Abstract
The construct of mild cognitive impairment (MCI) has evolved over the past 10 years since the publication of the new MCI definition at the Key Symposium in 2003, but the core criteria have remained unchanged. The construct has been extensively used worldwide, both in clinical and research settings, to define the grey area between intact cognitive functioning and clinical dementia. A rich set of data regarding occurrence, risk factors, and progression of MCI has been generated. Discrepancies between studies can be mostly explained by differences in the operationalization of the criteria, differences in the setting where the criteria have been applied, selection of subjects, and length of follow-up in longitudinal studies.
Major controversial issues that remain to be further explored are: algorithmic versus clinical classification, reliability of clinical judgment, temporal changes in cognitive performances, and predictivity of putative biomarkers.
Some suggestions to further develop the MCI construct include the tailoring of the clinical criteria to specific populations and to specific contexts. The addition of biomarkers to the clinical phenotypes is promising but requires deeper investigation. Translation of findings from the specialty clinic to the population setting, although challenging, will enhance uniformity of outcomes.
More longitudinal population-based studies on cognitive aging and MCI need to be performed to clarify all these issues.
Keywords: Alzheimer’s disease, dementia, memory impairment, mild cognitive impairment
Introduction
In the last decade much effort in the field of ageing and dementia has been devoted to the early clinical diagnosis of dementing disorders with the aim of identifying signs and symptoms that could be used as reliable predictive markers of disease development. Such identification, as within other areas of medicine, would allow the implementation of research to verify whether and which interventions at the early stages may change the natural history of the disorder. Multiple definitions have been proposed to capture the intermediate stage between healthy ageing with slight cognitive changes and dementia [1–4]. Of these clinical labels by far the most successful and enduring has been the term mild cognitive impairment (MCI) [3]. MCI was introduced as a clinical entity more than 20 years ago, and since then groups of individuals with this diagnosis have been intensively investigated from many perspectives including clinical, imaging, genetic, pathological and epidemiological [5].
The current articulation of the concept of MCI intends to identify this intermediate stage of cognitive impairment that is often, but not always, a transitional phase from cognitive changes of normal ageing to those typically found in dementia. The term MCI was introduced in the late 1980s by Reisberg and colleagues to characterize subjects who were at this intermediate stage; the identification of these subjects was based on the Global Deterioration Scale, when criteria for Stage 3 were fulfilled [5]. Petersen et al. in 1999 further developed the concept by proposing criteria based on an observational study of ageing [6]. This development was stimulated first by the clinical awareness of the existence of a grey zone of cognitive impairment that was not captured by any clinical definition and by the rising awareness of dementia as an important area of public health. Further, it was reinforced by the emerging clinical need of something beyond the binary diagnosis of the presence or absence of dementia, which could allow an earlier diagnosis and secondary prevention if new treatments were proved efficacious at these early stages.
Further development came some years later with the convening of a Key Symposium on this topic in 2003, which led to the publication of international criteria for MCI and broadening of the construct from earlier versions that had focused only on memory impairment [7, 8]. In response to a large body of evidence which suggested that a redefinition of the concept was required, the original articulation was revised. In the international criteria, MCI became a much broader construct referring to a clinical syndrome with multiple clinical profiles due to a variety of aetiologies. The assumption was that the new criteria would identify all individuals at the intermediate cognition stage and have a greater clinically utility. In the new definition, the initial purpose of MCI, directed specifically towards the detection of underlying Alzheimer’s disease (AD), was restricted to a subtype of MCI.
Since the publication of the international criteria, numerous studies have used the new MCI definition to collect or identify individuals in the early stages of cognitive impairment; these criteria have provided additional clinical features and genetic background, as well identified predictors of progression and pathological outcome [9, 10]. Many thousands of studies have been reported over the years including randomized controlled trials of a variety of medications mostly focused on MCI on the AD spectrum [11–14]. The concept has moved rapidly outside the research field providing clinicians with a helpful intermediate diagnosis, often for watchful waiting. Clinical research in this area has also influenced the development of new clinical criteria for practitioners [15].
In spite of all this attention, there has been and remains a considerable amount of controversy surrounding MCI. It has often been stated that MCI is heterogeneous and unstable, which are valid criticisms if the entity is only considered as a pre-AD dementia state. However, the current criteria are much broader with the consequence that heterogeneity will be much greater and predictive value lower, if specific subtypes are not taken into account. In addition, many other factors influence the meaning of the MCI label and its use in research and clinical activity, which has led to significant inconsistencies and disagreement with regard to the findings of different studies. Major factors include: (i) settings in which the criteria are used, (ii) age of subjects, (iii) operational criteria; (iv) implementation of the diagnostic criteria; (iv) retrospective versus prospective data collection; (v) algorithmic versus clinical application of the criteria; (vi) blindness with respect to previous diagnoses; (vii) length of follow-up of subjects when assessing outcome; and (viii) stability of the construct. These controversial issues will be the focus of the present review.
Definitions of MCI: evolution of a clinical concept
The first clinical criteria for MCI were proposed by a group of investigators from the Mayo Clinic in the late 1990s [6]. The criteria were derived from the clinical observation of signs and cognitive performance in patients in a longitudinal study of ageing and dementia in the community. While the study was designed to characterize normal ageing and dementia, it became apparent that a sizeable group of subjects were ‘in between’ and the concept of MCI was thus introduced to better describe these subjects. As shown in Table 1, the original Mayo Clinic MCI criteria required the presence of memory complaints corroborated by objective deficits on tests of episodic memory, in individuals who did not have dementia. Furthermore, to be classified as having MCI, general cognitive functioning needed to be preserved, as well as the capability to perform daily life activities independently (Table 1). This first definition of MCI was clearly focused on memory problems which were regarded as prodromal signs of incipient AD (parallelling the purpose of an early detection, as in the case of other medical disorders). Mild deficits in cognitive domains other than memory were allowed, but isolated deficits in non-memory domains were not taken into account. When these criteria were investigated by other researchers and in other settings, it became clear that not all forms of MCI evolve into AD and that other underlying causes can lead to MCI. Thus, a broader conceptualization became necessary.
Table 1.
Clinical characterization of mild cognitive impairment (MCI)
| Definitions | Original Mayo Clinic [6] | Expanded/Key Symposium [7, 8] | NIA-AA [16] | DSM-5 [15] |
|---|---|---|---|---|
| Criteria | ||||
| Self- or informant-reported memory complaint | x | |||
| Self- or informant-reported cognitive complaint | x | x | x | |
| Objective memory impairment | x | |||
| Objective cognitive impairment | x | x | x | |
| Essentially preserved general cognitive functioning | x | |||
| Preserved independence in functional abilities | x | x | x | x |
| No dementia | x | x | x | x |
Core clinical criteria according to major definitions are listed.
NIA-AA, National Institute on Aging-Alzheimers Association workgroup; DSM-V, fifth edition of the Diagnostic and Statistical Manual of Mental Disorders; X: criterion required..
To reach an agreement on the clinical characterization of MCI, an international consensus conference was held in 2003. The discussion at the first Key Symposium on MCI led to the formulation of revised core criteria for this condition [7]. The expanded Mayo Clinic criteria for MCI were no longer focused on memory impairment alone but were broadened to include impairment in other areas of cognitive functioning [8]. The additional cognitive syndromes now included in the definition of MCI also led to clarification of the ‘essentially preserved general cognitive functioning criterion (Table 1).
More recently, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) charged a workgroup with the task of re-discussing MCI criteria along the AD spectrum [16]. The NIA-AA proposed criteria for the specific definition of MCI due to AD (see following section), but the core clinical criteria overlap with those proposed by the 2003 MCI Key Symposium (Table 1).
Newly proposed entity
The American Psychiatric Association has recently published new criteria for dementia in the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5), which recognize the pre-dementia stage of cognitive impairment. The condition, which has many of the features of MCI, is termed mild neurocognitive disorder (NCD) [15]. Mild NCD recognizes subtle features of cognitive impairment that are distinct from ageing but do not represent dementia. Futhermore, mild NCD concerns the initial phases of cognitive disorders and precedes major NCD which is analogous to the previous diagnosis of dementia. The criteria for mild NCD closely resemble the expanded core MCI criteria outlined in Table 1, including the following features:(i) clinical concern raised by the patient or an informant, or observations made by the clinician, (ii) cognitive impairment in one or more cognitive domains preferably relative to appropriate normative data for that individual, (iii) preservation of functional independence and (iv) no dementia [15]. These criteria are in line with previously described MCI criteria and, while no definite neuropsychological cut-off scores are recommended, there is the implication that neuropsychological testing can be very helpful in making the diagnosis. The DSM-5 approach involves the characterization of the syndrome, mild or major NCD, and then a subsequent task of determining its aetiology, such as AD, frontotemporal degeneration, Lewy body disorders or vascular cognitive impairment. This approach suggests that biomarkers are likely to be incorporated into the decision process, but most are not validated at present for use in routine clinical practice and remain areas of major research interest.
Operationalization of MCI
According to current definitions [7, 8, 15, 16], clinical data suggesting a change in cognitive abilities is necessary for being classifed as MCI. This information is generally gathered through questions asked to the person examined or to the next of kin. The subjective cognitive complaint then needs to be confirmed by objective cognitive measures, such as neuropsychological test batteries. Objective cognitive impairment is defined as a poor performance in one or more cognitive measures, which suggests deficits in one or more cognitive areas or domains. There is no gold standard to specify which neuropsychological test battery to use, but it is important that all the main cognitive areas are examined. Typically, executive functions, attention, language, memory and visuospatial skills are taken into account. Regarding the report of cognitive complaints and objective cognitive impairment, the clinical diagnosis of MCI overlaps with the diagnosis of dementia. The two syndromes differ in the further requirement for MCI cases to have preserved independence in functional abilities. This is usually investigated by means of a thorough interview with the person and with the next of kin, and registered in terms of activities of daily living (ADL) and instrumental activities of daily living (IADL) scales. Very mild problems in instrumental ADL are generally consistent with MCI, while basic ADL should be preserved. Finally, individuals who are still functionally independent will also be classified as ‘not demented’, which is another prerequisite for the MCI classification. For a correct classification of MCI, it is also relevant that the diagnosis of dementia is accurate and follows standard criteria.
MCI subtypes
Although available instruments or markers to discriminate aetiological subtypes of MCI are still far from being accurate, several promising attempts to classify cognitive impairment according to its aetiology have been made. The first comprehensive clinical conceptualization of MCI subtypes was presented together with the revised Mayo Clinic criteria for MCI [8]. Patients with MCI ascertained according to the core clinical criteria (Table 1) could be classified into one of two categories: amnestic MCI (a-MCI) if performance on neuropsychological tests of episodic memory was poor, and non-amnestic MCI (na-MCI) in the case of poor performance on neuropsychological tests covering cognitive domains other than memory, such as executive functions, language or visuospatial abilities. The impairment could be restricted to one cognitive domain (MCI single domain) or to multiple domains (MCI multiple domains), and thus a patient could be classified in one of four possible clinical subtypes: (i) a-MCI–single domain, (ii) a-MCI–multiple domain, (iii) na-MCI–single domain and (iv) na-MCI–multiple domain. The clinical characterization could integrate information coming from the anamnesis as well as from laboratory tests and neuroimaging, when available, to guide the clinician in formulating hypotheses regarding the progression of the cognitive impairment syndromes. Specifically, the central idea was that, through the combination of clinical subtypes and putative aetiologies, it could be possible to predict the type of dementia that MCI patients would develop [8].
Recently, the NIA-AA workgroup defined research criteria for MCI due to AD to determine the aetiology of cases of MCI previously ascertained according to the core clinical criteria [16]. This should provide a guideline for investigators when using the suggested biomarkers of AD pathology in predicting possible MCI progression. The NIA-AA workgroup also clearly specified that biomarkers remain uninformative for the definition of MCI, which needs to be assessed based on core clinical criteria [7, 8, 15, 16]. However, for research purposes (e.g. in clinical trials) the use of biomarkers could aid in identifying aetiological MCI subtypes by differentiating between MCI due to AD and MCI that is unlikely to be due to AD. Currently two main sets of biomarkers can help in formulating this clinical judgment: biomarkers of amyloid-beta (Aβ) deposition and of neuronal injury. According to the NIA-AA workgroup [16], valid indicators of Aβ deposition are: (i) cerebrospinal fluid concentrations of Aβ42 (CSF Aβ42) and (ii) positron emission tomography (PET) amyloid imaging; valid indicators of neuronal injury are: (i) CSF tau/phosphorylated tau, (ii) hippocampal volume or medial temporal atrophy by volumetric measures or visual rating, (iii) rate of brain atrophy, (iv) fluoro-deoxyglucose (FDG) PET imaging and (v) SPECT perfusion imaging. The NIA-AA notes that some neuroimaging techniques such as functional magnetic resonance imaging (MRI), MRI perfusion, magnetic resonance spectroscopy, and diffusion tensor imaging are not sufficiently validated to be included as biomarkers at present. In conclusion, by combining information from these two types of biomarkers, evidence of amyloid deposition and neuronal injury, it will be possible to weight the probability of identifying MCI due to AD pathology (see Table 2).
Table 2.
NIA-AA MCI aetiological subtypes based on biomarkers
| MCI due to AD | Biomarkers of Aβ deposition | Biomarkers of neuronal injury |
|---|---|---|
| Intermediate likelihood | Positive | Untested |
| Untested | Positive | |
|
| ||
| High likelihood | Positive | Positive |
|
| ||
| Unlikely | Negative | Negative |
NIA-AA, National Institute on Aging-Alzheimers Association workgroup; MCI, mild cognitive impairment; AD, Alzheimer’s disease; Aβ, amyloid-beta.
Similarly to the aetiological approach proposed by Petersen in 2004 [8], the recent DSM-5 criteria [15] use a two-step procedure which includes first classifying the subjects clinically as having mild-NCD or major-NCD and then attempting to determine the underlying aetiology of the clinical syndrome. The subsequent aetiological categories include AD, frontotemporal dementia, vascular cognitive impairment, dementia with Lewy bodies, Parkinson’s disease, Huntington’s disease, HIV/AIDS, traumatic brain injury and substance abuse. There are various criteria to be employed in making the aetiological diagnoses of each of these categories. For AD, the biomarkers are considered but only for future possible use. Neuropathological population-based studies have long shown the development of considerable pathology in individuals who do not express clinical dementia (see below), and these findings are being suggested by emerging biomarker studies, therefore the validity of these biomarkers is still unproven.
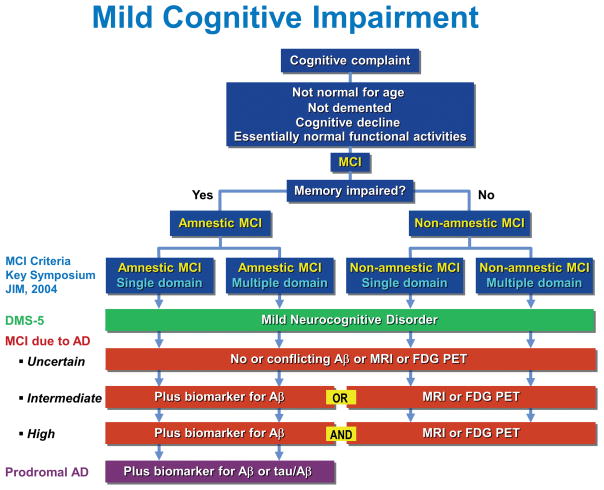
In summary, Fig. 1 shows a comparison of the current criteria for MCI. The original MCI clinical criteria from the 2003 Key Symposium [7, 8] are reproduced at the top of the figure and divide the clinical spectrum into a-MCI and na-MCI. The DSM-5 criteria for mild NCD are essentially the same as the Key Symposium criteria and encompass a-MCI and na-MCI [15]. MCI due to AD criteria are divided into three categories, uncertain, intermediate and high, reflecting the probability that the clinical syndrome, essentially outlined above as the MCI criteria from the Key Symposium, are due to underlying AD [16]. The uncertain category refers to the clinical criteria alone or to conflicting and unhelpful biomarkers, if available. At the intermediate level, in addition to the clinical syndrome of MCI from the Key Symposium, biomarker data are available and positive for either the presence of Aβ by PET or CSF or evidence of neurodegeneration as depicted by atrophy on MRI, hypometabolism on FDG PET or the presence of elevated total or phosphorylated tau in the CSF. The high probability of MCI due to AD results from a combination of both evidence of Aβ and neurodegeneration. Finally, prodromal AD relates to only the amnestic portion of the MCI clinical criteria, and it is augmented by evidence of Aβ either from PET scanning or CSF or the presence of an abnormal tau/Aβ ratio in the CSF.
Fig. 1.
Comparison of current criteria for mild cognitive impairment (MCI). The criteria outlined in blue were proposed at the Key Symposium in 2003 [7, 8]. Other criteria include those of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [15] and MCI due to Alzheimer’s disease (AD) [16]. Aβ amyloid-beta; MRI,magnetic resonance imaging; FDG PET, fluorodeoxyglucose positron emission tomography; tau, tau protein.
Current knowledge
Prevalence
During the last decade, an increasing number of studies have been conducted in an attempt to estimate the prevalence of MCI in the general population. Initially the frequency of MCI in the population was underestimated, as well as its incidence among individuals with healthy cognitive functioning. Indeed, the first round of epidemiological investigations of MCI adopted the original Mayo Clinic MCI criteria which were restricted to isolated memory impairment. In more recent studies the expanded MCI criteria have been used, producing considerably higher estimates. From the major population-based studies using the expanded Mayo Clinic criteria [17–32], the average prevalence of MCI is 18.9% (see Table 3), which is almost three times higher than the prevalence of 7% derived from the major population-based studies of a-MCI reported in a recent review [33]. Similarly, average incidence rates using the expanded MCI criteria are 47.9 (range 21.5–71.3) per 1000 person-years, which is more than three times higher than the value of 15.2 (range 8.5–25.9) per 1000 person-years derived by averaging incidence rates of a-MCI from major population-based studies [33].
Table 3.
Prevalence estimates of MCI defined according to expanded Mayo Clinic criteria in major population-based studies
| Study | Location | Reference | n | Age (years) | Prevalence % |
|---|---|---|---|---|---|
| CHS | USA | Lopez et al. 2003 [17] | 1690 | ≥75 | 22.0 |
| EPESE | USA | Purser et al. 2005 [18] | 3673 | ≥65 | 24.7 |
| LEILA 75+ | Germany | Busse et al. 2006 [19] | 980 | 75–79 | 19.3 |
| Kolkata | India | Das et al. 2007 [20] | 745 | ≥50 | 14.9 |
| ILSA | Italy | Di Carlo et al. 2007 [21] | 2830 | 65–84 | 16.1 |
| KP | Sweden | Palmer et al. 2008 [22] | 379 | ≥75 | 11.1 |
| 3C | France | Artero 2008 et al. [23] | 6892 | ≥65 | 42.0 |
| Manhattan | USA | Manly 2008 et al. [24] | 2364 | ≥65 | 21.8 |
| ADAMS | USA | Plassman et al. 2008 [25] | 856 | ≥71 | 22.2 |
| Mayo | USA | Petersen et al. 2010 [26] | 1969 | 70–89 | 14.8 |
| CSBA | Italy | Ravaglia et al. 2008 [27] | 1016 | ≥65 | 7.7 |
| Tone Town | Japan | Sasaki et al. 2009 [28] | 1888 | ≥65 | 18.9 |
| MYHAT | USA | Ganguli et al. 2010 [29] | 2036 | ≥65 | 16.6 |
| HNR | Germany | Dlugaj et al. 2010 [30] | 4145 | 50–80 | 7.8 |
| South Korea | Korea | Kim et al. 2011 [31] | 1673 | ≥65 | 24.1 |
| MemoVie | Luxemburg | Perquin et al. 2012 [32] | 1377 | 65–70 | 20.0 |
Risk factors
The study of the possible factors that can promote or predict the development of MCI and the different subtypes is relatively new to dementia research and most of the available information has been collected during the last 10 years. Also, it is often unclear whether identified factors are true hazards for MCI or merely predictors of its occurrence, including reverse causation.
As expected, the major risk factors associated with cognitive decline and dementia, i.e. older age and lower level of education, have also been repeatedly associated with MCI [34]. However, there is no strong agreement on the extent of the association. This is probably due to the fact that neuropsychological test scores used in the MCI classification are generally corrected for age and education. Moreover, MCI is by definition often a transitory state and the setting in which the case series are collected can also impact on risk profiles.
There is also no strong agreement regarding the effect of gender on MCI occurrence; however, some recent findings point towards a possible association with male gender [35, 36]. The association between MCI and the well-known ε4 allele of the AD susceptibility gene APOE has been widely investigated and findings have been generally consistent with results for dementia and AD [37–39]. However, a strong genetic base of MCI is not supported by concordance studies among twins, which showed no difference in level of concordance between monozygotic and dizygotic twins [40]. The presence of comorbidities is another widely studied variable in relation to MCI [41, 42]. In particular, the association between MCI and vascular diseases was assessed in several studies, with mixed results. Indeed, while cross-sectional studies have often demonstrated increased odds of MCI in individuals with vascular diseases such as stroke [43–46] or heart disease [43, 47], the majority of prospective studies showed no association [48–50]. A similar pattern can be observed for diabetes, with the findings of cross-sectional studies suggesting an association [45, 46, 51] and the majority of prospective studies showing no association with incident MCI [48, 50, 52]. A possible explanation for these discrepant findings can be identified in competing outcomes, i.e. the elevated risk among patients with vascular disease of death or progression to dementia during the follow-up interval and before MCI can be detected. Neuropsychiatric symptoms are common in individuals with MCI and in particular depression has been extensively studied as a possible risk factor/predictor of MCI [53–55]. Regarding environmental exposures/lifestyle, physical activity is currently considered a key factor in the prevention of MCI [56], as for dementia and cognitive decline, although the degree of preventive potential is unknown and as yet few prospective studies of physical activity and cognitive functioning have been conducted. Moreover, a careful review of the current evidence suggests that sustained physical, social and cognitive activities can all contribute to postponing or preventing MCI [57, 58], and therefore prospective studies to investigate the combined effect, as well as the individual contribution of each of these factors, are warranted.
Neuropathology
According to a comprehensive review including 162 studies of MCI, the pathological profile of MCI is heterogeneous and many lesions seem to be on a continuum between dementia and cognitively intact individuals [59]. Indeed, it has been frequently observed that neuropathological alterations are present even in persons who do not manifest any cognitive impairment, particularly in older age groups [59]. This can explain why no definitive profile that characterizes the state of MCI has been identified by neuropathological studies. In addition, the above discussion of MCI due to AD and more general MCI is relevant here insofar as uniform pathology would not be expected in the broader definition of MCI.
Pathological changes observed in MCI are similar to those observed in dementia and in the cognitively intact elderly. In fact, as noted above, a single neuropathological substrate would not be anticipated because MCI can represent a variety of conditions. This is similar to dementia itself; there is no single pathological explanation because it represents multiple substrates. Even in cases that do not fulfill all MCI criteria, often labelled pre-MCI, the neuropathological profile was similar to that observed in cases with confirmed AD, similar to findings reported in a-MCI and expanded MCI [59]. Selection bias may have been part of the difficulty in identifying MCI profiles. Studying the neuropathology of MCI, because of its transitory status and the phenomenon of terminal decline in cognition is very problematic. The largest population-based studies which include brain donation have only been able to identify small numbers of such intermediate states for study. Combining data from those who have died with a similar cognitive profile will allow to assemble larger sample sizes with standard neuropathological measures. This will be valuable to establish the degree of heterogeneity and hence utility of the clinical definitions in relation to the ambition to move towards prevention.
Neuroimaging
Neuroimaging with its various structural and functional modalities has provided evidence of neurobiological changes across the trajectory of normal ageing–MCI–dementia, and AD in particular [60]. Structural MRI studies have identified the key areas of atrophy: the medial temporal lobe, reflecting entorhinal and hippocampal volume loss, and the posterior cingulate [61]. Furthermore, longitudinal studies have shown that acceleration of the annual rate of hippocampal atrophy as well as rates of cortical atrophy and ventricular expansion are good predictors of AD progression in MCI subjects [62, 63]. However, a recent review of previous studies on the progression towards AD in MCI subjects revealed accuracy figures between 56% and 82% [64].
FDG PET studies have shown substantial impairment in temporoparietal and posterior cingulate association cortices in MCI subjects who progress rapidly to dementia, and AD in particular [65]. Using amyloid-labelling ligands, such as the most popular C-11 Pittsburgh compound B, PET enables molecular imaging of regional cerebral patterns of amyloid pathology and has shown increased Aβ burden in progressive MCI, particularly in the lateral frontal cortex, posterior cingulate cortex, regions of the medial and lateral parietal lobe, and the lateral temporal lobe [66, 67]. Both MRI and FDG PET are surrogate markers of neuronal degeneration in a model of temporal evolution of disease-specific pathology proposed by Jack et al. [68, 69]. According to this hypothesis, amyloid biomarkers show abnormalities earlier than markers for neuronal degeneration, possibly 10–20 years before first symptom occurrence. In comparative studies in which different biomarkers were combined in a prediction model of MCI, FDG-PET together with episodic memory test was a strong predictor of the clinical transition to AD, whereas CSF biomarkers primarily reflected rate of longitudinal cognitive decline independent of disease severity [70, 71]. In logistic regression models combining clinical information with MRI imaging, CSF proteins and FDG PET, the latter added the most prognostic information [72].
Finally, NIA-AA criteria were used recently in a population-based sample of 450 cognitively normal persons who underwent a complete assessment including neuroimaging and CSF [73]. While 43% of subjects did not fulfill criteria for preclinical AD and had negative markers, 31% were in preclinical stages 1–3 and 23% were classified as having suspected non-AD pathophysiology (SNAP), which is characterized by normal amyloid biomarkers but abnormal biomarkers of neuronal injury. In a recent study of MCI by the same group from the Mayo Clinic Study of Aging, 14% of all MCI subjects were biomarker negative, and 14% were positive for amyloid alone, 43% for amyloid plus neuronal injury and 29% for neuronal injury alone (MCI-SNAP) [74]. Most of the progression to dementia was predicted by the amyloid plus neuronal injury or neuronal injury alone. Correlative clinicopathological studies have revealed the existence of multiple pathologies which contribute to determining cognitive function during the life course [75–77]. Therefore, it is plausible to expect that a combination of different imaging modalities and biomarkers performs better in terms of diagnostic classification accuracy at the group but also at the individual level when interpreted in the context of presenting clinical symptoms.
Treatment
Currently, there is no pharmacological treatment that is recommended for MCI. Indeed, although during the last 10–15 years a great effort has been made to individuate compounds capable of slowing down cognitive decline in subjects with MCI, none of these agents has proven effective. A recent systematic review, including nine randomized clinical trials (RCTs) and a total of 5149 persons with MCI, reported essentially no effect of cholinesterase inhibitors (donezepil, galantamine and rivastigmine) on cognitive test scores or on the progression to dementia within 3 years [78]. The findings of one study of vitamin E and donepezil suggested a positive effect of donepezil up to 12 months, and up to 36 months in ApoE4 carriers, but overall the rate of progression to Alzheimer’s disease after three years was not lower among patients treated with donepezil than among those given placebo [11]. Similarly, a systematic review on the efficacy of vitamin E for the treatment of MCI, including three RCTs and a total of 1167 participants with MCI, reported no substantial evidence that vitamin E is of benefit in the treatment of MCI [79]. One of the studies included in the review actually found that, even in subjects for whom vitamin E was effective in lowering oxidative stress markers, there was no significant difference in the percentage change in mini-mental state examinationcore between those with MCI and control subjects [80].
Considering the negative results of multiple RCTs using cholinesterase inhibitors and the lack of RCTs using memantine in MCI, there is no support from regulatory or clinical practice guidelines for the use of cholinesterase inhibitors or memantine in MCI. This is in contrast to the common practice of prescribing these drugs to subjects with MCI in some parts of the world. The need to do something useful for the patient cannot replace the lack of evidence for benefit and potential harm of using such drugs.
There is some evidence of a possible benefit on MCI from non-pharmacological interventions, such as cognitive training and physical exercise, activities that may be neuroprotective or compensatory. A recent review showed how several studies demonstrated the efficacy of cognitive training in MCI measured as improved performances in tests of global cognitive functioning, memory and meta-memory [81, 82]. A limitation of these findings is the small sample sizes of the individual studies. Only seven RCTs were identified by a systematic review [82], with a total of 296 MCI subjects who were cognitively treated. Most of these studies in fact included samples of fewer than 50 individuals; therefore, replication of the findings in larger RCTs is warranted.
A rapidly growing body of evidence suggests that exercise, specifically aerobic exercise, may attenuate cognitive impairment [57]. A systematic review of the effect of aerobic exercise on cognitive performance in individuals with neurological disorders found modest improvements in attention and processing speed, executive function and memory [83]. Therefore, as for the case of cognitive training, larger RCTs specifically in subjects with MCI, are warranted to confirm or refute these preliminary results. In particular, there is a strong need for evidence regarding the combined effect of multiple non-pharmacological interventions on MCI evolution and ongoing multidomain RCTs of MCI are particularly relevant [84]. A further possibility is to combine pharmacological and non-pharmacological interventions and evaluate whether their joint effect has more therapeutic value than the individual treatments alone. Such studies could also be combined with therapeutic trials.
MCI: controversial issues
As demonstrated above, current knowledge of MCI is limited by inconsistent findings. In many ways the rapid uptake of this research diagnosis into clinical settings has been premature. However, this uptake does reflect a clinical need, given the high awareness of cognitive disorders in populations of the Western world. Clinicians are quick to recognize persons with such cognitive features but have not known how to classify them. Variability in epidemiological estimates, lack of agreement in the identification of risk and progression factors, lack of specific pathological and clinical markers and difficulties in finding effective treatments are good examples of the limitations of this field. Many of these differences are accounted for by the lack of a population framework in which to interpret the results and determine their true value for ageing populations [85]. We have tried to identify the major causes of these limitations, as discussed below and summarized in Table 4.
Table 4.
Summary of major controversial issues for mild cognitive impairment (MCI) research
| SUBJECTS | IMPLEMENTATION OF CRITERIA | DATA COLLECTION |
|---|---|---|
|
Source Memory clinic vs. community Age Middle aged vs. elderly |
What test battery? What cut-off? What norms? |
Prospective vs. retrospective case finding |
| CLASSIFICATION | BLINDEDNESS | FOLLOW-UP |
|
Standardized algorithm vs. clinical judgment |
Awareness vs. blindedness (to clinical history) |
Short-term vs. long-term predictivity |
Source of subjects
Several studies have shown that the characterization of subjects with MCI and their outcome can be influenced by the setting in which the criteria are applied. For example, subjects attending a memory disorders or dementia clinic or an AD centre are likely to have significant cognitive impairment at the time of assessment. That is, the likelihood that they will be cognitively impaired with either MCI or dementia is much higher than if subjects from the community were assessed in an epidemiological study [85]. As such, MCI prevalence is much higher in referral clinics than in the general population, and the rates of progression to greater degrees of cognitive impairment or dementia are also much higher [86]. Positive predictive value is strongly influenced by this prevalence. In general, the progression rate to dementia in many referral clinics is in the range of 10–15% per year, whereas progression rate in the general population, prospectively sampled in epidemiological studies, tends to be around 5–10% per year [86]. This not only implies the obvious lack of comparability among studies based on different types of population, but the issue of the breadth of the construct of MCI is also relevant here. In the clinic setting, the subset of individuals with MCI is more likely to be on the AD pathophysiological spectrum; whereas in the community setting, MCI due to any aetiology is more likely to be found. In addition, the MCI detected in the clinic setting is more likely to be due to a single entity, whereas multiple comorbidities are common in the community. In other words, it seems that the MCI construct identifies different clinical syndromes depending on the populations to which the criteria are applied. At the community level, a larger spectrum, probably of the same clinical syndrome, is captured by the current MCI criteria and this obviously will affect prognostic outcomes. Further, in the specialized clinical setting it is likely that the selection of MCI subjects is driven not only by severity of the symptoms, but also by other factors such as presence of other dementia cases in the family, educational level of the subjects, and presence of serious comorbidities. A major challenge for future research is to overcome this ambiguity by at least identifying the setting in which the criteria might be applied. This implies that researchers need to articulate clearly the potential value of MCI diagnosis, considering the evolving heterogeneous state in the general population and the more specific aims for individuals within diverse clinical settings.
Age of subjects
The age of the subjects involved in a research study can have an important impact on the nature of the underlying aetiology of the MCI and on the occurrence and progression rate [22, 26, 36]. For example, middle-aged subjects diagnosed with MCI are most likely to have a single aetiological entity such as early-onset AD. In even younger populations, conditions such as HIV/AIDS, traumatic brain injury and psychiatric disorders can be relatively more common. However, when MCI is detected in persons of 70 years of age and above – and even more so in those of 80 years and above – a degenerative or a mixed aetiology is more likely to be inferred. Most of the epidemiological studies focused on older populations have reported a preponderance of AD-type clinical characterization, and neuropathological data have shown that mixed AD/vascular features are more common than pure AD chartacteristics [87, 88]. Indeed, MCI criteria are less problematic when applied to younger patients but given that the younger age groups are at lower risk of incident dementia, any assessment will have reduced positive predictive value, despite good sensitivity and specificity.
Finally, in very old populations, MCI may be due to several causes including systemic disorders, brain tumours, subdural haematoma, multiple morbidities, medications, psychiatric disorders and terminal decline. This implies that the MCI construct should include a list of possible causes, preferably age-related, that should be excluded when the research aim is to estimate the likelihood of progression of MCI to AD or other dementias. However, for clinical purposes the inclusion of these forms in the MCI definition is essential and very relevant to the identification of those forms of MCI in the general population that can be successfully treated.
Implementation of MCI criteria
Several of the MCI studies conducted around the world have used a similar set of criteria, as outlined above. However, the manner in which the criteria have been implemented has varied greatly. This is not surprising as the characterization of subjects with MCI can depend heavily on the cognitive measurements employed, the norms used, age and education as well as cultural contexts. Therefore, if different instruments are used in various studies, prevalence and incidence estimates as well as rates of progression will differ considerably. Some studies, such as RCTs, use a specific cut-off score on a cognitive screening test for enrolment, and the chosen cut-off value can influence the estimated rate of progression [11–13]. Observational studies also employ variable criteria and therefore estimated figures can vary considerably. As an example, it is clear to both clinicians and researchers that the characterization of the groups is influenced by the specific cut-off scores that are employed to make the distinctions between a-MCI versus na-MCI or single- versus multiple-domain MCI. However this situation is not different from that of other clinical entities involving cognitive impairment. That is, there is no standard set of measuring instruments or cut-off scores for most of the cognitive disorders encountered in clinical practice including dementia, AD, frontotemporal degeneration, dementia with Lewy bodies and vascular cognitive impairment. Therefore, while this is a source of variability in MCI, it is not unique to MCI as a clinical diagnosis.
It is evident that lack of specific operational criteria leads easily to variability in research and even more when the MCI criteria are applied in the clinical daily routine. While it is difficult to identify a specific cognitive battery for MCI, it is reasonable to develop guidelines to better clarify which cognitive domains and how many tests for each domain should be used. Similar effort should be made with regard to functional impairment. It seems possible that in the near future there may be agreement between researchers and clinicians to identify a core, culturally generalizable, set of principles of specific and explicit value with further standardization and norm generation for particular populations and population subgroups.
Retrospective versus prospective data collection
In the early years of MCI research, databases from population-based studies including information already collected within the cohorts were used to identify MCI cases applying the available criteria for MCI derived from the clinical setting. The retrospective application of MCI criteria to already collected information has been hampered by the need to adopt a particular algorithm to categorize subjects, particularly with cognitive instruments. Many clinical cohorts were not recruited with an emphasis on the milder end of the cognitive spectrum, thus providing a less than ideal base for the study of MCI. Some earlier studies included MCI equivalents according to the Cambridge Mental Disorders of the Elderly Examination, CAMDEX) minimal dementia criteria. However, all these approaches have subtle differences and even a difference of a single point on the cut-off value will have major effects on prevalence estimates of this varying concept. This is not different from the dementia literature in which different diagnostic criteria show hugely different prevalence estimates depending on how and which criteria are used. Recent prospective cohort studies [89, 90] as well as follow-up studies from specialized memory clinics have enhanced the reliability of MCI diagnoses.
Algorithmic versus clinical application of criteria
As mentioned above, algorithmic categorizations of subjects with MCI was a necessity when applying the criteria retrospectively, i.e. to previously collected data from clinical cohorts. In addition, using algorithms prospectively can be useful but may also give rise to variability among different centres if different cut-off scores are used to categorize individual subjects. However, even the clinical application of the criteria without specified cut-off values is affected by the same, or sometimes even greater, imprecision. The use of a standardized procedure has the advantage of objectively characterizing subjects and promoting repeatability, features that are essential to clinical trials (and to any decision based on standardized criteria, such as eligibility for medications or insurance coverage), but is at odds with much usual clinical practice. That is, as mentioned above, clinical practice involves the incorporation of a clinical history, neurological examination, psychiatric examination and neuropsychological testing to make a final judgment. While cognitive and functional measures can be very helpful in making this determination, they often only influence the final clinical judgment; while clinical judgment may not be suitable for repeatability, it more closely simulates actual practice involving diagnosing individuals from a variety of backgrounds and with varying degrees of cognitive impairment. Also, it should be noted that in many of the RCTs cited above, there was good agreement between many centres in the diagnosis of MCI due to AD. However, research on the variability between medical practitioners in the clinical diagnosis of dementia even in the specialized setting is vast and it is likely the MCI diagnosis is not to be an exception. Clinical judgment is related to many factors: specialization within the medical and allied professions, subspecialization, length of experience and whether clinicians follow patients over extended periods to determine the disease course, and the settings in which individual practitioners work. The challenge for the future is to be able to incorporate all these important clinical aspects in a detailed structured and standardized procedure in which the clinical history and temporal changes are the major focus.
Blindedness
An important feature in evaluating the outcome of subjects with MCI relates to the degree to which the observers making the ultimate diagnosis were aware of previous diagnoses. The appraisal of previous clinical diagnoses is likely to influence subsequent diagnoses; in longitudinal prospective cohort studies, while this knowledge does simulate clinical practice more accurately, it also complicates the ability to develop independent predictors of subsequent progression. On the other hand, studies that are designed to treat each ‘subject encounter’ as an independent assessment, with no knowledge of previous evaluations (blindedness), allow for the independent assessment of each predictor variable. However, blindedness of the clinicians also leads to increased variability in following the longitudinal course of subjects. That is, subjects who are at the threshold of subtle impairment may be classified as having MCI on one occasion, normal on the next and subsequently MCI again. This is to be expected given the mild nature of the impairment. Similar determinations are seen in other areas of medicine, such as labile hypertension preceding the actual diagnosis of hypertension and glucose intolerance preceding the diagnosis of diabetes mellitus.
Length of follow-up
Several of the early studies focusing on the longitudinal outcome of subjects with mild MCI demonstrated a great deal of instability in rates of progression. Some studies showed as high as a 40% reversion to normal rate in patients diagnosed with MCI [91]. Most factors that can explain instability of the construct have been discussed above with respect to setting, recruitment, blindedness and the nature of the clinical diagnosis, but length of follow-up is also an important variable. Recently, it has been documented that the stability of the diagnosis improves with longer follow-up periods, which will be influenced by age, attrition and mortality effects [89]. That is, subjects who may ‘bounce around’ in the early diagnostic period may later declare themselves as having cognitive impairment. As noted above, these findings are not unexpected and, due to the subtle nature of the early impairment, the length of follow-up ultimately determines that many of the subjects who apparently ‘reverted’ in fact subsequently ‘converted’ and ultimately were shown to have cognitive impairment. Two recent studies showed that any diagnosis of MCI, even if followed by a reversion to normal, led to an increased risk of subsequently developing dementia [89, 92]. However, not all studies have been able to confirm this finding [93]. The reversion to intact cognitive function, when detected in older populations (older than middle age, 65–70) from the community, may also be the result of the inclusion of those MCI cases due to treatable causes of cognitive impairment such as side effects of drugs, depression and comorbidity. However, this is what would be expected considering that not all MCI conditions are predicted to progress. As stated above, better definition of exclusion criteria in the MCI construct depending on the aims of the research or the target of the clinical work will help to reduce such variability and disagreement.
Conclusions
Notwithstanding the known open controversies within the field of MCI research (see Table 4), at present several points of general consensus can be identified:
There is an interval between optimal cognitive functioning and clinical dementia when individuals experience cognitive decline and, more often than in the past, can seek medical advice and prognostic counselling;
Those subjects referring to specialized clinical settings, and to a lesser extent even to primary care physicians, are a selected group of individuals who are not representative of the large spectrum of cognitive deficits preceding dementia diagnosis which can be detected in the general population;
Not all those who experience cognitive decline, especially in advanced ages, will develop AD, and some classified as having MCI will not even progress to clinically defined dementia. A large proportion of these individuals may have cognitive decline due to causes other than neurodegeneration, such as depression, anxiety, drug use, medical comorbidities and other treatable conditions. The identification of different types of MCI, with their particular aetiological pathways, is made easier by new diagnostic criteria [15] and by some promising biomarkers [16] which are available for testing in different clinical settings.
There are numerous challenges for the future. Some suggestions for further development of the MCI concept include:
Evaluating all studies of longitudinal cognition, using state-based analysis imposed on continuous longitudinal data [94–96] to assess the consistencies;
Creating frameworks and models which incorporate the setting and recruitment practices so that the findings can be interpreted with regard to specific populations. This is particularly important for older populations, as the identification of ‘poor’ performance is subject to major challenges which include educational and comorbid conditions affecting performance, and use of test norms often established in younger populations;
Conducting frequent deep phenotyping studies of specific populations and integration in the population framework;
Testing clinical judgement in a blind manner to verify the inter- and intra-rater reliability taking into account recruitment, setting and attrition;
Assessing temporal changes in cognitive performances, posing follow-up of the individual as a crucial part of the clinical diagnosis of MCI;
Contextualizing the construct by identifying possible clinical and research utilization, including prognostic counselling, support and follow-up costs, healthcare planning, drug development, investigation and recruitment into specific trials;
Conducting more longitudinal population-based studies on cognitive ageing and MCI to clarify these issues.
Acknowledgments
This work was supported by funding from the National Institute on Aging P50 AG016574 (Mayo Clinic Alzheimer’s Disease Research Center), U01 AG006786 (Mayo Clinic Study of Aging), the Swedish Council for Working Life and Social Research, regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Brain Power Initiative and Stockholm University.
Footnotes
Conflict of interest statement
Dr Petersen declares the following: Pfizer, Inc: Chair, Data monitoring committee, Janssen Alzheimer Immunotherapy: Chair, Data monitoring committee, GE Heathcare: Consultatnt, Roche Inc: Consultant.
References
- 1.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–09. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 2.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–62. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephan BC, Savva GM, Brayne C, Bond J, McKeith IG, Matthews FE Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Optimizing mild cognitive impairment for discriminating dementia risk in the general older population. Am J Geriatr Psychiatry. 2010;18:662–73. doi: 10.1097/jgp.0b013e3181e0450d. [DOI] [PubMed] [Google Scholar]
- 5.Reisberg B, Ferris S, de Leon MJ, et al. Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the Alzheimer type. Drug Dev Res. 1988;15:101–14. [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 9.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–85. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 10.Palmer K, Bäckman L, Winglad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:603–11. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Thomas RG, Grundman M, et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–12. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 13.Thal LJ, Ferris SH, Kirby L, et al. Rofecoxib Protocol 078 study group. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–15. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 14.Winblad B, Gauthier S, Scinto L, et al. GAL-INT-11/18 Study Group. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–35. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 16.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–89. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 18.Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc. 2005;53:1966–72. doi: 10.1111/j.1532-5415.2005.53566.x. [DOI] [PubMed] [Google Scholar]
- 19.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment. Long-term course of four clinical subtypes. Neurology. 2006;67:2176–85. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 20.Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007;68:2019–26. doi: 10.1212/01.wnl.0000264424.76759.e6. [DOI] [PubMed] [Google Scholar]
- 21.Di Carlo A, Lamassa M, Baldereschi M, Inzitari M, Scafato E, Farchi G, Inzitari D. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–16. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 22.Palmer K, Bäckman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:603–11. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 23.Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–84. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 24.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravaglia G, Forti P, Montesi F, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56:51–8. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Kodama C, Hidaka S, et al. Prevalence of four subtypes of mild cognitive impairment and APOE in a Japanese community. Int J Geriatr Psychiatry. 2009;24:1119–26. doi: 10.1002/gps.2234. [DOI] [PubMed] [Google Scholar]
- 29.Ganguli M, Chang CH, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of Mild Cognitive Impairment by Multiple Classifications: The MYHAT Project. Am J Geriatr Psychiatry. 2010;18:674–83. doi: 10.1097/JGP.0b013e3181cdee4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dlugaj M, Weimar C, Wege N, Verde PE, Gerwig M, Dragano N, Moebus S. Prevalence of mild cognitive impairment and its subtypes in the Heinz Nixdorf Recall study cohort; Heinz Nixdorf Recall Study Investigative Group. Dement Geriatr Cogn Disord. 2010;30:362–73. doi: 10.1159/000320988. [DOI] [PubMed] [Google Scholar]
- 31.Kim KW, Park JH, Kim MH, Kim MD, Kim BJ, Kim SK, Kim JL. A nationwide survey on the prevalence of dementia and mild cognitive impairment in South Korea. J Alzheimers Dis. 2011;23:281–91. doi: 10.3233/JAD-2010-101221. [DOI] [PubMed] [Google Scholar]
- 32.Perquin M, Schuller AM, Vaillant M, Diederich N, Bisdorff A, Leners JC, D’Incau M. The epidemiology of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) in community-living seniors: protocol of the MemoVie cohort study, Luxembourg. BMC Public Health. 2012;12:519. doi: 10.1186/1471-2458-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2010;29:164–75. doi: 10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–51. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caracciolo B, Palmer K, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–85. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 37.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–55. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tervo S, Kivipelto M, Hänninen T, Vanhanen M, Hallikainen M, Mannermaa A, Soininen H. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 39.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–99. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 40.Caracciolo B, Gatz M, Xu W, Pedersen NL, Fratiglioni L. Differential distribution of subjective and objective cognitive impairment in the population: a nation-wide twin-study. J Alzheimers Dis. 2012;29:393–403. doi: 10.3233/JAD-2011-111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caracciolo B, Gatz M, Xu W, Marengoni A, Pedersen NL, Fratiglioni L. Relation of SCI and CIND to chronic disease and multimorbidity in a nation-wide twin study. J Alzheimers Dis. 2013;36:275–84. doi: 10.3233/JAD-122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephan BC, Brayne C, Savva GM, Matthews FE Medical Research Council Cognitive Function and Ageing Study. Occurrence of medical co-morbidity in mild cognitive impairment: implications for generalisation of MCI research. Age Ageing. 2011;40:501–7. doi: 10.1093/ageing/afr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Carlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48:775–82. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 44.Frisoni GB, Fratiglioni L, Fastbom J, Guo Z, Viitanen M, Winblad B. Mild cognitive impairment in the population and physical health: data on 1,435 individuals aged 75 to 95. J Gerontol A Biol Sci Med Sci. 2000;55:M322–8. doi: 10.1093/gerona/55.6.m322. [DOI] [PubMed] [Google Scholar]
- 45.Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–84. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 46.Atti AR, Forlani C, De Ronchi D, Palmer K, Casadio P, Dalmonte E, Fratiglioni L. Cognitive Impairment after Age 60: Clinical and Social Correlates in the “Faenza Project”. J Alzheimers Dis. 2010;21:1325–34. doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 47.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2010;31:1894–1902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–91. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 49.Monastero R, Palmer K, Qiu C, Winblad B, Fratiglioni L. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2007;15:60–9. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 50.Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig longitudinal study of the aged. J Am Geriatr Soc. 2010;58:1903–10. doi: 10.1111/j.1532-5415.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 51.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–73. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu W, Caracciolo B, Wang HX, Winblad B, Backman L, Qiu C, Fratiglioni L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59:2928–35. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24:461–72. doi: 10.1097/YCO.0b013e32834bb9d4. [DOI] [PubMed] [Google Scholar]
- 54.van der Linde RM, Stephan BC, Matthews FE, Brayne C, Savva GM Medical Research Council Cognitive Function and Ageing Study. The presence of behavioral and psychological symptoms and progression to dementia in the cognitively impaired older population. Int J Geriatr Psychiatry. 2013;28:700–9. doi: 10.1002/gps.3873. [DOI] [PubMed] [Google Scholar]
- 55.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18:11–30. doi: 10.3233/JAD-2009-1120. [DOI] [PubMed] [Google Scholar]
- 56.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–84. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller DI, Taler V, Davidson PS, Messier C. Measuring the impact of exercise on cognitive aging: methodological issues. Neurobiol Aging. 2012;33:622.e29–43. doi: 10.1016/j.neurobiolaging.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Marioni RE, Valenzuela MJ, van den Hout A, Brayne C, Matthews FE MRC Cognitive Function and Ageing Study. Active cognitive lifestyle is associated with positive cognitive health transitions and compression of morbidity from age sixty-five. PLoS One. 2012;7:e50940. doi: 10.1371/journal.pone.0050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephan BC, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, Brayne C. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17:1056–76. doi: 10.1038/mp.2011.147. [DOI] [PubMed] [Google Scholar]
- 60.Smith CD. Neuroimaging through the course of Alzheimer’s disease. J Alzheimers Dis. 2010;19:273–90. doi: 10.3233/JAD-2010-1217. [DOI] [PubMed] [Google Scholar]
- 61.Fennema-Notestine C, McEvoy LK, Hagler DJ, Jacobson MW, Dale AM. Alzheimer’sDisease Neuroimaging Initiative. Behav Neurol. 2009;21:3–12. doi: 10.3233/BEN-2009-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jack CR, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung KL, Bartlett JW, Barnes J, Manning EN, Ourselin S, Fox NC. Cerebral atrophy in mild cognitive impairment and Alzheimer disease. Rates and acceleration. Neurology. 2013;80:648–54. doi: 10.1212/WNL.0b013e318281ccd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eskildsen SF, Coupé P, Garcia-Lorenzo D, et al. Prediction of Alzheimer’s disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. NeuroImage. 2013;65:511–21. doi: 10.1016/j.neuroimage.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herholz K. Cerebral glucose metabolism in preclinical and prodromal Alzheimer’s disease. Expert Rev Neurother. 2010;10:1667–73. doi: 10.1586/ern.10.136. [DOI] [PubMed] [Google Scholar]
- 66.Koivunen J, Pirtillä T, Kemppainen N, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid beta-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:378–83. doi: 10.1159/000163927. [DOI] [PubMed] [Google Scholar]
- 67.Forsberg A, Engler H, Almkvist O, Forsber, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–65. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 68.Jack CR, Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–20. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–38. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choo IH, Ni R, Schöll M, Wall A, Almkvist O, Nordberg A. Combination of (18)F-FDG PET and cerebrospinal fluid biomarkers as a better predictor of the progression to Alzheimer’s disease in mild cognitive impairment patients. J Alzheimers Dis. 2013;33:929–39. doi: 10.3233/JAD-2012-121489. [DOI] [PubMed] [Google Scholar]
- 72.Shaffer JL, Petrella JR, Shelon FC, et al. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology. 2013;266:583–91. doi: 10.1148/radiol.12120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jack CR, Jr, Knopman DS, Weigand SD, et al. An Operational Approach to NIA-AA Criteria for Preclinical Alzheimer’s Disease. Ann Neurol. 2012;71:765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen RC, Aisen P, Boeve BF, et al. Criteria for mild cognitive impairment due to Alzheimer’s disease in the community. Ann Neurol. 2013 doi: 10.1002/ana.23931. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green M, Kaye JA, Ball MJ. The Oregon Brain Aging Study. Neurology. 2000;54:105–13. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 76.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–17. [PubMed] [Google Scholar]
- 77.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132. doi: 10.1002/14651858.CD009132.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farina N, Isaac MG, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2012;11:CD002854. doi: 10.1002/14651858.CD002854.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lloret A, Badía MC, Mora NJ, Pallardó FV, Alonso MD, Viña J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17:143–49. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 81.Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev. 2012;36:1163–78. doi: 10.1016/j.neubiorev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr. 2011;25;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDonnell MN, Smith AE, Mackintosh SF. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch Phys Med Rehabil. 2011;92:1044–52. doi: 10.1016/j.apmr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Lautenschlager NT, Cox K, Kurz AF. Physical activity and mild cognitive impairment and Alzheimer’s disease. Curr Neurol Neurosci Rep. 2010;10:352–58. doi: 10.1007/s11910-010-0121-7. [DOI] [PubMed] [Google Scholar]
- 85.Brayne C, Davis D. Making Alzheimer’s and dementia research fit for populations. Lancet. 2012;380:1441–3. doi: 10.1016/S0140-6736(12)61803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–7. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 89.Lopez OL, Becker JT, Chang YF, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79:1599–1606. doi: 10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 92.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz S, Christianson TJH. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. doi: 10.1212/WNL.0000000000000055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palmer K, Wang HX, Bäckman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–42. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 94.Muniz-Terrera G, van den Hout A, Piccinin AM, Matthews FE, Hofer SM. Investigating terminal decline: Results from a UK population-based study of aging. Psychol Aging. 2013;28:377–85. doi: 10.1037/a0031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matthews FE, Stephan BC, Bond J, McKeith I, Brayne C Medical Research Council Cognitive Function and Ageing Study. Operationalization of mild cognitive impairment: a graphical approach. PLoS Med. 2007;4:1615–9. doi: 10.1371/journal.pmed.0040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stephan BC, Matthews FE, McKeith IG, Bond J, Brayne C Medical Research Council Cognitive Function and Aging Study. Early cognitive change in the general population: how do different definitions work? J Am Geriatr Soc. 2007;55:1534–40. doi: 10.1111/j.1532-5415.2007.01386.x. [DOI] [PubMed] [Google Scholar]