Abstract
To enhance silencing and avoid off-target effects, siRNAs are often designed with an intentional bias to ensure that the end of the siRNA that contains the guide strand 5′ end is less stably hybridized relative to the end containing the passenger strand 5′ end. One means by which this is accomplished is to introduce a terminal mismatch, typically by changing the passenger strand sequence to impair its hybridization with the guide strand 5′ end. However, there are conflicting reports about the influence of terminal mismatches on the silencing efficacy of siRNAs. Here, the silencing efficiency of siRNAs with a terminal mismatch generated either by altering the guide strand (at the 5′ end, nucleotide 1) or the passenger strand (nucleotide 19 from the 5′ end) was examined. Subsequently, we studied the relationship between the silencing efficiency of the siRNAs and their binding to the RNA-induced silencing complex loading complex proteins HIV transactivating response RNA-binding protein and Dicer in H1299 cytoplasmic extracts. Binding of siRNA and the transactivating response RNA-binding protein was significantly reduced by terminal mismatches, which largely agrees with the reduction in eventual silencing efficacy of the siRNAs. Single terminal mismatches led to a small increase in Dicer binding, as expected, but this did not lead to an improvement in silencing activity. These results demonstrate that introduction of mismatches to control siRNA asymmetry may not always improve target silencing, and that care should be taken when designing siRNAs using this technique.
Keywords: Dicer, mismatches, RNA interference, short interfering RNA, TRBP
Introduction
Short interfering RNAs (siRNAs) can be designed to target and regulate the expression of any gene of interest. Gene silencing by RNA interference (RNAi) is mediated by endogenous proteins, resulting in target mRNA cleavage or translational inhibition [1]. In the cytoplasm of human cells, the dsRNA binding proteins HIV transactivating response RNA-binding protein (TRBP) and Dicer recognize and bind the siRNA and form RNA-induced silencing complex (RISC) loading complexes (RLCs) [2–4]. Argonaute 2 (Ago2), the catalytic core of the RISC [5,6], is then recruited by the RLC to form a holo-RISC [7]. Although other proteins such as protein activator of protein kinase R (PACT) might also be associated with the formation of holo-RISCs [8–12], in vitro experiments have shown that TRBP, Dicer and Ago2 alone are capable of forming an active minimal RLC [13].
Being double-stranded, either strand of the siRNA can be used as the guide strand of an active RISC. Loading of both strands results in reduced silencing efficiency due to competition for RISC components, and has the potential to result in off-target silencing [14]. Functional siRNAs and miRNAs have been shown to have greater asymmetries in their terminal hybridization stabilities compared to non-functional siRNAs [15–17]. In Drosophila, the protein R2D2 binds to the more stable end of the siRNA duplex and directs binding of Dicer-2 to the other, less stable, end, and hence the guide strand is selected through interaction of its 5′ end with Dicer-2 [18,19]. While the functions of the human proteins have not been firmly defined, it has been suggested that TRBP, a homolog of R2D2, senses siRNA asymmetry [4]. To ensure maximal specific silencing of the intended target, loading of the appropriate guide strand into the RISC is critical. Improved understanding of the interactions of siRNAs with TRBP and Dicer will enable improved design of siRNA therapeutics.
In current applications, siRNAs are typically designed with an intentional bias, to maximize preferential selection of the appropriate guide strand, by making its 5′ end less stably hybridized than the other end [17,20]. An end can be destabilized by introducing mismatches, wobble base pairs, or increasing the A–U content [17]. Some studies using siRNAs with a terminal mismatch showed improved activity [21,22], but not in all cases [26,27]. Typically, these studies used siRNAs that were initially found to be thermodynamically symmetric, with asymmetry subsequently induced by the mismatch. However, simultaneously changing sequence, structure and asymmetry potentially disguises the impacts of multiple variables.
Thus, in this study, we investigated the effects of introducing a terminal mismatch to siRNAs with preexisting thermodynamic asymmetry. In this way, the effects of structure and sequence changes were separated from changes in asymmetry. We found that a terminal mismatch at the 5′ end of the known guide strand, which enhances the natural bias of the siRNA, has an adverse impact on its binding to TRBP and generally reduces its silencing activity. Unlike terminal mismatches, internal mismatches enhanced siRNA binding by both Dicer and TRBP. These results highlight the importance of siRNA structure in the interactions with RNAi pathway proteins, and provide guidance for the design of highly active siRNAs.
Results and discussion
Design of siRNAs and EGFP silencing efficiency
Designing siRNAs with an intentional bias in hybridization stability is intended to maximize correct guide strand selection and loading into the RISC. This is beneficial both in achieving strong silencing and also minimizing off-target silencing by the passenger strand [45]. Thus the relative thermodynamic stability of the ends of the siRNA is an important design criterion for highly active siRNAs. Directing selection of the guide strand by chemical modifications has proven effective [25]. However, asymmetry is typically achieved by modification of either the passenger strand or the guide strand to generate a mismatch at the 5′ end of the guide strand [22,26]. Asymmetric siRNAs generated by introducing a terminal mismatch to an initially symmetric siRNA were found to be more active than the symmetric siRNA (Table S1) [22]. However, our goal was to test whether introducing a mismatch to an already asymmetric siRNA would also improve the silencing efficiency of the siRNA.
We tested an siRNA targeting position 396 of the enhanced green fluorescent protein (EGFP) mRNA (Table S2) [27]. Using mfold [28,29], we calculated the terminal stabilities of the siRNA (Table 1). For this siRNA, the known antisense strand 5′ end is located at the end that is predicted to be relatively thermodynamically unstable, as expected for correct loading into the RISC. Using this sequence as a basis, siRNAs with mismatches were generated by changing either the first nucleotide of the guide strand, 396-AG, 396-UG and 396-GG, or the 19th nucleotide of the passenger strand, 396-CA, 396-CU and 396-CC (changed nucleotides are shown in bold; Table S2). The predicted free energies confirmed that the mismatches show increased asymmetry relative to the fully paired duplex (Table 1), which should enhance the likelihood for correct guide strand incorporation into the RISC.
Table 1.
Difference in siRNA end stabilities. The passenger strand 5′ end ΔG values are −9.8 and −9.3 kcal·mol−1 for all the variations of duplexes 396 and 306, respectively. Stability at each end was calculated using mfold [29,30], by summing the contributions of the first four nearest neighbors and the overhang sequence.
| Sequence | Guide strand 5′ end ΔG (kcal·mol−1) |
Difference in end stability ΔΔG (kcal·mol−1) |
|---|---|---|
| 396 | −8.7 | 1.1 |
| 396-AG | −6.9 | 2.9 |
| 396-UG | −7.7 | 2.1 |
| 396-GG | −6.9 | 2.9 |
| 396-CA | −6.9 | 2.9 |
| 396-CU | −6.9 | 2.9 |
| 396-CC | −6.9 | 2.9 |
| 306 | −7.1 | 2.8 |
| 306-CC | −5.4 | 4.5 |
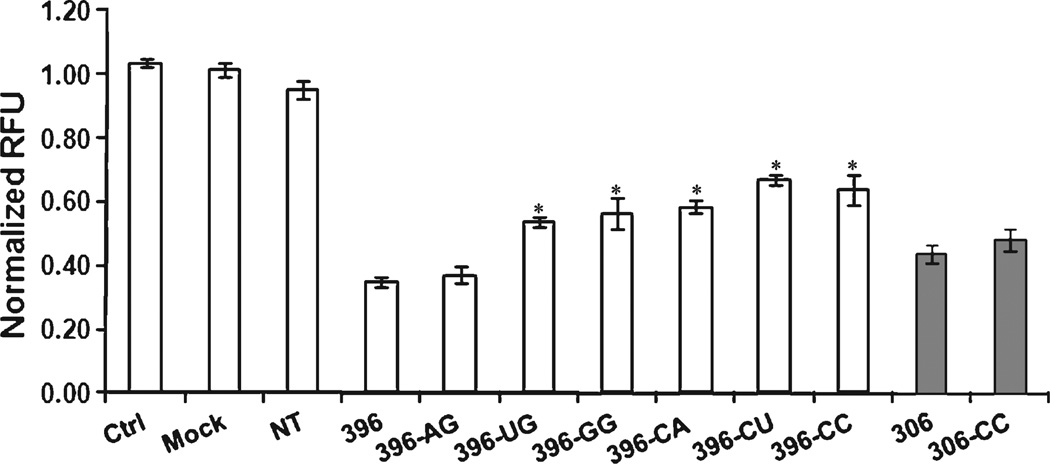
These siRNAs were then used to silence EGFP in H1299 cells constitutively expressing EGFP [30]. The silencing efficacy of the mismatched siRNAs was reduced, with the exception of 396-AG (Fig. 1). To confirm that this effect was not limited to sequence 396, silencing by siRNA 306 (targeting position 306) and a corresponding mismatched sequence, 306-CC, was tested. Introducing a mismatch that increased the natural asymmetry of the duplex (Table 1) did not increase the silencing activity of the siRNA (Fig. 1). Our results agree with those of previous studies in which introduction of terminal mismatches did not necessarily improve siRNA activity (Table S3) [24,26]. For selected siRNAs, we also examined the dose dependence of silencing, to ensure that the differences among the siRNAs that we observed at 10 nm were within the dose-responsive concentration range (Fig. S1).
Fig. 1.
Effect of guide strand 5′ end mismatch on silencing efficacy of siRNAs. EGFP-expressing H1299 cells were transfected with either an siRNA targeting the EGFP mRNA or a non-targeting (NT) siRNA at a final concentration of 10 nm. Fluorescence was measured 24 h after transfection. The mean and standard deviation are shown for each condition. Asterisks indicate that the two-tailed t test comparison of silencing efficacy of the siRNAs with guide strand 5′ end mismatches versus siRNA 396 was significant at P < 0.05. ‘Control’ and ‘mock’ refer to untreated cells and cells treated with the transfection reagent alone, respectively. White bars indicate siRNAs based on siRNA 396 and gray bars indicate siRNAs based on siRNA 306.
Effect of TRBP or Dicer knockdown on the silencing efficacy of mismatched siRNAs
We hypothesized that the reduction in the function of the mismatched siRNAs was a consequence of impaired interactions with TRBP and / or Dicer. While both proteins are part of the RLC and holo-RISC and are necessary for optimum silencing, RNAi-induced target silencing has been demonstrated in the absence of either Dicer [31–33] or TRBP [4]. Further, unlike the Drosophila RNAi pathway, in which R2D2 binding is a necessary precursor for Dicer-2 binding [18], Dicer by itself can bind siRNAs in humans [34,35].
To study the effect of these two proteins on the functionality of the siRNAs with and without a terminal mismatch, we knocked down either TRBP or Dicer protein in H1299 cells (Fig. S2). After knockdown of TRBP, silencing of EGFP by the fully paired duplex was reduced from more than 65% to less than 37%, with only one mismatched sequence being statistically significantly affected (396-UG, from 46% to 34%) (Fig. 2A). Notably, even with TRBP knocked down, siRNA 396-AG maintained essentially the same silencing capacity as in the presence of TRBP, actually becoming the most active of all the siRNAs under these conditions (Fig. 2A). In contrast, after knockdown of Dicer, only the silencing efficacy of 396-AG was significantly reduced (from 63% to 47%), making it significantly worse than that of the fully paired duplex in this case (Fig. 2B). The functionality of 396, together with all of the other mismatched sequences, was relatively unaffected by the reduction of Dicer protein (Fig. 2B).
Fig. 2.
Effect of TRBP or Dicer knockdown on the silencing efficacy of the EGFP targeting siRNAs. EGFP-expressing H1299 cells were co-transfected with EGFP-targeting siRNAs and either a non-targeting (NT) siRNA (white bars), a TRBP-targeting siRNA (A, gray bars) or Dicer-targeting siRNA (B, black bars). Total final siRNA concentrations were 20 nm (10 nm per siRNA). Fluorescence was measured 24 h after transfection. The mean and standard deviation are shown for each condition. The dollar symbol ($) indicates that the two-tailed t test comparison of silencing efficacy of the gray columns (EGFP-si + TRBP-si) versus the white columns (EGFP-si + NT-si) (A) or of the black columns (EGFPsi + Dicer-si) versus the white columns (EGFP-si + NT-si) (B) was significant at P < 0.05. The percentage symbol (%) indicates that the twotailed t test comparison of silencing efficacy of the EGFP-targeting siRNAs co-transfected with TRBP-targeting siRNA versus siRNA 396 (gray columns for mismatched sequences versus gray column for sequence 396) (A) or Dicer-targeting siRNA versus siRNA 396 (black columns for mismatched sequences versus black column for sequence 396) (B) was significant at P < 0.05. Control, mock, and NT refer to untreated cells, cells treated with the transfection reagent alone, and cells transfected with NT siRNA rather than EGFP-targeting siRNA, respectively.
TRBP–siRNA binding has been shown to be more critical for formation of the RLC than Dicer–siRNA binding [3,36]. Of all the sequences with a terminal mismatch, only 396-AG exhibited silencing efficacy that was comparable to that of the fully paired 396. In addition, knockdown of Dicer had the greatest impact on the activity of 396-AG. Dicer has been shown to prefer adenosine nucleotides at the terminal position during processing of long double-stranded RNAs to siRNAs [37]. Thus, the unique behavior of this sequence could be due to enhanced interactions with Dicer and a reduced need to interact with TRBP, relative to the other sequences.
Effect of guide strand 5′ end mismatch on TRBP and Dicer binding
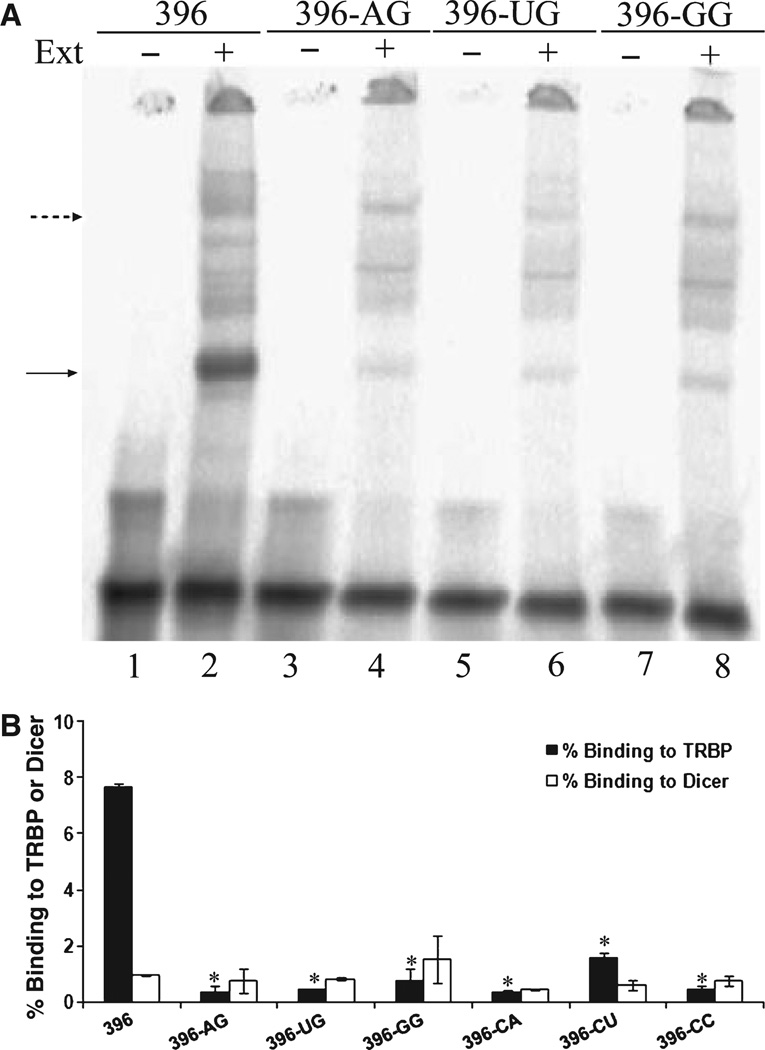
Having observed variability in the impact of silencing TRBP and Dicer on the function of the fully paired and mismatched sequences, we wished to examine whether the binding affinity of these proteins for the sequences is affected by sequence and structure differences. Radiolabeled siRNA was added to cytoplasmic extracts from human cells, and the complexes formed were detected by native electrophoretic mobility shift assay (EMSA) (Fig. 3A). This G/C-rich sequence (Table S2, NT and si-0) was used, as we had already determined that it would form easily discernable bands in the extracts (data not shown). As seen previously [34], we detected putative Dicer–siRNA complex formation in H1299 cell lysates (Fig. 3A, dashed arrow, lane 1; substantially equivalent data obtained with HepG2 and HeLa extracts not shown). To confirm the presence of Dicer in the complex, we performed the binding in the presence of Dicer antibody, TRBP antibody and nuclear factor κB (NF-κB) antibody (Fig. 3A, dashed arrow; compare lane 2 to lanes 1, 3 and 4), similarly to our previous experiments with purified Dicer protein [35]. As expected, the band was shifted in the presence of the Dicer antibody but not the other antibodies.
Fig. 3.
Characterization of siRNA–TRBP and siRNA–Dicer complexes. (A) EMSA of siRNA–protein complexes formed in H1299 cell extracts (lane 1), in the presence of Dicer antibody (lane 2), in the presence of TRBP antibody (lane 3), or in the presence of a control antibody against NF-κB (lane 4). The broken arrow indicates the position of the siRNA–Dicer complex, the double asterisks indicate the migration of the shifted siRNA– Dicer complex, and the solid arrow indicates the position of the siRNA–TRBP complex. ab, antibody. (B) Western blot analysis shows TRBP overexpression in H1299 cells transfected with TRBP plasmid (lane 3) compared to control cells (lane 2). (C) EMSA of the siRNA–protein complexes formed in H1299 cell extracts with TRBP overexpression (lane 2) and in control cells (lane 1).
We also wished to confirm the location of any TRBP-containing bands, if these could be visualized. In the presence of TRBP antibody, we noticed a reduction in the signal from a band at the appropriate position for the expected molecular weight of TRBP (Fig. 3A, solid arrow; compare lane 3 to lanes 1, 2 and 4; gel quantification indicated that the intensity was reduced by approximately 40% compared to lane 1), but we did not detect a shifted complex. To verify the presence of TRBP in this siRNA–protein complex, we overexpressed TRBP in H1299 cells, and confirmed the increase in expression by Western blot (Fig. 3B; compare lanes 2 and 3). Incubating the radiolabeled siRNAs with lysates of TRBP-overexpressing cells indicated a significant increase in formation of the putative TRBP complex (Fig. 3C; compare lanes 1 and 2), strongly supporting the antibody shift results and suggesting the presence of TRBP in this complex. Binding reactions performed in extracts after TRBP silencing showed a concomitant reduction in binding at the expected location (Fig. S3). Based on molecular weight, both the Dicer and TRBP complexes are assumed to contain only one molecule each of protein and siRNA. As further confirmation of the identities of the complexes, we showed that formation of both the protein–siRNA complexes was improved by the presence of ATP in the extracts (Fig. S4A,B), as shown previously [34]. Another siRNA-containing complex of unknown identity was also seen in these extracts (Fig. 3C, asterisk), which may be a result of the response of the cell to the presence of the plasmid and/or excess TRBP.
Identical binding reactions were performed with siRNA 396 and the mismatched siRNAs in H1299 cell extracts (Fig. 4A,B). Analysis of protein complexes formed by these siRNAs with TRBP showed that binding to TRBP was significantly lower for the siRNAs with a single terminal mismatch (Fig. 4A,B), including 396-AG. This trend agrees closely with our results from TRBP silencing experiments, in which the fully matched sequence appeared to depend most on the function of TRBP. The trend in TRBP binding was verified using two other siRNAs, 306 and 274 [27], which contained a mismatch, 306-CC (Fig. S5), or a U–G wobble, 274-UG (Fig. S6). There was no consistent behavior in Dicer binding for these sequences (Fig. 4A,B), even including 396-AG.
Fig. 4.
Effect of terminal mismatch at the guide strand 5′ end on siRNA–TRBP and siRNA–Dicer complex formation. (A) EMSA of siRNA–TRBP and siRNA–Dicer complexes formed in H1299 cell extracts using siRNAs 396 (lane 2), 396-AG (lane 4), 396-UG (lane 6) and 396-GG (lane 8). Separate gels were used for other siRNAs (results not shown). (B) Quantification of EMSA gel images. Percentage binding was calculated by normalizing the intensity of siRNA–protein complexes to the siRNA not exposed to extract (e.g. complexes in lane 2 versus free siRNA in lane 1). The mean and standard deviation are shown for triplicate binding experiments. Asterisks indicate that the two-tailed t test comparison of TRBP binding of various siRNAs versus siRNA 396 was significant at P < 0.05; the dollar symbol ($) indicates that the two-tailed t test comparison of Dicer binding of various siRNAs versus siRNA 396 was significant at P < 0.05.
Recent work by our group using purified TRBP protein has shown that it can bind siRNAs in an ATP-independent manner (J. A. Gredell, M. J. Dittmer and S. P. Walton, unpublished data). In those studies, TRBP protein by itself did not show a strong preference for binding of fully matched siRNAs over siRNAs with a terminal mismatch. These results indicate that recognition and binding of the siRNAs by Dicer and TRBP in cells might involve ATP as a co-factor, and hence, an in vitro assay using the purified proteins may not capture their behavior completely. That said, Dicer and TRBP complexes were only formed in the presence of siRNAs and not RNA–DNA heteroduplexes or DNA– DNA duplexes (Fig. S4C), similar to our results with recombinant TRBP protein in vitro. The sensitivity of TRBP binding to the terminal modifications suggests that it primarily binds at the siRNA termini, corroborating its proposed role as a sensor for siRNA asymmetry (Gredell, Dittmer and Walton, unpublished data).
It has also been shown that an immunopurified complex containing Dicer, TRBP and Ago2 has the ability to process pre-miRNAs, form active RISC upon selection of a guide strand, and direct Ago2-mediated silencing [7,38]. Active RISCs formed from Dicerprocessed pre-miRNAs were 10-fold more active than those formed from mature miRNAs targeting the same sequence [38]. This is different from the activity of in vitro constituted RLCs consisting of only Dicer, TRBP and Ago2 [13]. The silencing activity of the RISC formed from the in vitro complex is similar for both pre-miRNAs or miRNAs [13], suggesting that there might be other cellular co-factors associated with the RLC and RISC that affect their function in cells. Studying proteins such as MOV10 (Moloney leukemia virus 10 homolog) [10,11], TNRC6B (trinucleotide repeat-containing 6B) [10] and RHA (DEAH box polypeptide 9) [11] that are associated with Ago2 may elucidate the differences between in vitro and in vivo RLC/RISC formation and function.
Effect of siRNA structure and composition on siRNA–protein complexes
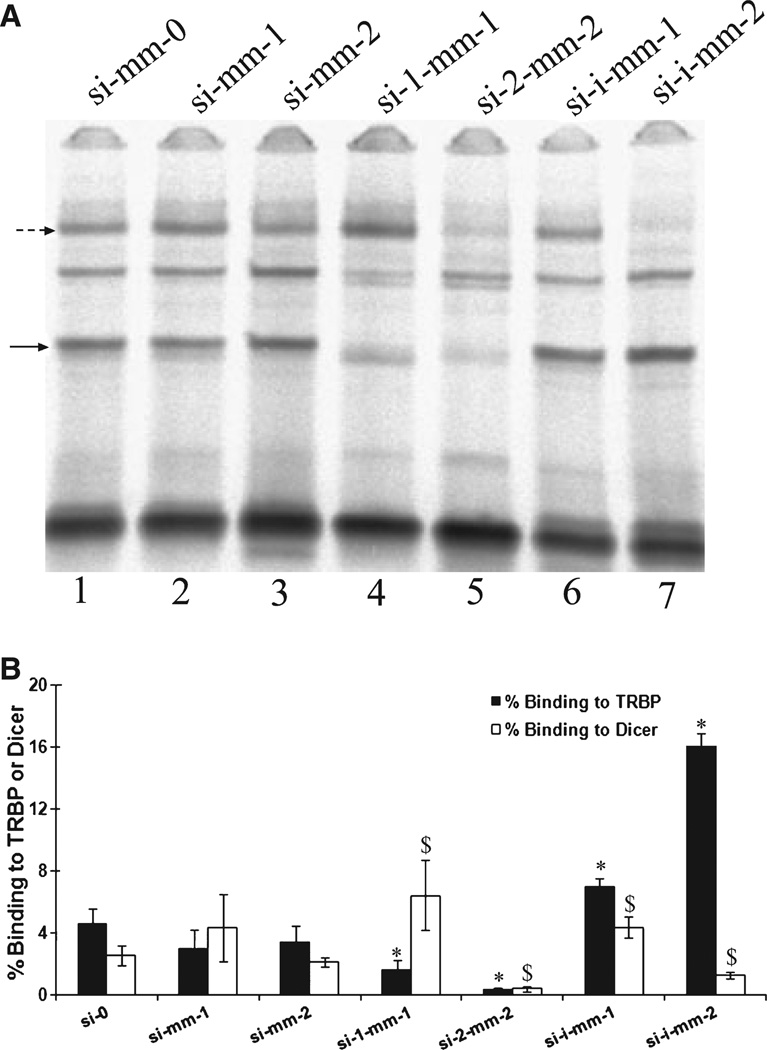
We wished to examine further the impact of terminal mismatches and also selected internal mismatches on the interactions of Dicer and TRBP with siRNAs (Table S2). We again used the G/C-rich sequence (used in Fig. 3) to give the clearest read-out for changes that occurred in formation of the complexes. A single or double mismatch at one end of the duplex appeared to decrease TRBP binding slightly, but not significantly (Fig. 5). For Dicer, binding improved slightly with a single mismatch, but weakened by the double mismatch. Again, neither of these changes was statistically significant. Simultaneous single or double mismatches at both ends of the duplex significantly reduced binding by TRBP, echoing what was seen with mismatches at only one end. As with one terminal mismatch, binding by Dicer was improved for simultaneous single mismatches but reduced for double mismatches. In all cases, terminal mismatches reduced TRBP binding, as above (Fig. 4), strongly suggesting that terminal mismatches should be avoided when attempting to generate siRNAs with maximal activity.
Fig. 5.
Effect of terminal and internal mismatches on siRNA–TRBP and siRNA–Dicer complexes. (A) EMSA of siRNA–TRBP and siRNA–Dicer complexes formed in H1299 cell extracts with siRNAs of varying terminal and internal structures (Fig. S7). Broken and solid arrows indicate the migration of the siRNA–Dicer and siRNA–TRBP complexes, respectively. (B) Quantification of EMSA gel images. Percentage binding was calculated by normalizing the intensity of the siRNA–protein complex to that of the respective unbound siRNAs (control lanes not shown). All sequences are listed in Table S2. The mean and standard deviation are shown for triplicate binding experiments. Asterisks indicate that the two-tailed t test comparison of TRBP binding of various siRNAs versus siRNA si-0 was significant at P < 0.05; the dollar symbol ($)indicates that the two-tailed t test comparison of Dicer binding of various siRNAs versus siRNA si-0 was significant at P < 0.05.
The efficiency of Dicer processing of long dsRNAs is known to depend on the overhang length of the substrates, with overhangs of two or three nucleotides being highly favorable compared to overhangs longer than three nucleotides [37]. In addition, the Piwi-Argonaute-Zwille (PAZ) domain, which is present in Dicer, is known to mediate binding with dsRNAs and siRNAs through 3′ overhangs [39–41]. The binding affinity of the human Ago2 PAZ domain to a siRNA duplex has been shown to be reduced by 5-fold and 50-fold by increasing the overhang length from two nucleotides to four and ten nucleotides, respectively [42]. Thus, we feel that the assays using cellular extracts accurately demonstrates the natural function of the proteins.
Both proteins showed higher affinity for a duplex with one internal mismatch (Fig. 5, si-i-mm-1). Binding by TRBP improves with two internal mismatches (Fig. 5, si-i-mm-2) but binding by Dicer is significantly reduced. In relation to Dicer binding, the two internal mismatches are located approximately where the doublestranded RNA binding domain (dsRBD) is positioned after the PAZ domain binds to one end of the duplex [36,41], thus the reduction in binding affinity may result from the inability of the double-stranded RNA binding domain (dsRBD) to bind the disrupted helix [43]. It is possible that the multiple dsRBDs of TRBP assist in its interaction with the sequences that contain internal mismatches [3,44]. However, it is not immediately clear why the binding would be improved for the internally mismatched sequence relative to the fully matched control. These structures do resemble miRNAs, and it may be that both Dicer and TRBP have higher affinity for the endogenous silencers compared to exogenous siRNAs. Also, functional siRNAs tend to have lower internal stability than non-functional siRNAs, particularly at positions 1–6 and 10–15 (with position 1 being the 5′ end of the guide strand) [15], exactly where the mismatches are located in our case. The effect of this reduced internal stability may result from an as yet uncharacterized function of TRBP in RNAi.
Here, we have characterized the interactions of siRNAs that contain terminal mismatches with TRBP and Dicer, and determined the impact of these interactions on their silencing activity. Primarily, we found that, for an asymmetric siRNA, introducing a terminal mismatch that further reduces the stability of the guide strand 5′ end does not enhance the functionality of the siRNAs. Based on comparison of the binding and silencing results, we believe that reduced TRBP binding is a probable reason for reduced silencing by mismatched siRNAs. That said, it appears that Dicer binding can have an impact on the silencing efficiency of some siRNAs in a terminal sequence-dependent manner. It is interesting to note that all of our mismatches were located at the end at which Dicer preferentially binds, based on the current model for RISC formation and siRNA asymmetry sensing [18]. Nonetheless, the binding by TRBP is more dramatically and consistently affected by the mismatches. Our assay does not indicate the location to which either TRBP or Dicer bind on the siRNA. We expect that TRBP can associate with equal likelihood at either end of the siRNA, but that its dissociation rate is faster with the less-stable end. As such, our mismatches probably enhance this dissociation rate and hence reduce the overall average affinity of TRBP for the mismatched siRNA relative to the fully paired sequence. Alternatively, this could be a reflection of the importance of the TRBP–Dicer interaction in binding to siRNAs, which would also help to explain the differences between binding with only purified TRBP or Dicer versus binding in extracts. It may also suggest that the role of human Dicer in selecting the guide strand and generating an active RISC is more prominent than that of Drosophila Dicer-2, which is controlled by R2D2 binding rather than actively participating in determining which end to bind [18]. Future work examining internal and terminal modifications will identify design rules for enhancing the activity of siRNA duplexes, and also provide a better understanding of the roles of TRBP and Dicer in controlling siRNA silencing activities.
Experimental procedures
General methods
siRNAs were purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA). Lyophilized RNAs were resuspended to 100 µm in TE (pH 8.0) and stored at −80 °C. RNAs were 5′ labeled using 33P-γ-ATP (Perkin-Elmer Life and Analytical Sciences, Boston, MA,USA) using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA, USA). Labeled strands were purified from unincorporated label using G-25 Sephadex columns (Roche Applied Science, Indianapolis, IN, USA). Cell cytoplasmic extracts were prepared as described previously [45]. Binding reactions in cell extracts with radiolabeled siRNAs were performed as described previously [34]. All binding reactions were performed for 1 h at 37 °C. The competency of all extracts for in vitro silencing was tested by measuring EGFP mRNA transcript levels in H1299 cell cytoplasmic extracts before and after addition of siRNAs (data not shown). EMSAs were performed as previously described [35], and the results were quantified using a Storm 860 imager (Amersham /GE Healthcare, Piscataway, NJ, USA). Percentage binding was calculated by normalizing the intensity of the siRNA– protein complex (Fig. 4A, lanes 2, 4, 6 and 8, complexes indicated by arrows) to that of the respective unbound siRNA (Fig. 4A, lanes 1, 3, 5 and 7). The sequences of all RNAs used in these studies are listed in Table S2. ATP depletion experiments were carried out in binding buffer lacking ATP, and containing glucose and hexokinase without creatine phosphate or creatine kinase [34].
Cell transfection and EGFP quantification
Human lung carcinoma cells (H1299) constitutively expressing EGFP were generously provided by Dr Jorgen Kjems (Department of Molecular Biology, University of Aarhus, Denmark). They were maintained in Dulbecco’s modified Eagle’s medium complemented with 10% v / v fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 100 mg·mL−1 of penicillin and 100 units·mL−1 streptomycin (Invitrogen). Twenty-four hours before transfection, cells were seeded at 50 000 cells per well in 24-well plates in antibiotic-free medium for siRNA transfection or seeded at 400 000 cells per well in six-well plates for TRBP plasmid DNA transfection. Cells were transfected using Lipofectamine 2000 (Invitrogen) (0.8 µL for siRNA transfection and 3 µL for plasmid transfection), according to the manufacturer’s recommendations. siRNA or TRBP plasmid DNA was diluted using Opti-MEM (Invitrogen), followed by addition of Lipofectamine and complex formation. siRNAs were used at final concentrations of 10 nm and TRBP plasmid DNA at 1 µg. When two siRNAs were transfected simultaneously, the final total siRNA concentration was 20 nm. Cells were treated with this transfection medium for 4 h at 37 °C, after which the transfection medium was replaced with normal cell culture medium. Twenty-four hours after transfection, the culture medium was aspirated and EGFP levels were quantified as described previously [27]. We have previously confirmed that the transfection efficiency using our established protocols provides essentially uniform siRNA loading across the various siRNA treatments [27]. For EGFP quantification, the fluorescence of each well of the 24-well plates was measured in nine locations within the well (three by three grid) using a Gemini fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA). The mean fluorescence for each well was calculated from these nine values. The mean fluorescence for each condition was calculated as the mean of multiple wells (typically three or four) on the same plate. Relative fluorescence units (RFU) (Figs 1 and 2) were calculated by normalizing the multi-well mean fluorescence for each condition to the multi-well mean fluorescence of mock-transfected wells from the same plate. At least three wells from at least six 24-well plates were measured for each condition (n ≥ 18).
Western blots
Cells were collected 24 h after plasmid or siRNA transfection. SDS loading buffer was added to samples, and heat-denatured at 95 °C for 5 min. The samples were immediately placed on ice, and the proteins were resolved on 4–20% gradient SDS–PAGE (Bio-Rad, Hercules, CA, USA) at 150 V for 90 min. Proteins were then transferred to a poly(vinylidene difluoride) membrane at 100 V for 1 h. The membrane was then incubated with blotting-grade milk (Bio-Rad) for 1 h, and then incubated overnight at 4 °C with either TRBP antibody (Abnova, Walnut, CA, USA) or Dicer antibody (Abcam, Cambridge, MA, USA) at 1 : 1000 dilution. Blots were then washed with TBS–Tween, and incubated with horseradish peroxidase-conjugated secondary antibody, and the proteins were detected using SuperSignal West Femto maximum sensitivity substrate (Pierce Biotechnology, Rockford, IL, USA). β-Actin was used as the loading control. Images were collected using a ChemiDoc XRS (Bio-Rad), and band intensities were quantified using bio-rad Quantity One software. Dicer and TRBP knockdowns were quantified by a ratio of ratios. Dicer and TRBP levels were each divided by the level of the β-actin loading control for each treatment, and these ratios were then divided by the ratio for control cells (no transfection).
Free energy calculations
The terminal stability (ΔG, kcal·mol−1) at each end of the siRNA duplex was calculated using mfold [28,29] by summing the nearest-neighbor contributions for the first five nucleotides (four nearest-neighbor energies) at the 5′ end [16]. Differential end stability (ΔΔG, kcal·mol−1) was calculated as the difference in thermodynamic stabilities at each end. For example, siRNA 396 has guide strand sequence of 5′-CAGGAUGUUGCCGUCCUCCTT-3′ and a passenger strand sequence of 5′-GGAGGACGGCAACAUCCUGT T-3′. Base pairing energies for the duplex were predicted using the mfold two-state hybridization server for RNA with default parameters. The four nearest neighbors at the guide strand 5′ end, CA:GU, AG:UC, GG:CC and GA:CU, have a cumulative base pairing energy of −8.7 kcal·mol−1. The four nearest neighbors at the passenger strand 5′ end, GG:CC, GA:CU, AG:UC and GG:CC, have a cumulative base pairing energy of −9.8 kcal·mol−1. Consequently the differential end stability (ΔΔG), i.e. the thermodynamic asymmetry, for the duplex is 1.1 kcal·mol−1. Positive values of ΔΔG indicate that the sequence is asymmetric in favor of selection of the appropriate guide strand.
Supplementary Material
Acknowledgements
We thank all the members of the Cellular and Biomolecular Laboratory at Michigan State University (http://www.egr.msu.edu/cbl/) for their advice and support, and Dr Jørgen Kjems (University of Aarhus, Denmark) for providing us with the EGFP cells. Financial support for this work was provided in part by Michigan State University, the National Science Foundation (0425821) and the National Institutes of Health (CA126136, GM079688 and RR024439).
Abbreviations
- Ago2
Argonaute 2
- EGFP
enhanced green fluorescent protein
- EMSA
electrophoretic mobility shift assay
- RISC
RNA-induced silencing complex
- RLC
RISC loading complex
- siRNA
short interfering RNA
- TRBP
HIV transactivating response RNA-binding protein
Footnotes
Supporting information
The following supplementary material is available:
Fig. S1. EGFP silencing efficacy of siRNAs at various concentrations.
Fig. S2. Western blot analysis of TRBP and Dicer levels in H1299 cells.
Fig. S3. Characterization of siRNA–TRBP complex formation after TRBP knockdown.
Fig. S4. Additional characterization of Dicer and TRBP complexes.
Fig. S5. Effect of a terminal mismatch at the guide strand 5′ end on siRNA–TRBP complex formation (sequence 306).
Fig. S6. Effect of a terminal mismatch at the guide strand 5′ end on siRNA–TRBP complex formation (sequence 274).
Fig. S7. Structures of high G/C content siRNAs with terminal and internal mismatches.
Table S1. siRNAs with terminal modifications [22].
Table S2. Sequence of siRNAs used to target EGFP.
Table S3. siRNAs with terminal modifications [24,26].
This supplementary material can be found in the online version of this article.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- 1.Filipowicz W. RNAi: the nuts and bolts of the RISC machine. Cell. 2005;122:17–20. doi: 10.1016/j.cell.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 7.Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev. 2005;19:2979–2990. doi: 10.1101/gad.1384005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with Dicer to facilitate the production of small interfering RNA. J Biol Chem. 2007;282:17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- 10.Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, Tuschl T. Identification of novel argonaute-associated proteins. Curr Biol. 2005;15:2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Hock J, Weinmann L, Ender C, Rudel S, Kremmer E, Raabe M, Urlaub H, Meister G. Proteomic and functional analysis of Argonaute-containing mRNA–protein complexes in human cells. EMBO Rep. 2007;8:1052–1060. doi: 10.1038/sj.embor.7401088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landthaler M, Gaidatzis D, Rothballer A, Chen PY, Soll SJ, Dinic L, Ojo T, Hafner M, Zavolan M, Tuschl T. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA. 2008;14:2580–2596. doi: 10.1261/rna.1351608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISCloading complex. Proc Natl Acad Sci USA. 2008;105:512–517. doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread siRNA ‘offtarget’ transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 18.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 19.Bramsen JB, Laursen MB, Damgaard CK, Lena SW, Babu BR, Wengel J, Kjems J. Improved silencing properties using small internally segmented interfering RNAs. Nucleic Acids Res. 2007;35:5886–5897. doi: 10.1093/nar/gkm548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Burchard J, Linsley PS, Aronin N, Xu Z, Zamore PD. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2006;2:e140. doi: 10.1371/journal.pgen.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohjoh H. Enhancement of RNAi activity by improved siRNA duplexes. FEBS Lett. 2004;557:193–198. doi: 10.1016/s0014-5793(03)01492-3. [DOI] [PubMed] [Google Scholar]
- 22.Ding H, Liao G, Wang H, Zhou Y. Asymmetrically designed siRNAs and shRNAs enhance the strand specificity and efficacy in RNAi. J RNAi Gene Silencing. 2007;4:12. [PMC free article] [PubMed] [Google Scholar]
- 23.Holen T. Mechanisms of RNAi: mRNA cleavage fragments may indicate stalled RISC. Journal of RNAi and Gene Silencing. 2005;1:21–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J, Wei N, Chalk A, Wang J, Song Y, Yi F, Qiao RP, Sonnhammer EL, Wahlestedt C, Liang Z, Du Q. Focusing on RISC assembly in mammalian cells. Biochem Biophys Res Commun. 2008;368:703–708. doi: 10.1016/j.bbrc.2008.01.116. [DOI] [PubMed] [Google Scholar]
- 25.Chen PY, Weinmann L, Gaidatzis D, Pei Y, Zavolan M, Tuschl T, Meister G. Strand-specific 5′-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA. 2008;14:263–274. doi: 10.1261/rna.789808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holen T, Moe SE, Sorbo JG, Meza TJ, Ottersen OP, Klungland A. Tolerated wobble mutations in siRNAs decrease specificity, but can enhance activity in vivo. Nucleic Acids Res. 2005;33:4704–4710. doi: 10.1093/nar/gki785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gredell JA, Berger AK, Walton SP. Impact of target mRNA structure on siRNA silencing efficiency: a large-scale study. Biotechnol Bioeng. 2008;100:744–755. doi: 10.1002/bit.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Howard KA, Dong M, Andersen MO, Rahbek UL, Johnsen MG, Hansen OC, Besenbacher F, Kjems J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 32.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci USA. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellino JL, Jaskiewicz L, Filipowicz W, Sontheimer EJ. ATP modulates siRNA interactions with an endogenous human Dicer complex. RNA. 2005;11:1719–1724. doi: 10.1261/rna.2102805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kini HK, Walton SP. In vitro binding of single- stranded RNA by human Dicer. FEBS Lett. 2007;581:5611–5616. doi: 10.1016/j.febslet.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoh T, Suzuki T. Specific residues at every third position of siRNA shape its efficient RNAi activity. Nucleic Acids Res. 2007;35:1–14. doi: 10.1093/nar/gkl1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen A, Behlen L, Reynolds A, Wolfson A, Marshall WS, Karpilow J, Khvorova A. The contributions of dsRNA structure to Dicer specificity and efficiency. RNA. 2005;11:674–682. doi: 10.1261/rna.7272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Lingel A, Simon B, Izaurralde E, Sattler M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat Struct Mol Biol. 2004;11:576–577. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- 40.Lingel A, Simon B, Izaurralde E, Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature. 2003;426:465–469. doi: 10.1038/nature02123. [DOI] [PubMed] [Google Scholar]
- 41.Song JJ, Liu J, Tolia NH, Schneiderman J, Smith SK, Martienssen RA, Hannon GJ, Joshua-Tor L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat Struct Biol. 2003;10:1026–1032. doi: 10.1038/nsb1016. [DOI] [PubMed] [Google Scholar]
- 42.Ma JB, Ye K, Patel DJ. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature. 2004;429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 44.Laraki G, Clerzius G, Daher A, Melendez-Pena C, Daniels S, Gatignol A. Interactions between the double-stranded RNA-binding proteins TRBP and PACT define the Medipal domain that mediates protein–protein interactions. RNA Biol. 2008;5:92–103. doi: 10.4161/rna.5.2.6069. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Zerivitz K, Akusjarvi G. Small-Scale Preparation of Nuclear Extracts from Mammalian Cells. London: Academic Press; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.