Abstract
Sepsis is a major cause of morbidity and mortality in critically ill patients, and despite advances in management, mortality remains high. In survivors, sepsis increases the risk for the development of persistent acquired weakness syndromes affecting both the respiratory muscles and the limb muscles. This acquired weakness results in prolonged duration of mechanical ventilation, difficulty weaning, functional impairment, exercise limitation, and poor health-related quality of life. Abundant evidence indicates that sepsis induces a myopathy characterized by reductions in muscle force-generating capacity, atrophy (loss of muscle mass), and altered bioenergetics. Sepsis elicits derangements at multiple subcellular sites involved in excitation contraction coupling, such as decreasing membrane excitability, injuring sarcolemmal membranes, altering calcium homeostasis due to effects on the sarcoplasmic reticulum, and disrupting contractile protein interactions. Muscle wasting occurs later and results from increased proteolytic degradation as well as decreased protein synthesis. In addition, sepsis produces marked abnormalities in muscle mitochondrial functional capacity and when severe, these alterations correlate with increased death. The mechanisms leading to sepsis-induced changes in skeletal muscle are linked to excessive localized elaboration of proinflammatory cytokines, marked increases in free-radical generation, and activation of proteolytic pathways that are upstream of the proteasome including caspase and calpain. Emerging data suggest that targeted inhibition of these pathways may alter the evolution and progression of sepsis-induced myopathy and potentially reduce the occurrence of sepsis-mediated acquired weakness syndromes.
Keywords: sepsis, myopathy, critical illness, diaphragm, peripheral muscles, mitochondria, contractile proteins, ICU-acquired weakness
Acquired weakness syndromes are a major cause of mortality and long-term morbidity in critically ill patients but the true occurrence rate and prevalence of these syndromes are not known (1–5). The classic descriptions of these syndromes have largely been based on electrophysiologic testing and defined by the presence of either a primary neuropathic process or a primary myopathic process, but it is now apparent that many patients who develop clinically recognizable weakness as a result of critical illness manifest findings consistent with both a neuropathy and a myopathy (1–6). Furthermore, a recent study examined muscle biopsies from a limited number of patients who were persistently weak after recovery from acute respiratory distress syndrome (7). Interestingly, all muscle biopsies were markedly abnormal, findings which could not be predicted based on the electrophysiologic diagnosis. As such, it remains unclear how to best diagnose intensive care unit (ICU)-acquired weakness because significant physiologic changes can develop in muscle without detectable electrophysiologic abnormalities. If these alterations occur in acutely ill patients in the ICU, it is reasonable to postulate that the prevalence of ICU-acquired myopathy may be much higher than previously reported.
The major clinical findings in ICU-acquired weakness are limb muscle weakness and respiratory muscle weakness. Long-term consequences of peripheral muscle weakness include functional impairment, exercise limitation, and lower than normal health-related quality of life, sometimes persisting for years after discharge from the ICU (8–10). Importantly, the presence of respiratory muscle weakness results in prolonged duration of mechanical ventilation, difficulty weaning patients from the ventilator, and recurrence of respiratory failure after extubation (11, 12). Although successful weaning depends on a number of factors, it is ultimately the balance between the load imposed on the respiratory muscles caused by alterations in lung and chest wall mechanics (e.g., increased airway resistance, decreased compliance) and the ability of the ventilatory pump to support this load that allows resumption of spontaneous ventilation (13, 14). Therefore, successful weaning is critically dependent on the strength and endurance of the respiratory muscles. Studies suggest that diaphragm fatigue does not contribute to weaning failure (15). However, when the respiratory muscles are weak, as is frequently the case, successful weaning can only occur when respiratory muscle strength and endurance improve.
Clinical Assessment of Skeletal Muscle Strength in Critically Ill Patients
Early clinical detection of either peripheral and/or respiratory muscle weakness is difficult. The complexities in performing electrophysiologic testing include the requirement for neuromuscular specialists with expertise in the technique, variabilities in interpretation of results, and technical limitations due to treatment modalities that may interfere with accurate placement of electrodes, such as wound dressings, arterial catheters, or peripheral edema (4). In addition, for maximum reliability, testing requires patient cooperation. These factors contribute to the lack of routine use of these techniques particularly because the availability of neurologists with this expertise is limited. As a result, electrodiagnostic testing is usually requested after clinical recognition of weakness, which typically occurs late in the evolution of the disease.
Peripheral muscle involvement is usually recognized clinically when patients are recovering from the acute phase of illness, and upon awakening, are noted to be weak. The severity of peripheral muscle weakness is widely variable and can be profound in some cases, manifesting symptoms of paralysis (16). The most widely used clinical tool to assess peripheral muscle strength at the bedside is the Medical Research Council examination, which incorporates strength testing of three muscle groups in each limb with assignment of scores on a scale of 1 to 5. A Medical Research Council score of <48 is indicative of clinically significant weakness (11). Although it has been suggested that these measurements are reliable and reproducible, patients must be awake and fully cooperative. This restricts the utility of this tool in determining the presence of weakness, particularly early in the course of acute illness.
Similarly, recognition of acquired respiratory muscle weakness most often occurs clinically when patients are difficult to wean from mechanical ventilation. Bedside assessment of respiratory muscle strength in critically ill patients typically involves measurements of maximal inspiratory pressure generation and maximal expiratory pressure generation (17). However, in mechanically ventilated patients, measurements are often inaccurate because they are highly dependent both on the operator as well as the volitional effort of the patient. Even when standardized techniques are used by trained investigators, these measurements are unreliable (18). As a result, it is difficult, using these methods, to diagnose acquired respiratory muscle weakness early in the course of critical illness.
On the other hand, it is important to recognize that magnetic stimulation techniques are available to objectively measure respiratory muscle strength and peripheral muscle strength in critically ill mechanically ventilated patients (19). These techniques can be performed reproducibly without the limitation of an awake cooperative patient and also provide the opportunity to detect early reductions in muscle strength. Several studies have utilized anterolateral magnetic phrenic nerve stimulation to measure diaphragm pressure generation in response to supramaximal twitch stimulation (TwPdi) in mechanically ventilated patients in the ICU (15, 20). In patients who were thought to be ready for weaning from mechanical ventilation, Laghi et al found marked decrements in TwPdi values which averaged 8 to 10 cm H2O (15). Watson et al also measured TwPdi in 33 critically ill mechanically ventilated patients with a variety of diagnoses and duration of ventilation, and found values averaged 10.14 cm H2O (20). These data reveal diaphragm strength is profoundly reduced in critically ill patients, with values averaging between 23% and 36% of normal (20, 21). In addition, these studies reported a wide range in the severity of respiratory muscle weakness with some patients showing TwPdi values as low as 1 to 2 cm H2O (20). These nonvolitional objective measurements have also been used to assess peripheral muscle strength in a number of patient populations including ICU patients. Harris et al assessed adductor pollicus strength in a small group of critically ill patient and noted severe peripheral muscle weakness, with values as low as 30% of controls (22). Magnetic stimulation has also been used to objectively measure the strength of lower limb muscles (19, 23). Use of such techniques is therefore likely to provide clinically relevant information regarding the physiologic evolution of weakness in critically ill patients, particularly in the early stages of acute illness.
Electrodiagnostic testing, use of the Medical Research Council examination, and measures of maximal inspiratory pressure and maximal expiratory pressure generation all require an awake cooperative patient, whereas magnetic stimulation techniques provide objective measurements of strength independent of these confounding variables. Nonetheless, there has been a fair amount of interest in developing more simplified approaches to assess weakness in critically ill patients (24–26). Recently, it has been argued that, because the therapeutic armamentarium for ICU-acquired weakness is virtually nonexistent, it is not essential to make a precise physiologic diagnosis (24). On the other hand, it is important to realize that no study has systematically employed objective nonvolitional functional measurements of muscle strength to determine the prevalence or the time course of development of respiratory muscle dysfunction and/or limb muscle weakness in critically ill patients. Emerging data suggest that use of pharmacologic agents and other interventions may very well impact the occurrence and progression of ICU-acquired weakness syndromes, and potentially improve recovery. Use of a simplistic, less sophisticated approach in clinical trials, however, is unlikely to result in sufficiently scientifically rigorous outcome measurements that would provide clear evidence as to whether or not these agents or interventions improve strength or prevent wasting. Furthermore, the fact that our progress in management and treatment of these syndromes is in its infancy provides a strong rationale for an approach that systematically incorporates both the available scientific evidence and best diagnostic tools to critically address these issues, rather than retreating to simplistic tactics. Failure to use more sophisticated methods may very well impede advancements in understanding and development of effective treatments for critical care-acquired weakness.
Sepsis Is a Major Risk for Acquired Weakness in Critically Ill Patients
Although a number of factors have been associated with prolonged weakness in critically ill patients, sepsis (including septic shock, systemic inflammatory response syndrome of infectious and noninfectious etiologies, multiple organ failure) is a major risk (4, 27). Although Bolton et al (28) were the first to suggest that critical illness-acquired weakness syndromes were associated with sepsis, systemic inflammatory response syndrome, and multiple organ failure, additional prospective and retrospective clinical studies confirm this observation (29–31). Importantly, the reported frequency of prolonged weakness in sepsis is extremely high, occurring in 70% to 100% of these patients (29–31). Furthermore, these studies provide clear evidence that sepsis produces profound decrements in both limb and respiratory muscle function.
In the last decade, much attention has focused on studies showing that mechanical ventilation per se induces deleterious effects on the diaphragm (32–35). However, the clinical importance of ventilator-induced diaphragm dysfunction and its relationship to ICU-acquired weakness is still unclear. For example, ventilator-induced diaphragm dysfunction does not produce peripheral muscle weakness (36). Furthermore, the mode of ventilation is a major factor in producing ventilator-induced diaphragm dysfunction (37–39). Although Levine et al elegantly validated that diaphragm atrophy develops in brain dead patients who were mechanically ventilated using a controlled mode (32), this study did not include assessments of diaphragm strength. In addition, an international survey suggested that controlled mechanical ventilation is not commonly used in the ICU (40). Finally, if mechanical ventilation is the major cause of respiratory muscle weakness in critically ill patients, ventilatory support theoretically would prohibit successful weaning in any patient who requires mechanical ventilation because of persistent ongoing respiratory muscle injury. As such, more studies are required to address the importance of ventilator-induced diaphragm dysfunction in patients and its relationship to ICU-acquired weakness. In contrast, both animal and human data showed that sepsis-induced respiratory and limb muscle weakness is a major risk for development of ICU-acquired weakness.
Manifestations of Sepsis-Induced Myopathy
There is abundant scientific evidence that sepsis induces a myopathy characterized by reductions in force-generating capacity, atrophy (loss of muscle mass), and altered bioenergetics. A variety of animal models including cecal ligation perforation (CLP), endotoxin administration, live bacteria injection, pneumonia, and cytokine induction have been used to assess skeletal muscle function in sepsis. Interestingly, most functional assessments of skeletal muscle in sepsis have examined the respiratory muscles; fewer data exist for limb muscles. The following sections will review the manifestations of sepsis-induced changes in muscle.
Sepsis Reduces Skeletal Muscle-Specific Force Generation
It is important to understand that muscle strength is dependent on two factors: muscle force generation for a given amount of muscle mass and total muscle mass. These factors represent two distinct aspects of muscle function and can alter muscle strength independently of each other. This is a critical concept because the processes that regulate and modulate muscle force generation for a given amount of muscle mass (force per cross-sectional area) and the processes that regulate and modulate total muscle mass are different. Most of the studies described in this section evaluated physiologic function in muscle by assessing muscle-specific force generation, a measure of muscle strength where force is normalized to muscle cross- sectional area, thereby taking into account any changes in muscle mass. Furthermore, muscle wasting can occur without producing changes in muscle-specific force generation. For example, in a model of prolonged nutritional deprivation, Lewis et al showed that diaphragm mass was reduced by 50%, but diaphragm-specific force generation was normal (41). In addition, Le Bourdelles et al showed that after 48 hrs of mechanical ventilation, limb muscle mass is reduced significantly without concomitant reductions in limb muscle-specific force generation (36). Therefore, although force reductions and muscle wasting often occur simultaneously, it is important to understand that reductions in muscle-specific force generation may exist in the absence of atrophy and atrophy may occur without reducing muscle-specific force generation. Likewise, enhancing muscle mass does not necessarily improve muscle-specific force generation, as demonstrated in studies that assessed muscle contractile performance in myostatin knockout animals (42). Finally, experimental evidence suggests that pharmacologic interventions can differentially affect muscle-specific force generation and wasting (43, 44).
In one of the first studies evaluating respiratory muscle contractile performance in sepsis, Hussain et al showed that injection of Escherichia coli endotoxin in spontaneously breathing dogs induced hypercapneic respiratory failure (45). Subsequently, a number of investigators demonstrated that diaphragm contractile performance is profoundly reduced in several different animal models of sepsis and infection, including CLP, endotoxin administration and pneumonia (46–53) (Fig. 1). Declines in respiratory muscle strength occur within hours of induction of sepsis and are rapidly progressive. Importantly, these decrements in diaphragm-specific force generation (force normalized per muscle cross-sectional area) occur before any evidence of reductions in diaphragm mass or diaphragm protein content (54).
Figure 1.
Diaphragm force-frequency relationships in endotoxin-treated animals. Diaphragm force-frequency curves for control (◯) and low (●), medium (▴), and high (┎) dose endotoxin groups. As shown, endotoxin elicited a dose-dependent reduction in respiratory muscle-specific force generation. *Control group significantly different from the three endotoxin groups. Modified and reproduced with permission from Supinski et al (55).
Similar data indicate that sepsis also alters peripheral muscle-specific force generation. Supinski et al examined the effects of varying doses of endotoxin and observed >50% reductions in force-generating capacities of both the diaphragm and limb muscles (55) (Fig. 2). Recently, Eikermann et al examined force generation and fatiguability in the adductor pollicus muscle of critically ill septic patients, comparing these functional parameters in patients with normal individuals before and after cast immobilization (56). Importantly, 2 wks of lower arm immobilization had no effects on force generation in controls, but force generation was severely reduced in septic patients (Fig. 3).
Figure 2.
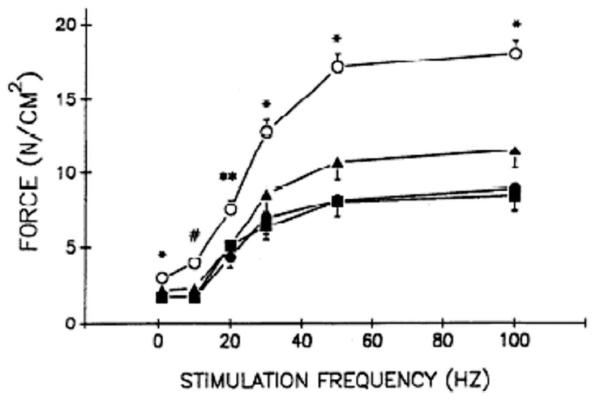
Limb muscle force-frequency relationships in endotoxin-treated animals. Limb muscle (flexor hallucis longus) force-frequency relationships for control (◯) and low (●), medium (▴), and high (■) dose endotoxin groups. Endotoxin reduced limb muscle-specific force generation in a dose-dependent fashion. *Control group significantly different from the three endotoxin groups; #control group significantly different from low and high endotoxin dose groups; **control group significantly different from low endotoxin dose group. Modified and reproduced with permission from Supinski et al (55).
Figure 3.
Force-frequency relationships in patients with sepsis and multiple organ failure. Force-frequency curves of adductor pollicis muscle during supramaximal ulnar nerve stimulation before performing the fatigue protocol (mean ± standard deviation). Force is significantly lower in patients with sepsis and multiple organ failure (full circles; n = 13) compared with healthy volunteers (triangles; n = 7) both before (open triangles) and after (solid triangles) 2 wks of immobilization of their lower arm and thumb. *p < .01 vs. volunteers before and after immobilization; #p < .05 vs. volunteers before immobilization. These data indicate that sepsis significantly reduced muscle force generation in peripheral muscle whereas immobilization had no effect. Reproduced with permission from Eikermann et al (56).
In contrast, pneumonia markedly impairs diaphragm force generation, but peripheral muscle force is preserved (51). Likewise, cardiac specific overexpression of tumor necrosis factor (TNF)-α reduces diaphragm strength without altering limb muscle function (57). Studies also indicate that sepsis induces more abundant cytokine levels in the diaphragm compared with limb muscle, raising the question as to whether or not the respiratory muscles are more susceptible to sepsis-induced injury (58). If this is true, it is conceivable that respiratory muscle weakness may represent the earliest manifestation of sepsis-induced myopathy.
Sepsis Reduces Skeletal Muscle Mass
The earliest important physiologic alteration in response to acute sepsis is a reduction in muscle-specific force-generating capacity. However, in more prolonged models of sepsis, muscle wasting is a prominent feature and is thought to result from increased proteolysis and decreased protein synthesis. A number of recent reviews have highlighted the current knowledge regarding the mechanisms underlying muscle atrophy in a variety of conditions including sepsis (59–63). For example, in the CLP model, data indicate that limb muscle protein content diminishes due to increased proteolysis (64). Rossignol et al also found reductions in limb muscle force generation and mass, using a prolonged model of CLP (65). Wasting also occurs in both respiratory and limb muscles with prolonged endotoxemia (66, 67) and in other models of protracted infection (68). Although experimental models of pneumonia have not completely addressed atrophy and protein loss, Langen et al described significant muscle wasting and impaired muscle regeneration in a transgenic mouse model of chronic pulmonary inflammation (69). These data, therefore, provide evidence that loss of muscle mass is also an important aspect of sepsis-induced muscle weakness.
Sepsis Alters Skeletal Muscle Bioenergetics
Alterations in mitochondrial bioenergetic function are thought to play a critical role in sepsis-induced organ failure and there are a number of recent reviews on this topic (70–75). Both human and animal data indicate that sepsis induces mitochondrial dysfunction in respiratory and limb muscles, and these alterations are thought to be important in the pathogenesis of sepsis-induced muscle dysfunction. Brealey et al demonstrated decreased respiratory chain complex I activity and lower adenosine triphosphate (ATP) levels in skeletal muscle biopsies taken from critically ill patients in septic shock (76). Furthermore, they showed a significant correlation between muscle mitochondrial dysfunction and death in sepsis. In follow-up studies, this group reported similar alterations in complex I activity and ATP levels in hind-limb muscle of rodents as well as significant decrements in bioenergetics in other tissues (77). Interestingly, they also demonstrated that, despite severe metabolic disturbances in muscle, gross histologic damage or evidence of cell death is absent; this underscores the importance of performing physiologic assessments when evaluating the effects of sepsis in muscle. Although bioenergetic function in sepsis is markedly abnormal, the extent to which these mitochondrial derangements participate in the development and progession of sepsis-induced prolonged ICU-acquired weakness, or in the recovery process, however, are yet to be determined.
Subcellular Sites of Skeletal Muscle Dysfunction in Sepsis
Sepsis-induced myopathy may produce muscle dysfunction by effecting a number of sites involved in excitation contraction coupling including the sarcolemma, the sarcoplasmic reticulum, the contractile apparatus, and the mitochondria.
Sarcolemma
Much attention has been given to the discovery that muscle becomes electrically inexcitable during the development of acquired weakness in critically ill patients (78–81). This has led to the concept that ICU-associated weakness represents an acquired channelopathy involving dysregulation of sodium channels. It is important to note, however, that much of these data with respect to critical illness myopathy has been generated in animal models using denervation with concomitant administration of high-dose steroids and should not be extrapolated to sepsis. However, Rossignol et al recently showed evidence of decreased muscle membrane excitability and up-regulation of Nav 1.5 in a chronic sepsis model, corroborating findings in the denervation steroid model (82). Additional studies will be needed to determine whether this process plays a central role in the pathogenesis of sepsis-induced myopathy.
On the other hand, there is clear evidence that sepsis causes marked sarcolemmal injury in muscles (83–85). Using a fluorescent tracer dye (procion orange) which does not enter cells with intact membranes, Lin et al found that diaphragm myofibers of both lipopolysaccharide treated and CLP animals demonstrated a markedly greater level of sarcolemmal damage than in control animals (83) (Fig. 4). In addition, sarcolemmal damage also increased in the soleus muscles of the CLP group. Furthermore, these alterations were linked to excessive nitric oxide generation and alterations in diaphragm myofiber membrane potential (83). Importantly, institution of mechanical ventilation in septic animals prevented sarcolemmal damage in the diaphragm (84), suggesting that mechanical ventilation under these conditions was not harmful but, in fact, protective.
Figure 4.
Sepsis induces skeletal muscle sarcolemmal injury. Representative micrographs illustrating the effects of sepsis on sarcolemmal integrity in the diaphragm. a, Control group. Very little tracer dye uptake within individual myofibers was found, although intense staining of extracellular connective tissue indicates good dye diffusion throughout the tissue. b, Lipopolysaccharide group. Note the presence of numerous myofibers with sarcolemmal damage, as indicated by their inability to prevent entry of the low molecular weight tracer dye. c, Cecal ligation perforation group. Myofibers with sarcolemmal damage are characterized by variable degrees of intracellular fluorescent staining, similar to the findings in the lipopolysaccharide group. Reproduced with permission from Lin et al (83).
Although it is not known if sepsis-induced sarcolemmal injury occurs in patients, this is nevertheless an important issue to consider. For example, the beneficial effect of exercise on skeletal muscle are well described; however, exercise increases cytokines and free-radical generation in muscle (86). In addition, Dumont et al recently showed that reloading muscles induces inflammatory cell infiltration and worsens sarcolemmal injury (87). As a result, it is conceivable that exercising patients with damaged muscles could potentially propagate muscle inflammation and injury, or delay recovery. Such issues should be carefully examined in the context of early mobilization and exercise interventions, and particularly in patients with sepsis-induced acquired weakness.
Sarcoplasmic Reticulum
The skeletal muscle ryanodine receptor (RyR1) is a highly redox-senstive ion channel, modulated by hydrogen peroxide and nitric oxide (NO) under physiologic conditions (88 –90). However, a number of studies suggest that resting intracellular calcium (Ca++) levels are increased in muscle in sepsis, and although most studies have examined this issue in cardiac muscle, there are data for skeletal muscle. For example, Benson et al showed that Ca++ uptake and content in limb skeletal muscle are increased during sepsis and that these high Ca++ concentrations are critically involved in regulation of muscle protein breakdown in sepsis (91). On the other hand, Liu investigated the in vivo effects of lipopolysaccharide on the Ca++-dependent mechanical activity and ryanodine response in isolated sarcoplasmic reticulum membrane vesicles from the mouse diaphragm, demonstrating that Ca++ release and [3H]ryanodine binding in sarcoplasmic reticulum membrane vesicles of lipopolysaccharide-treated animals were significantly depressed (92) (Fig. 5). Taken together, these data suggest that complex alterations in calcium homeostasis are present in septic skeletal muscle, with evidence that calcium increases in some subcellular compartments and decreases in others. Additional data will be needed to determine whether sarcoplasmic reticulum alterations are important in the pathogenesis of sepsis-induced myopathy and ICU-acquired weakness.
Figure 5.

Sepsis induces alterations in skeletal muscle sarcomplasmic reticulum calcium handling. Ca2+ release and [3H]ryanodine binding were studied using sarcoplasmic reticulum membrane vesicles isolated from skeletal muscles of control and lipopolysaccharide (LPS) (7.5 mg/kg)-treated animals. Some rats were pretreated with polymyxin B (PMB), the polycationic antibiotic that neutralizes LPS before the application of LPS. A) Ryanodine (2 μM)-induced Ca2+ release from sarcoplasmic reticulum was significantly reduced in muscles from LPS-treated animals compared with controls. B) [3H]ryanodine binding was also significantly reduced in animals treated with LPS. Pretreatment with polymyxin B abated LPS-induced changes in Ca2+ release and [3H]ryanodine binding. Data are presented as mean ± standard error of the mean. *p < .05 as compared with control; **p < .05 as compared with LPS group. Reproduced with permission from Liu et al (92).
Contractile Proteins
One mechanism by which sepsis reduces skeletal muscle force generation in sepsis is by directly altering contractile protein function. In support of this possibility, we have used permeabilized skinned fiber techniques to examine the force-pCa relationship in respiratory and limb muscles in sepsis (93). This powerful technique directly assesses the ability of the contractile proteins per se to generate force (mitochondria and the sarcoplasmic reticulum are absent in these preparations). We previously showed that incubation of contractile proteins with either superoxide-generating solutions, hydroxyl radical-generating solutions, or peroxynitrite rapidly leads to loss of contractile protein function, reducing force generation in single diaphragm muscle fibers (94, 95). Using similar techniques, we found that sepsis also significantly reduces diaphragm (Fig. 6) and limb muscle (Fig. 7) force-pCa relationships (93). Furthermore, administration of either a superoxide scavenger (i.e., PEG-SOD) or an inhibitor of NO synthesis (NG-nitro-L-arginine methyl ester) significantly attenuated endotoxin-induced reductions in muscle contractile protein force generation, providing evidence that free radicals are linked to sepsis-induced contractile protein dysfunction (96). In addition, TNF-α has been shown to decrease tetanic force due to changes in myofilament function (97, 98).
Figure 6.
Endotoxin alters contractile protein force generation in the diaphragm. Single diaphragm fibers were isolated from control and endotoxin-treated animals, followed by permeabilization with Triton × 100 to remove the sarcolemma, sarcoplasmic reticulum, and mitochondria. Fibers were mounted on a force transducer and exposed to a series of solutions containing increasing calcium concentrations. Force per cross-sectional area was determined and expressed in kPa. The pCa is negative log of the calcium concentration (i.e., as pCa decreases, Ca2+ concentrations increase). Mean data presenting averaged absolute (Abs) force vs. pCa relationship for diaphragmatic fibers from control (◯) and endotoxemic (●) animals. Error bars represent standard error of the mean. As shown, endotoxin significantly decreased contractile force generation in single diaphragm fibers. Reproduced with permission from Supinski et al (93).
Figure 7.
Endotoxin alters contractile protein force generation in limb muscle. Single fibers from the soleus and extensor digitorum longus were isolated from endotoxin-treated animals, permeabilized and force vs. pCa relationships determined. A) Mean data presenting averaged absolute force vs. pCa relationship for soleus fibers from control (◻) and endotoxin (■)-treated animals. B) Mean data presenting averaged absolute force vs. pCa relationship for extensor digitorum longus fibers from control (Δ) and endotoxin (▴)-treated animals. Endotoxin alters myofilament function in limb skeletal muscle. Reproduced with permission from Supinski et al (93).
Taken together, these observations provide substantial evidence that sepsis induces contractile dysfunction at the level of the myofilaments and that free radicals modulate these alterations. Importantly, these reductions in single fiber force generation correlate closely with the observed reductions in muscle-specific force generation in intact muscles (e.g., single fiber force generation and intact muscle force generation are reduced to the same degree), and therefore can explain much of the contractile dysfunction in sepsis. Alterations in myofilament function, therefore, represent a major site of involvement in septic myopathy.
Mitochondria
Sepsis is also associated with significant alterations in skeletal muscle mitochondrial function. We and others have demonstrated significant decrements in oxidative phosphorylation in the respiratory muscles in sepsis (99–101). For example, in animal studies, we showed that oxidative phosphorylation is impaired in diaphragm mitochondrial isolates with significant reductions in state 3 respiratory rates (99, 100) (Fig. 8). Similar alterations in oxidative phosphorylation have been reported in limb muscle and in human muscle samples from septic patients (102). We have also found that administration of either a free-radical scavenger (PEG-SOD) or a nitric oxide synthase inhibitor (L-NAME) prevented both endotoxin-mediated physiologic alterations in state 3 respiration as well as depletion and modification of selective mitochondrial proteins, implicating peroxynitrite (the reaction product of superoxide and NO) in the pathogenesis of sepsis-induced diaphragm mitochondrial dysfunction (100). In addition, sepsis up-regulates a mitochondrial-specific NO synthase (mtNOS) and inhibition of inducible mtNOS with melatonin restores mitochondrial function in sepsis (103–105). These data indicate that sepsis produces marked alterations in oxidative phosphorylation, reducing mitochondrial ATP-generating capacity and also provide evidence that free radicals mediate mitochondrial dysfunction in septic muscle.
Figure 8.
Sepsis produces decrements in oxidative phosphorylation in diaphragm mitochondria. Oxidative phosphorylation was assessed in diaphragm mitochondrial isolates from control and endotoxin-treated animals. In addition, to determine whether the defects in oxidative phosphorylation were due to changes at the level of the electron transport chain per se, the nicotinamide adenine dinucleotide (NADH) oxidase assay was also performed. A) State 3 oxygen consumption rates for diaphragm mitochondrial isolates taken from (left to right), control animals and groups of animals treated for 12, 24, 36, or 48 hrs with endotoxin. State 3 rates for 24 hrs, 36 hrs, and 48 hrs in endotoxin-treated groups were significantly lower than rates for control animals (*p < .02). Error bars indicate 1 standard error of the mean (SEM). B) Upper portion of the graph presents rates of NADH, reduced form, consumed per minute per milligram protein for diaphragm mitochondrial isolates taken from control (time zero) and (left to right) samples from animals treated with endotoxin for 12, 24, 36, or 48 hrs. The bottom portion of the graph represents state 3 oxygen consumption rates for diaphragm mitochondrial isolates taken from control and animals treated with endotoxin for 12, 24, 36, or 48 hrs (n = 4 for each group). Error bars represent 1 sem. NADH oxidase rates for groups treated with endotoxin for 36 or 48 hrs were significantly lower than rates for control animals (*p < .02 for both comparisons). The time course of reductions in NADH oxidase and state 3 respiraory rates paralleled each other, suggesting that defects in the electron transport chain per se account for most of the reduction in mitochondrial adenosine triphosphate-generating capacity. Reproduced with permission from Callahan and Supinski (99).
In addition to changes in oxidative phosphorylation, we have shown that sepsis induces selective depletion of several electron transport chain proteins in the respiratory muscles (99). The reductions in protein content parallel reductions in state 3 respiratory rates, and interestingly, many of these complex subunit proteins contain iron-sulfur centers, which are known to be susceptible to oxidative stress. Sepsis also reduces protein content and physiologic activity of the mitochondrial sarcomeric creatine kinase, the critical enzyme responsible for transmitochondrial ATP transport in skeletal and cardiac muscle. This enzyme is highly sensitive to oxidative stress (106). As such, decreases in sarcomeric mitochondrial creatine kinase activity would limit restoration of cytosolic ATP stores, limiting contractile performance. Sepsis also down-regulates gene expression of several diaphragm electron transport chain subunit proteins and significantly alters the activity of phosphofructokinase, the rate-limiting enzyme in glycolysis (107). These data provide evidence that sepsis can produce profound alterations in respiratory muscle mitochondrial function.
The reports of sepsis-induced mitochondrial alterations in limb muscle are variable. Whereas Porta et al reported no changes in skeletal muscle oxidative phosphorylation in prolonged endotoxemia (108), Trumbeckaite and Gellerich and colleagues showed significant reductions in complex I/III activity in the vastus lateralis of endotoxin-treated rabbits (109). Others have shown that, in the CLP model, sepsis decreases complex I activity and ATP levels in hind-limb muscle, mirroring previous findings reported in muscle biopsies from septic patients (77).
Studies from muscles in septic patients have provided a link between low ATP levels and subsequent death (76). Other human data reveal depleted muscle levels of reduced glutathione, impaired complex I activity, elevated nitrate/nitrite (indicator of reactive nitrogen species), increased electron paramagnetic resonance-detected radical species, and decreased complex I iron-sulfur centers (76, 110). Fredriksson et al showed that patients with sepsis have decreased mitochondrial content and lower concentrations of energy-rich phosphates in limb muscle biopsies (111). Recently, this group also reported loss of coordination of key elements of mitochondrial biogenesis and loss of muscle-specific genes accompanied by an oxidative stress response in muscle biopsies from septic patients (112, 113). These data indicate that sepsis elicits skeletal muscle mitochondrial dysfunction in patients and, importantly, the severity of derangements correlate with multiple organ failure and death. Whether or not these sepsis-induced mitochondrial abnormalities persist and are responsible for ICU-acquired weakness syndromes is not known.
Mechanisms Responsible for Skeletal Muscle Dysfunction in Sepsis
The mechanisms responsible for sepsis-mediated derangements in skeletal muscle function are complex, and many of these processes are likely to be more important at different times in the evolution of sepsis-induced myopathic changes. Processes that have been identified in playing crucial roles in modulating sepsis-induced muscle injury include localized elaboration of cytokines within skeletal muscle, excessive free-radical generation from a number of different sources, enhanced proteolytic degradation involving upstream activation of caspases and calpains, and decreased protein synthesis.
Role of Cytokines
Systemic inflammation is the major factor initiating organ dysfunction in sepsis. Elevated circulating levels of various cytokines and chemokines are present in patients with sepsis and the systemic inflammatory response syndrome (infectious and noninfectious), and the levels of this early cytokine response are predictive of organ failure and mortality. Although this primary cytokine surge is part of the normal immune response, these cytokines initiate secondary responses in organs that propagate tissue damage and dysfunction. There is sufficient evidence to support this scenario in skeletal muscle in sepsis. For example, human skeletal cell cultures constitutively express low levels of proinflammatory cytokines including interleukin-1 and interleukin-6 (113, 114) and exposure of these cells to exogenous cytokines further up-regulates and induces additional proinflammatory cytokines. Furthermore, Shindoh et al reported that TNF-α expression increases in the diaphragm muscle after in vivo administration of endotoxin (46). Others have reported up-regulation of proinflammatory cytokine messenger ribonucleic acid and protein levels in vivo and in vitro for both diaphragm and limb muscles in response to lipopolysaccharide, with exaggerated levels in the diaphragm, suggesting that the respiratory muscles may be predisposed to proinflammatory responses (58) (Fig. 9). These data provide evidence that the systemic cytokine response in sepsis results in local amplification of proinflammatory cytokines in muscle.
Figure 9.

Endotoxin up-regulates cytokine and chemokine protein levels in skeletal muscle. Quantification of cytokine/chemokine protein levels in the diaphragm and limb muscle after lipopolysaccharide administration in vivo. Protein levels of selected proinflammatory mediators (tumor necrosis factor [TNF]-α, interleukin [IL]-6, and maximal inspiratory pressure [MIP]-2) were measured in the diaphragm (filled bars) and tibialis anterior (open bars) at 6 hrs after sham (S) or lipopolysaccharide (L) treatment. All data are group mean ± standard error of the mean (n = 6 mice/group). *p < .05 for comparisons between diaphragm and tibialis within the same condition (lipopolysaccharide or saline); †p < .05 for comparisons between sham- and lipopolysaccharide-treated mice within the same type of muscle (diaphragm or tibialis). Reproduced with permission from Demoule et al (58).
In addition to these data, there is abundant experimental evidence linking the actions of proinflammatory cytokines to skeletal muscle weakness and wasting in vitro and in vivo (97, 115–117). For example, implantation of tumors producing TNF-α induces significant atrophy of the limb musculature (118). Acharyya et al have shown, in a murine model of colon cancer where interleukin-6 is over-expressed, that muscle wasting is prominent and there is selective down-regulation of specific myofibrillar proteins (119). Other studies have examined the effect of exposure of isolated muscles (115) or muscle cell lines to TNF-α or a combination of cytokines in vitro (98, 119–122) and found that in vitro exposure (over several hours) to TNF-α reduces muscle-specific force without changing muscle mass or protein levels in isolated skeletal muscle bundles, but prolonged exposure of cultured muscle cells to cytokines reduces both cell size and protein content (122).
Sepsis-Enhanced Free-Radical Generation in Muscle
For several decades, it has been known that free radicals modulate muscle contraction and that alterations in the cellular redox balance of muscle can produce decrements in muscle force generation. A number of outstanding reviews have highlighted the importance of free radicals in redox regulation and pathogenic dysregulation of skeletal muscle performance in a variety of conditions (86, 123–126). It is now clear that cytokines elicit excessive generation of free-radical species (including superoxide, NO, peroxynitrite, hydrogen peroxide, and hydroxyl radicals) in multiple organs in response to sepsis including skeletal muscle. There is overwhelming evidence that excessive free-radical generation plays a pivotal role in the induction of sepsis-induced myopathy.
Tissue markers of free radical-mediated protein and lipid modifications, such as malondialdehyde, 8-isoprostane, 4-hydroxynonenol, protein carbonyls, and nitrotyrosine (127–129), are increased in skeletal muscle in sepsis. A number of investigations have used two-dimensional electrophoresis, immunoblotting, and mass spectrometry to identify free-radical adduct formation in the diaphragm in response to sepsis and found modifications in multiple proteins involved in mitochondrial bioenergetic function and contractile function, identifying these proteins as intracellular targets of oxidative stress (127–129).
In addition to these tissue indices of oxidative stress, there is substantial evidence that free radicals modulate sepsis-induced skeletal muscle contractile dysfunction at several subcellular sites as outlined above. For example, sepsis-induced diaphragm sarcolemmal injury is mediated by excessive NO generation (83), as administration of a nitric oxide synthase (NOS) inhibitor prevented membrane damage. A number of reports indicate that administration of free-radical scavengers (such as polyethylene glycol adsorbed superoxide dismutase, catalase, or N-acetylcysteine) prevents declines in muscle-specific force generation in animal models of sepsis (47, 48, 52, 53). Furthermore, we have shown that administration of free-radical scavengers also ameliorates sepsis-induced reductions in myofilament function, a major mechanism by which muscle force generation is reduced in sepsis (96). Sepsis-induced mitochondrial derangements are also free radical mediated (130) as administration of agents that prevent peroxynitrite formation ablates sepsis-induced skeletal muscle mitochondrial derangements (100).
Data from both animal models and humans showed that sepsis increases proteolysis through the activation of the ubiquitin proteasomal degradation pathway (131, 132), but no studies have directly linked free-radical generation to activation of the ubiquitin proteasomal degradation pathway in sepsis. However, recent studies demonstrate that free radicals are likely to be involved in upstream activation of caspase and calpain proteolytic pathways, which have now been shown to be important modulators of muscle force generation and catabolism in sepsis. For instance, Jiang et al showed that hydrogen peroxide incubation of C2C12 cells results in caspase 3 activation (133). In addition, McClung et al have shown that exposure of C2C12 cells to low levels of H2O2 induces myotube atrophy and protein loss via activation of μ-calpain, providing a link between free radicals and calpain-induced muscle wasting (134). Recently, studies from our laboratory also provide a link between free radicals, calpain activation, and sepsis-induced respiratory muscle weakness (135).
There are multiple potential sources of free-radical generation in skeletal muscle and at least three of these sources are important in sepsis–the mitochondria; the sarcolemmal nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; and NOS. Increased skeletal muscle mitochondrial NOS (mtNOS) activity in sepsis has been linked to mitochondrial dysfunction (102, 136, 137). Others have shown that skeletal muscle mitochondria generate excessive superoxide/hydrogen peroxide in response to sepsis (101, 138). In addition to mitochondrial sources, Javesghani et al identified a constitutively expressed superoxide-generating nonphagocytic NADPH oxidase in the sarcolemma of the diaphragm, and showed that sepsis induces excessive NADPH oxidase-derived superoxide generation in the diaphragm (139). Furthermore, in vivo administration of an NADPH oxidase inhibitor, apocynin, ameliorated sepsis-induced reductions in diaphragm-specific force generation, providing additional evidence that activation of NADPH oxidase plays a role in sepsis-induced myopathy (140). Up-regulation of inducible NOS (iNOS) activity has also been linked to the early decrements in diaphragm-specific force generation, as several groups have shown that inhibition of iNOS partially ameliorates sepsis-induced deficits in diaphragm-specific force generation (83, 141). These data demonstrate that the mitochondria, the sarcolemmal skeletal muscle NADPH oxidase, and several isoforms of NOS (specifically iNOS and mtNOS) are important sources of free-radical species in skeletal muscle in sepsis.
There is a paucity of data related to regulation of endothelial NOS (eNOS) and neuronal NOS (nNOS) isoforms in septic muscle. In early sepsis, it seems that eNOS and nNOS do not contribute significantly to the alterations in contractile force generation (129). However, eNOS and nNOS can generate superoxide when the enzyme becomes uncoupled. It is not known if NOS uncoupling occurs in skeletal muscle or if these NOS isoforms are sources of excessive free-radical generation in sepsis. Interestingly, Capasso et al reported marked reductions in nNOS and eNOS content in muscle biopsies from patients who were diagnosed with critical illness myopathy, suggesting that dysregulation of these NOS isoforms may be important in the pathogenesis of ICU-acquired weakness (142). Furthermore, Kobayashi et al have recently shown that, in various myopathic models, an exaggerated postexercise fatigue response in skeletal muscle is due to lack of contraction-induced signaling from sarcolemma-localized nNOS, which is responsible for pathogenic disruption of postexercise vasodilatation (143). This raises the question as to whether or not eNOS- or nNOS-regulated processes are important in sepsis-induced myopathy or sepsis-induced-acquired critical care weakness; future investigations will be required to consider these possibilities.
Sepsis Increases Muscle Proteolysis
It is now widely recognized that enhanced proteolytic degradation occurs in many forms of muscle wasting including disuse, cancer, nutritional deprivation, fasting, uremia, immobilization, and sepsis. Numerous studies have shown that several components of the proteasome proteolytic degradation system (atrogin, other E-3 ligases, the 20S proteasome subunit, etc.) are up-regulated in skeletal muscle in both infected patients and in animal models of systemic inflammation (62, 131, 132, 144, 145). This led to the concept that infection or inflammation produces generalized skeletal muscle protein degradation and that reductions in skeletal muscle force generation can be explained entirely by proteasomal-mediated protein loss. However, the proteasome cannot degrade intact myofibrillar proteins. Furthermore, proteolysis in muscle requires an initial step that disrupts the contractile matrix due to activation of either calpain or caspase, or both, followed by a second step whereby disrupted proteins undergo ubiquitin conjugation followed by proteasomal-mediated degradation (63). A number of reviews provide further details regarding the role of the proteasome in muscle wasting (59, 60, 146). The following paragraphs, therefore, will focus on the involvement of caspase and calpain activation in sepsis, as studies indicate that activation of these upstream proteolytic processes are particularly important in sepsis-induced contractile dysfunction.
Sepsis Activates Caspase in Skeletal Muscle
Almost a decade ago, Hotchkiss et al provided evidence for caspase 3 activation in human tissues from patients with sepsis (147). An extensive literature now exists regarding the role of caspase 3-mediated apoptosis of lymphocytes in modulating the immune response in sepsis. Recently, Du and colleagues identified a role for caspase 3 activation in muscle wasting syndromes (148). It is important to realize that caspase 3 activation can occur through the intrinsic (mitochondrial) pathway, which involves activation of caspase 9 and subsequent release of cytochome c from mitochondria, with subsequent apoptosis. In addition, caspase 3 can be activated through extrinsic (nonmitochondrial) pathways, including the death receptor-linked caspase 8 pathway, and produce cellular damage without apoptosis (149–151).
To our knowledge, the only studies that have evaluated the role of caspase activation in the respiratory muscles in sepsis were performed in our laboratory (54, 152). We discovered that endotoxin administration markedly increased caspase 3 activation in the diaphragm (54) (Fig. 10). In addition, diaphragm-specific force generation fell progressively over time in response to endotoxin with 50% reductions in force after 24 hrs. Administration of a broad-spectrum caspase inhibitor, Z-Val-Ala-Asp(OCH3)-fluoromethylketone (zVAD-fmk), or a selective caspase 3 inhibitor, N-acetyl-Asp-Glu-Val-Asp-al (DEVD-CHO), ameliorated the effects of endotoxin-induced diaphragm weakness (Fig. 11). Importantly, caspase 3 activation preceded declines in diaphragm-specific force generation and reductions in diaphragm force generation occurred without decreasing diaphragm mass or protein content, indicating that contractile dysfunction precedes muscle wasting in sepsis. In follow-up studies, we found that activation of the extrinsic pathway via caspase 8 is the principal upstream pathway responsible for decrements in sepsis-induced contractile dysfunction in the diaphragm (152). These data show that activation of caspase 3 via the extrinsic pathway significantly contributes to the early contractile protein dysfunction in sepsis (54, 152).
Figure 10.
Sepsis activates caspase 3 in the diaphragm. A) Representative Western blot for procaspase 3 and active caspase 3 proteins, comparing diaphragm samples from control and endotoxin-treated (24 hrs) animals. Procaspase 3 bands were similar for samples from control and endotoxin-treated animals (top bands), but active caspase 3 protein (bottom band, top gel) was greater for samples from the endotoxin-treated group. Blotting against α-tubulin (bottom) with these samples was employed as a loading control. B) Mean densitometry data for procaspase 3 and active caspase 3 protein bands. Procaspase 3 levels were similar for samples from control and endotoxin (24 hrs)-treated animals, whereas active caspase 3 was significantly greater for samples from endotoxin-treated animals (p < .003). *Statistical difference compared with controls. LPS, lipopolysaccharide. Reproduced with permission from Supinski and Callahan (54).
Figure 11.
Inhibition of caspase 3 restores diaphragm-specific force generation in sepsis. Mean diaphragm force-frequency curves comparing control animals, endotoxin-treated animals (24 hrs), animals given both zVAD-fmk (a broad spectrum caspase inhibitor) and endotoxin, and animals given DEVD-CHO (a selective inhibitor of caspase 3) and endotoxin. DEVD-CHO was equivalent to zVAD-fmk in preventing endotoxin-induced reductions in diaphragm force (not significant for comparison of DEVD-CHO plus endotoxin and zVAD-fmk plus endotoxin group forces at all frequencies). *Statistical difference compared with controls. LPS, lipopolysaccharide. Reproduced with permission from Supinski and Callahan (54).
Whereas the above studies indicate that caspase activation via the extrinsic pathway is important in the genesis of sepsis-induced muscle weakness, it is also possible that activation of the intrinsic pathway may play some role in skeletal muscle dysfunction in sepsis. For example, a number of studies that have examined cardiac function in sepsis suggest that mitochondrially mediated apoptotic pathways are important in the genesis of cardiac contractile and mitochondrial dysfunction (153, 154). Because sepsis increases skeletal muscle mitochondrial free-radical generation and induces profound physiologic derangements in mitochondrial function, it is reasonable to postulate that the instrinsic pathway of caspase activation is also critically important in sepsis-induced skeletal muscle dysfunction. Future studies are needed to elucidate additional roles for caspase activation in sepsis-induced myopathy and prolonged ICU-acquired weakness.
Sepsis Activates Calpain in Skeletal Muscle
There is extensive evidence that calpain activation is involved in muscle atrophy including sepsis-induced muscle wasting (61). Calpains are calcium-dependent cysteine proteases and the two most characterized isoforms in skeletal muscle are μ-calpain and m-calpain. Although the regulation of calpain activity is complex, calcium is known to be the most important activator of calpain; however, calpain activity is also regulated by the endogenous inhibitor, calpastatin. In skeletal muscle, calpains participate in sarcomeric protein release by cleaving cytoskeletal elements, such as titin and nebulin, which anchor contractile elements. In support of the role of calpain activation in sepsis, Bhattacharyya et al showed that muscle calpain activity was increased in rats undergoing CLP (155). In addition, in limb muscle, sepsis increases gene expression of μ-calpain and m-calpain, disrupts Z bands, enhances release of myofilaments from myofibrillar proteins, and inhibits calpastatin activity (156, 157). These observations support a role for calpain activation in sepsis-induced muscle wasting.
On the other hand, despite the numerous reports of the effects of calpain activation on muscle wasting in sepsis, none of these studies have addressed the possibility that calpain activation might be important in mediating muscle contractile dysfunction in sepsis. To examine this critical issue, we recently performed studies and found that endotoxin significantly increased diaphragm calpain activity, protein levels of active μ-calpain and active m-calpain (the autocatalytic product of calpain activation), levels of calpain-specific talin degradation products, and reduced diaphragm-specific force generation (135). Importantly, administration of a calpain inhibitor prevented endotoxin-induced calpain activation and attenuated reductions in diaphragm specific force generation (Fig. 12). We also determined if free radicals participate in modulating calpain activation in the respiratory muscles in sepsis, and found that administration of a superoxide scavenger ablated endotoxin-induced calpain activation, providing a link between sepsis-induced free-radical generation, calpain activation, and reductions in specific force generation in skeletal muscle (135).
Figure 12.
Calpain inhibition improves diaphragm-specific force generation in endotoxin-induced sepsis. Mean diaphragm force-frequency curves comparing control animals, endotoxin-treated animals (24 hrs), animals given both endotoxin and calpain inhibitor III, and animals given calpain inhibitor III alone. Force generation was significantly lower at stimulation frequencies from 10 to 150 Hz for diaphragms from lipopolysaccharide (LPS)-treated animals (filled circles) than for control animals (open squares). Diaphragms from animals given both calpain inhibitor III and lipopolysaccharide generated forces significantly higher than diaphragms from animals given endotoxin alone for frequencies from 50 to 150 Hz. Force generation for muscles taken from animals given calpain inhibitor III alone were similar to levels for control animals. *Significant statistical difference between control and endotoxin; #statistical significance between endotoxin and endotoxin plus calpain inhibitor III groups. Reproduced with permission from Supinski and Callahan (135).
Although these data provide evidence for the role of calpain in sepsis-induced muscle weakness and wasting, there are several other aspects to consider. For example, traditionally it has been thought calpains are localized in the cytosolic compartment, but Badugu et al have recently reported that μ-calpain is also present in the mitochondrial intermembrane space (158). Whether or not mitochondrially localized μ-calpain participates in sepsis-induced mitochondrial dysfunction has not been examined. In addition, calpains are thought to be critical for localized remodeling of the cytoskeleton after plasma membrane damage and essential for muscle regeneration and growth (159, 160). Therefore, one must consider that, although calpain inhibition clearly retards sepsis-mediated reductions in muscle contractile function and loss of muscle mass, nonspecific calpain inhibition in the later stages of septic myopathy may be less effective and could theoretically impair recovery of muscle function. This highlights the importance for understanding of the detailed mechanisms underlying skeletal muscle weakness and atrophy in sepsis. Nevertheless, promising data are emerging indicating that use of more targeted calpain inhibitors in other myopathic syndromes may be highly effective, and future studies are needed to examine the utility of such agents in sepsis.
Sepsis Decreases Skeletal Muscle Protein Synthesis
Sepsis produces a net effect of loss in muscle mass, and as much of this has been attributed to enhanced proteolytic degradation, it is also important to note that sepsis clearly alters protein synthesis in skeletal muscle (161). For example, Vary et al have shown that protein synthesis is decreased by 50% in hind-limb muscle of septic animals (162). In addition, sepsis preferentially inhibits synthesis of both myofibrillar and sarcoplasmic proteins in fast muscle and, in severe sepsis, decreases overall muscle protein synthesis regardless of fiber type composition (162). The majority of the work that has examined alterations in muscle protein synthesis in sepsis has identified that the major point of control is at the level of translation initiation via effects which lower the amount of eIF4G bound to eIF4E (161). Studies by Vary et al identified a cytokine-dependent decrease in the steady-state phosphorylation of eIF4G during sepsis, with additional findings suggesting that interleukin-1β is the proinflammatory mediator responsible for these reductions (163). In addition, sepsis decreases mammalian target of rapamycin phosphorylation, and imparts leucine resistance in skeletal muscle by mechanisms that have not been clearly identified, but which seem to be independent of effects mediated by insulin and insulin-like growth factor (161). Despite the fact that much more attention has focused on the proteolytic aspects of sepsis-induced muscle wasting, these data provide important information regarding the complexity of this process. A more thorough understanding of the mechanisms involved in regulation of skeletal muscle protein synthesis in sepsis is essential, as this information is likely to provide significant insights into aspects related to muscle remodeling, particularly as it relates to recovery of muscle function after sepsis.
Implications and Future Directions
Sepsis (including septic shock, systemic inflammatory response syndrome of infectious and noninfectious etiologies, multiple organ failure) is a major cause of mortality and long-term morbidity in ICU patients. Sepsis induces a myopathy involving both the respiratory and limb muscles. Persistence of these sepsis-induced myopathic alterations likely contributes significantly to ICU-acquired weakness. In the past, our clinical approach to weakness and wasting has been to support these patients with adequate nutrition and physical therapy, hoping that this will result in functional recovery, but it is clear that this approach has not impacted the prevalence or outcome of patients who develop ICU-acquired weakness. Perhaps this is because we have not addressed the role of many other important factors.
Further basic science research is required to elucidate the pathologic sequences involved at the cellular level as well as their interactions and importance at different stages in the evolution of sepsis-induced contractile dysfunction, muscle wasting, and bioenergetic failure. Investigations into the mechanisms involved in recovery of muscle function after injury are also needed. This should provide key information for developing specific muscle-targeted and/or organelle-targeted pharmacologic therapies to prevent ICU-acquired muscle dysfunction and to expedite recovery. In addition, clinical trials should systematically incorporate the best scientific evidence and make use of the best diagnostic tools to critically address these issues in our patients. Finally, timing of therapeutic interventions will be crucial. By using such approaches, we will be poised to develop rational therapies for prevention and treatment of ICU-acquired myopathies.
Acknowledgments
The authors have received funding from the National Institutes of Health (HL 080609 and HL 069821 to LAC; HL 080429, HL 081525, and HL 063698 to GSS).
Footnotes
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.De Jonghe B, Lacherade JC, Durand MC, et al. Critical illness neuromuscular syndromes. Neurol Clin. 2008;26:507–520. doi: 10.1016/j.ncl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Batt J, Hopkins RO. The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin. 2008;24:179–199. doi: 10.1016/j.ccc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Hermans G, De Jonghe B, Bruyninckx F, et al. Clinical review: Critical illness polyneuropathy and myopathy. Crit Care. 2008;12:238. doi: 10.1186/cc7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan J, Harrison TB, Rich MM. Mechanisms of neuromuscular dysfunction in critical illness. Crit Care Clin. 2008;24:165–177. doi: 10.1016/j.ccc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latronico N, Guarneri B. Critical illness myopathy and neuropathy. Minerva Anestesiol. 2008;74:319–323. [PubMed] [Google Scholar]
- 6.Bolton CF. The discovery of critical illness polyneuropathy. Eur J Anaesthesiol Suppl. 2008;42:66–67. doi: 10.1017/S0265021508003530. [DOI] [PubMed] [Google Scholar]
- 7.Angel MJ, Bril V, Shannon P, et al. Neuromuscular function in survivors of the acute respiratory distress syndrome. Can J Neurol Sci. 2007;34:427–432. doi: 10.1017/s0317167100007307. [DOI] [PubMed] [Google Scholar]
- 8.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy DW, Eid MP, Sedrakyan A, et al. Quality of life in adult survivors of critical illness: A systematic review of the literature. Intensive Care Med. 2005;31:611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 12.Latronico N, Guarneri B, Alongi S, et al. Acute neuromuscular respiratory failure after ICU discharge. Report of five patients. Intensive Care Med. 1999;25:1302–1306. doi: 10.1007/s001340051062. [DOI] [PubMed] [Google Scholar]
- 13.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 14.MacIntyre NR, Epstein SK, Carson S, et al. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest. 2005;128:3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 15.Laghi F, Cattapan SE, Jubran A, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 16.Latronico N, Rasulo FA, Recupero D, et al. Acute quadriplegia with delayed onset and rapid recovery. Case report. J Neurosurg. 1998;88:769–772. doi: 10.3171/jns.1998.88.4.0769. [DOI] [PubMed] [Google Scholar]
- 17.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 18.Aldrich TK, Spiro P. Maximal inspiratory pressure: Does reproducibility indicate full effort? Thorax. 1995;50:40–43. doi: 10.1136/thx.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man WD, Moxham J, Polkey MI. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J. 2004;24:846–860. doi: 10.1183/09031936.04.00029004. [DOI] [PubMed] [Google Scholar]
- 20.Watson AC, Hughes PD, Louise Harris M, et al. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Polkey MI, Duguet A, Luo Y, et al. Anterior magnetic phrenic nerve stimulation: laboratory and clinical evaluation. Intensive Care Med. 2000;26:1065–1075. doi: 10.1007/s001340051319. [DOI] [PubMed] [Google Scholar]
- 22.Harris ML, Luo YM, Watson AC, et al. Adductor pollicis twitch tension assessed by magnetic stimulation of the ulnar nerve. Am J Respir Crit Care Med. 2000;162:240–245. doi: 10.1164/ajrccm.162.1.9902073. [DOI] [PubMed] [Google Scholar]
- 23.Swallow EB, Gosker HR, Ward KA, et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol. 2007;103:739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 24.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 25.Latronico N, Bertolini G, Guarneri B, et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: The Italian multi-centre CRIMYNE study. Crit Care. 2007;11:R11. doi: 10.1186/cc5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali NA, O'Brien JM, Jr., Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 27.Visser LH. Critical illness polyneuropathy and myopathy: Clinical features, risk factors and prognosis. Eur J Neurol. 2006;13:1203–1212. doi: 10.1111/j.1468-1331.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 28.Bolton CF, Gilbert JJ, Hahn AF, et al. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47:1223–1231. doi: 10.1136/jnnp.47.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan J, Harrison TB, Rich MM, et al. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology. 2006;67:1421–1425. doi: 10.1212/01.wnl.0000239826.63523.8e. [DOI] [PubMed] [Google Scholar]
- 30.Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–184. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- 31.Tennila A, Salmi T, Pettila V, et al. Early signs of critical illness polyneuropathy in ICU patients with systemic inflammatory response syndrome or sepsis. Intensive Care Med. 2000;26:1360–1363. doi: 10.1007/s001340000586. [DOI] [PubMed] [Google Scholar]
- 32.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 33.McClung JM, Kavazis AN, DeRuisseau KC, et al. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175:150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 35.Powers SK, Shanely RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 36.Le Bourdelles G, Viires N, Boczkowski J, et al. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994;149:1539–1544. doi: 10.1164/ajrccm.149.6.8004310. [DOI] [PubMed] [Google Scholar]
- 37.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 38.Gayan-Ramirez G, Testelmans D, Maes K, et al. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33:2804–2809. doi: 10.1097/01.ccm.0000191250.32988.a3. [DOI] [PubMed] [Google Scholar]
- 39.Futier E, Constantin JM, Combaret L, et al. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care. 2008;12:R116. doi: 10.1186/cc7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–1458. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 41.Lewis MI, Lorusso TJ, Zhan WZ, et al. Interactive effects of denervation and malnutrition on diaphragm structure and function. J Appl Physiol. 1996;81:2165–2172. doi: 10.1152/jappl.1996.81.5.2165. [DOI] [PubMed] [Google Scholar]
- 42.Mendias CL, Marcin JE, Calerdon DR, et al. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matuszczak Y, Arbogast S, Reid MB. Allopurinol mitigates muscle contractile dysfunction caused by hindlimb unloading in mice. Aviat Space Environ Med. 2004;75:581–588. [PubMed] [Google Scholar]
- 44.Whidden MA, McClung JM, Falk DJ, et al. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol. 2009;106:385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussain SN, Simkus G, Roussos C. Respiratory muscle fatigue: A cause of ventilatory failure in septic shock. J Appl Physiol. 1985;58:2033–2040. doi: 10.1152/jappl.1985.58.6.2033. [DOI] [PubMed] [Google Scholar]
- 46.Shindoh C, Hida W, Ohkawara Y, et al. TNF-alpha mRNA expression in diaphragm muscle after endotoxin administration. Am J Respir Crit Care Med. 1995;152:1690–1696. doi: 10.1164/ajrccm.152.5.7582314. [DOI] [PubMed] [Google Scholar]
- 47.Shindoh C, Dimarco A, Nethery D, et al. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis. 1992;145:1350–1354. doi: 10.1164/ajrccm/145.6.1350. [DOI] [PubMed] [Google Scholar]
- 48.Supinski G, Nethery D, DiMarco A. Effect of free radical scavengers on endotoxin-induced respiratory muscle dysfunction. Am Rev Respir Dis. 1993;148:1318–1324. doi: 10.1164/ajrccm/148.5.1318. [DOI] [PubMed] [Google Scholar]
- 49.Boczkowski J, Dureuil B, Branger C, et al. Effects of sepsis on diaphragmatic function in rats. Am Rev Respir Dis. 1988;138:260–265. doi: 10.1164/ajrccm/138.2.260. [DOI] [PubMed] [Google Scholar]
- 50.Nin N, Cassina A, Boggia J, et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–2278. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 51.Divangahi M, Matecki S, Dudley RW, et al. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med. 2004;169:679–686. doi: 10.1164/rccm.200307-949OC. [DOI] [PubMed] [Google Scholar]
- 52.Fujimura N, Sumita S, Aimono M, et al. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med. 2000;162:2159–2165. doi: 10.1164/ajrccm.162.6.9912144. [DOI] [PubMed] [Google Scholar]
- 53.Fujimura N, Sumita S, Narimatsu E. Alteration in diaphragmatic contractility during septic peritonitis in rats: Effect of polyethylene glycol-absorbed superoxide dismutase. Crit Care Med. 2000;28:2406–2414. doi: 10.1097/00003246-200007000-00036. [DOI] [PubMed] [Google Scholar]
- 54.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol. 2006;100:1770–1777. doi: 10.1152/japplphysiol.01288.2005. [DOI] [PubMed] [Google Scholar]
- 55.Supinski G, Nethery D, Stofan D, et al. Comparison of the effects of endotoxin on limb, respiratory, and cardiac muscles. J Appl Physiol. 1996;81:1370–1378. doi: 10.1152/jappl.1996.81.3.1370. [DOI] [PubMed] [Google Scholar]
- 56.Eikermann M, Koch G, Gerwig M, et al. Muscle force and fatigue in patients with sepsis and multiorgan failure. Intensive Care Med. 2006;32:251–259. doi: 10.1007/s00134-005-0029-x. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–1696. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 58.Demoule A, Divangahi M, Yahiaoui L, et al. Endotoxin triggers nuclear factor-kappaB-dependent up-regulation of multiple proinflammatory genes in the diaphragm. Am J Respir Crit Care Med. 2006;174:646–653. doi: 10.1164/rccm.200509-1511OC. [DOI] [PubMed] [Google Scholar]
- 59.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 60.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 61.Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab. 2008;295:E762–E771. doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasselgren PO, Menconi MJ, Fareed MU, et al. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–2168. doi: 10.1016/j.biocel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 64.Tiao G, Fagan JM, Samuels N, et al. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest. 1994;94:2255–2264. doi: 10.1172/JCI117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossignol B, Gueret G, Pennec JP, et al. Effects of chronic sepsis on contractile properties of fast twitch muscle in an experimental model of critical illness neuromyopathy in the rat. Crit Care Med. 2008;36:1855–1863. doi: 10.1097/CCM.0b013e318176106b. [DOI] [PubMed] [Google Scholar]
- 66.Choo JJ, Horan MA, Little RA, et al. Muscle wasting associated with endotoxemia in the rat: Modification by the beta 2-adrenoceptor agonist clenbuterol. Biosci Rep. 1989;9:615–621. doi: 10.1007/BF01119805. [DOI] [PubMed] [Google Scholar]
- 67.Chai J, Wu Y, Sheng ZZ. Role of ubiquitin-proteasome pathway in skeletal muscle wasting in rats with endotoxemia. Crit Care Med. 2003;31:1802–1807. doi: 10.1097/01.CCM.0000069728.49939.E4. [DOI] [PubMed] [Google Scholar]
- 68.Drew JS, Farkas GA, Pearson RD, et al. Effects of a chronic wasting infection on skeletal muscle size and contractile properties. J Appl Physiol. 1988;64:460–465. doi: 10.1152/jappl.1988.64.1.460. [DOI] [PubMed] [Google Scholar]
- 69.Langen RC, Schols AM, Kelders MC, et al. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol. 2006;35:689–696. doi: 10.1165/rcmb.2006-0103OC. [DOI] [PubMed] [Google Scholar]
- 70.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: A primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 71.Rudiger A, Stotz M, Singer M. Cellular processes in sepsis. Swiss Med Wkly. 2008;138:629–634. doi: 10.4414/smw.2008.12319. [DOI] [PubMed] [Google Scholar]
- 72.Singer M. Cellular dysfunction in sepsis. Clin Chest Med. 2008;29:655–660. doi: 10.1016/j.ccm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Singer M. Mitochondrial function in sepsis: Acute phase versus multiple organ failure. Crit Care Med. 2007;35:S441–S448. doi: 10.1097/01.CCM.0000278049.48333.78. [DOI] [PubMed] [Google Scholar]
- 74.Protti A, Singer M. Strategies to modulate cellular energetic metabolism during sepsis. Novartis Found Symp. 2007;280:7–16. [PubMed] [Google Scholar]
- 75.Brealey D, Singer M. Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep. 2003;5:365–371. doi: 10.1007/s11908-003-0015-9. [DOI] [PubMed] [Google Scholar]
- 76.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dys-function and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 77.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–R497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 78.Rich MM, Teener JW, Raps EC, et al. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46:731–736. doi: 10.1212/wnl.46.3.731. [DOI] [PubMed] [Google Scholar]
- 79.Teener JW, Rich MM. Dysregulation of sodium channel gating in critical illness myopathy. J Muscle Res Cell Motil. 2006;27:291–296. doi: 10.1007/s10974-006-9074-5. [DOI] [PubMed] [Google Scholar]
- 80.Trojaborg W, Weimer LH, Hays AP. Electrophysiologic studies in critical illness associated weakness: Myopathy or neuropathy—A reappraisal. Clin Neurophysiol. 2001;112:1586–1593. doi: 10.1016/s1388-2457(01)00572-7. [DOI] [PubMed] [Google Scholar]
- 81.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: The electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–1514. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- 82.Rossignol B, Gueret G, Pennec JP, et al. Effects of chronic sepsis on the voltage-gated sodium channel in isolated rat muscle fibers. Crit Care Med. 2007;35:351–357. doi: 10.1097/01.CCM.0000254335.88023.0E. [DOI] [PubMed] [Google Scholar]
- 83.Lin MC, Ebihara S, El Dwairi Q, et al. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med. 1998;158:1656–1663. doi: 10.1164/ajrccm.158.5.9803112. [DOI] [PubMed] [Google Scholar]
- 84.Ebihara S, Hussain SN, Danialou G, et al. Mechanical ventilation protects against diaphragm injury in sepsis: Interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165:221–228. doi: 10.1164/ajrccm.165.2.2108041. [DOI] [PubMed] [Google Scholar]
- 85.Comtois AS, Barreiro E, Huang PL, et al. Lipopolysaccharide-induced diaphragmatic contractile dysfunction and sarcolemmal injury in mice lacking the neuronal nitric oxide synthase. Am J Respir Crit Care Med. 2001;163:977–982. doi: 10.1164/ajrccm.163.4.9912057. [DOI] [PubMed] [Google Scholar]
- 86.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dumont N, Bouchard P, Frenette J. Neutrophil-induced skeletal muscle damage: A calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1831–R1838. doi: 10.1152/ajpregu.90318.2008. [DOI] [PubMed] [Google Scholar]
- 88.Sun J, Xu L, Eu JP, et al. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 89.Aghdasi B, Zhang JZ, Wu Y, et al. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J Biol Chem. 1997;272:3739–3748. doi: 10.1074/jbc.272.6.3739. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton SL, Reid MB. RyR1 modulation by oxidation and calmodulin. Antioxid Redox Signal. 2000;2:41–45. doi: 10.1089/ars.2000.2.1-41. [DOI] [PubMed] [Google Scholar]
- 91.Benson DW, Hasselgren PO, Hiyama DT, et al. Effect of sepsis on calcium uptake and content in skeletal muscle and regulation in vitro by calcium of total and myofibrillar protein breakdown in control and septic muscle: Results from a preliminary study. Surgery. 1989;106:87–93. [PubMed] [Google Scholar]
- 92.Liu SH, Lai JL, Yang RS, et al. Nitric oxide is not involved in the endotoxemia-induced alterations in Ca2+ and ryanodine responses in mouse diaphragms. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:327–334. doi: 10.1007/s00210-002-0597-z. [DOI] [PubMed] [Google Scholar]
- 93.Supinski G, Nethery D, Nosek TM, et al. Endotoxin administration alters the force vs. pCa relationship of skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2000;278:R891–R896. doi: 10.1152/ajpregu.2000.278.4.R891. [DOI] [PubMed] [Google Scholar]
- 94.Supinski G, Stofan D, Callahan LA, et al. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol. 1999;87:783–791. doi: 10.1152/jappl.1999.87.2.783. [DOI] [PubMed] [Google Scholar]
- 95.Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- 96.Callahan LA, Nethery D, Stofan D, et al. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24:210–217. doi: 10.1165/ajrcmb.24.2.4075. [DOI] [PubMed] [Google Scholar]
- 97.Hardin BJ, Campbell KS, Smith JD, et al. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694–699. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- 98.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 99.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dys-function and protein depletion. Am J Respir Crit Care Med. 2005;172:861–868. doi: 10.1164/rccm.200410-1344OC. [DOI] [PubMed] [Google Scholar]
- 100.Callahan LA, Stofan DA, Szweda LI, et al. Free radicals alter maximal diaphragmatic mitochondrial oxygen consumption in en dotoxin-induced sepsis. Free Radic Biol Med. 2001;30:129–138. doi: 10.1016/s0891-5849(00)00454-8. [DOI] [PubMed] [Google Scholar]
- 101.Boczkowski J, Lisdero CL, Lanone S, et al. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999;13:1637–1646. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- 102.Boveris A, Alvarez S, Navarro A. The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic Biol Med. 2002;33:1186–1193. doi: 10.1016/s0891-5849(02)01009-2. [DOI] [PubMed] [Google Scholar]
- 103.Lopez LC, Escames G, Ortiz F, et al. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol Lett. 2006;27:623–630. [PubMed] [Google Scholar]
- 104.Escames G, Lopez LC, Tapias V, et al. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res. 2006;40:71–78. doi: 10.1111/j.1600-079X.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 105.Lopez LC, Escames G, Tapias V, et al. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol. 2006;38:267–278. doi: 10.1016/j.biocel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 106.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J Appl Physiol. 2007;102:44–53. doi: 10.1152/japplphysiol.01204.2005. [DOI] [PubMed] [Google Scholar]
- 107.Callahan LA, Supinski GS. Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol. 2005;99:1120–1126. doi: 10.1152/japplphysiol.01157.2004. [DOI] [PubMed] [Google Scholar]
- 108.Porta F, Takala J, Weikert C, et al. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit Care. 2006;10:R118. doi: 10.1186/cc5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trumbeckaite S, Opalka JR, Neuhof C, et al. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem. 2001;268:1422–1429. doi: 10.1046/j.1432-1327.2001.02012.x. [DOI] [PubMed] [Google Scholar]
- 110.Svistunenko DA, Davies N, Brealey D, et al. Mitochondrial dysfunction in patients with severe sepsis: An EPR interrogation of individual respiratory chain components. Biochim Biophys Acta. 2006;1757:262–272. doi: 10.1016/j.bbabio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Fredriksson K, Hammarqvist F, Strigard K, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–E1050. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 112.Fredriksson K, Tjader I, Keller P, et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE. 2008;3:e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bartoccioni E, Michaelis D, Hohlfeld R. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. Immunol Lett. 1994;42:135–138. doi: 10.1016/0165-2478(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 114.Nagaraju K, Raben N, Merritt G, et al. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998;113:407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilcox PG, Wakai Y, Walley KR, et al. Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med. 1994;150:1368–1373. doi: 10.1164/ajrccm.150.5.7952566. [DOI] [PubMed] [Google Scholar]
- 116.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 117.Reid MB, Li YP. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol Scand. 2001;171:225–232. doi: 10.1046/j.1365-201x.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 118.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 119.Acharyya S, Ladner KJ, Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li YP, Reid MB. Effect of tumor necrosis factor-alpha on skeletal muscle metabolism. Curr Opin Rheumatol. 2001;13:483–487. doi: 10.1097/00002281-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 122.Li YP, Schwartz RJ, Waddell ID, et al. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 123.Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33:371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 124.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem. 1998;179:99–110. doi: 10.1023/a:1006859920875. [DOI] [PubMed] [Google Scholar]
- 126.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- 127.Hussain SN, Matar G, Barreiro E, et al. Modifications of proteins by 4-hydroxy-2-nonenal in the ventilatory muscles of rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L996–L1003. doi: 10.1152/ajplung.00337.2005. [DOI] [PubMed] [Google Scholar]
- 128.Barreiro E, Gea J, Di Falco M, et al. Protein carbonyl formation in the diaphragm. Am J Respir Cell Mol Biol. 2005;32:9–17. doi: 10.1165/rcmb.2004-0021OC. [DOI] [PubMed] [Google Scholar]
- 129.Barreiro E, Comtois AS, Gea J, et al. Protein tyrosine nitration in the ventilatory muscles: Role of nitric oxide synthases. Am J Respir Cell Mol Biol. 2002;26:438–446. doi: 10.1165/ajrcmb.26.4.4634. [DOI] [PubMed] [Google Scholar]
- 130.Boczkowski J, Lisdero CL, Lanone S, et al. Peroxynitrite-mediated mitochondrial dysfunction. Biol Signals Recept. 2001;10:66–80. doi: 10.1159/000046876. [DOI] [PubMed] [Google Scholar]
- 131.Rabuel C, Renaud E, Brealey D, et al. Human septic myopathy: Induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology. 2004;101:583–590. doi: 10.1097/00000542-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 132.Klaude M, Fredriksson K, Tjader I, et al. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci (Lond) 2007;112:499–506. doi: 10.1042/CS20060265. [DOI] [PubMed] [Google Scholar]
- 133.Jiang B, Xiao W, Shi Y, et al. Role of Smac/DIABLO in hydrogen peroxide-induced apoptosis in C2C12 myogenic cells. Free Radic Biol Med. 2005;39:658–667. doi: 10.1016/j.freeradbiomed.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 134.McClung JM, Judge A, Talbert EE, et al. Calpain-1 is required for hydrogen peroxide induced myotube atrophy. Am J Physiol Cell Physiol. 2009;296:C363–C371. doi: 10.1152/ajpcell.00497.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Supinski GS, Callahan LA. Calpain activation conributes to endotoxin induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2009 Mar 27; doi: 10.1165/rcmb.2008-0275OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carreras MC, Franco MC, Finocchietto PV, et al. The biological significance of mtNOS modulation. Front Biosci. 2007;12:1041–1048. doi: 10.2741/2124. [DOI] [PubMed] [Google Scholar]
- 137.Alvarez S, Boveris A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Radic Biol Med. 2004;37:1472–1478. doi: 10.1016/j.freeradbiomed.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 138.Supinski GS, Callahan LA. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am J Respir Crit Care Med. 2006;173:1240–1247. doi: 10.1164/rccm.200410-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Javesghani D, Magder SA, Barreiro E, et al. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 140.Supinski G, Stofan D, Nethery D, et al. Apocynin improves diaphragmatic function after endotoxin administration. J Appl Physiol. 1999;87:776–782. doi: 10.1152/jappl.1999.87.2.776. [DOI] [PubMed] [Google Scholar]
- 141.Sambe A, Ungureanu-Longrois D, Danialou G, et al. Role of nitric oxide on diaphragmatic contractile failure in Escherichia coli endotoxemic rats. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:167–175. doi: 10.1016/s1095-6433(97)00422-4. [DOI] [PubMed] [Google Scholar]
- 142.Capasso M, Di Muzio A, Pandolfi A, et al. Possible role for nitric oxide dysregulation in critical illness myopathy. Muscle Nerve. 2008;37:196–202. doi: 10.1002/mus.20907. [DOI] [PubMed] [Google Scholar]
- 143.Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 145.Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007;13:1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. [DOI] [PubMed] [Google Scholar]
- 146.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 147.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 148.Du J, Wang X, Miereles C, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maelfait J, Beyaert R. Non-apoptotic functions of caspase-8. Biochem Pharmacol. 2008;76:1365–1373. doi: 10.1016/j.bcp.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 150.Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: The physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lamkanfi M, Festjens N, Declercq W, et al. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 152.Supinski GS, Ji X, Wang W, et al. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol. 2007;102:1649–1657. doi: 10.1152/japplphysiol.00377.2006. [DOI] [PubMed] [Google Scholar]
- 153.Hassoun SM, Marechal X, Montaigne D, et al. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med. 2008;36:2590–2596. doi: 10.1097/CCM.0b013e3181844276. [DOI] [PubMed] [Google Scholar]
- 154.Larche J, Lancel S, Hassoun SM, et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol. 2006;48:377–385. doi: 10.1016/j.jacc.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 155.Bhattacharyya J, Thompson K, Sayeed MM. Calcium-dependent and calcium-independent protease activities in skeletal muscle during sepsis. Circ Shock. 1991;35:117–122. [PubMed] [Google Scholar]
- 156.Wei W, Fareed MU, Evenson A, et al. Sepsis stimulates calpain activity in skeletal muscle by decreasing calpastatin activity but does not activate caspase-3. Am J Physiol Regul Integr Comp Physiol. 2005;288:R580–R590. doi: 10.1152/ajpregu.00341.2004. [DOI] [PubMed] [Google Scholar]
- 157.Williams AB, Decourten-Myers GM, Fischer JE, et al. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J. 1999;13:1435–1443. doi: 10.1096/fasebj.13.11.1435. [DOI] [PubMed] [Google Scholar]
- 158.Badugu R, Garcia M, Bondada V, et al. N terminus of calpain 1 is a mitochondrial targeting sequence. J Biol Chem. 2008;283:3409–3417. doi: 10.1074/jbc.M706851200. [DOI] [PubMed] [Google Scholar]
- 159.Mellgren RL, Zhang W, Miyake K, et al. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 160.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 161.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 162.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol. 1992;262:C1513–C1519. doi: 10.1152/ajpcell.1992.262.6.C1513. [DOI] [PubMed] [Google Scholar]
- 163.Vary TC, Deiter G, Lang CH. Cytokine-triggered decreases in levels of phosphory-lated eukaryotic initiation factor 4G in skeletal muscle during sepsis. Shock. 2006;26:631–636. doi: 10.1097/01.shk.0000230299.78515.2c. [DOI] [PubMed] [Google Scholar]