlying the use of these tests in the clinical trial, as well as the baseline neuropsychological performance of all subjects, have been described (Martins et al., 2005; Townes et al., 2008).
Table 1 lists the 22 neurobehavioral tests that were assessed and presents their means and standard deviations at their last year of administration (yr 7). Tests are organized within the behavioral domains that were evaluated in the clinical trial (DeRouen et al., 2006). Arrows depict whether the test score increases or decreases in magnitude with improved performance. Diminished or adversely affected performance associated with Hg exposure or gene variant status is described as occurring in the direction of impaired performance, whereas enhanced or beneficially affected performance associated with either of these variables is described as occurring in the direction of improved performance. The Comprehensive Test Of Nonverbal Intelligence (CTONI; Portuguese translation) was given to each child at the beginning of the clinical trial to obtain a measure of IQ at baseline. Social and cultural issues associated with measurements and interpretation of IQ test results among children in cross-cultural contexts have been previously described (Martins et al., 2005; Woods et al., 2013).
TABLE 1.
Neurobehavioral Tests Assessed With Mean Scores for Year 7 (Final Year of Clinical Trial)
| Test/domain | Test abbreviation | Measurea | Boys, n = 120, mean (SD) | Girls, n = 119, mean (SD) |
|---|---|---|---|---|
| Attention/Concentration (7 tests) | ||||
| Stroop Test–Color, | Stroop-Color | Number correct ↑ | 66.16 (11.67) | 69.25 (10.39) |
| Word | Stroop-Word | Number correct ↑ | 89.93 (15.17) | 91.54 (15.19) |
| Color-Word | Stroop-ColWd | Number correct ↑ | 41.74 (9.76) | 43.93 (8.73) |
| WAIS-III–Digit Span | Digit Span | Number correct ↑ | 14.30 (3.67) | 14.14 (2.76) |
| WAIS-III–Spatial Span | Spatial Span | Number correct ↑ | 15.83 (3.03) | 15.61 (3.12) |
| Adult Trails A | Trails A | Time (sec) ↓ | 26.43 (10.63) | 30.25 (11.42) |
| Adult Trails B | Trails B | Time (sec) ↓ | 65.97 (26.94) | 63.10 (23.80) |
| Visual-Spatial (3 tests) | ||||
| Standard Reaction Time | SRT | Time (sec) ↓ | 0.74 (0.15) | 0.77 (0.13) |
| WAIS III–Digit Symbol | Digit Symbol | Number correct ↑ | 72.02 (16.48) | 76.58 (13.85) |
| WAIS-III–Symbol Search | Symbol Search | Number correct ↑ | 32.99 (8.82) | 34.59 (8.01) |
| Learning & Memory (8 tests) | ||||
| RAVLT Trial 1–List A | RAVLT 1 | Number correct ↑ | 5.62 (1.49) | 6.12 (1.86) |
| Trial 5–List A (fifth repetition) | RAVLT 5 | Number correct ↑ | 11.23 (2.20) | 11.55 (2.23) |
| Trial 6–List B | RAVLT 6 | Number correct ↑ | 4.73 (1.38) | 5.28 (1.56) |
| Trial 7–List A/Post B | RAVLT 7 | Number correct ↑ | 9.85 (2.57) | 10.23 (2.48) |
| Trial 8–List A after 20′ | RAVLT 8 | Number correct ↑ | 9.29 (2.73) | 10.08 (2.76) |
| Trials 1–5 | RAVLT 1–5 | Number correct ↑ | 45.41 (9.58) | 48.01 (9.37) |
| WMS-III–Visual Reproductions–Immediate | VisRep-Imm | Number correct ↑ | 34.69 (4.66) | 36.62 (2.97) |
| WMS-III–Visual Reproductions–Delayed | VisRep-Del | Number correct ↑ | 32.01 (6.98) | 34.93 (4.02) |
| Motor (4 tests) | ||||
| WRAVMA–Pegs–Dominant Hand | Pegs-Dom | Number Pegs ↑ | 47.35 (8.51) | 49.92 (6.28) |
| WRAVMA–Pegs–Non Dominant Hand | Pegs-NonDom | Number Pegs ↑ | 44.37 (7.59) | 45.03 (6.10) |
| Finger Tapping–Dominant Hand | FT-Dom | Number Taps ↑ | 52.66 (5.53) | 48.55 (5.83) |
| Finger Tapping–Non Dominant Hand | FT-NonDom | Number Taps ↑ | 46.54 (5.79) | 42.53 (5.78) |
Arrows show direction of improved performance.
Genotyping Assays
Genotyping was performed on DNA extracted from buccal cell samples that were obtained from study subjects following completion of the clinical trial (n = 199) or from blood samples that were acquired at baseline for blood lead (Pb) assessments (n = 152). Genotyping was performed by the Functional Genomics Laboratory of the NIEHS Center for Ecogenetics and Environmental Health at the University of Washington, using commercially available TaqMan Detection System-based genotyping assays (Applied Biosystems, Inc., Hercules, CA) to characterize COMT rs4680, rs4633, rs4818, and rs6269 alleles. Subjects evaluated for COMT rs4680 were categorized as wild-type (GG), heterozygous (GA) or homozygous mutant (AA); for rs4633 as wild-type (CC), heterozygous (CT), or homozygous mutant (TT); for rs4818 as wild-type (CC), heterozygous (CG), or homozygous mutant (GG); and for rs6269 as wild-type (GG), heterozygous (GA), or homozygous mutant (AA). Substantial numbers of subjects were genotyped as wild-type (WT) or as full homozygous mutant for all four COMT variants under evaluation (Table 2). Therefore, allelic status was dichotomized as either wild-type (WT) or homozygous mutant (Mut) for most analyses, both to limit the number of comparisons and to maximize the potential impact of these variants. SNP descriptions are found at http://www.ncbi.nlm.nih.gov/snp/?term=rs4680, http://www.ncbi.nlm.nih.gov/snp/?term=rs4818, http://www.ncbi.nlm.nih.gov/snp/?term=rs4633, or http://www.ncbi.nlm.nih.gov/snp/?term=rs6269, respectively (National Center for Biotechnology Information, 2013).
TABLE 2.
Study Population Characteristics for Participants at Entry (Baseline) and in Year 2 and Year 7
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| Entry. |
Year 2. |
Year 7. |
Entry. |
Year 2. |
Year 7. |
|
| Characteristic | mean (SD) | mean (SD) | mean (SD) | mean (SD) | mean (SD) | mean (SD) |
| Age | 10.17 (.82) | 12.22 (.84) | 17.15 (.85) | 10.14 (.93) | 12.15 (.96) | 17.06 (1.03) |
| School year | 4.05 (1.05) | 5.78 (1.21) | 9.40 (2.05) | 4.14 (1.07) | 5.91 (1.16) | 9.86 (1.51) |
| Non-Verbal IQ (at entry only) | 85.96 (9.96) | — | — | 85.54 (10.23) | — | — |
| Urinary mercury concentrations | ||||||
| Unadjusted urinary Hga | 1.45 (1.13) | 2.17 (2.14) | 1.86 (5.35) | 1.52 (1.13) | 2.83 (3.02) | 2.37 (2.61) |
| Range | (0.04, 7.70) | (0.03, 11.5) | (.01, 58.3) | (0.16, 7.09) | (0.01, 15.6) | (.01, 15.6) |
| Ln HgU (creatinine corrected)b | 0.89 (0.42) | 1.02 (0.49) | 0.62 (0.48) | 0.94 (0.48) | 1.18 (0.57) | 0.83 (0.56) |
| Range | (0.13, 2.15) | (0.04, 2.53) | (.005, 3.49) | (0.10, 3.20) | (.01, 2.81) | (.01, 2.79) |
| Calculated cumulative Hg measurec | — | — | 2.48 (0.50) | — | — | 2.74 (0.55) |
| Range | — | — | (1.32, 4.03) | — | — | (1.44, 4.13) |
| Distribution | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) |
|---|---|---|---|---|---|---|
| Total subjects (n) | 163 | 159 | 120 | 167 | 152 | 119 |
| Caucasian, % (n) | 74.2% (121) | 74.2% (118) | 71.7% (86) | 71.3% (119) | 69.1% (105) | 69.7% (83) |
| COMT–rs4680 | ||||||
| Wild-type (G/G) | 33% (54) | 33% (53) | 33% (40) | 37% (61) | 37% (56) | 38% (45) |
| Heterozygous (G/A) | 50% (82) | 50% (79) | 48% (57) | 48% (80) | 49% (74) | 49% (58) |
| Homozygous mutant (A/A) | 17% (27) | 17% (27) | 19% (23) | 16% (26) | 14% (22) | 13% (16) |
| COMT–rs4633 | ||||||
| Wild-type (C/C) | 31% (51) | 31% (50) | 32% (38) | 38% (63) | 38% (58) | 39% (46) |
| Heterozygous (C/T) | 53% (87) | 53% (84) | 51% (61) | 47% (78) | 47% (72) | 48% (57) |
| Homozygous mutant (T/T) | 15% (25) | 16% (25) | 18% (21) | 16% (26) | 14% (22) | 13% (16) |
| COMT–rs4818 | ||||||
| Wild-type (C/C) | 36% (59) | 36% (57) | 39% (47) | 37% (61) | 36% (55) | 36% (43) |
| Heterozygous (C/G) | 49% (80) | 50% (79) | 48% (58) | 46% (76) | 46% (70) | 45% (54) |
| Homozygous mutant (G/G) | 15% (24) | 14% (23) | 13% (15) | 18% (30) | 18% (27) | 18% (22) |
| COMT–rs6269 | ||||||
| Wild-type (A/A) | 21% (35) | 21% (34) | 22% (26) | 24% (40) | 24% (36) | 25% (30) |
| Heterozygous (A/G) | 52% (85) | 52% (83) | 50% (60) | 52% (87) | 53% (81) | 51% (61) |
| Homozygous mutant (G/G) | 26% (43) | 26% (42) | 28% (34) | 24% (40) | 23% (35) | 24% (28) |
µg Hg/L.
ln(µg Hg/g creatinine + 1).
ln[Σµg Hg/g creatinine) + 1].
Human Subjects Considerations
All parents or guardians of children who participated in the clinical trial gave written consent, and all children provided signed assent, for the treatments and assessments made during the course of the trial, including collection of blood samples. Written consent was also obtained from all participants who provided buccal cell samples for genotyping subsequent to completion of the clinical trial. The study protocols for both the clinical trial and the present genotyping study were approved by the institutional review boards at the University of Lisbon and the University of Washington.
Urinary Mercury Analysis
A urine sample was collected from each child at baseline of the clinical trial and at each subsequent annual visit to the University of Lisbon School of Dental Medicine for dental, neurologic, and neurobehavioral evaluations. Strictly maintained sterile conditions and handling procedures precluded contamination of urine samples by Hg or any other substance. Analysis of total mercury (Hg) was performed by continuous-flow, cold-vapor spectrofluorometry, as previously described (Pingree et al., 2001). Urinary creatinine concentrations were measured using a standard colorimetric procedure (Sigma number 555-A; Sigma-Aldrich, St. Louis, MO). Urinary Hg concentrations (HgU) were calculated as micrograms per gram creatinine (µg/g creatinine).
Assessment of Hg Exposure
In this study, the clinical trial approach was modified in 2 essential ways. First, rather than using the assignment to Hg amalgam or composite treatment groups, a cumulative measure of urinary Hg concentrations (HgU) measured annually for all participants was employed as the measure of Hg exposure. This allowed us to capture the effects of all Hg exposure, whether or not related to dental amalgam. This decision was based on the fact that assignment group accounted for, at most, 17% of the variation in HgU among boys (r2 = .171) and 15% among girls (r2 = .154), both occurring in yr 2 of the clinical trial, and indicating considerable background Hg exposure unrelated to dental amalgam. In this regard, because urine may include inorganic Hg derived from dealkylation of ingested methylmercury (Sherman et al. 2013), fish consumption could constitute a source of urinary Hg observed among subjects in this study. Second, the potential modifying effects of COMT genotype variants on the dose-response effects of Hg exposure, as represented by urinary Hg levels, on neurobehavioral performance were evaluated. Because previous studies (Woods et al., 2007, 2012, 2013) suggested possible sex-related differences in Hg handling and susceptibility to Hg toxicity, these assessments were conducted independently in boys and girls.
Study Design
This study evaluated whether COMT allelic status affected the dose-response relationship between urinary Hg concentration and tests of neurobehavioral functions among children who were evaluated annually from baseline through 7 yr of follow-up after initial placement of dental amalgam or composite resin tooth fillings. The wide range in ages of subjects at the beginning of the clinical trial (8–12 yr), duration of the study (which included passage through puberty for most subjects), and associated change in specific tests administered to subjects based upon their age group during the course of the trial (e.g., child versus adult versions of some tests) introduced significant complexity into the interpretation of repeated measures analyses for this study. It was therefore decided to evaluate the acute effects of Hg exposure on performance using the concurrent urinary Hg concentrations (HgU) and neurobehavioral test performance measures at the end of the second year of follow-up (yr 2), where mean HgU reached a peak among both boys and girls in the clinical trial cohort and where cumulative effects of Hg would be minimal. Similarly, it was decided to estimate the chronic effects of Hg exposure by examining the relationship between a cumulative measure of HgU over the entire study period and performance outcomes during the final study year (yr 7).
The measure of acute Hg exposure was calculated as the natural log of HgU adjusted by 1 (ln[HgU + 1]). The natural log best accommodates how exposures are distributed biologically, whereas adding 1 minimizes the influence of small changes in HgU at low levels (which could have otherwise dominated the analyses). The measure of chronic Hg exposure was evaluated using cumulative HgU calculated as the natural log of the sum of HgU from baseline and each year of follow-up also adjusted by 1 (ln[ΣHgU) + 1]). A small number of subjects (28) who were followed for the full 7 years of the clinical trial had missed one or more intermediate annual evaluations. Subjects missing 3 or more evaluations (n = 2, both female) were eliminated from the chronic exposure analyses. Those missing 1 (n = 22, 9 female) or 2 (n = 4, 3 female) annual evaluations were included in the chronic Hg exposure analyses with their mean HgU concentrations substituted for the missing years. Chronic Hg exposure analyses conducted with and without these subjects did not significantly differ. Of note, acute and chronic measures of Hg exposure have a Pearson correlation of 0.36, indicating that they share only 13% common variance (r2) and, hence, are not highly correlated.
Statistical Analyses
The effect of COMT gene variants on the dose-response association between Hg exposure and neurobehavioral performance was the principal focus of this study. Thus, our analytical protocol focused on Hg–gene interactions, independently evaluating the impact of allelic status of the COMT gene individually and in combination on performance of each behavioral test. In all cases, boys and girls were evaluated independently, as were the effects of acute and chronic Hg exposures. Statistical analyses were performed using SPSS Version 19 (IBM®SPSS, Chicago, IL).
Initial analysis was conducted using a full model consisting of Hg exposure (either acute or chronic as defined earlier), dichotomous allelic status for each gene (WT or Mut), and their interaction terms. Covariates in this model included age at assessment, race, and nonverbal IQ (determined at baseline). All behavioral tests with significant interaction terms were reevaluated for dose-response associations between Hg exposure and test performance independently for subjects with WT or Mut allelic status for each SNP. In these analyses, p ≤ .05 was established as the measure of significance. This strategy provides a clear description of Hg dose-response relationships within each genotypic group, where the differences (interactions) can be directly observed.
For those behavioral tests where the initial full model analysis indicated no significant interactions, interaction terms were dropped from the model, and the main effects of Hg exposure and allelic status were evaluated independently for the full cohort of boys or girls.
Additional analyses were conducted to evaluate the possibility that the observed effects of Hg on neurobehavioral functions among subjects genotyped as COMT WT or Mut might be affected by the allelic status of other genes, that is, coproporphyrinogen oxidase (CPOX, rs1131857), metallothionein 1M (MT1M, rs2270837), and metallothionein 2A (MT2A, rs10636), for which previous studies (Woods et al., 2012, 2013) demonstrated increased sensitivity to Hg exposure, with a concern being that these effects might be associated with colinearity among the three gene status variables or shared mechanisms of effect. These analyses were performed as described above but included dummy variables for CPOX WT, CPOX Het/Mut, MT1M WT, MT1M Het/Mut, MT2A WT, and MT2A Het/Mut as additional covariates in the regression models. Using the same analytic approach, all behavioral tests with significant interaction terms were reevaluated for dose-response associations between Hg exposure and test performance independently for subjects with WT or Mut allelic status for each COMT SNP.
Prior to fitting the regression models, the assumptions of the model were examined by scrutinizing the distributions and variances of all cognitive tests and ln(HgU). Most distributions had no significant deviation from normality or inflated variance. After fitting each model, the standardized residuals were examined for statistical outliers. In the rare event that an outlier was found, it was removed and the model was refitted.
Haplotype Construction and Analysis
Haplotype analyses were restricted to Caucasians, owing to significant differences in allelic frequencies across population groups, particularly those of European and African origin (Gabriel et al., 2002; Mukherjee et al., 2010). SNP by SNP linkage disequilibrium (LD) was measured using D′ statistics between each pairwise combination of all four COMT SNPs using Haploview V3.32 (www.broad.mit.edu/mpg/haploview). Haploview was employed to create a graphical representation of LD structure (Barrett et al., 2005). Haplotypes were estimated from genotype data using the PHASE software, with default settings (Stephens et al., 2001). The order of SNPs in haplotype construction was rs6269, rs4633, rs4818, rs4680, reflecting the order of occurrence from 5′ to 3′ in the COMT gene (Diatchenko et al., 2005).
RESULTS
The study cohort consisted of 330 children (164 boys and 166 girls) for whom COMT rs4680, rs4633, rs4818, and rs6269 allelic status were available from among 507 total subjects enrolled at the start of the clinical trial. Excluded subjects either did not provide a blood sample at the initiation of the study, or were lost to follow-up for acquisition of a buccal cell sample following completion of the trial. Table 2 presents the characteristics of the cohort at entry as well as at yr 2 and 7 of the clinical trial. Both boys and girls averaged 10.1 yr of age, and most were in fourth grade at entry into the study. At entry, 74% of boys and 71% of girls were Caucasian, and both genders had an average nonverbal IQ score of 86. Table 2 also displays the mean “raw” values for urinary Hg concentrations (HgU) and the natural log calculations for the HgU at entry, yr 2, and yr 7 of the clinical trial, as well as the calculated cumulative chronic exposure measure for both boys and girls at yr 7, along with the range for each measure. The change in number of total subjects between entry and yr 7 reflects the overall 14% loss to follow-up over the course of the clinical trial. The frequencies of the homozygous common (wild-type, WT), heterozygous (Het), and homozygous mutant (Mut) alleles for boys and girls for each of the four COMT variants are also presented.
To preclude the possibility of selection bias in those who provided DNA samples for the present study, neurobehavioral test scores for subjects at baseline and at clinical trial years 2 and 7 for those included in the present analyses were compared, using t tests, to all those participating in the clinical trial from which present subjects were drawn. No significant differences at any time point between groups were observed when adjusting for age and gender distribution, IQ, and grade level. These findings militate strongly against selection bias in the present population.
Individual COMT SNP Effects in Relation to Acute Hg Exposure
Table 3 presents results of the acute Hg dose-response analyses among boys and girls for each of the four COMT SNPs by allelic status (WT and Mut) for each test that (1) had a significant interaction term in the full model analyses and (2) remained significant when reevaluated for dose-response associations between Hg exposure and test performance for subjects who were homozygous for allelic status for each SNP. Results that are significant at p ≤ .05 are indicated in bold. It is apparent that there are no significant results among either boys or girls having WT status for any of the four SNPs evaluated. Significant interaction terms, then, are defined by significant dose responses among subjects only with Mut status for each SNP.
TABLE 3.
Acute Hg Dose-Response Effects in Year 2 Among Boys and Girls by WT or Mut Allelic Status for COMT SNPs
| rs4680 |
rs4633 |
rs4818 |
rs6269 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT |
Mut |
WT |
Mut |
WT |
Mut |
WT |
Mut |
|||||||||
| Measure | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) |
| Boys | ||||||||||||||||
| Attention/Concentration | ||||||||||||||||
| Stroop Test: Color | −1.58 (2.50) | −.09 (.53) | −9.32 (4.09) | −.44 (.03) | −1.58 (2.56) | −.09 (.54) | −7.60 (3.70) | −.42 (.05) | ||||||||
| Stroop Test: Word | −3.83 (3.57) | −.15 (.29) | −12.43 (6.21) | −.39 (.06) | ||||||||||||
| Stroop Test: Color-Word | 1.22 (1.94) | .09 (.54) | −7.92 (2.76) | −.52 (.009) | 1.35 (1.96) | .10 (.49) | −7.98 (2.73) | −.52 (.01) | 3.43 (2.42) | .25 (.17) | −5.31 (2.59) | −.32 (.05) | ||||
| WAIS-III – Digit Span | −.56 (.65) | −.12 (.40) | −2.11 (.93) | −.44 (.03) | −.58 (.68) | −.13 (.40) | −2.04 (.98) | −.42 (.05) | −.74 (.86) | −.16 (.40) | −1.72 (.79) | −.34 (.04) | ||||
| Learning & Memory | ||||||||||||||||
| RAVLT–Trial 5: List A | .25 (.64) | .06 (.32) | −2.66 (1.08) | −.49 (.02) | .18 (.66) | .04 (.79) | −2.70 (1.06) | −.49 (.02) | −.01 (.78) | −.00 (.99) | −2.00 (.78) | −.39 (.01) | ||||
| RAVLT–Trial 6: List B | .29 (.37) | .11 (.44) | −1.68 (.69) | −.49 (.02) | .22 (.37) | .09 (.56) | −1.65 (.69) | −.49 (.02) | ||||||||
| RAVLT–Trial 7: List A post B | .29 (.76) | .06 (.71) | −2.83 (1.19) | −.48 (.03) | .24 (.79) | .04 (.76) | −2.80 (1.16) | −.48 (.03) | −.02 (1.02) | −.00 (.98) | −1.96 (.94) | −.32 (.04) | ||||
| RAVLT–Trial 8: A post 20′ | −.07 (.71) | −.02 (.92) | −3.70 (1.48) | −.50 (.02) | −.17 (.72) | −.03 (.82) | −3.69 (1.44) | −.50 (.02) | −.23 (.95) | −.04 (.81) | −2.63 (1.04) | −.38 (.02) | ||||
| RAVLT–Trials 1–5: Sum | −1.17 (2.13) | −.08 (.58) | −12.21 (4.26) | −.55 (.01) | −1.52 (2.13) | −.11 (.48) | −12.38 (4.20) | −.55 (.008) | −3.71 (2.47) | −.27 (.14) | −8.41 (3.00) | −.42 (.008) | ||||
| Motor | ||||||||||||||||
| Finger Tapping – Dominant | −1.40 (1.60) | −.12 (.38) | 4.39 (1.71) | .51 (.02) | ||||||||||||
| Girls | ||||||||||||||||
| Attention/Concentration | ||||||||||||||||
| Stroop Test: Color | −.37 (1.84) | −.03 (.84) | 8.39 (3.16) | .49 (.01) | ||||||||||||
| Stroop Test: Word | −2.83 (3.58) | −.11 (.43) | 11.90 (4.68) | .48 (.02) | ||||||||||||
| Stroop Test: Color-Word | −1.06 (1.53) | .10 (.49) | 4.84 (1.85) | .49 (.02) | ||||||||||||
| Trails A | 3.03 (2.05) | .20 (.15) | 7.49 (1.51) | .77 (.0001) | 2.53 (1.95) | .18 (.20) | 6.18 (2.00) | .60 (.007) | ||||||||
| Trails B | 2.53 (3.50) | .10 (.47) | 6.52 (2.81) | .49 (.03) | 2.53 (3.50) | .10 (.47) | 6.52 (2.81) | .49 (.03) | ||||||||
| Learning & Memory | ||||||||||||||||
| RAVLT–Trial 6: List B | .73 (.46) | .27 (.12) | .81 (.37) | .37 (.04) | ||||||||||||
Note. Values in bold signify p ≤ 0.05 in the direction of impaired performance with increasing Hg exposure. Values in bold italics signify p ≤ .05 in direction of improved performance.
Among boys, those with Mut versions of rs4680 and rs4633 displayed significantly impaired performance on the same eight tests in response to increasing acute Hg exposure. These included all five scales of the RAVLT verbal learning tests in the Learning & Memory domain, and three tests in the domain of Attention/Concentration, including Stroop color and color-word tests and the WAIS Digit Span test. Similar significant results were observed among boys genotyped as rs6269 Mut in a subset of six of these tests. Among boys genotyped as rs4818 Mut, only one significant result (Finger Tapping-Dominant), in the Motor domain, was observed, and this result was in the direction of improved performance with Hg exposure (value in bold italics).
The similarity of the results among boys genotyped as having three of these four SNPs can be viewed as consistent with the strong association between occurrences of their variants. As seen in Table 4, there were 25 boys with the rs4633 Mut variant, all of whom shared the rs4680 Mut variant, which occurred in 27 boys. Thus, there was a difference of only two subjects between these groups. These same 25 boys also shared the Mut variant of rs6269, but an additional 18 boys genotyped as rs6269 Mut were not included in groups sharing rs4680 and rs4633 Mut. However, there was not a strong association among boys genotyped as having the rs4818 variant with those having any of these other SNPs.
TABLE 4.
Distribution of Three COMT Variants Among Boys
| COMTrs4680 |
|||||
|---|---|---|---|---|---|
| Boys at entry | G/G | G/A | A/A | Total | |
| COMTrs4633 | C/C | 51 | 0 | 0 | 51 |
| C/T | 3 | 82 | 2 | 87 | |
| T/T | 0 | 0 | 25 | 25 | |
| Total | 54 | 82 | 27 | 163 | |
| COMTrs6269 | A/A | 35 | 0 | 0 | 35 |
| A/G | 16 | 67 | 2 | 85 | |
| G/G | 3 | 15 | 25 | 43 | |
| Total | 54 | 82 | 27 | 163 | |
As presented in Table 3, substantially fewer significant effects of COMT genotype were observed among girls in terms of modification of neurobehavioral test results in response to acute Hg exposure. No significant associations were observed for WT status and acute Hg exposure for any of the 4 SNPs evaluated. In contrast to boys, girls genotyped as having Mut versions of rs4680 or rs4633 displayed significantly impaired Trails A and Trails B test results (Attention/Concentration) with increasing acute Hg exposure. Conversely, significant modification of acute Hg effects on all three Stroop test results in the direction of improved performance were observed among girls genotyped as rs4818 Mut. Significant but improved performance with increasing acute Hg exposure was also observed among girls genotyped as rs6262 Mut for the RAVLT Trial 6 test of Learning & Memory (values in bold italics).
Individual COMT SNP Effects in Relation to Chronic Hg Exposure
Table 5 presents results of the chronic Hg dose-response analyses among boys and girls for each of the four COMT SNPs by allelic status (WT and Mut) using the same criteria as for Table 3. Results that are significant at p ≤ .05 are indicated in bold. Similar to findings assessing the effects of genotype in relation to acute Hg exposure, almost all significant associations were restricted to subjects having homozygous Mut status for each of the four COMT SNPs. Notable, however, were two significant associations among subjects with WT status: one among boys with WT rs4818 (WRAVMA pegs dominant test in the Motor domain), showing impaired performance with chronic Hg exposure, and one among girls genotyped as WT rs6269 (Stroop color test in the Attention/Concentration domain) showing improved performance with increasing Hg exposure (value in bold italics). The majority of significant interaction terms, however, are defined by significant dose responses only among subjects with Mut status.
TABLE 5.
Chronic Hg Dose-Response Effects in Year 7 Among Boys and Girls by WT or Mut Allelic Status for COMT SNPs
| rs4680 |
rs4633 |
rs4818 |
rs6269 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | WT |
Mut |
WT |
Mut |
WT |
Mut |
WT |
Mut |
||||||||
| Measure | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) | Beta (SE) | |
| Boys | ||||||||||||||||
| Attention/Concentration | ||||||||||||||||
| Stroop Test: Color | −7.20 (3.94) | −.23 (.18) | −13.79 (6.23) | −.48 (.04) | ||||||||||||
| WAIS–III – Spatial Span | −1.24 (.97) | −.21 (.21) | −5.05 (1.95) | −.52 (.02) | −1.27 (.99) | −.22 (.21) | −4.58 (1.84) | −.53 (.02) | ||||||||
| Visual–Spatial | ||||||||||||||||
| WAIS–III – Digit Symbol | −6.74 (5.58) | −.20 (.24) | −32.46 (11.28) | −.56 (.01) | −6.76 (5.74) | −.20 (.25) | −35.71 (10.87) | −.64 (.005) | −7.96 (8.45) | −.21 (.36) | −17.92 (6.80) | −.44 (.01) | ||||
| WAIS–III – Symbol Search | −2.89 (2.28) | −.21 (.21) | −16.49 (5.52) | −.58 (.008) | −2.92 (2.30) | −.22 (.21) | −16.19 (5.53) | −.59 (.01) | −5.35 (3.11) | −.35 (.10) | −8.48 (3.83) | −.38 (.04) | ||||
| Motor | ||||||||||||||||
| WRAVMA –Pegs – Dominant | −6.15 (2.50) | −.36 (.02) | 3.72 (4.84) | .23 (.46) | ||||||||||||
| Girls | ||||||||||||||||
| Attention/Concentration | ||||||||||||||||
| Stroop Test: Color | 8.61 (2.70) | .54 (.004) | 6.60 (3.70) | .36 (.09) | ||||||||||||
| Adult Trails A | −3.06 (2.57) | −.18 (.24) | 9.24 (6.21) | .41 (.16) | −3.08 (2.73) | −.17 (.27) | 10.62 (6.46) | .44 (.13) | ||||||||
| Learning & Memory | ||||||||||||||||
| RAVLT – Trial 8: A post 20′ | −.58 (.61) | −.15 (.35) | −2.29 (1.06) | −.55 (.05) | ||||||||||||
Note. Values in bold signify p ≤ 0.05 in the direction of impaired performance with increasing Hg exposure. Values in bold italics signify p ≤ .05 in direction of improved performance with increasing Hg exposure.
Among boys, those with Mut versions of rs4680 or rs4633 showed significantly impaired performance of the same four tests that are observed in response to increasing acute Hg exposure. These included the Stroop color test and the WAIS Spatial Span test in the Attention/Concentration domain and the Digit-Symbol and Symbol Search tests in the domain of Visual-Spatial acuity. In addition, boys genotyped as having rs6269 Mut showed significantly impaired performance on the Digit Symbol and Symbol Search tests of Visual-Spatial acuity. In contrast, boys genotyped as rs4818 Mut displayed no significant adverse effects on any neurobehavioral test in response to chronic Hg exposure.
Among girls, those genotyped as rs4680 Mut displayed significantly impaired performance on the RAVLT Trial 8 test in the Learning-Memory domain. This association, however, was of only borderline significance (p = .05).
Analysis of the Potential Contribution of Other Genes to Hg Effects on Neurobehavioral Functions Among Subjects With COMT WT or Mut Allelic Status
Previous studies (Woods et al., 2012, 2013) reported that boys genotyped as having Het/Mut status with respect to CPOX4, MT1M, and/or MT2M demonstrated increased sensitivity to the adverse effects of Hg exposure on many of the same neurobehavioral tests as observed here among boys with Mut forms of COMT SNPs The possibility was therefore tested that Het/Mut variants of CPOX or MT might alter the observed dose-response effects of Hg observed on neurobehavioral performance among subjects with COMT Mut status. As shown in Table S1 (Supplementary Materials), inclusion of CPOX WT, CPOX Het/Mut, MT1M WT, MT1M Het/Mut, MT2A WT, and MT2A Het/Mut as covariates in the regression models had only modest effects when evaluated in relation to acute Hg exposure on neurobehavioral performance, with the principal effect being a decrease the significance of the interactions terms for two tests of Attention/Concentration (Stroop Color-Word, Digit Span) and one test of Learning & Memory (RAVLT Trial 7) among boys with Mut COMT rs4680, rs4633 and/or rs6269 status. In addition, girls genotyped as having Mut versions of COMT rs4680 or rs4633 had less significantly impaired Trails A and Trails B test results in response to acute Hg exposure when the allelic status of CPOX and MT genes was included in the regression model. Similar slight changes were observed when evaluating the effects of CPOX and MT allelic status on chronic Hg dose-response effects on neurobehavioral functions among boys and girls with or without COMT variants (Table S2, Supplementary Materials).
Haplotype Analysis
Haploview was used to create a graphical representation of linkage disequilibrium (LD) structure. As shown in Figure 1, the four COMT SNPs were found to be in strong LD with each other, forming a single haplotype block. Seven haplotypes were detected within this block (GCGGval, ATCAmet, ACCGval, GCCGval, GCCAmet, GTGGval, and GTCGval), having frequencies (SE) of 0.444 (0.032), 0.435 (0.032), 0.078 (0.018), 0.038 (0.013), 0.004 (0.004), 0.001 (0.001), and 0.001 (0.001), respectively. These haplotypes correspond to those previously identified (Diatchenko et al., 2005; Nackley et al., 2006) and are consistent with haplotypes associated with populations of European descent (Gabriel et al., 2002). The two haplotypes of highest frequency (GCGGval and ATCAmet) were evaluated with respect to modification of Hg effects on neurobehavioral test performance among boys and girls. Of note, COMT activity in cells transfected with COMT cDNA clones corresponding to GCGGval was 4.8-fold higher than that in cells transfected with clones corresponding to ACTAmet (Diatchenko et al., 2005). As shown in Table 6, we observed no modification of acute Hg effects on neurobehavioral test performance among either boys or girls genotyped as haplotype GCGGval-GCGGval (Group A). In contrast, among subjects genotyped as haplotype ATCAmet-ATCAmet (Group B), significant adverse effects were noted in response to acute Hg exposure within domains of Attention/Concentration and Learning & Memory among boys, and within domains of Attention/Concentration and Motor Function among girls. Similarly, when effects of haplotypes A and B were evaluated in relation to chronic Hg exposure (Table 7), no significant modification of chronic Hg effects were found on neurobehavioral test performance among either boys or girls genotyped as GCGGval-GCGGval (Group A), but significant impairment of tests of both Attention/Concentration and Visual-Spatial acuity among boys genotyped as ATCAmet-ATCAmet was noted. The Group B haplotype, therefore, appears to be associated with increased sensitivity to the adverse effects of both acute and chronic Hg exposure on neurobehavioral functions, principally among boys.
FIGURE 1.
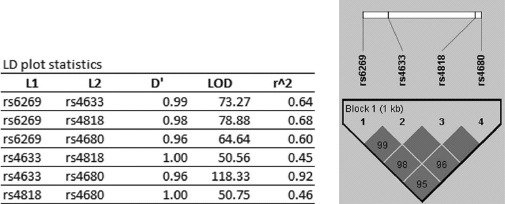
Haplotype block structure of the COMT gene in study subjects. Linkage disequilibrium (LD) plot statistics of rs6269, rs4633, rs4818, and 4680. L1 = Locus 1; L2 = Locus 2; D′ = D prime, a measure of pairwise LD; LOD (logarithm of odds) = LOD score; r2 = goodness of fit. Haplotype block structure is as depicted by Haploview. Values for D′ (×100) are shown in shaded cells; cells with D′ = 1.00 are shaded without values indicated.
TABLE 6.
Acute Hg Dose-Response Effects Among Boys and Girls With ACGG (Group A) or GTCA (Group B) COMT haplotype
| Haplotype |
||||
|---|---|---|---|---|
| GCGGval-GCGGval (Group A) |
ATCAmet-ATCAmet (Group B) |
|||
| Measure | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) |
| Boys | ||||
| Attention/Concentration | ||||
| Stroop Test: Color-Word | 4.62 (2.95) | .34 (.13) | −8.38 (3.25) | −.53 (.02) |
| WAIS–III Digit Span | −.92 (.77) | −.27 (.24) | −2.43 (1.01) | −.50 (.03) |
| Learning & Memory | ||||
| RAVLT–Trial 5: List A | .84 (.84) | .23 (.32) | −2.55 (1.16) | −.47 (.04) |
| RAVLT–Trial 6: List B | −.07 (.53) | −.03 (.90) | −1.48 (.70) | −.46 (.05) |
| RAVLT–Trial 8: A post 20′ | 1.03 (1.01) | .23 (.32) | −3.17 (1.51) | −.45 (.05) |
| RAVLT–Trials 1–5: Sum | −.28 (2.93) | −.02 (.92) | −11.79 (4.61) | −.53 (.02) |
| Girls | ||||
| Attention | ||||
| Trails A | 3.32 (3.47) | .21 (.36) | 6.96 (1.78) | .74 (.002) |
| Motor | ||||
| Finger Tapping–Non-Dominant Hand | −.27 (1.93) | −.03 (.89) | −4.18 (1.30) | −.66 (.007) |
| WRAVMA–Pegs–Dominant Hand | 1.84 (2.06) | .21 (.38) | −.60 (2.27) | −.07 (.80) |
Note. Values in bold signify p ≤ .05 with increasing Hg exposure.
TABLE 7.
Chronic Hg Dose-Response Effects Among Boys and Girls With ACGG (Group A) or GTCA (Group B) COMT Haplotype
| Haplotype |
||||
|---|---|---|---|---|
| GCGGval-GCGGval (Group A) |
ATCAmet-ATCAmet (Group B) |
|||
| Measure | Beta (SE) | rpart (p) | Beta (SE) | rpart (p) |
| Boys | ||||
| Attention/Concentration | ||||
| Stroop Test: Color | −3.87 (7.11) | −.16 (.60) | −13.08 (6.39) | −.47 (.06) |
| WAIS–III–Spatial Span | .56 (1.29) | .13 (.87) | −4.89 (1.84) | −.57 (.02) |
| Visual-Spatial | ||||
| WAIS-III–Digit Symbol | .80 (11.16) | .02 (.94) | −32.90 (10.25) | −.64 (.006) |
| WAIS-III–Symbol Search | −.30 (4.16) | −.02 (.94) | −16.70 (5.71) | −.60 (.01) |
Note. Values in bold signify p ≤ .05 with increasing Hg exposure.
Figures 2 and 3 demonstrate graphically the effects of Group B haplotype on representative tests of Learning & Memory (RAVLT Trials Sum 1–5) and Visual-Spatial acuity (WAIS-III Adult Digit Symbol) in relation to acute and chronic Hg exposure, respectively, among boys. These analyses demonstrate that for either test, Hg exposure accounted for less than 1% of performance variance among boys with Not Group B haplotype status, but for 12.6% (RAVLT Sum Trials 1–5) and 27.0% (Digit Symbol) of performance variance among boys with the Group B haplotype (based on regression r2).
FIGURE 2.
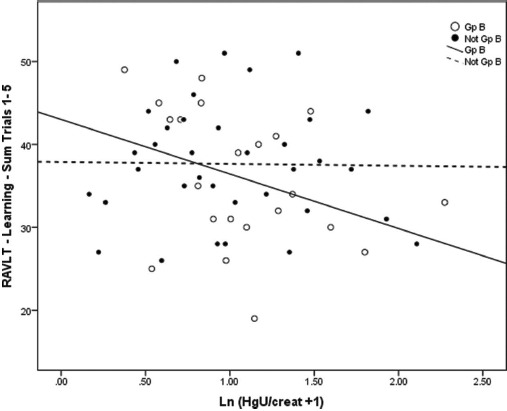
Associations between performance on the Rey Auditory Verbal Learning Test (RAVLT) Trials 1–5: Sum of Learning & Memory and acute Hg exposure among boys. Scatter plots and simple linear regression fit lines of RAVLT Trials 1–5: Sum test scores by acute Hg exposure (ln[HgU + 1]) are plotted to distinguish boys with the COMT Group B haplotype Mut (ATCAmet-ATCAmet) (open circles, solid line) versus those with Not Group B haplotype status (closed circles, dashed line). The linear slope r2 values for Group B and Not Group B are .126 and 2.562E-4, respectively (p < .02). Thus, while acute Hg exposure explains 12.6% of the performance variation among boys with the Group B haplotype, Hg explains virtually no variation among those with Not Group B status.
FIGURE 3.
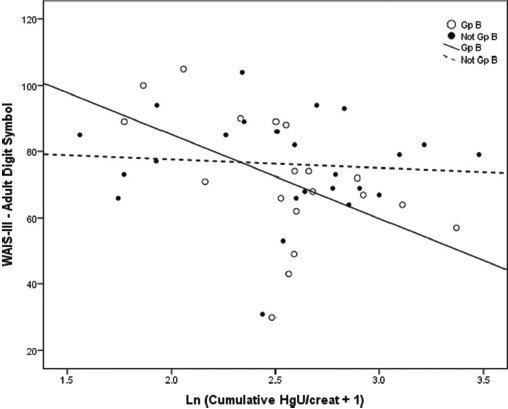
Associations between performance on the WAIS-III Digit Symbol test of Visual-Spatial acuity and chronic cumulative Hg exposure among boys. Scatter plots and simple linear regression fit lines of WAIS-III Digit Symbol test scores by chronic cumulative Hg exposure (ln[(ΣHgU) + 1]) are plotted to distinguish boys with the COMT Group B haplotype Mut (ATCAmet-ATCAmet) (open circles, solid line) versus those with Not Group B haplotype status (closed circles, dashed line). Linear slope r2 values for Group B and Not Group B are .270 and .007, respectively (p < .006). Thus, while chronic Hg exposure explains 27% of the performance variation among boys with the Group B haplotype, it explains less than 1% of variation among those with Not Group B status.
Summary of Findings
COMT rs4680 gene status, whether individually or in haplotype configuration, was found to modify the adverse effects of Hg exposure on a wide range of neurobehavioral test rests among boys. All of these effects were in the direction of impaired performance and spanned three of four neurobehavioral domains, including Attention/Concentration, Learning & Memory and Visual Spatial acuity. These highly consistent associations, affecting half of the tests evaluated, along with the absence of any significant interactions between Hg exposure and COMT wild-type status, are highly compelling and argue strongly against chance observations.
DISCUSSION
Recent studies (Woods et al., 2012, 2013) described polymorphisms of coproporphyrinogen oxidase (CPOX4 rs1131857) and metallothionein (MT1M rs2270837 and MT2A rs10636) that significantly modified the adverse effects of Hg exposure on a wide variety of neurobehavioral functions in children, principally boys. The present studies provide further evidence of genetic susceptibility to Hg toxicity in children in describing significant modification of Hg effects on multiple neurobehavioral functions among boys with the common rs4680 mutant variant of the COMT gene, as well as among those sharing a haplotype comprising the mutant allele of rs4680, that is, ATCAmet-ATCAmet, common to approximately 17% of subjects in the present study population. This report is the first to our knowledge to describe significant modification of the adverse effects of Hg exposure on multiple neurobehavioral functions by genetic variants of COMT in children and adolescents.
Comparable distributions of the three most common haplotypes (GCGGval, ATCAmet, and ACCGval) as defined by Nackley et al. (2006) were found in this population, although low numbers of subjects precluded our evaluation of ACCGval as a modifier of Hg effects on neurobehavioral performance. Analysis of Hg effects associated with haplotypes GCGGval and ATCAmet confirmed significantly increased susceptibility to Hg neurotoxicity among subjects having the ATCAmet-ATCAmet (Group B) haplotype, but not the GCGGval-GCGGval (Group A), consistent with effects associated with the Mut form of rs4680. Moreover, the effects of SNPs rs4633 and rs6269 (but not rs4818) were largely comparable to those of rs4680 when evaluated individually as modifiers of Hg effects on neurobehavioral functions. Because rs4633 and rs6269 are in high LD with rs4680 (Figure 1), these SNPs are likely to be highly statistically correlated with rs4680 and might therefore be considered as biomarkers of susceptibility to Hg toxicity rather than as directly causal in these effects. Together, these observations suggest that although the other SNPs comprising the ATCAmet-ATCAmet haplotype may affect the level of COMT expression, the rs4680 met158 met is predominantly involved in modification of the Hg effects on neurobehavioral functions. This might be expected if such effects occur as a consequence of Hg2+ binding with thiol constituents of the met enzyme, as suggested by the dose-response effects observed. Of note, no significant main effects of Hg, that is, effects in the absence of association with the met rs4680 allele, were found.
The mechanisms by which genetic variants of COMT modify behavior and/or exacerbate the adverse effects of Hg on neurobehavioral functions remain to be delineated. The adverse effects of COMT allelic variants, particularly the homozygous met158 variant of rs4680, on the association of neurobehavioral test performance and Hg exposure were observed primarily on tests of working memory, attentional control, and perceptual cognition—functions that are most predominantly associated with the prefrontal cortex, in which COMT-mediated enzymatic methylation is principally responsible for reduction in extracellular DA levels (Yavich et al., 2007). Because of this greater dependence of the prefrontal cortex on COMT to deactivate DA in comparison with other brain regions, the met allele of COMT exerts a predominant effect on the DA system of the prefrontal cortex (Diamond et al., 2004). Moreover, studies in humans and other species demonstrated that there is an inverted U-shaped function of optimal dopaminergic signaling (Goldman-Rakic, 1998), such that too little or too much DA results in diminished performance on cognitive tasks that are dependent on the prefrontal cortex (Farrell et al., 2012; Mattay et al., 2003). In this context, children who are homozygous for the COMT rs4680 met158 allele were found to perform better on prefrontal-dependent tasks than those homozygous for the val158 version (Diamond et al., 2004), suggesting a beneficial effect of increased DA associated with the slower deactivating met enzyme. This so-called “met advantage” can be disrupted by administration of chemicals or stressors that move met allele carriers over the apex of the inverted U-shaped function of optimal DA signaling (Barnett et al., 2009). As suggested earlier, preferential inhibition of the S-containing met form of COMT by Hg, as might be expected in light of the extraordinarily high thiol-binding affinity of Hg2+ (Dong et al., 2011), might produce comparable adverse effects, as observed here on tests of neurobehavioral function among met-met carriers in relation to Hg exposure. Of note, direct inhibition of COMT in rodent brain by Hg compounds in vitro was reported (Tsuzuki, 1981; Tunnicliff and Wood, 1973), although differential effects of Hg on expressed COMT genetic variants have not been described. In addition, although not included herein, few significant effects of Hg on neurobehavioral functions were found among subjects genotyped as heterozygous for rs4680 (val158 met), consistent with the view that susceptibility to Hg toxicity, at the level of exposure experienced by subjects in this study, is a function of the availability of the fully mutant (met158 met) form of COMT.
Alternatively, Hg might act independently of inhibition of COMT enzyme activity via effects on other components of the cortical dopaminergic system. Studies show that met COMT enhances the stability of working memory traces via dopamine D1 receptors in the dorsolateral prefrontal cortex and that local injection of selective D1 receptor antagonists impairs performance on tasks that are dependent on this region (Cohen et al., 2002). Hg may act similarly as a D1 receptor antagonist to mediate the effects observed. Of note, Coccini et al. (2011) demonstrated decreased D1 receptor density in rat cortex following prolonged perinatal exposure to Hg as MeHg, consistent with this view. While these observations provide scientific rationale for the diminished neurobehavioral performance observed here among boys with variants of COMT and Hg exposure, further studies are required to define the specific mechanisms underlying this association.
Notably, no substantial modification of Hg effects on neurobehavioral functions among subjects genotyped as COMT WT or Mut was found when controlling for CPOX4 and MT1M/MT2A allelic status in the analysis. These observations suggest that these other variants are not confounding the observed results and hence do not act in concert to modify the effects of Hg on neurobehavioral functions observed among subjects genotyped as COMT Mut. These findings are not altogether unexpected in the case of coproporphyrinogen oxidase, inasmuch as deficits in heme signaling associated with CPOX4 Het/Mut that are further exacerbated by Hg exposure are implicated in the impaired neurological functions observed (Li and Woods, 2010; Woods et al., 2012). It is noted also that among boys genotyped as CPOX4 Het/Mut, a substantially greater number of significantly impaired test results were observed in relation chronic as compared with acute Hg exposure, whereas among those genotyped as COMT Mut, substantially more impaired findings were seen among those with acute rather than chronic Hg exposure. These findings suggest a clear delineation of mechanisms underlying the adverse effects of Hg on neurobehavioral functions, by age and/or duration of Hg exposure, among subjects with CPOX4 Het/Mut versus those with COMT Mut status, respectively. Similarly, MT effects were found predominantly in relation to chronic Hg exposure, again delineated from those observed among subjects genotyped as COMT Mut. A comparable lack of substantial modification of Hg effects on neurobehavioral functions was found among subjects genotyped as CPOX4 Het/Mut when controlling for COMT and MT1M/MT2A allelic status, or among subjects genotyped as either MT1M/MT2A when controlling for CPOX and COMT allelic status in the analysis (data not presented). Overall, these findings do not support confounding associated with any of the genotypes evaluated, nor do they suggest a common pathway through which these variants act to modulate the adverse neurobehavioral effects observed. Further studies utilizing genome-wide genotyping and pathway enrichment analyses are planned to more fully define the genetic basis of Hg exposure risk and to identify core pathways and/or neural networks underlying this effect.
As in previous studies (Woods et al., 2012, 2013), notable differences were observed between boys and girls in the effects of Hg exposure and genetic variants of COMT on neurobehavioral test performance in this study. In this regard, clear gender differences in COMT activities and DA levels in the prefrontal cortex and other tissues have been described (Harrison and Tunbridge, 2011). Moreover, COMT is transcriptionally downregulated by estrogen (Xie et al., 1999), such that overall COMT activity in prefrontal cortex and other tissues is about 30% lower in females than in males (Chen et al., 2004). This diminished COMT activity translates to about 30% higher baseline DA levels in females than males. Diamond (2007) has suggested that this gender differential may correspond to females having near optimal baseline DA levels, that is, being close to the apex of the inverted U-shaped dopaminergic signaling curve, but males having somewhat too low baseline DA, such that male performance improves when DA levels are slightly increased, whereas female performance does not. In this context, having the met form of COMT, which elevates cortical DA levels, would benefit males but not females on DA-dependent tasks. Consistent with this suggestion, males genotyped as COMT met do, in fact, demonstrate improved performance on tasks dependent on the prefrontal cortex, whereas met females do not (Diamond, 2007). The present findings of impaired performance with Hg exposure among boys but not girls genotyped as met-met are also consistent with this suggestion, since under these circumstances, inhibition of COMT met enzyme activity by Hg should be detrimental to boys (by inhibiting the met enzyme associated with improved performance), but of no adverse consequence or even beneficial to girls (by lowering DA concentrations to more optimal levels). Further studies are required to confirm this possibility. The sexually divergent responses to Hg exposure and genetic disposition observed in the present study highlight the importance of considering such differences in development of strategies aimed at risk assessment and prevention, especially in children.
In conclusion, the present studies demonstrate significant adverse effects of a relatively common genetic variant of COMT rs4680, either individually or within haplotype configuration, on neurobehavioral functions associated with Hg exposure among children, principally boys. These findings extend previous observations describing altered genetic susceptibility to the adverse neurobehavioral effects of Hg exposure in children, and may have important public health implications for future strategies aimed at protecting children and adolescents from the potential health risks associated with Hg exposure.
FUNDING
Research reported in this publication was supported by grants (P42ES04696, P30ES07033, R21ES019632) to the University of Washington from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health. Additional funding was provided by the Wallace Research Foundation. We thank Jasmine Wilkerson, Functional Genomics Laboratory, University of Washington, for excellent technical assistance in the conduct of this study.
SUPPLEMENTAL DATA
Supplemental data for this article, Tables S1 and S2, can be accessed at DOI: 10.1080/15287394.2014.867210
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Barnett J. H., Heron J., Ring S. M., Golding J., Goldman D., Xu K., Jones P. B. Gender-specific effects of the catechol-O-methyltransferase Val108/158 Met polymorphism on cognitive function in children. Am. J. Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- Barnett J. H., Heron J., Goldman D., Jones P. B., Xu K. Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. Am. J. Psychiatry. 2009;166:909–916. doi: 10.1176/appi.ajp.2009.08081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J., Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bilder R. M., Volavka J., Malhotra A. K., Kennedy J. L., Ni X., Goldman R. S., Hoptman M. J., Sheitman B., Lindenmayer J. P., Citrome L., McEvoy J. P., Kunz M., Chakos M., Cooper T. B., Lieberman J. A. Neurocognitive correlates of the COMT val158met polymorphism in chronic schizophrenia. Biol. Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Chen J., Lipska B. K., Halim N., Ma Q. D., Matsumoto M., Melhem S., Kolachana B. S., Hyde T. M., Herman M. M., Apud J., Egan M. F., Kleinman J. E., Weinberger D. R. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccini T., Roda E., Castoldi A. F., Poli D., Goldoni M., Vettori M. V., Mutti A., Manzo L. Developmental exposure to methylmercury and 2,2#65533;,4,4#65533;5,5#65533;-hexachlorobiphenyl (PCB153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch. Toxicol. 2011;85:1281–1294. doi: 10.1007/s00204-011-0660-y. [DOI] [PubMed] [Google Scholar]
- Cohen J., Braver T., Brown J. Computational perspective on dopamine function in prefrontal cortex. Curr. Opin. Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Counter S. A., Buchanan L. H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- DeRouen T. A., Leroux B. G., Martin M. D., Townes B. D., Woods J. S., Leitão J., Castro-Caldas A., Braveman N. Issues in the design and analysis of a randomized clinical trial to assess the safety of dental amalgam restorations in children. Controlled Clin. Trials. 2002;23:301–320. doi: 10.1016/s0197-2456(01)00206-9. [DOI] [PubMed] [Google Scholar]
- DeRouen T. A., Martin M. D., Leroux B. G., Townes B. D., Woods J. S., Leitão J., Castro-Caldas A., Luis H., Bernardo M., Rosenbaum G., Martins I. P. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. J. Am. Med. Assoc. 2006;295:1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- Diamond A. Consequences of variations in genes that affect dopamine in prefrontal cortex. Cerebral Cortex. 2007;17:i161–i170. doi: 10.1093/cercor/bhm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Briand L., Fossella J., Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am. J. Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diatchenko L., Slade G. D., Nackley A. G., Bhalang K., Sigurdsson A., Belfer I., Goldman D., Xu K., Shabalina S. A., Shagin D., Max M. B., Makarov S. S., Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dong W., Bian Y., Liang L., Gu B. Binding constants of mercury and dissolved organic matter determined by a modified ion exchange technique. Environ. Sci. Technol. 2011;45:3576–3583. doi: 10.1021/es104207g. [DOI] [PubMed] [Google Scholar]
- Farrell S. M., Tunbridge E., Braeutigam S., Harrison P. J. COMT val158 met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol. Psychiatry. 2012;71:538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman E. M., Silbernagel S. M., Fenske R. A., Burbacher T. M., Ponce R. A. Mechanisms underlying children's susceptibility to environmental toxicants. Environ. Health Perspect. 2000;108((Suppl. 1)):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S. B., Schaffner S. F., Nguyen H., Moore J. M., Roy J., Blumenstiel B., Higgins J., DeFelice M., Lochner A., Faggart M., Lui-Cordero S. N., Rotimi C., Adeyemo A., Cooper R., Ward R., Lander E. A., Daly M. J., Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Garris P. A., Wrightman R. M. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. J. Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. The cortical dopamine system: Role in memory and cognition. Adv. Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Tunbridge E. M. Catechol-O-methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Heyer N. J., Echeverria D., Martin M. D., Farin F. M., Woods J. S. Catechol-O-methyltransferase (COMT) val158met functional polymorphism, dental mercury exposure, and self-reported symptoms and mood. J. Toxicol. Environ. Health A. 2009;72:599–609. doi: 10.1080/15287390802706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Papolos D. F., Saito T., Yu Y. M., Szumlanski C. L., Weinshilboum R. M. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–350. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Landrigan P. J., Goldman L. R. Children's vulnerability to toxic chemicals: A challenge and opportunity to strengthen health and environmental policy. Health Affairs. 2011;30:842–850. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- Li T., Woods J. S. Cloning, expression, and biochemical properties of CPOX4, a genetic variant of coproporphyrinogen oxidase that affects susceptibility to mercury toxicity in humans. Toxicol. Sci. 2009;109:228–236. doi: 10.1093/toxsci/kfp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri A., Goveia M., Balbus J., Parkin R. Children's susceptibility to chemicals: A review by developmental stage. J. Toxicol. Environ. Health B. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Malhotra A. K., Kestler L. J., Mazzanti C., Bates T., Goldberg T., Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am. J. Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Martins I. P., Castro-Caldas A., Townes B. D., Ferreira G., Rodrigues P., Marques S., Rosebaum G. Age and sex differences in neurobehavioral performance: A study of Portuguese elementary school children. Int. J. Neurosci. 2005;115:1687–1709. doi: 10.1080/00207450590958556. [DOI] [PubMed] [Google Scholar]
- Mattay V. S., Goldberg T. E., Fera F., Hariri A. R., Tessitore A., Egan M. F., Kolachana B., Callicott J.H., Weinberger D. R. CatecholO-methyltransferase val158met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N., Kidd K. K., Pakstis A.J., Speed W. C., Li H., Tarnok Z., Barta C., Kajuna S. L. B., Kidd J. R. The complex global pattern on genetic variation and linkage disequilibrium at catechol-O-methyltransferase. Mol. Psychiat. 2010;15:216–225. doi: 10.1038/mp.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley A. G., Shabalina S. A., Tchivileva I. E., Satterfield K., Korchynskyi O., Makarov S. S., Maixner W., Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Nackley A. G., Shabalina S. A., Lambert J. E., Conrad M. S., Gibson D. G., Spiridonov A. N., Satterfield S. K., Diatchenko L. Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: Evidence for marker SNPs. PloS One. 2009;4:e5237. doi: 10.1371/journal.pone.0005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. SNP linked to COMT SNPs via contig annotation. 2012. http://www.ncbi.nlm.nih.gov/snp/?term=rs4680, http://www.ncbi.nlm.nih.gov/snp/?term=rs4818, http://www.ncbi.nlm.nih.gov/snp/?term=rs4633, or http://www.ncbi.nlm.nih.gov/snp/?term=rs6269, respectively (accessed September 13, 2013).
- Pingree S. D., Simmonds P. L., Woods J. S. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol. Sci. 2001;61:224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- Sherman L. S., Blum J. D., Franzblau A., Basu N. New insight into biomarkers of human mercury exposures using naturally occurring mercury isotopes. Environ. Sci. Technol. 2013;47:3403–3409. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Stephens M., Smith N., Donnelly P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen J., Salminen M., Jalanko A., Ukkonen I., Ulmanen I. Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol. 1993;12:253–263. doi: 10.1089/dna.1993.12.253. [DOI] [PubMed] [Google Scholar]
- Tenhunen J., Salminen M., Lundstrom K., Kiviluoto T., Savolainen R., Ulmanen I. Genomic organization of the human catechol-O-methyltransferase gene and its expression from two distinct promoters. Eur. J. Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Townes B. D., Martins I. P., Castro-Caldas A., Rosenbaum G., DeRouen T. Repeat test scores on neurobehavioral measures over an eight-year period in a sample of Portuguese children. Int. J. Neurosci. 2008;118:79–93. doi: 10.1080/00207450601042102. [DOI] [PubMed] [Google Scholar]
- Tsuzuki Y. Effects of chronic methylmercury exposure on activities of neurotransmitter enzymes in rat cerebellum. Toxicol. Appl. Pharmacol. 1981;60:379–381. doi: 10.1016/0041-008x(91)90241-6. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G., Wood J. D. The inhibition of mouse brain neurotransmitter enzymes by mercury compounds and a comparison with the effects of hyperbaric oxygen. Comp. Gen. Pharmacol. 1973;4:101–105. [Google Scholar]
- Weinshilboum R. M., Otterness D. M., Szumlanski C. L. Methylation pharmacogenetics: CatecholO-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu. Rev. Pharmacol. Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Woods J. S., Heyer N. J., Echeverria D., Russo J. E., Martin M. D., Bernardo M. F., Luis H. S., Vaz L., Farin F. M. Modification of neurobehavioral effects of mercury by a genetic polymorphism of coproporphyrinogen oxidase in children. Neurotoxicol. Teratol. 2012;34:513–521. doi: 10.1016/j.ntt.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Heyer N. J., Russo J. E., Martin M. D., Pillai P. B., Farin F. M. Modification of neurobehavioral effects of mercury by genetic polymorphisms of metallothionein in children. Neurotoxicol. Teratol. 2013;39:36–44. doi: 10.1016/j.ntt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Martin M. D., Leroux B. G., DeRouen T. A., Leitão J. G., Bernardo M., Luis H. S., Simmonds P. L., Kushleika J. V., Huang Y. The contribution of dental amalgam to urinary mercury excretion in children. Environ. Health Perspect. 2007;115:527–1531. doi: 10.1289/ehp.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Ho S. L., Ramsden D. B. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 1999;56:3–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yavich L., Porsberg M. M., Karayiorgou M., Gogos J. A., Mannisto P. T. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


