ABSTRACT
The pathogenesis of malaria, an insect-borne disease that takes millions of lives every year, is still not fully understood. Complement receptor 1 (CR1) has been described as a receptor for Plasmodium falciparum, which causes cerebral malaria in humans. We investigated the role of CR1 in an experimental model of cerebral malaria. Transgenic mice expressing human CR1 (hCR1+) on erythrocytes were infected with Plasmodium berghei ANKA and developed cerebral malaria. No difference in survival was observed in hCR1+ mice compared to wild-type mice following infection with P. berghei ANKA; however, hCR1 detection was significantly diminished on erythrocytes between days 7 and 10 postinfection. hCR1 levels returned to baseline by day 17 postinfection in surviving animals. Immunoblot assays revealed that total erythrocyte hCR1 levels were diminished, confirming that immune complexes in association with erythrocyte hCR1 were likely removed from erythrocytes in vivo by clearance following immune adherence. Decreases in hCR1 were completely dependent on C3 expression, as mice treated with cobra venom factor (which consumes and depletes C3) retained hCR1 on erythrocytes during C3 depletion through day 7; erythrocyte hCR1 decreases were observed only when C3 levels recovered on day 9. B-cell-deficient mice exhibit a marked increase in survival following infection with P. berghei ANKA, which suggests that immune complexes play a central role in the pathogenesis of experimental cerebral malaria. Together, our findings highlight the importance of complement and immune complexes in experimental cerebral malaria.
IMPORTANCE
Cerebral malaria is a deadly complication of infection with Plasmodium falciparum. Despite its high prevalence, relatively little is understood about its pathogenesis. We have determined that immune complexes are generated and deposited on erythrocytes specifically expressing human complement receptor 1 in a mouse model of cerebral malaria. We also provide evidence demonstrating the importance of immunoglobulins in the pathogenesis of cerebral malaria in mice. These findings may have important implications in human cerebral malaria.
INTRODUCTION
Approximately half of the world’s population is at risk of malaria. According to World Health Organization estimates, malaria infects more than 200 million people worldwide each year, kills over 600,000 people, and is a public health issue in more than 90 countries (http://www.who.int/malaria/media/world_malaria_report_2013). Annual deaths from malaria may in fact be twice as high (1). The incubation period of malaria ranges from 9 to 14 days for Plasmodium falciparum. Symptoms of malaria include fever, chills, headache, fatigue, muscular pains, mild diarrhea, nausea, and vomiting and can be mistaken for influenza or gastrointestinal infection. Cerebral malaria and severe malarial anemia are two complications of P. falciparum infection. Malaria can lead to impairment of brain or spinal cord function, seizures, or loss of consciousness. Cerebral malaria death is not well understood (2, 3). Heavy parasite sequestration and extravascular pathological findings in the brain, retina, gastrointestinal tract, and subcutaneous fat are seen with cerebral malaria (4–6). The understanding of cerebral malaria is limited because of the low frequency of autopsies in most areas in which malaria is endemic. Severe anemia occurs during the P. falciparum blood stage due to an increase in clearance of uninfected cells and a failure of an adequate bone marrow response. The degree of anemia also depends on the immune status of the patient, nutritional background, and other complicating factors (7–10).
Murine infections with Plasmodium species are widely used as surrogate models to study malaria. Mouse models of malaria are clearly divided into two groups, those resistant to and those susceptible to cerebral disease (11, 12). Certain strains of mice infected with Plasmodium berghei ANKA exhibited neurological signs, sharing characteristics with human cerebral malaria (13). Parasitized red cells are responsible for lesions in various organs in humans and can also be found in different organs in mice (6, 14–17). While cell-mediated immunity protects against the parasite, an imbalance in immune responses may contribute to the pathogenesis of human cerebral malaria (18). As an example, a robust humoral response with high serum levels of IgG and IgM antibodies can result in the deposition of immune complexes and can contribute to inflammation in cerebral microvessels (19).
The role of the complement system in the pathogenesis of several diseases has been increasingly recognized (20–24). Complement proteins or receptors may modulate the course of malaria in distinct ways. C5−/− mice have a slight survival advantage in cerebral malaria (25), while others found that C3−/− mice have no survival advantage (26). Hematin has been shown to activate the alternative pathway on erythrocytes (27). Human complement receptor 1 (hCR1) has been reported to serve as a receptor for Plasmodium falciparum invasion via direct binding of the parasite ligand (28). Erythrocyte CR1 is also involved in the rosette formation of uninfected erythrocytes with P. falciparum-infected erythrocytes (29). CR1 plays an important role in the control of complement activation and also serves as the “immune adherence receptor” to facilitate clearance of immune complexes (ICs), as IC-coated erythrocytes traverse macrophage-lined liver and spleen sinusoids. ICs activate complement, which results in the deposition of C3 and C4 fragments on the ICs. By virtue of its ability to bind to C3 and C4 fragments, erythrocyte CR1 binds to complement-coated ICs, and CR1 and complement-coated ICs together are cleared from the circulation as erythrocytes traverse the liver and spleen. Following removal of complement-coated ICs and CR1, erythrocytes return to the circulation. ATP release by the IC-coated erythrocyte may promote CR1 clustering to increase avidity of binding of complement-coated ICs with CR1 and also facilitate the phagocytosis of immune-adherent ICs (30).
We investigated whether murine models of Plasmodium infection could be used to address the roles of complement, ICs, and erythrocyte CR1 during malaria. Because normal murine erythrocytes do not express CR1, we employed transgenic mice that express hCR1 on their erythrocytes (31) to elucidate the role of human erythrocyte CR1 and circulating immune complexes (CICs) during experimental cerebral malaria. We found that infecting either wild-type or human CR1 transgenic mice with P. berghei ANKA results in equal rates of lethal cerebral malaria. Strikingly, a transient but reproducible reduction in erythrocyte CR1 levels is observed following infection. We sought to determine the mechanism by which this decrease in erythrocyte CR1 occurs.
RESULTS
The presence of erythrocyte CR1 does not influence the disease course in murine malaria.
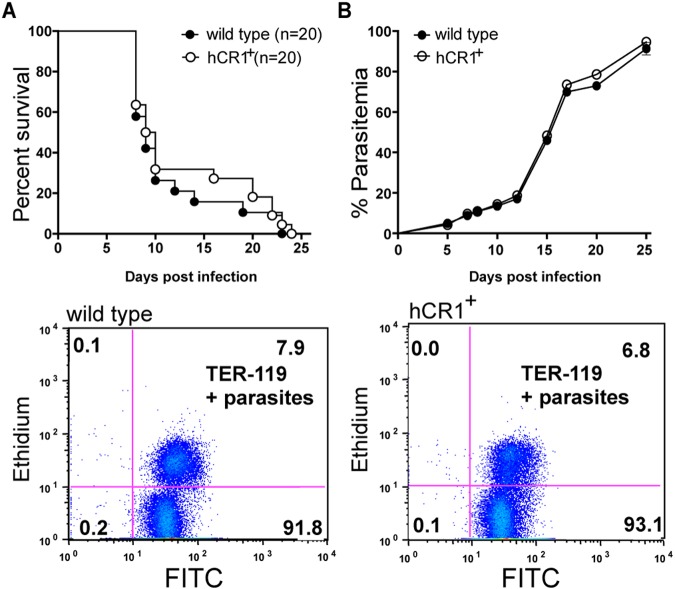
Infections with P. berghei ANKA are typically established by an intraperitoneal injection of 104 to 105 infected erythrocytes simultaneously exhibiting all of the parasite developmental stages in the blood. Experimental cerebral malaria (ECM) develops in susceptible mice between 6 and 8 days postinfection and is a major cause of mortality. Following infection with 105 infected erythrocytes, the following signs of cerebral malaria were used to score disease severity in wild-type C57BL/6 and hCR1 transgenic (hCR1+) mice: ruffled fur, abnormal posture, disturbances in balance, limb paralysis, convulsion, coma, and death. No significant differences were observed in either morbidity or survival in hCR1+ mice versus wild-type mice (Fig. 1) or disease severity (data not shown). In addition, parasitemia levels were similar between hCR1+ and wild-type mice (Fig. 1B). Erythrocytes were monitored by expression of the TER-119 antigen, a 52-kDa glycophorin A-associated protein that is expressed from the early proerythroblast stage to mature erythrocytes (32), and parasites were stained with ethidium bromide according to previously published methods (33).
FIG 1 .
Transgenic hCR1 mice infected with Plasmodium berghei ANKA develop parasitemia and cerebral malaria. (A) hCR1+ mice and wild-type mice equally succumbed to cerebral malaria infection. hCR1+ mice (n = 20) and wild-type mice (n = 20) were intraperitoneally infected with 105 P. berghei ANKA-infected erythrocytes and monitored for survival. Animals with severe cerebral malaria were sacrificed by CO2 asphyxiation. (B) Parasitemia levels were comparable between hCR1+ mice and wild-type C57BL/6 mice following infection with P. berghei ANKA. Parasitemia was assessed every 2 to 4 days by microscopy of Giemsa-stained thin blood smears or by flow cytometry using a monoclonal antibody against TER-119 at 1:100 and ethidium bromide to stain the nuclei (parasites). Representative scatter plots from day 7 postinfection are shown for wild-type and hCR1+ animals. The x axis shows FITC (TER-119), and the y axis shows ethidium bromide (parasites).
hCR1 on erythrocytes decreases after mice are infected with P. berghei ANKA.
Flow cytometric analysis of hCR1 levels during the course of P. berghei ANKA infection revealed an apparent loss of CR1 expression on erythrocytes that is greatest between 7 and 10 days postinfection, though some decreases can be detected as early as day 5 postinfection (Fig. 2A). Detection of hCR1 was restored by day 17 postinfection in surviving mice. By day 17, the presence of a second high-CR1-staining peak suggested that new erythrocytes entered the circulation during infection, likely in response to infection-related anemia (Fig. 2A). The average values of hCR1 detected in mice through day 25 following infection are plotted in Fig. 2B. As expected, some animals succumbed to disease over time.
FIG 2 .
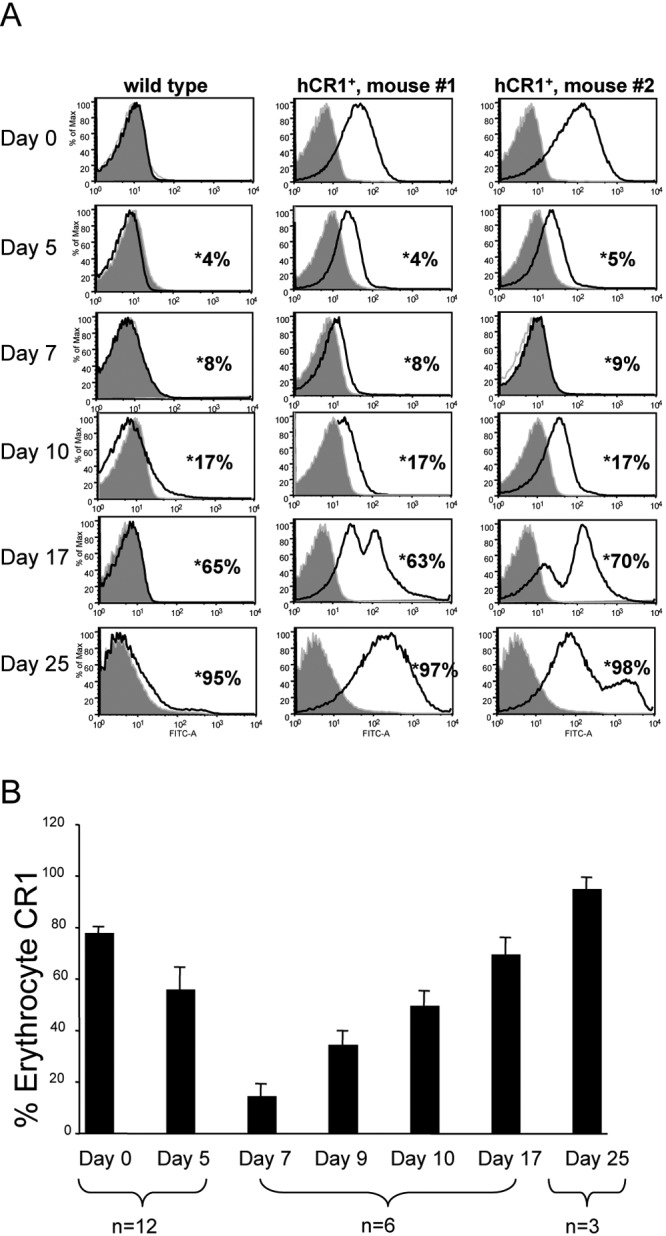
Infection of hCR1+ mice with P. berghei ANKA results in diminished detection of hCR1 on erythrocytes at 7 to 10 days postinfection. (A) Flow cytometry histograms from an experiment with one representative wild-type and two representative hCR1+ mice that survived 25 days following P. berghei ANKA infection are shown over a time course. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 5, 7, 9, 10, 17, and 25. The expression of cell surface hCR1 was analyzed. The histograms show the fluorescence intensity of erythrocyte CR1 detected (the x axis indicates FITC or CR1, and the y axis represents the percentage of the maximum); 1 × 105 cells were analyzed in each event. Percent parasitemia of each mouse, also measured by flow cytometry (data not shown), is indicated with asterisks. (B) Average values of CR1 detection for each time point for available mice are shown. Error bars indicate the standard errors of the means.
To ensure that the diminished erythrocyte CR1 levels observed by flow cytometry were not the result of ICs binding to CR1, thereby restricting access of the anti-CR1 antibody to its epitope on CR1, we performed immunoblotting assays on erythrocytes from hCR1+ mice infected with P. berghei ANKA. Decreases in hCR1 were observed at day 9 postinfection compared to baseline (day 0) (Fig. 3). hCR1 levels rose significantly by day 15 postinfection compared to baseline levels in surviving mice, which correspond to the flow cytometry data observations (Fig. 2A) and could be attributed to new erythrocyte generation.
FIG 3 .
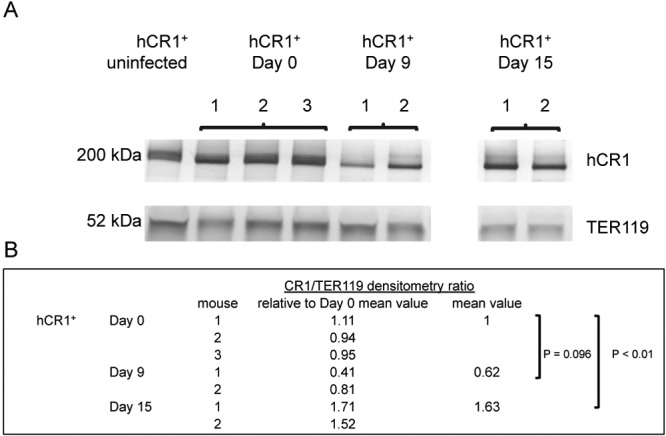
hCR1+ mice had decreased protein levels of erythrocyte hCR1 at day 7 after infection with P. berghei ANKA. (A) Representative immunoblots of CR1 and TER-119 in erythrocytes from hCR1+ mice infected with P. berghei ANKA. CR1 bands (200 kDa) were quantified by densitometry and normalized to TER-119 (52 kDa). Decreases in hCR1 normalized to TER-119 were observed at day 9 postinfection relative to baseline (day 0). CR1 levels rose significantly by day 15 compared to baseline. A second independent experiment revealed decreases in erythrocyte CR1 in three mice at day 7 compared to baseline (day 0). (B) Densitometry ratios of CR1 to TER-119, relative to day 0 baseline values.
Decreases in hCR1 following P. berghei ANKA infection are dependent on C3.
hCR1 binds to C3b via its long homologous repeat B (LHR-B) and LHR-C, which facilitate the clearance of complement-coated immune complexes (“immune adherence”-mediated clearance). Thus, we sought to establish if the apparent decrease in hCR1 during P. berghei ANKA infection would occur in the absence of C3. Treatment of mice with cobra venom factor (CoVF) leads to a decrease in C3 with a nadir observed approximately 24 h postinjection. A single therapeutic dose of CoVF results in low hemolytic activity for 4 to 6 days (34) and low C3 levels for 5 to 8 days (35). To maximize C3 depletion for as long a time as possible in this experimental system, we used four doses of CoVF, with the knowledge that CoVF is highly immunogenic and antibodies elicited after a week will block CoVF activity (34). We found that control hCR1+ mice not treated with CoVF showed the typical decline in erythrocyte hCR1 levels at day 7 after infection with P. berghei ANKA, whereas hCR1+ mice treated with CoVF did not show decreased erythrocyte hCR1 levels until day 9 postinfection. Average hCR1 levels are plotted in Fig. 4; individual histograms are shown in Fig. S1 in the supplemental material.
FIG 4 .
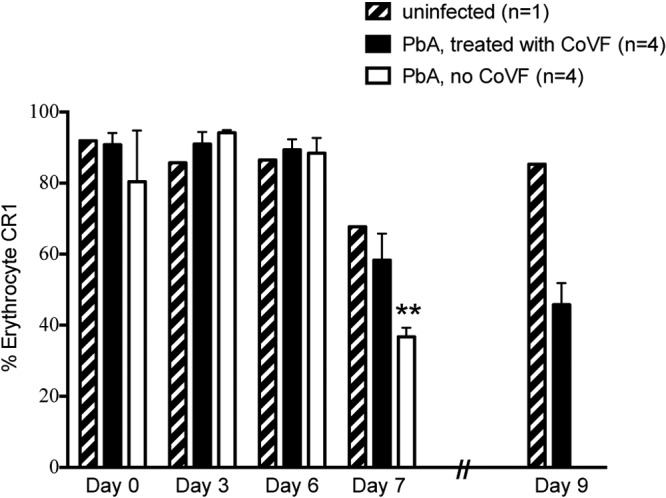
Treatment with cobra venom factor delays the kinetics of hCR1 decline. hCR1+ mice were infected with P. berghei ANKA (PbA) (intraperitoneally with 105 infected erythrocytes). Mice were treated with either cobra venom factor (CoVF, n = 4) or saline control (n = 4) on the day of infection and on days 3, 5, and 7 postinfection and monitored for survival. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 3, 6, 7, and 9. All mice treated with saline control exhibited the characteristic decrease in erythrocyte CR1 detection on day 7 postinfection by flow cytometry using antibody 7G9. On day 8 postinfection, these same mice all experienced convulsions and were euthanized. Mice treated with CoVF also had a decrease in erythrocyte hCR1 detection, but the decrease was delayed until day 9 postinfection, which was consistent with the kinetics of CoVF activity. Average values of CR1 detection for each time point for mice are shown. Error bars indicate the standard errors of the means. **, P < 0.01.
Circulating immune complexes are detected during the course of infection of P. berghei ANKA.
We next confirmed the presence of ICs in serum during the course of P. berghei ANKA infection. Serum samples from wild-type and hCR1+ mice were collected at days 8 and 10 postinfection. In both wild-type and transgenic mice, CICs peaked at day 8 and began to decline at day 10 (Fig. 5). This was consistent with the data above and suggested that CICs bind to erythrocyte CR1 between days 7 and 10 postinfection. At day 10, the IgG and IgM levels were significantly lower in hCR1+ mice than in wild-type mice, presumably because CICs are cleared more rapidly in these animals.
FIG 5 .
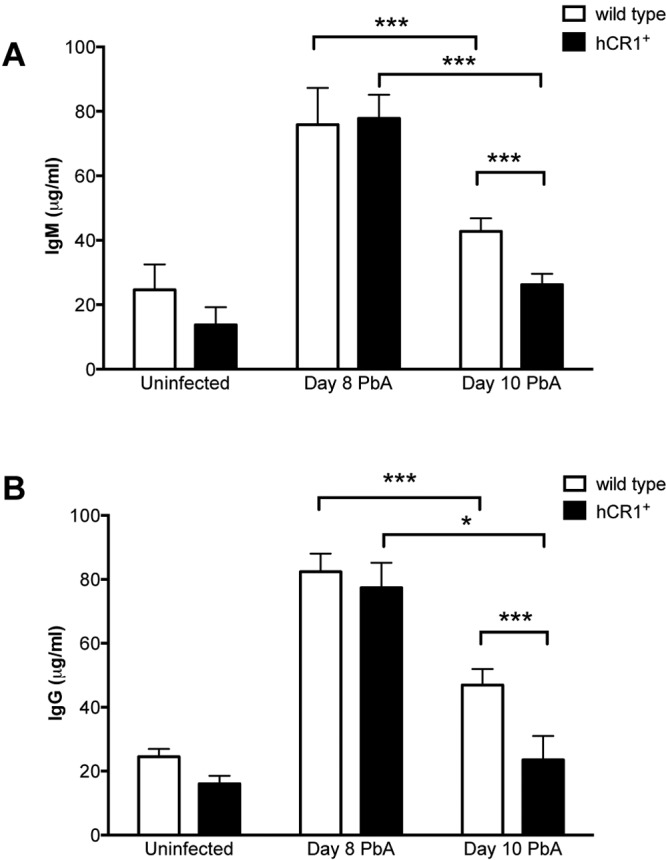
Circulating immune complexes develop during the course of P. berghei ANKA infection. Immune complexes with IgM (A) or IgG (B) are detected in sera from infected wild-type (n = 6) and hCR1+ (n = 6) mice at days 8 and 10 after infection with P. berghei ANKA. Levels are significantly lower at day 10 than at day 8 for both wild-type and hCR1+ mice. In addition, at day 10, levels are slightly but significantly lower in hCR1+ mice than in wild-type mice. Values for uninfected wild-type and hCR1+ mouse sera are shown for reference. *, P < 0.05; ***, P < 0.001.
B-cell-deficient mice display increased survival following P. berghei ANKA infection.
In light of our findings suggesting that ICs formed during ECM and were associated with hCR1 on erythrocytes, we sought to determine if IC formation impacts survival of mice infected with P. berghei ANKA. Thus, we infected wild-type C57BL/6 and B-cell-deficient mice, which lack mature B cells and cannot generate immunoglobulins (36), with P. berghei ANKA and monitored survival in four independent experiments. The combined results of these four studies, each of which independently demonstrated significance, are shown in Fig. 6A. The B-cell-deficient mice had significantly increased survival, with a median survival of 12.5 days compared to 10 days in wild-type mice (P < 0.0001; χ2 = 47.18, log rank test). Multiple cohorts did not result in any confounding effect, as unadjusted and adjusted hazard ratios from the Cox model were similar. Only 2% of wild-type mice (1 of 44) escaped cerebral malaria, versus 50% of B-cell-deficient mice (21 of 42). Histopathologic analysis of brains of moribund, infected B-cell-deficient mice revealed distinct microvascular lesions (Fig. 6B). The most striking observation was that blood vessels of B-cell-deficient mice had significant leukocyte plugging and cellular infiltration with minimal hemorrhage. In contrast, wild-type mice consistently had moderate hemorrhage with minimal leukocyte plugging of the microvasculature. Vascular lesions were similar in number and distribution between wild-type and B-cell-deficient mice. Infected red blood cells (iRBCs) were present in both groups without significant differences in parasitemia at day 11 postinfection; levels in wild-type mice were 25% ± 2% (n = 16) and levels in B-cell-deficient mice were 23% ± 1% (n = 16). Of note, B-cell-deficient mice exhibited distinct signs of illness at terminal stages of disease. These mice did not have the convulsions typically seen in wild-type mice with ECM. B-cell-deficient mice presented with hind limb paralysis during end-stage disease, whereas wild-type animals had unilateral hemiplegia.
FIG 6 .
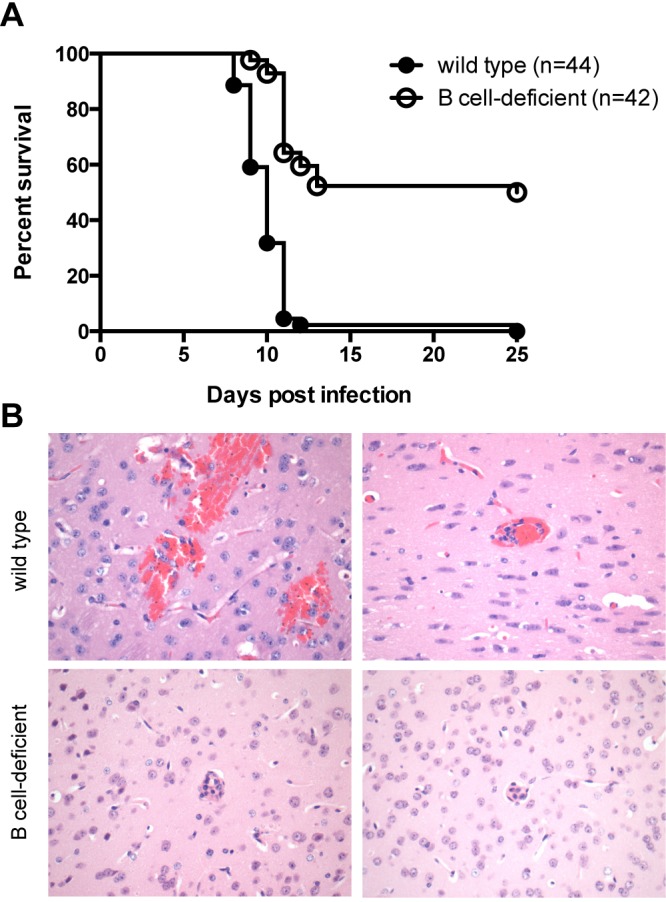
B-cell-deficient mice display tempered courses of experimental cerebral malaria. B-cell-deficient mice were intraperitoneally infected with 105 P. berghei ANKA-infected erythrocytes and monitored for survival. (A) Survival was significantly increased for B-cell-deficient mice (n = 42) compared to wild-type mice (n = 44) (P < 0.0001) (χ2 = 47.18 using a log rank test). Combined data from four independent experiments are shown. (B) B-cell-deficient mice exhibit morphologically different vascular lesions in brains compared with those of wild-type mice in experimental cerebral malaria. Wild-type and B-cell-deficient mice were challenged with P. berghei ANKA and euthanized on day 9 (wild-type) or day 10 (B-cell-deficient) postinfection, when animals were moribund. Wild-type mouse brains (upper panels) showed mild to moderate hemorrhage involving the forebrain and cerebellum with occasional neuronal cell death. Mild leukocyte plugging of microvasculature was observed. Brains from B-cell-deficient mice (lower panels) had minimal to mild hemorrhage with notable leukocyte plugging of the microvasculature. Malarial forms were present in erythrocytes in both wild-type and B-cell-deficient mice. Sections from seven wild-type and six B-cell-deficient mice at terminal stages of illness were compared, with consistent findings within each group. All sections were stained with hematoxylin-eosin. Magnification, ×400.
DISCUSSION
We have made several important findings with P. berghei ANKA ECM in transgenic mice expressing human CR1 on erythrocytes. We have identified the generation of ICs during P. berghei ANKA infection, which is accompanied by decreases in erythrocyte hCR1 levels between days 7 and 10 following infection. However, erythrocyte CR1 did not appear to affect parasitemia, disease course, or survival. The transient decrease in CR1 was detected by flow cytometry and validated by immunoblot assay. In mice that survived the infection, erythrocyte CR1 levels eventually recovered and surpassed baseline levels both by flow cytometry (manifested as a second peak) and by immunoblot assay, and that recovery probably is the result of introduction of new erythrocytes into the circulation. The decreased CR1 levels noted by flow cytometry and by immunoblotting confirm true loss of CR1 from the erythrocyte, rather than “masking” of CR1 epitopes for the detecting antibody by ICs. The decreases in erythrocyte CR1 during ECM are dependent on C3, as demonstrated by the delay in kinetics following treatment of mice with CoVF. A schematic of erythrocyte CR1 clearance following immune adherence is shown in Fig. 7.
FIG 7 .
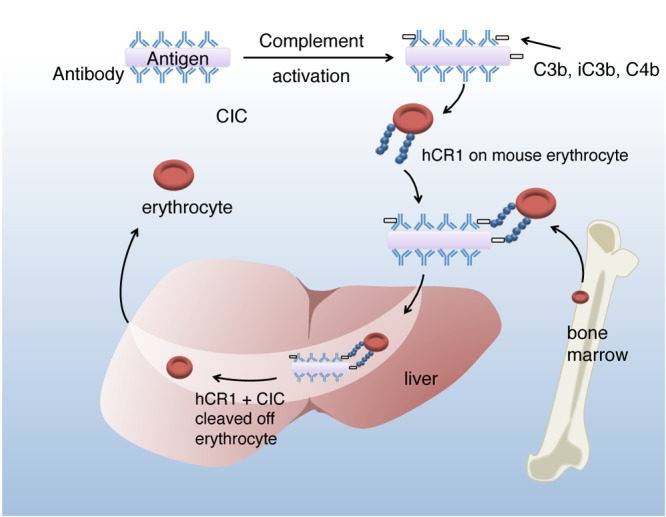
Summary figure of immune complex clearance in the P. berghei ANKA-infected hCR1+ transgenic mouse. CIC, circulating immune complex; hCR1, human complement receptor 1.
The role of ICs in the pathogenesis and severity of autoimmune diseases such as systemic lupus erythematosus is well established. ICs also contribute to the pathogenesis of several infectious diseases. Examples include IC-mediated vasculitis and renal damage in streptococcal infections, hepatitis B virus, HIV-1 infection, bacterial endocarditis, and cryoglobulinemia associated with hepatitis C virus (37). The interplay between erythrocyte CR1 and ICs could contribute to the complications of malaria. In humans, polymorphisms can dictate the size (length) and the expression levels of CR1 on erythrocytes. Four different size variants have been described (CR1*1 through 4) that result from deletions or duplications of LHRs during unequal crossover events (38, 39). The level of CR1 expressed is regulated by the H (high-expression) and L (low-expression) codominant alleles: LL, HL, and HH genotypes give rise to low, intermediate, and high CR1 expression, respectively (40). In humans, CR1 levels also vary with age—levels are high at birth, followed by low levels between 6 and 24 months of age (41), a period at which children are most susceptible to malaria.
Complement activation triggered by ICs may precipitate severe malarial anemia (42), and in this instance, individuals with low CR1 levels (and therefore with decreased ability to degrade C3b and C4b to their hemolytically inactive fragments) could conceivably be at a greater risk of developing severe malarial anemia (43–45). It is not clear whether the low CR1 levels observed in these instances are the result of removal of the CR1 from erythrocytes (as we described here in our model) or the result of genetically determined low CR1 levels. Evidence supporting the former hypothesis stems from a study which showed normalization of CR1 levels following resolution of infection (44). Other studies have not shown a correlation between severe disease and CR1 levels (46).
Rosetting, a phenomenon where a specific variant PfEMP1 expressed by parasitized erythrocytes adheres to CR1 on uninfected erythrocytes to form clumps, is believed to play a central role in the pathophysiology of P. falciparum cerebral malaria (29). Rosetting was also demonstrated in 14 of 15 clones of Plasmodium chabaudi tested in a murine model using wild-type C57BL/6J mice (47), suggesting that this phenomenon may occur independently of erythrocyte CR1 in mice. One would expect CR1 polymorphisms that are associated with reduced ability to form rosettes (e.g., Sl2 and Sl2/2) to be associated with a lower incidence of cerebral malaria, but studies addressing this issue have yielded conflicting results (48, 49).
ICs have proinflammatory properties, and indeed, studies currently being carried out by our group have shown that human peripheral blood mononuclear cells stimulated with ICs isolated from the serum of individuals with Plasmodium vivax malaria secrete interleukin-6, tumor necrosis factor, and IL-1β (D. Golenbock and R. Gazzinelli, unpublished observations). The loss of CR1 that we have observed coincides with a rise in IgG- and IgM-containing ICs and may represent an attempt to clear these proinflammatory complexes from the circulation.
Fernandez-Arias et al. recently reported diminished levels of surface CR1 on monocytes/macrophages both in a rodent malaria model and in patients infected with either P. falciparum or P. vivax (50). These data together illustrate the importance of IC deposition and clearance during malaria infection.
Our data led us to investigate the role of immunoglobulins in the pathogenesis of ECM. B-cell-deficient mice, devoid of immunoglobulins, exhibited increased survival and delayed onset of disease. Histopathology revealed striking differences, with a lower degree of microvascular hemorrhage in the B-cell-deficient mice. To our knowledge, only one other study reports data with P. berghei ANKA ECM using B-cell-deficient mice having the targeted deletion in the μ region of the IgM locus (also known as B-cell-null, BKO, μMT, or Igh-6null mice). Yañez et al. reported that 3 of 4 μMT mice and 8 of 8 wild-type mice developed cerebral malaria in a single experiment; the limited numbers presumably precluded achieving statistical significance (51). Infections of JHD mice, which have a targeted deletion of the JH region of the IgM locus, were more suggestive of a role of B cells in disease; in three experiments, only 8 of 31 JHD mice developed cerebral malaria, whereas 24 of 27 wild-type mice developed cerebral malaria (51). In our studies, the pathological findings of decreased hemorrhage and increased leukocyte plugging in the microvasculature of brains of the B-cell-deficient mice with ECM were striking. In ECM, P. berghei ANKA parasites do not infect the brain parenchyma; rather, a fraction of infected erythrocytes accumulates intravascularly, including in the brain (52). CD4+ and CD8+ T cells have both been implicated in the development of ECM (51, 53–55). Mice deficient in T cells are ECM resistant and lack microvascular lesions, endothelial cell death, and mononuclear cell infiltration. It would be interesting to determine if the decreased hemorrhage in the microvasculature is directly associated with a lack of IC formation, as this could have important implications for the pathogenesis of cerebral malaria.
Of note, we did not observe any decreases in hCR1 when hCR1+ transgenic mice were challenged with either P. chabaudi, which results in parasite clearance, or P. berghei NK65, which causes severe anemia (data not shown). Disease was comparable between transgenic and wild-type mice in both infection models in terms of parasitemia, anemia, and clinical signs. Thus, the decreases in erythrocyte CR1 appear to be specific for P. berghei ANKA infection and/or ECM. Altogether, we propose that CIC plays a major role in the pathogenesis of ECM and that immunoglobulin deficiency alters the disease outcome following P. berghei ANKA infection. Future studies to define the antigenic content of malarial IC and to determine how ICs may specifically contribute to microvascular damage and hemorrhage are merited.
MATERIALS AND METHODS
Ethics.
All experiments involving animals were in accordance with guidelines set by the American Association for Laboratory Animal Science (AALAS). All protocols related to this work were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Massachusetts Medical School.
Mice.
All mice were bred and maintained under specific-pathogen-free conditions in the animal facilities at the University of Massachusetts Medical School in accordance with the University of Massachusetts Medical School’s IACUC. B-cell-deficient mice (IgH6−) on the C57BL/6 background as well as control wild-type C57BL/6 mice were originally purchased from Jackson Laboratories. Transgenic mice expressing human CR1 on erythrocytes (hCR1+) were generated on the C57BL/6 background as previously described (31). Age- and sex-matched groups of mice were used in all experiments.
Plasmodium infection.
Mice (5 to 10 weeks old) were infected with the frozen stock of Plasmodium berghei ANKA (gift of A. Luster, Massachusetts General Hospital, Boston, MA). Parasitemia of infected RBCs (iRBCs) was assessed every 2 or 3 days by microscopy of Giemsa-stained thin blood smears, and 7 to 8 days later, when the parasitemia showed mostly ring stages and the mice suffered from cerebral malaria symptoms, blood was drawn and used for infection studies.
Wild-type, transgenic, and knockout mice were each infected with 105 iRBCs in 200 µl phosphate-buffered saline (PBS) by intraperitoneal injection. Survival and signs of disease were monitored daily. Animals that showed neurological signs, such as convulsions, ataxia, and paralysis, followed by death, between 7 and 12 days after infection were considered to have cerebral malaria (56). Whole blood was collected periodically in hirulin for assessment of CR1.
Cobra venom factor treatment.
For some experiments, animals received either four doses of cobra venom factor (CompTech, Tyler, TX), each dose at 200 µg/kg of body weight, administered intraperitoneally prior to infection and on days 3, 5, and 7 postinfection to deplete complement, or sterile normal saline (control animals).
Immunoblot assays.
Red blood cell lysates in NuPAGE LDS sample buffer (4×) were separated and then transferred to an Immobilon polyvinylidene difluoride (PVDF) membrane (Millipore) (57). Membranes were blocked with PBS-1% milk and probed with a polyclonal rabbit IgG, anti-CR1 affinity-purified antibody that was provided by J. P. Atkinson (Washington University, St. Louis, MO). CR1-reactive bands were detected with goat anti-rabbit IgG conjugated to alkaline phosphatase (Sigma-Aldrich) followed by the addition of 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (BCIP/NBT)-Purple Liquid substrate (Promega, Madison, WI). Rat anti-mouse TER-119 antibody was obtained from eBioscience, and TER-119 bands were detected with anti-rat IgG-alkaline phosphatase antibody produced in goat (Sigma-Aldrich).
Flow cytometry.
The relative expression of mouse erythrocyte CR1 was determined using the anti-CR1 monoclonal antibody 7G9 (provided by Ron Taylor, University of Virginia School of Medicine). Either 7G9 was directly labeled with Alexa Fluor 647 according to the manufacturer’s directions (Molecular Probes, Eugene, OR, USA), or a fluorescein isothiocyanate (FITC)-labeled secondary antibody was used for detection. The erythrocyte population was identified by staining with FITC-conjugated anti-TER-119 antibody. Subsequently, the cells were washed twice, and 100,000 cells were analyzed using an LSRII flow cytometer (BD Bioscience). Data were acquired with DIVA software (BD Bioscience) and analyzed with FlowJo (Tree Star).
Immune complex ELISA.
Plates were coated with purified human C1q at 5 µg/ml in PBS overnight at 4°C and then blocked with 1% bovine serum albumin (BSA) in PBS for 1 h. Serum from infected and uninfected animals was diluted 1:100 and added for 1 h at 37°C. Captured immune complexes were detected with anti-mouse IgG and anti-mouse IgM alkaline phosphatase (1:1,000 dilution in PBS-0.05% Tween 20).
Histopathology.
Eight days following P. berghei ANKA infection, brains were carefully removed and fixed in formaldehyde solution (4%, vol/vol). Tissue sections were prepared and stained with hematoxylin and eosin as described elsewhere (9). Slides were reviewed independently by a veterinary pathologist.
Statistical analysis.
All data were analyzed using GraphPad Instat 4.0 software. Unless stated otherwise, all comparisons were performed using a two-tailed Student t test. Mann-Whitney U testing was used for nonparametric analysis when data did not fit a Gaussian distribution. A P value of ≤0.05 was considered to be statistically significant.
SUPPLEMENTAL MATERIAL
(A) Infection of hCR1+ mice with P. berghei ANKA results in diminished detection of hCR1 on erythrocytes at day 7 postinfection. Flow cytometry histograms from experiments with four hCR1+ mice infected with P. berghei ANKA are shown over a time course. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 3, 6, 7, and 9. All infected hCR1+ mice were dead by day 9. (B) Treatment of hCR1+ mice with cobra venom factor (CoVF) results in a delay in the diminished detection of hCR1 to day 9 postinfection. Flow cytometry histograms from experiments with four hCR1+ mice infected with P. berghei ANKA and treated with CoVF are shown over a time course. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 3, 6, 7, and 9. A greater decrease in detection was observed at day 9 than at day 7 relative to uninfected hCR1+ mouse levels of erythrocyte CR1. The x axis for each panel indicates APC (hCR1); the y axis shows the percent of the maximum. Download
ACKNOWLEDGMENTS
We thank Ronald Taylor for his generous donation of antibodies and other reagents used in these studies and especially for his invaluable intellectual input. We thank Anna Cerny for animal husbandry, Nancy Nowak for assistance with immunoblotting assays, and Melanie Trombly with graphics.
This work was supported by Public Health Service grants NIH R01 AI079293 from the National Institute of Allergy and Infectious Diseases to D.T.G., NIH R01 AI054544 to S.R., and NIH R01 AI092105 to J.P.W.
Footnotes
Citation de Oliveira RB, Wang JP, Ram S, Gazzinelli RT, Finberg RW, Golenbock DT. 2014. Increased survival in B-cell-deficient mice during experimental cerebral malaria suggests a role for circulating immune complexes. mBio 5(2):e00949-14. doi:10.1128/mBio.00949-14.
REFERENCES
- 1. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431. 10.1016/S0140-6736(12)60034-8 [DOI] [PubMed] [Google Scholar]
- 2. Miller LH, Baruch DI, Marsh K, Doumbo OK. 2002. The pathogenic basis of malaria. Nature 415:673–679. 10.1038/415673a [DOI] [PubMed] [Google Scholar]
- 3. Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. 1996. Seizures and status epilepticus in childhood cerebral malaria. QJM 89:591–597. 10.1093/qjmed/89.8.591 [DOI] [PubMed] [Google Scholar]
- 4. Aikawa M. 1988. Human cerebral malaria. Am. J. Trop. Med. Hyg. 39:3–10 [DOI] [PubMed] [Google Scholar]
- 5. Aikawa M, Iseki M, Barnwell JW, Taylor D, Oo MM, Howard RJ. 1990. The pathology of human cerebral malaria. Am. J. Trop. Med. Hyg. 43:30–37 [DOI] [PubMed] [Google Scholar]
- 6. Pongponratn E, Riganti M, Punpoowong B, Aikawa M. 1991. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am. J. Trop. Med. Hyg. 44:168–175 [DOI] [PubMed] [Google Scholar]
- 7. Mukherjee AP, White JC, Lau KS. 1971. Falciparum malaria associated with jaundice, renal failure and anaemia. Trans. R. Soc. Trop. Med. Hyg. 65:808–814. 10.1016/0035-9203(71)90096-4 [DOI] [PubMed] [Google Scholar]
- 8. Akinosoglou KS, Solomou EE, Gogos CA. 2012. Malaria: a haematological disease. Hematology 17:106–114. 10.1179/102453312X13221316477336 [DOI] [PubMed] [Google Scholar]
- 9. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. 2011. Severe malarial anemia: innate immunity and pathogenesis. Int. J. Biol. Sci. 7:1427–1442. 10.7150/ijbs.7.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quintero JP, Siqueira AM, Tobón A, Blair S, Moreno A, Arévalo-Herrera M, Lacerda MV, Valencia SH. 2011. Malaria-related anaemia: a Latin American perspective. Mem. Inst. Oswaldo Cruz 106(Suppl 1):91–104. 10.1590/S0074-02762011000900012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delahaye NF, Coltel N, Puthier D, Flori L, Houlgatte R, Iraqi FA, Nguyen C, Grau GE, Rihet P. 2006. Gene-expression profiling discriminates between cerebral malaria (CM)-susceptible mice and CM-resistant mice. J. Infect. Dis. 193:312–321. 10.1086/498579 [DOI] [PubMed] [Google Scholar]
- 12. Delahaye NF, Coltel N, Puthier D, Barbier M, Benech P, Joly F, Iraqi FA, Grau GE, Nguyen C, Rihet P. 2007. Gene expression analysis reveals early changes in several molecular pathways in cerebral malaria-susceptible mice versus cerebral malaria-resistant mice. BMC Genomics 8:452. 10.1186/1471-2164-8-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grau GE, Craig AG. 2012. Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 7:291–302. 10.2217/fmb.11.155 [DOI] [PubMed] [Google Scholar]
- 14. Claser C, Malleret B, Gun SY, Wong AY, Chang ZW, Teo P, See PC, Howland SW, Ginhoux F, Rénia L. 2011. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One 6:e18720. 10.1371/journal.pone.0018720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ, Ferguson DJ, Pongponratn E. 2007. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop. Med. Int. Health 12:1037–1050. 10.1111/j.1365-3156.2007.01881.x [DOI] [PubMed] [Google Scholar]
- 16. Sitprija V, Indraprasit S, Pochanugool C, Benyajati C, Piyaratn P. 1967. Renal failure in malaria. Lancet i:185–188 [DOI] [PubMed] [Google Scholar]
- 17. Spitz S. 1946. The pathology of acute falciparum malaria. Mil. Surg. 99:555–572 [PubMed] [Google Scholar]
- 18. Stevenson MM, Riley EM. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169–180. 10.1038/nri1311 [DOI] [PubMed] [Google Scholar]
- 19. Maeno Y, Perlmann P, Perlmann H, Kusuhara Y, Taniguchi K, Nakabayashi T, Win K, Looareesuwan S, Aikawa M. 2000. IgE deposition in brain microvessels and on parasitized erythrocytes from cerebral malaria patients. Am. J. Trop. Med. Hyg. 63:128–132 [DOI] [PubMed] [Google Scholar]
- 20. Möller T. 2010. Neuroinflammation in Huntington’s disease. J. Neural Transm. 117:1001–1008. 10.1007/s00702-010-0430-7 [DOI] [PubMed] [Google Scholar]
- 21. Sturfelt G, Truedsson L. 2012. Complement in the immunopathogenesis of rheumatic disease. Nat. Rev. Rheumatol. 8:458–468. 10.1038/nrrheum.2012.75 [DOI] [PubMed] [Google Scholar]
- 22. Diamond B, Volpe BT. 2012. A model for lupus brain disease. Immunol. Rev. 248:56–67. 10.1111/j.1600-065X.2012.01137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liszewski MK, Atkinson JP. 2011. Too much of a good thing at the site of tissue injury: the instructive example of the complement system predisposing to thrombotic microangiopathy. Hematology Am. Soc. Hematol. Educ. Program 2011:9–14. 10.1182/asheducation-2011.1.9 [DOI] [PubMed] [Google Scholar]
- 24. Figueroa JE, Densen P. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel SN, Berghout J, Lovegrove FE, Ayi K, Conroy A, Serghides L, Min-oo G, Gowda DC, Sarma JV, Rittirsch D, Ward PA, Liles WC, Gros P, Kain KC. 2008. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J. Exp. Med. 205:1133–1143. 10.1084/jem.20072248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramos TN, Darley MM, Weckbach S, Stahel PF, Tomlinson S, Barnum SR. 2012. The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria. J. Biol. Chem. 287:24734–24738. 10.1074/jbc.C112.378364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pawluczkowycz AW, Lindorfer MA, Waitumbi JN, Taylor RP. 2007. Hematin promotes complement alternative pathway-mediated deposition of C3 activation fragments on human erythrocytes: potential implications for the pathogenesis of anemia in malaria. J. Immunol. 179:5543–5552 [DOI] [PubMed] [Google Scholar]
- 28. Spadafora C, Awandare GA, Kopydlowski KM, Czege J, Moch JK, Finberg RW, Tsokos GC, Stoute JA. 2010. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog. 6:e1000968. 10.1371/journal.ppat.1000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rowe JA, Moulds JM, Newbold CI, Miller LH. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292–295. 10.1038/40888 [DOI] [PubMed] [Google Scholar]
- 30. Melhorn MI, Brodsky AS, Estanislau J, Khoory JA, Illigens B, Hamachi I, Kurishita Y, Fraser AD, Nicholson-Weller A, Dolmatova E, Duffy HS, Ghiran IC. 2013. CR1-mediated ATP release by human red blood cells promotes CR1 clustering and modulates the immune transfer process. J. Biol. Chem. 288:31139–31153. 10.1074/jbc.M113.486035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Repik A, Pincus SE, Ghiran I, Nicholson-Weller A, Asher DR, Cerny AM, Casey LS, Jones SM, Jones SN, Mohamed N, Klickstein LB, Spitalny G, Finberg RW. 2005. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1). Clin. Exp. Immunol. 140:230–240. 10.1111/j.1365-2249.2005.02764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. 2000. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109:280–287. 10.1046/j.1365-2141.2000.02037.x [DOI] [PubMed] [Google Scholar]
- 33. Tippett E, Fernandes LA, Rogerson SJ, Jaworowski A. 2007. A novel flow cytometric phagocytosis assay of malaria-infected erythrocytes. J. Immunol. Methods 325:42–50. 10.1016/j.jim.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 34. Cochrane CG, Müller-Eberhard HJ, Aikin BS. 1970. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 105:55–69 [PubMed] [Google Scholar]
- 35. Pryjma J, Humphrey JH. 1975. Prolonged C3 depletion by cobra venom factor in thymus-deprived mice and its implication for the role of C3 as an essential second signal for B-cell triggering. Immunology 28:569–576 [PMC free article] [PubMed] [Google Scholar]
- 36. Kitamura D, Roes J, Kühn R, Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426. 10.1038/350423a0 [DOI] [PubMed] [Google Scholar]
- 37. Naicker S, Fabian J, Naidoo S, Wadee S, Paget G, Goetsch S. 2007. Infection and glomerulonephritis. Semin. Immunopathol. 29:397–414. 10.1007/s00281-007-0088-x [DOI] [PubMed] [Google Scholar]
- 38. Holers VM, Chaplin DD, Leykam JF, Gruner BA, Kumar V, Atkinson JP. 1987. Human complement C3b/C4b receptor (CR1) mRNA polymorphism that correlates with the CR1 allelic molecular weight polymorphism. Proc. Natl. Acad. Sci. U. S. A. 84:2459–2463. 10.1073/pnas.84.8.2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vik DP, Wong WW. 1993. Structure of the gene for the F allele of complement receptor type 1 and sequence of the coding region unique to the S allele. J. Immunol. 151:6214–6224 [PubMed] [Google Scholar]
- 40. Wilson JG, Wong WW, Schur PH, Fearon DT. 1982. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N. Engl. J. Med. 307:981–986. 10.1056/NEJM198210143071604 [DOI] [PubMed] [Google Scholar]
- 41. Waitumbi JN, Donvito B, Kisserli A, Cohen JH, Stoute JA. 2004. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J. Infect. Dis. 190:1183–1191. 10.1086/423140 [DOI] [PubMed] [Google Scholar]
- 42. Owuor BO, Odhiambo CO, Otieno WO, Adhiambo C, Makawiti DW, Stoute JA. 2008. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol. Med. 14:89–97. 10.2119/2007-00093.Owuor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. 2000. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood 95:1481–1486 [PubMed] [Google Scholar]
- 44. Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. 2003. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J. Infect. Dis. 187:522–525. 10.1086/367712 [DOI] [PubMed] [Google Scholar]
- 45. Ansar W, Habib SK, Roy S, Mandal C, Mandal C. 2009. Unraveling the C-reactive protein complement-cascade in destruction of red blood cells: potential pathological implications in Plasmodium falciparum malaria. Cell. Physiol. Biochem. 23:175–190. 10.1159/000204106 [DOI] [PubMed] [Google Scholar]
- 46. Sinha S, Jha GN, Anand P, Qidwai T, Pati SS, Mohanty S, Mishra SK, Tyagi PK, Sharma SK, Venkatesh V, Habib S. 2009. CR1 levels and gene polymorphisms exhibit differential association with falciparum malaria in regions of varying disease endemicity. Hum. Immunol. 70:244–250. 10.1016/j.humimm.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 47. Mackinnon MJ, Walker PR, Rowe JA. 2002. Plasmodium chabaudi: rosetting in a rodent malaria model. Exp. Parasitol. 101:121–128. 10.1016/S0014-4894(02)00103-0 [DOI] [PubMed] [Google Scholar]
- 48. Zimmerman PA, Fitness J, Moulds JM, McNamara DT, Kasehagen LJ, Rowe JA, Hill AV. 2003. CR1 Knops blood group alleles are not associated with severe malaria in the Gambia. Genes Immun. 4:368–373. 10.1038/sj.gene.6363980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thathy V, Moulds JM, Guyah B, Otieno W, Stoute JA. 2005. Complement receptor 1 polymorphisms associated with resistance to severe malaria in Kenya. Malar. J. 4:54. 10.1186/1475-2875-4-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fernandez-Arias C, Lopez JP, Hernandez-Perez JN, Bautista-Ojeda MD, Branch O, Rodriguez A. 2013. Malaria inhibits surface expression of complement receptor 1 in monocytes/macrophages, causing decreased immune complex internalization. J. Immunol. 190:3363–3372. 10.4049/jimmunol.1103812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yañez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC. 1996. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157:1620–1624 [PubMed] [Google Scholar]
- 52. Amante FH, Haque A, Stanley AC, Rivera FDL, Randall LM, Wilson YA, Yeo G, Pieper C, Crabb BS, de Koning-Ward TF, Lundie RJ, Good MF, Pinzon-Charry A, Pearson MS, Duke MG, McManus DP, Loukas A, Hill GR, Engwerda CR. 2010. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J. Immunol. 185:3632–3642. 10.4049/jimmunol.1000944 [DOI] [PubMed] [Google Scholar]
- 53. Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. 1997. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 114:7–12. 10.1017/S0031182096008293 [DOI] [PubMed] [Google Scholar]
- 54. Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, Mazier D, Combadiere B. 2003. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J. Immunol. 170:2221–2228 [DOI] [PubMed] [Google Scholar]
- 55. Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH. 1986. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 137:2348–2354 [PubMed] [Google Scholar]
- 56. Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E. 2006. Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol. Appl. Neurobiol. 32:177–188. 10.1111/j.1365-2990.2006.00706.x [DOI] [PubMed] [Google Scholar]
- 57. Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, Rice PA. 2008. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect. Immun. 76:339–350. 10.1128/IAI.00613-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Infection of hCR1+ mice with P. berghei ANKA results in diminished detection of hCR1 on erythrocytes at day 7 postinfection. Flow cytometry histograms from experiments with four hCR1+ mice infected with P. berghei ANKA are shown over a time course. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 3, 6, 7, and 9. All infected hCR1+ mice were dead by day 9. (B) Treatment of hCR1+ mice with cobra venom factor (CoVF) results in a delay in the diminished detection of hCR1 to day 9 postinfection. Flow cytometry histograms from experiments with four hCR1+ mice infected with P. berghei ANKA and treated with CoVF are shown over a time course. Freshly isolated erythrocytes from infected or uninfected mice were analyzed on days 0, 3, 6, 7, and 9. A greater decrease in detection was observed at day 9 than at day 7 relative to uninfected hCR1+ mouse levels of erythrocyte CR1. The x axis for each panel indicates APC (hCR1); the y axis shows the percent of the maximum. Download