Abstract
Imaging has become a cornerstone for medical diagnosis and the guidance of patient management. A new field called Image Guided Drug Delivery (IGDD) now combines the vast potential of the radiological sciences with the delivery of treatment and promises to fulfill the vision of personalized medicine. Whether imaging is used to deliver focused energy to drug-laden particles for enhanced, local drug release around tumors, or it is invoked in the context of nanoparticle-based agents to quantify distinctive biomarkers that could risk-stratify patients for improved targeted drug delivery efficiency, the overarching goal of IGDD is to use imaging to maximize effective therapy in diseased tissues and to minimize systemic drug exposure in order to reduce toxicities. Over the last several years innumerable reports and reviews covering the gamut of IGDD technologies have been published, but inadequate attention has been directed towards identifying and addressing the barriers limiting clinical translation. In this consensus opinion, the opportunities and challenges impacting the clinical realization of IGDD-based personalized medicine were discussed as a panel and recommendations were proffered to accelerate the field forward.
Over the last several years, the concept of the “magic therapeutic bullet” has come much closer to realization in the lab but these results have been slow to reach the clinic.1 Individualized targeting of drugs with the intent of improving safety and efficacy has evolved along two parallel paths with biomedical imaging playing a major role. The field of Image Guided Drug Delivery (IGDD), which takes advantage of the strengths of imaging to optimize drug therapy, has emerged with promises to fulfill the vision of personalized medical treatment. Along one path, imaging is used to visualize the target lesion and affect the local release or activation of drugs through image guided deposition of exogenous energy. As an example, the biodistribution of drug may be altered by focused energy disruption of temperature sensitive drug-laden liposomes to preferentially release free drug at the target. 2–6 Another example is image-guided hyperthermia, where particles bound near or in the target tissue are heated via light, magnetic, or acoustic energy to affect cell death. 7–16
The other path of IGDD technologies involves so-called theranostic agents, i.e., a pharmaceutical with drug delivery and targeted diagnostic imaging features. Theranostic platform technologies may be used diagnostically to characterize a patient’s disease and biomarkers and then for the appropriate subset of those individuals, the same platform can be functionalized to deliver treatment. 4, 6, 7, 17–84 In some instances, the agent may engender both imaging and therapeutic features simultaneously providing image-based confirmation and quantification of the delivered drug, so called rational dosimetry. Image-based rational dosimetry helps to assure adequacy of treatment and informs further medical care plan decisions immediately. It can eliminate undesirable delays in determining poor outcomes, which result from underdosing or ineffective treatments. In each circumstance, molecular imaging can provide longitudinal information about the biochemical and microanatomic response to treatments, including the early recrudescence of the underlying disease.
Regardless of approach, IGDD offers significant opportunity as a partner in medical management beyond the traditional diagnostic imaging role. While reports and reviews covering the gamut of technologies related to IGDD have touted the exciting opportunities, this opinion focuses on the perceived barriers limiting clinical translation of these achievements. This panel of informed scientists was assembled by the National Cancer Institute (NCI) to consider the issues impeding the “bench to bedside” transition of these technologies. Comments as to the direction of research and development efforts to address these unique challenges presented are not necessarily endorsed by the NCI or NIH.
CHALLENGES AND RECOMMENDATIONS FOR IMAGE-GUIDED DRUG DELIVERY
1. EFFICACY AND SAFETY ISSUES SURROUNDING IMAGE-GUIDED DRUG DELIVERY
1.1 Challenge: Optimizing drug concentrations delivered to the target cells mediating the disease
Opinion
Consistent with a “walk before you run” perspective, the first generation of nano- and microparticle technologies now reaching the clinic are primarily non-targeted or “vascularly targeted” applications, which address diseases like cancer, arthritis, atherosclerosis, and macular degeneration. Most of the non-targeted agents, whether liposomal, polymeric, emulsions, or micelles are generally extensions of traditional prolonged release drug delivery strategies intended to alter the pharmacokinetic profile of drugs in vivo and to a lesser extent to alter the biodistribution.
IGDD liposomal- or microbubble-based agents alter free drug pharmacokinetics and afford increased localized release when exogenous focused energy, such as high-intensity focused ultrasound, is applied. Therefore, locally increased concentrations of free drug will increase the percentage of the injected dose delivered. The penetration and target cell uptake of even small molecules must traverse several barriers and the rapidity of drug washout in blood from lesion can diminish the expected benefit. Exogenous energy can mechanically weaken or destroy the biological barriers giving improved access to the extravascular space, but still the issues of free drug cellular uptake versus washout can detract from the potential benefit.
From a nanoparticle molecular imaging perspective, vascular-constrained agents targeted to biomarkers expressed differentially by endothelial cells can aid patient diagnosis, therapeutic risk stratification and longitudinal management. However, from a treatment perspective, drug, gene, or biological, vascular-targeted approaches only impact the endothelium directly and influence the underlying pathology usually through secondary effects. Thus many vascular-targeted agents may best be used adjunctively to improve efficacy of current systemic regimens. 85 However, growing evidence suggests that vascular-targeted agents can be actively transported into lesions quickly and against the blood to tissue concentration gradient.
IGDD technologies, whether related to image-localized release of drug from nontargeted particles or targeted nanobased molecular imaging and therapy, will benefit from deeper penetration of particles into the disease site. Mechanical disruption of drug-laden particles within lesions using image-guided focused energy would increase compound bioavailablity to target cells and reduce washout of free drug. Microbubble systems undergoing intratumoral disruption would offer further synergistic effect by improving the biodistribution of free drug and by sonically impacting target cell permeability. 80, 86
Most investigators studying systemically targeted and non-targeted nanoparticles rely on the purported “enhanced permeability and retention” (EPR), 87, 88 a phenomenon primarily observed with subcutaneous xenografts mouse tumors. This effect is muted in less promiscuous models such as orthotopic transplants in mice or larger species. Ultimately, particulate agents larger than modestly sized proteins are poorly exchanged into vascular periphery of tumors, arthritic joints, or atherosclerotic plaques where deep drug penetration is desired. The natural receptors or “door keys” that selectively regulate endothelial uptake and trafficking of blood-borne constituents into the interstitium are known only to a limited extent. 89–121 The regulatory communication signals emanating from normal and pathological extravascular cells, that modulate endothelial cellular functions are a mystery with only fragmentary clues. The concept of targeted delivery through natural endothelial transcytosis systems, such as the caveolae system, 122–128 has been demonstrated for smaller agents, such as antibodies and very small nanoparticles with at least one caveolae-specific marker, i.e., a modified aminopeptidase 2 (APP 2). Using a monoclonal antibody against APP2, the Schnitzer laboratory has delivered radiolabeled payloads and small gold nanoparticles (10nm) into lung parenchyma firmly demonstrating the principle. For caveolae exploited transport mechanisms, antibody transport (pumping) into the extravascular space can be rapid with up to 70% of the injected dose delivered in a few minutes, against the blood-to-tissue concentration gradient. Ultimately, these investigators injected increasingly lower doses to avoid saturating the delivery mechanism, while maximizing lung parenchymal delivery. Co-opting caveolae transcytosis mechanisms for some treatment regimens will accelerate targeted delivery and reduce total drug dose exposure. Moreover, utilizing a caveolae transcytosis approach would obviate the need to pursue avoidance of the reticulo-endothelial system (RES), since the clearance of untargeted agent be desirable. Decreasing the whole body particle burden would improve safety profiles, including a reduction in “flu-like” symptoms associated with cytokines released by an activated RES.
Caveolae likely serve both constitutive and specialized transport roles. While the component parts of the system are defined mechanistically to a great extent, virtually no specific information concerning the physiological regulation (internal and external) of the “machine”, the cargo, and the transendothelial throughput exists in the cell biology literature. Discovery of organ or pathology specific caveolae markers with supportive characterization is minimal to date and far from the needed caveolae vascular map required to propel IGDD development along this pathway. A better understanding of basic cell biology specifically delineating the dynamic and biophysical constraints of caveolae transport using nanotechnology based probes is needed.
As mentioned, the transmigration of large cells, such as macrophages, neutrophils, and lymphocytes occurs through the endothelial cell itself, and is ongoing constantly to mediate inflammation responses to infection, atherosclerosis, cancer, arthritis, and more. Several participatory biomarkers involved in attracting and concentrating these cells along the apical endothelium lumen from where Intercellular adhesion molecule (ICAM)-mediated transcytosis to basal and lateral membranes release agents into the extravascular space. 129–133 The importance of this pathway is highlighted by the development of small molecule antagonists of lymphocyte function-associated antigen, αLβ2 (LFA-1) to prevent LFA-1/ICAM-mediated leukocyte transcytosis. 134–137
The Muro laboratory has conducted enlightening early studies demonstrating that the ICAM pathway can be usurped to transcytose 100nm polystyrene nanospheres electrostatically coated with anti-ICAM antibody through Caco-2 epithelial cells (a continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells) in vitro, providing convincing data using transmission electron microscopy and cell trans-wells. She has extended the characterization endothelial ICAM cell biology biochemical mechanisms from cells to nanoparticles with detailed proof of concept studies. Yet, little interest within the endothelial cell biologist community in the IGDD problem has been forthcoming. Perhaps the particle transcytosis topic is relatively unknown among those scientists or the issue is not effectively elevated for study by targeted funding opportunities on the topic. While ICAM is an important element for larger particles to enter lesions, it is only one of what may be many pathways. Other mechanisms exist, such as the iRGD approach (RGD refers to the recognition amino acid sequence for integrin binding to many extracellular matrix proteins) proffered by the Ruoslahti laboratory138–141, and natural pathways by which lipoproteins, like HDL, enter the extravascular space of tumors and plaques. 94, 142, 143 Certainly, more pathways for communicating from the blood to the extravascular space and the reverse exist.
Today much effort continues to be expended to chemically optimize particles for passive particle delivery and entrapment with delayed washout (EPR). Unfortunately, the penetration via leaky vasculature has proven to be highly limiting or ineffective in many situations. For efficient delivery of payloads into tumors, plaques or joints, a much greater understanding of how nature has evolved to achieve these same goals with extraordinary precision, speed and efficiency is needed. In the interim, substantive clinical therapeutic improvements can be achieved through image-guided focused energy release of drugs and vascular-targeted therapies that may be effective alone or act synergistically as adjuvants with current medical management regimens. 85, 144, 145
1.2 Challenge: Avoiding premature clearance of therapeutic particles before effective drug delivery is achieved
Opinion
Nano- and microparticles are typically cleared by RES system, which is composed of phagocytic cells in the lung, spleen, liver, and marrow. The RES system is currently conceived as a hindrance to the efficacy of targeted particles, since the rapid clearance of particles offsets the high concentration gradient needed as a driving force for passive transport and delivery. Indeed, the need for high mass loading and prolonged circulatory times for EPR to have any impact can only be achieved with improved RES avoidance. 147–149 Pegylation (i.e., PEG, polyethylene glycol) of particles has been used to create “stealthy” agents and slow RES clearance rates, but pegylation of particles can both impair ligand directed targeting due to steric interference. Moreover, PEG, once thought to be a benign surface modifier, because it diminished complement activation, can induce adaptive immune responses with repeat usage. 146, 150–152
Another approach to the RES issue has been to make particles very small, even approaching the size of large proteins. While the lung, liver, and spleen are all well known RES constituents acting in a coordinated sieve-like manner particularly on larger particles (>20 nm), the marrow is generally overlooked but is a depot for very small particles. The marrow has many phagocytic cells, and large (300 nm) and small particles (20 nm) are found to collect there. The marrow, which weighs 2.6kg in adults (by comparison the liver is 1.5kg), constantly maintains and replenishes platelets, leukocytes, and erythrocytes in addition to its clearance functions, and it may be functionally sensitive to particle engorgement. Regardless, the RES clearance in the marrow will be challenging to overcome.
On the other hand, RES clearance can be beneficial. The removal of therapeutic particles from circulation reduces off-target effects, which is typically reflected as decreased drug toxicity with IGDD treatments. For imaging, the removal of contrast agent from the circulation decreases background blood pool interference and improves contrast-to-noise ratios for targeted pathologies. The key to RES problem will likely resolve when faster and more efficient extravascular targeting of disease is achieved by utilizing natural cell transport mechanisms, which will allow much lower drug dosing levels and leave the RES to clear unneeded drug and prevent off-target effects.
1.3. Challenge: Designing particles to avoid complement activation and adaptive immune responses
Opinion
Unlike drugs, which are typically small molecules, particulate-based technologies can elicit host blood contact responses, including hemolysis, complement activation, or immune response. The relevance of particle shape, charge, and size are coming to the fore, but informed de novo design guidance of nano and microparticles to avoid these issues is not available. Nature’s “rules” governing the acute and adaptive immune responses to particle surfaces remain poorly delineated and understudied.
Animal immune responses to particle challenges need to be conducted to define the response with acute and repeat administration. 153 Assay methodology for complement activation (CA) and adaptive immunity assessments must be developed to clearly assess clinically relevant signal with minimal false positives. Clear guidance must be established to distinguish results that would elicit low-level, subclinical responses from those results reflecting clinical meaningful risk that warrant concern and reformulation. An easily available set of nanoparticle standards and simply executed method kits must be demonstrated and validated through inter-laboratory testing and standardized through ASTM (American Society for Testing and Materials) or similar organization. Importantly, techniques developed must be readily performed by any laboratory routinely synthesizing new agents. Public access databases documenting appropriate physical, chemical, and biological characterization of test particles using these standardized methods should be established and easily interrogated.
Pooled serum animal or human serum can blunt individual biological variation estimations. Serum from asymptomatic people as well as those with select patients with specific underlying pathologies should be obtained and developed into standardized panels to gain insight into the expected variability of responses. Since no exogenous material introduced into humans will be completely safe, we should not expect such to be the case for particle-based technology. A common clinical example of high benefit with acceptable risk involves microbubble acoustic diagnostics. Complement activation triggered by microbubbles can lead to transient (few minutes) episodes of back pain or neurological symptoms in echocardiography laboratory. While this is sometimes momentarily uncomfortable for the occasional patient, the overall health risk is very low and outweighed by the vastly improved diagnostic benefit. 154 Appropriate product labeling and monitoring should be anticipated until a sufficient clinical experience warrants a revision.
2. CLINICAL VALIDATION OF BIOMARKERS AND QUANTITATIVE IMAGING
2.1 Challenge: Biomedical imaging results are reported in qualitative, relativistic, and descriptive terms. However, molecular imaging for the purpose of patient risk stratification and longitudinal management should be quantitative and repeatable overtime and across institutions
Opinion
Too little work has considered how image contrast signals might be used clinically, particularly when used diagnostically for rational dosimetry, patient stratification, or longitudinal medical management. To utilize molecular imaging or blood pool signals serially in the same patient to support medical decisions requires accurate, precise, and repeatable quantitation rather than the relativistic measures typically reported in preclinical studies. Robust quantification that can be normalized across exams and between institutions would support the implementation of guidelines and the development of algorithmic patient management decision trees. Such IGDD uniformity will require the creation and distribution of reference phantoms for instrument and image calibration. To this end, appropriate quality control procedures and reference standards could be established and validated through NIST (National Institute of Standards and Technology). Importantly, such reference standards will allow manufacturers of instruments and software to achieve and report comparable outcome data while still allowing vendor unique algorithms for quantification. Ultimately, IGDD quantification must be easily and reproducibly adopted by imaging and pharmaceutical laboratories engaging in these advanced services to patients and physicians.
2.2 Challenge: Current models provide limited understanding of the temporal and spatial variation in receptor expression relative to the natural progression of disease, clinical status, and prognosis in humans
Opinion
Nature reuses the same or closely related proteins on many cell-types for related purposes. Although homing ligands with high affinity and specificity are implicitly required, targeted therapies also must be validated to bind specifically to the proper subset of cells to avoid misinterpretations and avoid off-target toxicity. Moreover, given the current limited understanding of the temporal and spatial variation in receptor expression in man or its relevance to the natural progression disease, clinical status, and prognosis in patients, a serious effort to characterize the time-course expression of potential pathological biomarkers in man is essential.
Cost-efficient preclinical models that better recapitulate human disease need to be established and broadly available. Today most preclinical models only provide modest confidence that a compound or nanoagent is biologically active in vivo and only offers gross indications of toxicity. Since animal models must be used to correlate imaging data, ideally with clinically relevant instrumentation, newer preclinical models beyond mice are needed, However, such models require supportive immunohistochemical and molecular biology reagents, which are generally lacking. It is clear that imaging and therapeutic success in mice must be confirmed and augmented in secondary species to improve the odds of clinical translation. Perhaps alternative models such as the rat, rabbit, guinea pig, hamster, or even avian species should be explored more aggressively. Programmatic impetus to further develop these models with complementary reagents for specific disease applications would be welcome.
In the nearer-term, we should consider new regulatory pathways to acquire human IGDD data safely sooner. Perhaps a more flexible extension to Phase 0 feasibility testing paradigm at higher doses applicable to micro and nanotechnologies could be envisioned for research studies using Good Laboratory Practice (GLP) produced agents that have completed a reduced essential battery of preclinical testing. If these agents have only minor issues at low doses in a few patients, then they could be stepped into Phase 1 as is or with additional supporting data. If an unexpected event occurs, it would be known earlier and transition to Phase 1 would be dependent on further clarification of that issue and safety impact.
Today, the current development cycle is too long and too risky for nanomedicines, particularly IGDD technologies, and this has suppressed innovation and translation by decreasing financial investment. Broadly speaking, an accelerated regulatory program would have a significant positive impact on the US biotechnology and pharmaceutical industries, particularly for the myriad small companies dependent on limited private equity and governmental funding.
3. INCREASING PHYSICIAN INVOLVEMENT IN NANOTECHNOLOGY AND IGDD RESEARCH
3.1 Challenge: In recent years, the development of IGDD technologies has been driven predominantly by basic and applied scientists and engineers who need the insight into the unmet clinical needs and perspectives that can be provided by physician-scientists
Opinion
Ultimately, IGDD technologies must be “pulled” into the clinic by end-users inspired to address previously intractable medical problems. Many IGDD concepts are developed without adequate consideration of clinical unmet need for a specific application. The practical insight of progressive, technology savvy physicians, radiologists, and surgeons engaged in IGDD projects, program advisory boards and grant review panels would enlighten other research scientists as well as provide an increasing pool of informed key influence physician/scientists to communicate the opportunities and limitation of these technologies to their colleagues. A greater effort to address medical and scientific constituencies beyond our IGDD colleagues is required, both to create enthusiasm for developing these new concepts and to preempt adverse messaging based on myths, conjectures, hyperbole, and bias. An educated medical community would be a primary resource to answer the questions of curious patients and interested parties at all levels of organization.
4. SYNERGIZING ACADEMIC COMMUNITIES, GOVERNMENT AND PRIVATE INDUSTRIAL RESOURCES
4.1 Challenge: The multidisciplinary nature of imaging and drug delivery research presents expertise-based barriers from academic discovery through commercial development
Opinion
The drug delivery and imaging communities are historically disjoint as scientific societies, as funding review panels and within educational programs. Significant expertise in medical pharmacology has developed within the delivery community in parallel to expertise in physics and biology within the imaging community. Bridging these communities remains a challenging problem and the educational infrastructure required to unite these fields has not yet been created.
With regard to the divide between academics and industry, the strength of academia lies in the formulation of imaginative concepts, the development of research prototypes, and the pursuit of rigorous experimentation. Academics lack expertise in the formal development of complicated drugs and imaging agents. Pharmaceutical and medical imaging companies, while clearly not lacking in creative or scientific potential, must currently focus on development projects with high potential for translation to the clinic.
Efforts to conjoin the imaging and delivery communities are now emerging through combined scientific sessions and new funding review panels. A continued effort to create such venues for discussion and focus will be important. Moreover, educational programs specifically aimed at creating the next generation of scientists who are formally trained in both the imaging and delivery disciplines have not yet emerged. The fundamental challenge is to incorporate sufficient training in chemistry, biology, mathematics and physics within such a program to guarantee that trainees master the core competencies of both the imaging and delivery communities. While molecular imaging training programs have recently emerged, the challenges of therapeutic delivery require additional training materials and expertise.
Further, clinical translation of IGDD research has always been limited by technical and cultural gaps between the biology-chemistry driven pharmaceutical and physics-engineering based medical instrumentation industries. Combination imaging and therapy product concepts are outside the mainstream expertise of either industry. Although academia continues to conceive of new IGDD innovations, this community lacks product development, regulatory, management and marketing experience. The translational prospects of new concepts need to be evaluated through the eyes of experience practitioners, and too few academics have adequate industry or regulatory experience to self-evaluate their own technologies with confidence.
Academic scientists often develop early stage technology as an individual or as part of a small start-up company, but generally such technologies must be transferred to a company or institution to develop, market, distribute, and support the new technology in the medical community. What type of company can best develop IGDD products, imaging or pharmaceutical companies? Likely the answer is a pharmaceutical company with an imaging collaborator(s) but the corollary is reasonable in some circumstances.
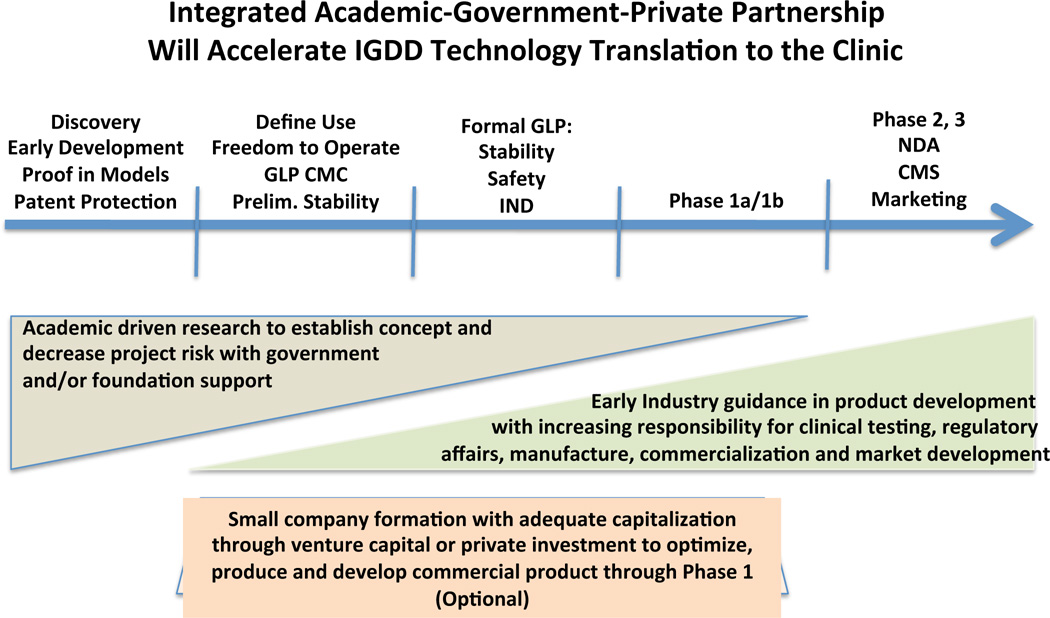
With financial resources limited in all sectors of research and development, programs that encourage corporations to engage and guide promising IGDD technology at early development stages while maintaining a low overall economic risk profile are desired. Industrial expertise, applied as consultation under a joint development program, could guide preclinical decisions into desirable directions compatible with long-term business plans of a corporate partner. Being in sync with the business goals of a corporate partner from the start is highly preferred over seeking a relationship when the product application is at the IND stage. Small start-up companies may be useful to de-risk new technology between academic discovery and clinical proof in humans stages. In todays economic environment, acquiring the capital needed to bridge this gap is more likely invested when a larger company has committed to the clinical pathway and accepted increasing responsibility and control as the product concept clears phase 1 and is poised to expand into phase 2 and beyond. (Figure 1) Such a relationship allows the interim investors to monetize their interests within a reasonable time interval for investment and equally offers the larger corporation a more de-risked product concept congruent with its long-term business plan. The challenge to the IGDD community remains how to provide incentives for these partnerships. One mechanism may evolve through programmatic changes in healthcare reimbursement.
FIGURE 1.
Potential paradigm for increasing the efficiency of “Bench to the Clinic” translation of IGDD technology achieved by synergizing the creativity of academia under government or foundation support with the product development and marketing capability of industry. Small companies capitalized by venture capital or private funds may serve to convert academic technology into pharmaceutically suitable, commercially scalable technologies that are produced under GLP to conduct preclinical stability and safety and GMP to open an IND for initiating Phase 1 human clinical trials. Involvement of industry provides smooth transition into later phase clinical studies and the market. CMC = chemistry, manufacturing, and controls; IND = investigational new drug application; GLP = Good Laboratory Practices; GMP = Good Manufacturing Practices; NDA = New Drug Application; CMS = Center for Medicare and Medicaid Services.
Recently, the New York Times published an article by A. Pollack reporting on the revolt of oncologists over the cost of drugs exceeding $100,000 /year (April 25, 2013). While the cost of developing sophisticated drugs demands high returns to recoup investment, often only a fraction of patients respond as expected while many spend the money and accept the adverse medical event risk without benefit. Cost-effective pre-evaluation to qualify patients for these expensive therapeutic regimens should be required. For many diseases, IGDD approaches offer a relatively inexpensive, direct study capable of yielding substantially improved outcomes in patients selected for treatment. Moreover, IGDD stratification could help avoid unnecessary exposures to adverse drug effects and save $100,000/patient for the majority of cases in which therapeutically benefit is unlikely.
SUMMARY
While the concept of personalized medicine is often tangentially inferred in many contexts, IGDD is a direct path to this goal. Treatments can be individualized through visualizing pathology and controlling the local delivery of therapy through focused energy or by stratifying a patient cohort with imaging to better ensure responsiveness to treatment. While numerous challenges face all new technologies, materializing the opportunities presented by IGDD continues to require addressing the significant interdisciplinary challenges and biological barriers. Vascular-targeted delivery of drug and imaging agents are feasible today, but penetration into lesions with particles passively has not succeeded. New concepts to co-opt natural transport mechanisms are emerging and some have substantial proof of concept, but too little detailed understanding of these biological transcytosis mechanisms with regard to their triggers, capacities, constraints, and biological control mechanisms exist. Partnerships of cell biologists and IGDD researchers should be encouraged programmatically to discover and exploit these cellular functionalities. The added complexity of developing IGDD technologies requires new approaches to create economic incentives for partnerships between commercial and the academic/government communities. Perhaps the single biggest incentive may arise for the healthcare payers insisting that the use of highly expensive personalized medicines be predicated upon effective documentation that a patient has a 60% or better chance of a successful outcome. For therapies with low overall benefit in all-comers treatment approach, imaging guided technologies could make the difference by improving effective drug delivery into the lesion with energy or by predetermining those patients most likely to respond treatment.
BIBLIOGRAPHY
- 1.Strebhard K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Bos C, Lepetit-Coiffé M, Quesson B, Moonen C. Simultaneous monitoring of temperature and T1: methods and preliminary results of application to drug delivery using thermosensitive liposomes. Magn Reson Med. 2005;54:1020–1024. doi: 10.1002/mrm.20635. [DOI] [PubMed] [Google Scholar]
- 3.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti S, Li K, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castle J, Butts M, Healey A, Kent K, Marino M, Feinstein SB. Ultrasound-mediated targeted drug delivery: Recent success and remaining challenges. American Journal of Physiology - Heart and Circulatory Physiology. 2013;304:H350–H357. doi: 10.1152/ajpheart.00265.2012. [DOI] [PubMed] [Google Scholar]
- 5.Deckers R, Rome C, Moonen C. The role of ultrasound and magnetic resonance in local drug delivery. J Magn Reson Imaging. 2008;27:400–409. doi: 10.1002/jmri.21272. [DOI] [PubMed] [Google Scholar]
- 6.Zhao YZ, Du LN, Lu CT, Jin YG, Ge SP. Potential and problems in ultrasound-responsive drug delivery systems. International Journal of Nanomedicine. 2013;8:1621–1633. doi: 10.2147/IJN.S43589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Zderic V, Frenkel V. Extracorporeal, low-energy focused ultrasound for noninvasive and nondestructive targeted hyperthermia. Future Oncology. 2010;6:1497–1511. doi: 10.2217/fon.10.101. [DOI] [PubMed] [Google Scholar]
- 8.Moroz P, Jones S, Winter J, Gray B. Targeting liver tumors with hyperthermia: ferromagnetic embolization in a rabbit liver tumor model. J Surg Oncol. 2001;78:22–29. doi: 10.1002/jso.1118. [DOI] [PubMed] [Google Scholar]
- 9.Nikawa Y, Okada F. Deep and localized heating for hyperthermia using ferrimagnetic resonance; Proceedings of the ninth annual conference of the IEEE Engineering in medicine and biology Society (Cat No. 87ch2513-0); Boston, MA. 1987. [Google Scholar]
- 10.Salomir R, Palussière J, Fossheim S, Rogstad A, Wiggen U, Grenier N, Moonen C. Local delivery of magnetic resonance (MR) contrast agent in kidney using thermosensitive liposomes and MR imaging-guided local hyperthermia: a feasibility study in vivo. J Magn Reson Imaging. 2005;22:534–540. doi: 10.1002/jmri.20416. [DOI] [PubMed] [Google Scholar]
- 11.Freddi S, Sironi L, D'Antuono R, Morone D, Dona A, Cabrini E, D'Alfonso L, Collini M, Pallavicini P, Baldi G, et al. A molecular thermometer for nanoparticles for optical hyperthermia. Nano Lett. 2013;13:2004–2010. doi: 10.1021/nl400129v. [DOI] [PubMed] [Google Scholar]
- 12.Patel RH, Wadajkar AS, Patel NL, Kavuri VC, Nguyen KT, Liu H. Multifunctionality of indocyanine green-loaded biodegradable nanoparticles for enhanced optical imaging and hyperthermia intervention of cancer. J Biomed Opt. 2012;17:046003. doi: 10.1117/1.JBO.17.4.046003. [DOI] [PubMed] [Google Scholar]
- 13.Kuo WS, Chang CN, Chang YT, Yang MH, Chien YH, Chen SJ, Yeh CS. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew Chem Int Ed Engl. 2010;49:2711–2715. doi: 10.1002/anie.200906927. [DOI] [PubMed] [Google Scholar]
- 14.Hu KW, Liu TM, Chung KY, Huang KS, Hsieh CT, Sun CK, Yeh CS. Efficient near-IR hyperthermia and intense nonlinear optical imaging contrast on the gold nanorod-in-shell nanostructures. J Am Chem Soc. 2009;131:14186–14187. doi: 10.1021/ja9062772. [DOI] [PubMed] [Google Scholar]
- 15.Svaasand LO, Boerslid T, Oeveraasen M. Thermal and optical properties of living tissue: application to laser-induced hyperthermia. Lasers Surg Med. 1985;5:589–602. doi: 10.1002/lsm.1900050607. [DOI] [PubMed] [Google Scholar]
- 16.Vaguine VA, Christensen DA, Lindley JH, Walston TE. Multiple sensor optical thermometry system for application in clinical hyperthermia. IEEE Trans Biomed Eng. 1984;31:168–172. doi: 10.1109/TBME.1984.325383. [DOI] [PubMed] [Google Scholar]
- 17.Santra S, Kaittanis C, Santiesteban OJ, Perez JM. Cell-specific, activatable, and theranostic prodrug for dual-targeted cancer imaging and therapy. J Am Chem Soc. 2011;133:16680–16688. doi: 10.1021/ja207463b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadajkar AS, Menon JU, Kadapure T, Tran RT, Yang J, Nguyen KT. Design and Application of Magnetic-based Theranostic Nanoparticle Systems. Recent Pat Biomed Eng. 2013;6:47–57. doi: 10.2174/1874764711306010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Alberti C. From molecular imaging in preclinical/clinical oncology to theranostic applications in targeted tumor therapy. Eur Rev Med Pharmacol Sci. 2012;16:1925–1933. [PubMed] [Google Scholar]
- 22.McCarthy JR. The future of theranostic nanoagents. Nanomedicine (Lond) 2009;4:693–695. doi: 10.2217/nnm.09.58. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Gold nanocages: from synthesis to theranostic applications. Acc Chem Res. 2011;44:914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC, Lee WJ, Sun IC, Kwon IK. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials. 2012;33:856–866. doi: 10.1016/j.biomaterials.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 25.Shrestha R, Elsabahy M, Luehmann H, Samarajeewa S, Florez-Malaver S, Lee NS, Welch MJ, Liu Y, Wooley KL. Hierarchically assembled theranostic nanostructures for siRNA delivery and imaging applications. J Am Chem Soc. 2012;134:17362–17365. doi: 10.1021/ja306616n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner DS, Delk NA, Lukianova-Hleb EY, Hafner JH, Farach-Carson MC, Lapotko DO. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials. 2010;31:7567–7574. doi: 10.1016/j.biomaterials.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J Am Chem Soc. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JI, Lee BS, Chun C, Cho JK, Kim SY, Song SC. Long-term theranostic hydrogel system for solid tumors. Biomaterials. 2012;33:2251–2259. doi: 10.1016/j.biomaterials.2011.11.083. [DOI] [PubMed] [Google Scholar]
- 30.Ambrogio MW, Thomas CR, Zhao YL, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc Chem Res. 2011;44:903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Lovell JF. Porphyrins as theranostic agents from prehistoric to modern times. Theranostics. 2012;2:905–915. doi: 10.7150/thno.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Tian M, Ignasi C, Cheng Z, Shen LH, Yang DJ. Molecular image-guided theranostic and personalized medicine. J Biomed Biotechnol. 2011;2011:673697. doi: 10.1155/2011/673697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 35.Puri A, Blumenthal R. Polymeric lipid assemblies as novel theranostic tools. Acc Chem Res. 2011;44:1071–1079. doi: 10.1021/ar2001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Wang L, Wang J, Jiang X, Li X, Hu Z, Ji Y, Wu X, Chen C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater. 2012;24:1418–1423. doi: 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- 37.Gu Z, Yan L, Tian G, Li S, Chai Z, Zhao Y. Recent Advances in Design and Fabrication of Upconversion Nanoparticles and Their Safe Theranostic Applications. Adv Mater. 2013 doi: 10.1002/adma.201301197. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013 doi: 10.1016/j.copbio.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res. 2011;44:1061–1070. doi: 10.1021/ar2001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulte JW. Science to practice: can theranostic fullerenes be used to treat brain tumors? Radiology. 2011;261:1–2. doi: 10.1148/radiol.11111636. [DOI] [PubMed] [Google Scholar]
- 41.Wan Q, Xie L, Gao L, Wang Z, Nan X, Lei H, Long X, Chen ZY, He CY, Liu G, et al. Self-assembled magnetic theranostic nanoparticles for highly sensitive MRI of minicircle DNA delivery. Nanoscale. 2013;5:744–752. doi: 10.1039/c2nr32438e. [DOI] [PubMed] [Google Scholar]
- 42.Bhojani MS, Van Dort M, Rehemtulla A, Ross BD. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol Pharm. 2010;7:1921–1929. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacKay JA, Li Z. Theranostic agents that co-deliver therapeutic and imaging agents? Adv Drug Deliv Rev. 2010;62:1003–1004. doi: 10.1016/j.addr.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Fernandez A, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Appl Biochem Biotechnol. 2011;165:1628–1651. doi: 10.1007/s12010-011-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed N, Fessi H, Elaissari A. Theranostic applications of nanoparticles in cancer. Drug Discov Today. 2012;17:928–934. doi: 10.1016/j.drudis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Borden M, Rege K. Theranostic biocolloids: soft matter colloids for imaging and therapy. Theranostics. 2012;2:1115–1116. doi: 10.7150/thno.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiessler M, Hennrich U, Pipkorn R, Waldeck W, Cao L, Peter J, Ehemann V, Semmler W, Lammers T, Braun K. Theranostic cRGD-BioShuttle Constructs Containing Temozolomide- and Cy7 For NIR-Imaging and Therapy. Theranostics. 2011;1:381–394. doi: 10.7150/thno/v01p0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feshitan JA, Vlachos F, Sirsi SR, Konofagou EE, Borden MA. Theranostic Gd(III)-lipid microbubbles for MRI-guided focused ultrasound surgery. Biomaterials. 2012;33:247–255. doi: 10.1016/j.biomaterials.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penet MF, Chen Z, Kakkad S, Pomper MG, Bhujwalla ZM. Theranostic imaging of cancer. Eur J Radiol. 2012;81(Suppl 1):S124–S126. doi: 10.1016/S0720-048X(12)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthu MS, Feng SS. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin Drug Deliv. 2013;10:151–155. doi: 10.1517/17425247.2013.729576. [DOI] [PubMed] [Google Scholar]
- 51.Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44:863–874. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 52.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3:137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 53.Pan D. Theranostic nanomedicine with functional nanoarchitecture. Mol Pharm. 2013;10:781–782. doi: 10.1021/mp400044j. [DOI] [PubMed] [Google Scholar]
- 54.Ma X, Zhao Y, Liang XJ. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc Chem Res. 2011;44:1114–1122. doi: 10.1021/ar2000056. [DOI] [PubMed] [Google Scholar]
- 55.Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi KY, Liu G, Lee S, Chen X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale. 2012;4:330–342. doi: 10.1039/c1nr11277e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44:936–946. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arbustini E, Gambarin FI. Theranostic strategy against plaque angiogenesis. JACC Cardiovasc Imaging. 2008;1:635–637. doi: 10.1016/j.jcmg.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Schmieder AH, Caruthers SD, Zhang H, Williams TA, Robertson JD, Wickline SA, Lanza GM. Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22:4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Liu TW, Chen J, Green D, Jaffray D, Wilson BC, Wang F, Zheng G. Transforming a Targeted Porphyrin Theranostic Agent into a PET Imaging Probe for Cancer. Theranostics. 2011;1:363–370. doi: 10.7150/thno/v01p0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson CJ, Bulte JWM, Chen K, Chen X, Khaw BA, Shokeen M, Wooley KL, VanBrocklin HF. Design of targeted cardiovascular molecular imaging probes. Journal of Nuclear Medicine. 2010;51:3S–17S. doi: 10.2967/jnumed.109.068130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binsalamah ZM, Paul A, Prakash S, Shum-Tim D. Nanomedicine in cardiovascular therapy: Recent advancements. Expert Review of Cardiovascular Therapy. 2012;10:805–815. doi: 10.1586/erc.12.41. [DOI] [PubMed] [Google Scholar]
- 63.Chacko AM, Hood ED, Zern BJ, Muzykantov VR. Targeted nanocarriers for imaging and therapy of vascular inflammation. Current Opinion in Colloid and Interface Science. 2011;16:215–227. doi: 10.1016/j.cocis.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Oliveira H, Thevenot J, Lecommandoux S. Smart polymersomes for therapy and diagnosis: Fast progress toward multifunctional biomimetic nanomedicines. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2012;4:525–546. doi: 10.1002/wnan.1183. [DOI] [PubMed] [Google Scholar]
- 65.Grimm J, Scheinberg DA. Will Nanotechnology Influence Targeted Cancer Therapy? Seminars in Radiation Oncology. 2011;21:80–87. doi: 10.1016/j.semradonc.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluza E, Strijkers GJ, Nicolay K. Multifunctional magnetic resonance imaging probes. 2013;Vol. 187:151–190. doi: 10.1007/978-3-642-10853-2_5. [DOI] [PubMed] [Google Scholar]
- 67.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Hu G, Schmieder AH, Wickline SA. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010;13:189–202. doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy JR, Bhaumik J, Karver MR, Sibel Erdem S, Weissleder R. Targeted nanoagents for the detection of cancers. Molecular Oncology. 2010;4:511–528. doi: 10.1016/j.molonc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair SB, Dileep A, Rajanikant GK. Nanotechnology based diagnostic and therapeutic strategies for neuroscience with special emphasis on ischemic stroke. Current Medicinal Chemistry. 2012;19:744–756. doi: 10.2174/092986712798992138. [DOI] [PubMed] [Google Scholar]
- 70.O'Farrell AC, Shnyder SD, Marston G, Coletta PL, Gill JH. Non-invasive molecular imaging for preclinical cancer therapeutic development. British Journal of Pharmacology. 2013;169:719–735. doi: 10.1111/bph.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Power S, Slattery MM, Lee MJ. Nanotechnology and its relationship to interventional radiology. Part II: Drug delivery, thermotherapy, and vascular intervention. CardioVascular and Interventional Radiology. 2011;34:676–690. doi: 10.1007/s00270-010-9967-y. [DOI] [PubMed] [Google Scholar]
- 72.Shin SJ, Beech JR, Kelly KA. Targeted nanoparticles in imaging: Paving the way for personalized medicine in the battle against cancer. Integrative Biology (United Kingdom) 2013;5:29–42. doi: 10.1039/c2ib20047c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silindir M, Erdogan S, Özer AY, Maia S. Liposomes and their applications in molecular imaging. Journal of Drug Targeting. 2012;20:401–415. doi: 10.3109/1061186X.2012.685477. [DOI] [PubMed] [Google Scholar]
- 74.Smith BA, Smith BD. Biomarkers and molecular probes for cell death imaging and targeted therapeutics. Bioconjugate Chemistry. 2012;23:1989–2006. doi: 10.1021/bc3003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Susa M, Milane L, Amiji MM, Hornicek FJ, Duan Z. Nanoparticles: A promising modality in the treatment of sarcomas. Pharmaceutical Research. 2011;28:260–272. doi: 10.1007/s11095-010-0173-z. [DOI] [PubMed] [Google Scholar]
- 76.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced Drug Delivery Reviews. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wheatley MA, Cochran M. Ultrasound contrast agents. Journal of Drug Delivery Science and Technology. 2013;23:57–72. [Google Scholar]
- 78.Yu Y, Sun D. Superparamagnetic iron oxide nanoparticle 'theranostics' for multimodality tumor imaging, gene delivery, targeted drug and prodrug delivery. Expert Review of Clinical Pharmacology. 2010;3:117–130. doi: 10.1586/ecp.09.39. [DOI] [PubMed] [Google Scholar]
- 79.Caskey CF, Hu X, Ferrara KW. Leveraging the power of ultrasound for therapeutic design and optimization. Journal of Controlled Release. 2011;156:297–306. doi: 10.1016/j.jconrel.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geis NA, Katus HA, Bekeredjian R. Microbubbles as a vehicle for gene and drug delivery: Current clinical implications and future perspectives. Current Pharmaceutical Design. 2012;18:2166–2183. doi: 10.2174/138161212800099946. [DOI] [PubMed] [Google Scholar]
- 81.Jin CS, Zheng G. Liposomal nanostructures for photosensitizer delivery. Lasers in Surgery and Medicine. 2011;43:734–748. doi: 10.1002/lsm.21101. [DOI] [PubMed] [Google Scholar]
- 82.Kiessling F, Bzyl J, Fokong S, Siepmann M, Schmitz G, Palmowski M. Targeted ultrasound imaging of cancer: An emerging technology on its way to clinics. Current Pharmaceutical Design. 2012;18:2184–2199. doi: 10.2174/138161212800099900. [DOI] [PubMed] [Google Scholar]
- 83.Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. Journal of Nuclear Medicine. 2012;53:345–348. doi: 10.2967/jnumed.111.099754. [DOI] [PubMed] [Google Scholar]
- 84.Lammers T, Kiessling F, Hennink WE, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm. 2010;7:1899–1912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 85.Winter P, Caruthers S, Zhang H, Williams T, Wickline S, Lanza G. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. J Am Coll Cardiol Img. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lum A, Borden M, Dayton P, Kruse D, Simon S, Ferrara K. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release. 2006;111:128–134. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advan. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 88.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 89.Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Okamoto T, Kanouchi H, Mohri S, Tsujioka K, Kajiya F. Live-cell visualization of the trans-cellular mode of monocyte transmigration across the vascular endothelium, and its relationship with endothelial PECAM-1. J Physiol Sci. 2012;62:63–69. doi: 10.1007/s12576-011-0181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gane J, Stockley R. Mechanisms of neutrophil transmigration across the vascular endothelium in COPD. Thorax. 2012;67:553–561. doi: 10.1136/thoraxjnl-2011-200088. [DOI] [PubMed] [Google Scholar]
- 91.Williams MR, Sakurai Y, Zughaier SM, Eskin SG, McIntire LV. Transmigration across activated endothelium induces transcriptional changes, inhibits apoptosis, and decreases antimicrobial protein expression in human monocytes. J Leukoc Biol. 2009;86:1331–1343. doi: 10.1189/jlb.0209062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarantos MR, Zhang H, Schaff UY, Dixit N, Hayenga HN, Lowell CA, Simon SI. Transmigration of neutrophils across inflamed endothelium is signaled through LFA-1 and Src family kinase. J Immunol. 2008;181:8660–8669. doi: 10.4049/jimmunol.181.12.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Issa Y, Nummer D, Seibel T, Muerkoster SS, Koch M, Schmitz-Winnenthal FH, Galindo L, Weitz J, Beckhove P, Altevogt P. Enhanced L1CAM expression on pancreatic tumor endothelium mediates selective tumor cell transmigration. J Mol Med (Berl) 2009;87:99–112. doi: 10.1007/s00109-008-0410-7. [DOI] [PubMed] [Google Scholar]
- 94.Schrage A, Wechsung K, Neumann K, Schumann M, Schulzke JD, Engelhardt B, Zeitz M, Hamann A, Klugewitz K. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology. 2008;48:1262–1272. doi: 10.1002/hep.22443. [DOI] [PubMed] [Google Scholar]
- 95.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 96.Zozulya AL, Reinke E, Baiu DC, Karman J, Sandor M, Fabry Z. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Hu M, Lin X, Du Q, Miller EJ, Wang P, Simms HH. Regulation of polymorphonuclear leukocyte apoptosis: role of lung endothelium-epithelium bilayer transmigration. Am J Physiol Lung Cell Mol Physiol. 2005;288:L266–L274. doi: 10.1152/ajplung.00209.2004. [DOI] [PubMed] [Google Scholar]
- 100.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104:3849–3857. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- 101.Koskinen K, Vainio PJ, Smith DJ, Pihlavisto M, Yla-Herttuala S, Jalkanen S, Salmi M. Granulocyte transmigration through the endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (VAP-1) Blood. 2004;103:3388–3395. doi: 10.1182/blood-2003-09-3275. [DOI] [PubMed] [Google Scholar]
- 102.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci U S A. 2002;99:1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 104.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 105.Weber KS, Draude G, Erl W, de Martin R, Weber C. Monocyte arrest and transmigration on inflamed endothelium in shear flow is inhibited by adenovirus-mediated gene transfer of IkappaB-alpha. Blood. 1999;93:3685–3693. [PubMed] [Google Scholar]
- 106.Allavena P, Del Maschio A. Leukocyte transmigration through vascular endothelium. An in vitro method. Methods Mol Biol. 1999;96:171–176. doi: 10.1385/1-59259-258-9:171. [DOI] [PubMed] [Google Scholar]
- 107.Predescu D, Predescu S, McQuistan T, Palade GE. Transcytosis of alpha1-acidic glycoprotein in the continuous microvascular endothelium. Proc Natl Acad Sci U S A. 1998;95:6175–6180. doi: 10.1073/pnas.95.11.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yong KL, Watts M, Shaun Thomas N, Sullivan A, Ings S, Linch DC. Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31) Blood. 1998;91:1196–1205. [PubMed] [Google Scholar]
- 109.Broadwell RD, Baker-Cairns BJ, Friden PM, Oliver C, Villegas JC. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp Neurol. 1996;142:47–65. doi: 10.1006/exnr.1996.0178. [DOI] [PubMed] [Google Scholar]
- 110.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Predescu D, Horvat R, Predescu S, Palade GE. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci U S A. 1994;91:3014–3018. doi: 10.1073/pnas.91.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villegas JC, Broadwell RD. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. II. Adsorptive transcytosis of WGA-HRP and the blood-brain and brain-blood barriers. J Neurocytol. 1993;22:67–80. doi: 10.1007/BF01181571. [DOI] [PubMed] [Google Scholar]
- 113.Moser R, Fehr J, Bruijnzeel PL. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992;149:1432–1438. [PubMed] [Google Scholar]
- 114.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol. 1992;262:H246–H254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 115.May MJ, Ager A. ICAM-1-independent lymphocyte transmigration across high endothelium: differential up-regulation by interferon gamma, tumor necrosis factor-alpha and interleukin 1 beta. Eur J Immunol. 1992;22:219–226. doi: 10.1002/eji.1830220132. [DOI] [PubMed] [Google Scholar]
- 116.Galis Z, Ghitescu L, Simionescu M. Fatty acids binding to albumin increases its uptake and transcytosis by the lung capillary endothelium. Eur J Cell Biol. 1988;47:358–365. [PubMed] [Google Scholar]
- 117.Balin BJ, Broadwell RD. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. I. Choroid plexus and the blood-cerebrospinal fluid barrier. J Neurocytol. 1988;17:809–826. doi: 10.1007/BF01216708. [DOI] [PubMed] [Google Scholar]
- 118.Pawlowski NA, Kaplan G, Abraham E, Cohn ZA. The selective binding and transmigration of monocytes through the junctional complexes of human endothelium. J Exp Med. 1988;168:1865–1882. doi: 10.1084/jem.168.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Predescu D, Simionescu M, Simionescu N, Palade GE. Binding and transcytosis of glycoalbumin by the microvascular endothelium of the murine myocardium: evidence that glycoalbumin behaves as a bifunctional ligand. J Cell Biol. 1988;107:1729–1738. doi: 10.1083/jcb.107.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Milici AJ, Watrous NE, Stukenbrok H, Palade GE. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987;105:2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 123.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 124.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Massey KA, Schnitzer JE. Targeting and imaging signature caveolar molecules in lungs. Proc Am Thorac Soc. 2009;6:419–430. doi: 10.1513/pats.200903-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chrastina A, Valadon P, Massey KA, Schnitzer JE. Lung vascular targeting using antibody to aminopeptidase P: CT-SPECT imaging, biodistribution and pharmacokinetic analysis. J Vasc Res. 2010;47:531–543. doi: 10.1159/000313880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Massey KA, Schnitzer JE. Caveolae and cancer. Recent Results Cancer Res. 2010;180:217–231. doi: 10.1007/978-3-540-78281-0_13. [DOI] [PubMed] [Google Scholar]
- 128.Chrastina A, Massey KA, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:421–437. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- 129.Papademetriou J, Garnacho C, Serrano D, Bhowmick T, Schuchman EH, Muro S. Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J Inherit Metab Dis. 2013;36:467–477. doi: 10.1007/s10545-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mane V, Muro S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int J Nanomedicine. 2012;7:4223–4237. doi: 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ghaffarian R, Bhowmick T, Muro S. Transport of nanocarriers across gastrointestinal epithelial cells by a new transcellular route induced by targeting ICAM-1. J Control Release. 2012;163:25–33. doi: 10.1016/j.jconrel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Control Release. 2012;157:485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105:650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 134.Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121:2704–2714. doi: 10.1182/blood-2012-08-448332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta A, Le A, Belinka BA, Kachlany SC. In vitro synergism between LFA-1 targeting leukotoxin (Leukothera) and standard chemotherapeutic agents in leukemia cells. Leuk Res. 2011;35:1498–1505. doi: 10.1016/j.leukres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 136.Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, Kaur M, Mei Y, Rao J. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk Res. 2010;34:777–785. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woessner S, Asensio A, Florensa L, Pedro C, Besses C, Sans-Sabrafen J. Expression of lymphocyte function-associated antigen (LFA)-1 in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 1994;13:457–461. doi: 10.3109/10428199409049635. [DOI] [PubMed] [Google Scholar]
- 138.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamzah J, Kotamraju VR, Seo JW, Agemy L, Fogal V, Mahakian LM, Peters D, Roth L, Gagnon MKJ, Ferrara KW, et al. Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7154–7159. doi: 10.1073/pnas.1104540108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haspel N, Zanuy D, Nussinov R, Teesalu T, Ruoslahti E, Aleman C. Binding of a C-end rule peptide to the neuropilin-1 receptor: A molecular modeling approach. Biochemistry. 2011;50:1755–1762. doi: 10.1021/bi101662j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, Hamzah J, Ruoslahti E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2011 doi: 10.1038/onc.2011.537. [DOI] [PubMed] [Google Scholar]
- 142.Sun SW, Zu XY, Tuo QH, Chen LX, Lei XY, Li K, Tang CK, Liao DF. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacologica Sinica. 2010;31:1336–1342. doi: 10.1038/aps.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cavelier C, Ohnsorg PM, Rohrer L, Von Eckardstein A. The Î2-chain of cell surface F0F1 ATPase modulates ApoA-I and HDL transcytosis through aortic endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:131–139. doi: 10.1161/ATVBAHA.111.238063. [DOI] [PubMed] [Google Scholar]
- 144.Zhou HF, Hu G, Wickline SA, Lanza GM, Pham CT. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomedicine (Lond) 2010;5:1065–1074. doi: 10.2217/nnm.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou HF, Yan H, Senpan A, Wickline SA, Pan D, Lanza GM, Pham CT. Suppression of inflammation in a mouse model of rheumatoid arthritis using targeted lipase-labile fumagillin prodrug nanoparticles. Biomaterials. 2012;33:8632–8640. doi: 10.1016/j.biomaterials.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Armstrong J, Hempel G, Koling S, Chan L, Fisher T, Meiselman H, Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 147.Liu D, Mori A, Huang L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta. 1992;1104:95–101. doi: 10.1016/0005-2736(92)90136-a. [DOI] [PubMed] [Google Scholar]
- 148.He Q, Zhang Z, Gao F, Li Y, Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7:271–280. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 149.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Semete B, Booysen LI, Kalombo L, Venter JD, Katata L, Ramalapa B, Verschoor JA, Swai H. In vivo uptake and acute immune response to orally administered chitosan and PEG coated PLGA nanoparticles. Toxicol Appl Pharmacol. 2010;249:158–165. doi: 10.1016/j.taap.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 151.Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25:3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 152.Rojas-Espinosa O, Oltra A, Arce P. Circulating immune complexes in patients with advanced pulmonary tuberculosis detected by a polyethylene glycol precipitation-complement consuming test (PEG-CC test) Rev Latinoam Microbiol. 1988;30:25–29. [PubMed] [Google Scholar]
- 153.Pham CT, Mitchell LM, Huang JL, Lubniewski CM, Schall OF, Killgore JK, Pan D, Wickline SA, Lanza GM, Hourcade DE. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286:123–130. doi: 10.1074/jbc.M110.180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aggeli C, Giannopoulos G, Lampropoulos K, Pitsavos C, Stefanadis C. Adverse bioeffects of ultrasound contrast agents used in echocardiography: true safety issue or "much ado about nothing"? Curr Vasc Pharmacol. 2009;7:338–346. doi: 10.2174/157016109788340695. [DOI] [PubMed] [Google Scholar]
- 155.Bawa R. Regulating nanomedicine - can the FDA handle it? Curr Drug Deliv. 2011;8:227–234. doi: 10.2174/156720111795256156. [DOI] [PubMed] [Google Scholar]
- 156.Bawa R. FDA and nanotech: Baby steps lead to regulatory uncertainty. In: Bagchi D, Bagchi M, Moriyama H, Shahidi F, editors. Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences. Oxford, UK: Blackwell Publishing Ltd; 2013. pp. 720–732. [Google Scholar]