Abstract
TNF superfamily ligands play a critical role in the regulation of adaptive immune responses, including the costimulation of dendritic cells, T cells, and B cells. This costimulation could potentially be exploited for the development of prophylactic vaccines and immunotherapy. Despite this, there have been only a limited number of reports on the use of this family of molecules as gene-based adjuvants to enhance DNA and/or viral vector vaccines. In addition, the molecule latent membrane protein 1 (LMP1), a viral mimic of the TNF superfamily receptor CD40, provides an alternative approach for the design of novel molecular adjuvants. Here, we discuss advances in the development of recombinant TNF superfamily ligands as adjuvants for HIV vaccines and as cancer immunotherapy, including the use of LMP1 and LMP1-CD40 chimeric fusion proteins to mimic constitutive CD40 signaling.
Keywords: CD40L, TNF Superfamily, LMP1, 4-1BBL, OX40L, BAFF, APRIL
Introduction
Immunological danger signals play a critical role in the induction of an adaptive immune response [1–3]. Dendritic cells (DCs) and other antigen-presenting cells (APCs) recognize pathogen-associated molecular patterns (PAMPS) using a variety of host-derived pattern recognition receptors (PRRs). In addition, both APCs and adaptive immune cells (T and B cells) respond to receptor signaling generated by costimulatory ligands encoded by host cells [4–6]. These costimulatory ligand-induced signals complement PRR signaling and provide a mechanism for the host to communicate immunological danger to the adaptive immune system.
A number of costimulatory molecules are members of the TNF superfamily (TNFSF). One example is the receptor CD40 and its cognate ligand CD40 ligand (CD40L). CD40 expression by DCs is upregulated by PRR signaling, while CD40L expression by T cells is upregulated by interaction with DC-presenting antigen on MHC and costimulation via B7.1 and B7.2 [7–9]. Similarly, the TNFSF receptors 4-1BB, OX40, GITR, and CD27 are expressed on T cells and respond to costimulation by ligands expressed on APC, lymphocytes, and innate immune cells [4, 10–13]. TNFSF receptors BAFF-R, TACI, and BCMA are expressed on B cells and respond to the TNFSF ligands BAFF and APRIL. B cells also express CD40, and response to CD40L plays a critical role in B-cell activation and proliferation [14–16].
A key question for vaccine development has been whether costimulation by TNFSF ligands can be successfully engineered for use as vaccine adjuvants. Work on CD40 agonistic antibodies has shown promise in pre-clinical and clinical trials [17–19]. In a parallel approach, Immunex Inc. developed soluble single trimers of CD40L [20]. However, it has become recognized that CD40L must be assembled to form two or more trimers (multi-trimers) for optimal activity [21, 22]. Similarly, other TNFSF ligands have been shown to require multi-trimerization for optimal activity, including GITRL and 4-1BBL [23, 24]. This has led to the work using a variety of self-assembling protein scaffolds to generate multi-trimers of TNFSF ligands.
In this review, we summarize recent data from our laboratory and other groups on the use of protein engineering techniques to generate multi-trimer forms of TNFSF ligands. We present a summary of published work on multi-trimeric TNFSF ligands as adjuvants for DNA and viral vector vaccines against HIV and cancer. We also discuss recent work on the use of LMP1, a viral mimic of signaling by the TNFSF receptor CD40, as a novel molecular adjuvant approach.
Conjugating CD40L to multi-trimer scaffolds
It has become recognized in the field that CD40L and other TNFSF ligands generally must be multimerized in order to fully activate responding cells [21, 22, 25]. Similar to findings with CD40L, studies with the TNFSF ligand Fas ligand (FasL, CD95) found that single trimers of FasL were unable to induce a biological response, while cross-linking the trimers using an anti-Flag antibody gave biological activity comparable with membrane-bound FasL [25]. Another TNFSF ligand, TRAIL, could also be enhanced by cross-linking, while the TNFSF ligand TWEAK was not enhanced further by multimerization beyond the single trimer. This led to the conception by Dr. Richard Kornbluth that active soluble TNFSF ligands could be generated by fusing the extracellular head group of CD40L to the body of naturally multimerizing proteins in the collectin family, such as surfactant protein D (SP-D) and ACRP30 (adiponectin) [26]. SP-D is a water-soluble protein that is composed of four trimeric collagenous arms linked to a central hub by disulfide bonds [27]. Initial studies replaced the carbohydrate recognition domain of SP-D with the extracellular domain of CD40L [21]. This molecule generates four CD40L trimers per molecule (dodecamer) and is therefore multi-trimeric (i.e., many CD40L trimers) (Fig. 1). The dodecameric form of CD40L was found to be more effective than soluble single trimers of CD40L, triggering higher levels of B-cell proliferation and inducing CD86 upregulation [21]. This biological activity appeared to be unrelated to the binding affinity, with both single-trimer and SP-D 4-trimer versions of CD40L showing a similar KD for CD40 by BIAcore™ analysis [21]. These data suggest that activity is dependent on clustering of CD40 at the membrane surface. In support of this, Grassme et al. [28] showed that CD40 clustering was necessary and sufficient to generate CD40 signaling and could be induced simply by the clustering of CD40L. Similarly, disruption of lipid rafts by pre-treatment of cells expressing CD40L could prevent CD40 aggregation, suggesting that it is clustering of CD40 ligand that leads to CD40 receptor signaling.
Fig. 1.
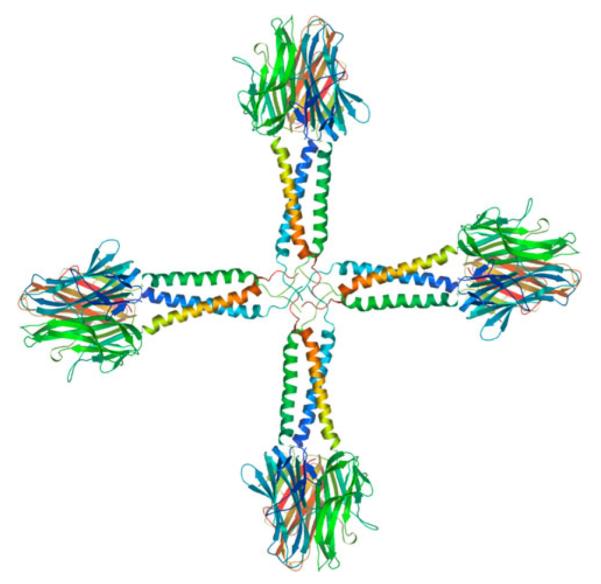
Theoretical model of the SP-D-CD40L multi-trimer complex. This theoretical model was developed using the SWISS-MODEL Workspace [63–66]. A model of the 12mer (4-trimer) SP-D-CD40L complex is shown, corresponding to the major population of SP-D-CD40L observed in previously published biochemical studies [21]
As an alternative approach to SP-D, the collectin family molecule ACRP30 has also been evaluated for the multi-trimerization of CD40L [22]. Studies by Holler et al. [22] compared CD40L with ACRP30 conjugation to the TNFSF ligand member Fas ligand (FasL). Interestingly, FasL showed optimal activity as a 2-trimer ACRP30 fusion, while the biological activity of ACRP30-CD40L could be further augmented by cross-linking two or more ACRP30-CD40L constructs.
Multi-trimer TNFSF ligands as DNA vaccine adjuvants
The enhanced activity of CD40L as a multi-trimer raised the question of whether this technique could be used to generate novel molecular vaccine adjuvants. Initially, we decided to test whether SP-D-CD40L or ACRP30-CD0L fusions would enhance a murine HIV DNA vaccine model [29]. We also evaluated GITRL, another member of the TNF superfamily, which is involved in the costimulation of T cells. Mammalian expression plasmids were constructed that encoded full-length membrane-bound CD40L, single-trimer CD40L (using a GCN4 leucine zipper motif), two-trimer CD40L (ACRP30-CD40L), and 4-trimer CD40L (SP-D-CD40L). Mice were vaccinated with HIV-1 Gag antigen plasmid with or without a CD40L adjuvant. Consistent with the work of Holler et al. [22], mice vaccinated with Gag plus SP-DCD40L generated the highest Gag-specific cytotoxic T-lymphocyte activity, interferon-gamma secretion as measured by ELISPOT assay, and the highest proportion of Gag-specific tetramer-positive CD8+ T cells in the spleen [29]. Activity was proportional to the valence of CD40L, with 4-trimer>2-trimer>1-trimer. Activity required the co-injection of antigen and adjuvant, and injection at separate sites did not enhance the immune response, confirming that SP-D-CD40L was directly impacting on antigen presentation and costimulation within the draining lymph node. Overall, this data confirmed the improved activity of CD40L in vivo when expressed as a soluble 4-trimer complex. Similar to SP-D-CD40L, the 4-trimer construct SP-D-GITRL enhanced T-cell responses in mice vaccinated with Gag and SP-D-GITRL expression plasmids. In addition, SP-DGITRL enhanced antibody responses, increasing endpoint titers>10-fold compared to Gag antigen alone.
More recently, our laboratory has evaluated a series of additional TNFSF ligands involved in the costimulation of innate and adaptive immune responses. SP-D fusions were constructed containing the molecules 4-1BBL, OX40L, CD70, LIGHT, BAFF, and RANKL, in addition to previously evaluated CD40L and GITRL. Molecules 4-1BBL, OX40L, CD70, and LIGHT are involved in the costimulation of T cells [30]. BAFF is well characterized for its role in B-cell costimulation [31–33], while RANKL is a costimulator of DCs and also involved in osteoblast development [34, 35]. In mouse HIV vaccine experiments, a number of these SP-D-TNFSF ligand fusion constructs showed activity as measured by interferon-gamma ELISPOT and proliferation assay [36]. Of particular interest, SP-D versions of 4-1BBL, OX40L, BAFF, and LIGHT enhanced T-cell avidity, measured by the ability of CD8+ T cells to secrete interferon-gamma in response to APC pulsed with low levels of antigen peptide (~1 ng/ml). SP-D fusions with OX40L, BAFF, and LIGHT enhanced IL-2 secretion by CD4+ and CD8+ memory T cells in response to antigen stimulation, and these adjuvants as well as SP-D-4-1BBL increased CD4+ and CD8+ T-cell proliferation in response to antigen stimulation. Overall, these data showed that the SP-D scaffold is a versatile technology providing a method to generate a diversity of gene-based vaccine adjuvants derived from number of TNFSF ligand molecules.
Cancer immunotherapy with multi-trimer CD40L and TLR agonists
The role of CD40 in cell-mediated immunity also has implications for cancer immunotherapy. CD40 stimulation is involved in the induction of IL-12p70 secretion and can potently augment immune stimulation when used in combination with one or more TLR agonists [37, 38]. Combining CD40 stimulation with TLR agonists has been used successfully for cancer immunotherapy. For example, Ahonen et al. [37] were able to significantly reduce tumor growth and increase survival in a mouse model through the use of anti-CD40 agonistic antibodies in combination with the TLR7 agonist S-27609.
Similarly, we have explored the use of SP-D-CD40L for cancer immunotherapy [39]. SP-D-CD40L was provided as a DNA plasmid expression vector (pSP-D-CD40L) and tested in combination with the TLR agonists CpG (TLR9) and poly(I:C) (TLR3). The peritumoral injection of pSP-D-CD40L into AB1 mesothelioma tumors was not effective on its own, but led to reduced tumor growth and increased survival when pSP-D-CD40L was combined with both CpG and poly(I:C) [39]. This response appeared to be antigen specific. Mice cured of AB1 tumor challenge by SP-D-CD40L treatment were able to resist AB1 rechallenge, but not challenge with a different tumor (A20 lymphoma). We also evaluated SP-D-CD40L combined with interferon-gamma treatment, which is known to increase the secretion of IL-12p70 when combined with a CD40 agonist. Surprisingly, this combination was not effective, highlighting the in vivo efficacy of CD40 stimulation when combined with TLR agonists such as CpG compared to interferon. One explanation is the limited effectiveness of IL-12p70 secretion at the tumor site relative to the draining lymph node.
A critical question for cancer immunotherapy with SPD-CD40L is which immune cells are involved in tumor regression in SP-D-CD40L + TLR agonist-treated animals. We observed an increase in CD8+ T lymphocytes and in particular F4/80+ macrophages in tumors treated with pSP-D-CD40L + CpG + poly(I:C), but not control tumors treated with PBS [39]. These results suggest that following SP-D-CD40L and TLR treatment, CD8+ T lymphocytes and macrophages are able to enter the tumor bed where one or more of these cell types eradicated the tumor.
In a second study, we used a nanoparticle delivery system to evaluate peritumoral injection of pSP-D-CD40L with or without the TLR agonists CpG and poly(I:C) in a B16-F10 melanoma tumor model [40]. Peritumoral injection of pSP-D-CD40L had only a modest effect on the growth of B16-F10 tumors, but was superior to single-trimer CD40L, consistent with previous reports that CD40L requires multi-trimerization for optimal activity [21, 22]. A number of TLR agonists combined with pSP-D-CD40L, including Pam3CSK, FSL1, MPL, Malp2, and imiquimod, were unable to further inhibit tumor growth. In contrast, CpG and poly(I:C) reduced tumor growth when combined with pSP-D-CD40L, and the combination of pSP-D-CD40L + CpG + poly(I:C) significantly delayed B16-F10 growth kinetics [40]. Similar to our AB1 model, we observed an increase in CD8+ lymphocytes in tumors treated with the combination of pSP-D-CD40L + CpG + poly(I:C). We next evaluated the use of the nanoparticles polyethylenimine (PEI) and C32 (provided by Dr. Robert Langer). Plasmid pSP-D-CD40L coated with PEI delayed tumor growth and enhanced survival. Addition of CpG and poly(I:C) to the PEI-coated pSP-D-CD40L resulted in 40 % survival of mice carrying palpable B16-F10 tumors, a remarkable activity against this highly aggressive cancer. Similarly, C32-coated pSP-D-CD40L delayed tumor growth and enhanced survival, but only when used in combination with CpG and poly(I:C). Overall, these studies suggest that SP-D-CD40L can be used as a DNA construct for the treatment of cancer, especially when combined with TLR agonists.
Multi-trimer TNFSF ligands as viral vector vaccine adjuvants
A major benefit of generating a secreted soluble form of TNFSF ligands is the ability to use these adjuvants in a number of vaccine formulations. Potential uses include purified recombinant SP-D-TNFSF ligands as an adjuvant for protein vaccines, expression plasmids encoding SP-D-TNFSF ligands as DNA vaccine adjuvants, and encoding SP-D-TNFSF ligands within viral vectors for use as viral vector vaccine adjuvants. TNFSF ligand adjuvants can also be used in heterologous prime/boost vaccines, a strategy being evaluated for the current generation of HIV vaccines [41–44]. SP-D-TNFSF ligands could potentially be used in both the prime and boost phases, incorporating SP-D-TNFSF ligand adjuvants in both prime and boost (DNA/viral vector, DNA/protein, viral vector/viral vector, etc.).
To evaluate the efficacy of SP-D-TNFSF ligands as adjuvants for viral vectors, Liu et al. [45] expressed SP-D-CD40L using a canarypox (ALVAC) viral vector and evaluated ALVAC-SP-D-CD40L adjuvant in combination with ALVAC-Gag antigen. In this context, full-length membrane-associated CD40L was superior to SP-D-CD40L as an adjuvant. However, this may reflect the unique tissue expression characteristics of the canarypox vector.
In more recent work, we evaluated SP-D-TNFSF ligand constructs expressed from replication defective-adenoviral vector (Ad5). In this context, SP-D-4-1BBL and SP-D-BAFF were particularly effective at enhancing the in vivo activity of an HIV-1 Gag vaccine, protecting mice from challenge with a recombinant vaccinia virus expressing HIV-1 Gag (unpublished data). Overall, these preliminary data suggested that SP-D-TNFSF ligands can enhance viral vector vaccines, but only when expressed from particular viral vector constructs such as adenovirus.
Activity of multi-trimer CD40L for rhesus macaque studies
Given the ability of CD40L, GITRL, and other TNFSF ligand multi-trimer constructs to enhance vaccine efficacy in murine models, we have also evaluated whether multi-trimers of TNFSF ligands such as CD40L and GITRL are biologically active as rhesus macaque constructs. If active, these constructs could then be tested in rhesus macaque simian immunodeficiency virus (SIV) vaccine models. Rhesus macaque lung and adipose tissue were used to generate cDNA encoding SP-D, ACRP30, and GITRL [46]. These genes, together with a previously described rhesus macaque CD40L (Genbank AF344859), were used to construct rhesus macaque versions of pSP-D-CD40L, pACRP30-CD40L, and pSP-D-GITRL. Protein expressed by these constructs was able to induce human B-cell proliferation in vitro, though at less than half of the activity of human SP-D-CD40L [46]. We also observed that rmSP-D-CD40L induced rhesus macaque B-cell proliferation in vitro, based on CFSE dilution of a CD20+ B-cell population from CFSE-labeled rhesus macaque PBMC. Similarly, rmSP-D-GITRL protein was able to induce proliferation of human anti-CD3-activated CD4+ T cells, confirming biological activity of this construct [46]. A unique characteristic of GITRL is the ability to reduce Treg-mediated inhibition of CD4+ T cells. To explore this, we cultured rmSP-D-GITRL protein with a mixture of human CD4 + CD25 + Treg and CD4 + CD25- effector T cells. In the presence of Treg, rmSP-D-GITRL was able rescue effector T-cell proliferation as measured by thymidine incorporation [46]. These data support a model whereby SP-D-GITRL costimulates effector T cells and blocks the inhibitory activity of Treg [47, 48]. These rhesus macaque SP-D constructs provided an excellent animal model system to explore the use of multi-trimeric TNFSF ligands as vaccine adjuvants. SP-D-CD40L and SP-D-GITRL are expected to enhance vaccine responses and to abrogate the effects of Treg inhibition in rhesus macaques, a response especially useful for immunotherapy approaches that require the inhibition of Treg function.
LMP1 as a viral mimic of CD40 signaling
The immunostimulatory membrane-bound activator protein, latent membrane protein 1 (LMP1), from Epstein–Barr virus (EBV) has a unique set of properties. LMP1 consists of a 6-segment transmembrane domain and a cytoplasmic signaling domain (Fig. 2) [49, 50]. EBV preferentially infects human B lymphocytes and persists in these cells by encoding a number of mimics of cellular proteins, including LMP1. LMP1 mimics the protein clustering and intracellular signaling induced by the interaction of CD40 ligand and the CD40 receptor [49]. This induces a constitutive costimulatory signal leading to the proliferation of infected B cells [51]. Researchers have previously expressed LMP1 in B cells to compare CD40 and LMP1 signaling [52]. In addition, the cytoplasmic activation domain of LMP1 has been replaced by the activation domain of CD40 [49, 53]. This chimeric protein, LMP1-CD40, has been shown to act as an immunostimulatory molecule in B cells, in a manner similar to LMP1. Both CD40 receptor signaling and LMP1 expression induce the activation, proliferation, and survival of B cells [54]. This phenotype is accomplished by the interaction of the LMP1 and CD40 intracellular domains with an overlapping series of signaling molecules [55]. Both LMP1 and CD40 interact with distinct molecules, but have a number of factors in common, including tumor necrosis factor receptor-associated factors (TRAFs) that activate signaling pathways for NFκB, c-Jun N-terminal kinase (JNK), p38/MAPK, and extracellular signal-related kinase (ERK). Common factors include TRAFs 1, 2, 3, and 5 [56, 57]. Distinct factors associated with LMP1 and CD40 cytoplasmic signaling domains include TRAF6 for CD40 and tumor necrosis factor receptor-associated death domain (TRADD) and receptor-interacting protein (RIP) for LMP1 [58]. Overall, LMP1 provides a unique method to stimulate CD40-like signaling. Both LMP1 and LMP1-CD40 are able to signal in a constitutive manner without the need for a ligand [49], a characteristic that is of particular interest for their use as gene-based vaccine adjuvants.
Fig. 2.
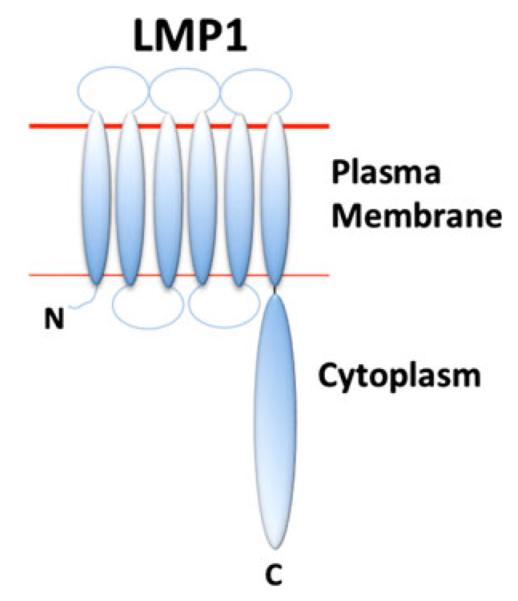
Model of the LMP1 transmembrane structure. The LMP1 N-terminal transmembrane region consists of six transmembrane domains. The cytoplasmic domain at the C-terminus is involved in intracellular signaling and activation of B cells, dendritic cells, and macrophages
LMP1 and LMP1-CD40 function as molecular adjuvants
While LMP1 has been studied as an immunostimulatory molecule in B cells for more than a decade, only recently it has been proposed that LMP1 could be used to enhance vaccines as a molecular adjuvant. We evaluated LMP1 in the context of HIV-1 infection by incorporating the LMP1 gene into the HIV-1 genome in front of the nef gene [59]. An IRES sequence allowed the expression of both LMP1 and Nef by this recombinant virus. We also introduced the LMP1-CD40 chimeric gene into HIV-1. To construct LMP1-CD40, the LMP1 cytoplasmic domain was replaced by the cytoplasmic domain of human CD40 [49]. These recombinant HIV-1 viruses were able to infect human monocyte-derived macrophages and dendritic cells (DCs), allowing us to explore the role of LMP1 and LMP1-CD40 in antigen-presenting cell (APC) maturation and activation. As has been observed by others [60], native HIV-1 virus or a HIV-GFP control virus (expressing GFP in place of LMP1) failed to induce maturation and activation of DCs and macrophages. In contrast, HIV-LMP1 and HIV-LMP1-CD40 were able to significantly enhance markers of activation and maturation including CD80, CD40, and CD83 surface expression on both DCs and macrophages [59]. This was accompanied by a significant increase in the secretion of pro-inflammatory cytokines IL-6, IL-8, IL-1β, and TNF-α, suggesting that LMP1 and LMP1-CD40 are potent immune stimulators of DCs and macrophages.
We next confirmed that immunostimulation by LMP1 and LMP1-CD40 was independent of activation provided by HIV-1 itself. RNA encoding LMP1 or LMP1-CD40 was used to transfect monocyte-derived DCs. Similar to our HIV constructs, this led to increase in surface expression of CD40, CD83, and CD40, as well as increased secretion of IL-6, IL-8, and TNF-α (data not published).
These data suggested that LMP1 might be able to function as a molecular adjuvant, enhancing the adaptive immune response against HIV-1 antigens. To test this, we infected human DCs with HIV-1 constructs expressing LMP1 or LMP1-CD40 and cocultured the DCs with autologous T cells for 12 days to generate HIV-1 Gag antigen-specific T cells (as measured by an interferon-γ ELISPOT assay). While culture of DCs infected with native HIV-1 or HIV-GFP induced only a small population of HIV Gag-specific T cells (<300 per million cells), HIV-LMP1 generated >1,000 cells secreting interferon-γ per million cells. HIV-LMP1-CD40 also increased the number of Gag-specific T cells by ELISPOT assay, but did not reach statistical significance [59]. These studies provided the first evidence that LMP1 functions as a potent molecular adjuvant in dendritic cells, either as a full-length protein or as a chimera with CD40.
LMP1 enhances the activity of single-cycle lentiviral vaccines
To further explore the use of LMP1 and LMP1-CD40 as vaccine adjuvant, we constructed SIV-based lentiviral vector vaccines encoding GFP, LMP1, or LMP1-CD40 [61]. The vaccine vector was derived from strain SIVmac239, with deletions and mutations that prevented viral replication beyond a single round. The nef gene (involved in MHC-I downregulation) was deleted and replaced with GFP, LMP1, or the LMP1-CD40 chimera [49]. Infection of human DCs and macrophages with SIV-LMP1 or SIV-LMP1-CD40 led to dramatic morphological changes characteristic of cellular activation and maturation. Consistent with these morphological changes, transduced DCs and macrophages significantly upregulated CD40, CD83, and CD80 surface expression [61]. Within 12–48 h post-infection, SIV-LMP1 infected DCs secreted high levels of IL-6, IL-8, and TNF-α, and low but increased levels of IL-1β and the cytokine IL-12p70. We also observed increased expression of the chemokines MIP-1α, MIP-1β, and RANTES in infected macrophages, consistent with enhanced anti-viral activity.
To confirm that our vaccine vectors SIV-LMP1 and SIV-LMP1-CD40 could function as molecular adjuvants, infected human DCs were cocultured with autologous T cells for 12 days. Both SIV-LMP1 and SIV-LMP1-CD40 significantly increased the number of SIV Gag-specific T cells secreting interferon-γ, suggesting these genes are effective molecular adjuvants in this viral vector vaccine model.
LMP1 future directions
LMP1 provides an intriguing alternative to the current series of molecular adjuvants available for DNA and viral vector vaccines, including IL-12, IL-15, Flt3L, GM-CSF, and our own work on TNFSF ligands [29, 36, 40]. Importantly, LMP1 does not require the expression of a receptor on DCs or T cells, since it is constitutively active [59, 62]. This allows LMP1 to activate DCs independently of receptors such as CD40. LMP1 can be encoded together with antigen(s) of interest, either as part of an attenuated virus (i.e., single-cycle SIV) or through the co-expression of antigen and LMP1 using an IRES system or dual-promoter construct. Our laboratory is currently evaluating the co-expression of HIV-1 antigen Gag and LMP1 using an IRES system for DNA and adenoviral vector HIV vaccines. Studies are also ongoing to determine whether LMP1 can adjuvant anti-tumor immune responses when encoded as a DNA or viral vector vaccine or when used to transfect DCs for dendritic cell immunotherapy.
Conclusion
TNF superfamily ligands are critical mediators of adaptive immune function and provide a wide variety of immune modulators for use as vaccine adjuvants. Conjugation of these ligands to a multi-trimer scaffold (SP-D, ACRP30) provides a novel method to exploit these TNFSF ligands as vaccine adjuvants. In particular, SP-D fusion proteins have been used to generate TNFSF ligand multi-trimers that can induce anti-HIV immune responses and protect animals from melanoma and mesothelioma cancers. SP-D-TNFSF ligand fusions of particular interest include CD40L, GITRL, 4-1BBL, OX40L, BAFF, and LIGHT. In addition to TNFSF ligands, we have also explored the use of the viral protein LMP1 as a gene-based vaccine adjuvant. Use of LMP1 as an adjuvant exploits the similarities between LMP1 and CD40 immune signaling, allowing us to use LMP1 as a mimic of constitutive CD40 immune activation. Both full-length LMP1 and a chimeric fusion of the LMP1 transmembrane domain with the CD40 cytoplasmic domain enhanced DC activation and generated adaptive immune responses in human in vitro studies. Overall, both of these technologies (SP-D-TNFSF ligands and LMP1 chimeras) provide novel approaches for the enhancement of vaccines against infectious diseases and cancer.
Acknowledgments
This study was supported by US National Institutes of Health grants R21 AI093294, R21 AI078834, and K22 AI068489 to G. Stone, Grant P30 AI073961 to the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine (PI Dr. Savita Pahwa), Florida Biomedical Bankhead-Coley Cancer Research Grant 1BF02 to G. Stone, American Cancer Society Institutional Research Grant 98-277-10, Sylvester Comprehensive Cancer Center (PI Dr. Joseph Rosenblatt) to G. Stone, Stanley J. Glaser Foundation Grant to G. Stone, and the NIH AIDS Reagent Program, Division of AIDS, NIAID.
Biography

Geoffrey W. Stone
References
- 1.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511–9. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Michallet MC, Rota G, Maslowski K, Guarda G. Innate receptors for adaptive immunity. Curr Opin Microbiol. 2013;16(3):296–302. doi: 10.1016/j.mib.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 5.Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol Rev. 2013;255(1):125–48. doi: 10.1111/imr.12086. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 8.Summers deLuca L, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12(5):339–51. doi: 10.1038/nri3193. [DOI] [PubMed] [Google Scholar]
- 9.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–44. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 13.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37(5):1165–9. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 14.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–33. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolduc A, Long E, Stapler D, Cascalho M, Tsubata T, Koni PA, et al. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. J Immunol. 2010;185(1):220–30. doi: 10.4049/jimmunol.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J Immunol. 1994;153(10):4357–66. [PubMed] [Google Scholar]
- 17.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 18.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035–43. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5(5):548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 20.Morris AE, Remmele RL, Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) J Biol Chem. 1999;274(1):418–23. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- 21.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001;31(10):3094–100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23(4):1428–40. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Song X, Berezov A, Zhang G, Li Y, Zhang H, et al. Human glucocorticoid-induced TNF receptor ligand regulates its signaling activity through multiple oligomerization states. Proc Natl Acad Sci USA. 2008;105(14):5465–70. doi: 10.1073/pnas.0711350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29(8):383–90. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187(8):1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornbluth RS. Inventor multimeric forms of the TNF superfamily ligands. PCT patent WO 01/42298A1. 2001 06/14/01.
- 27.Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D) J Biol Chem. 1994;269(25):17311–9. [PubMed] [Google Scholar]
- 28.Grassme H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to form a functional contact with CD40. J Biol Chem. 2002;277:30289–99. doi: 10.1074/jbc.M200494200. [DOI] [PubMed] [Google Scholar]
- 29.Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006;80(4):1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 31.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183(6):3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 32.Mackay F, Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23(3):113–5. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 33.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17(3):282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 35.Mackay F, Kalled SL. TNF ligands and receptors in autoimmunity: an update. Curr Opin Immunol. 2002;14(6):783–90. doi: 10.1016/s0952-7915(02)00407-7. [DOI] [PubMed] [Google Scholar]
- 36.Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, et al. Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine. 2012;30(4):691–702. doi: 10.1016/j.vaccine.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, et al. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111(6):3116–25. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone GW, Barzee S, Snarsky V, Santucci C, Tran B, Kornbluth RS. Regression of established AB1 murine mesothelioma induced by peritumoral injections of CpG oligodeoxynucleotide either alone or in combination with poly(I:C) and CD40 ligand plasmid DNA. J Thorac Oncol. 2009;4(7):802–8. doi: 10.1097/JTO.0b013e3181a8634d. [DOI] [PubMed] [Google Scholar]
- 40.Stone GW, Barzee S, Snarsky V, Santucci C, Tran B, Langer R, et al. Nanoparticle-delivered multimeric soluble CD40L DNA combined with toll-like receptor agonists as a treatment for melanoma. PLoS ONE. 2009;4(10):e7334. doi: 10.1371/journal.pone.0007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson HL. Prime boost vaccines power up in people. Nat Med. 2003;9(6):642–3. doi: 10.1038/nm0603-642. [DOI] [PubMed] [Google Scholar]
- 42.Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, et al. Enhanced control of pathogenic simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85(18):9578–87. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26(8):1098–110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ, et al. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med. 2006;203(6):1533–41. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Yu Q, Stone GW, Yue FY, Ngai N, Jones RB, et al. CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals. Vaccine. 2008;26(32):4062–72. doi: 10.1016/j.vaccine.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone GW, Barzee S, Snarsky V, Spina CA, Lifson JD, Pillai VK, et al. Macaque multimeric soluble CD40 ligand and GITR ligand constructs are immunostimulatory molecules in vitro. Clin Vaccine Immunol. 2006;13(11):1223–30. doi: 10.1128/CVI.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169(11):6210–7. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3(2):135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 49.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, et al. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16(20):6131–40. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fennewald S, van Santen V, Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984;51(2):411–9. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90(19):9150–4. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stunz LL, Busch LK, Munroe ME, Sigmund CD, Tygrett LT, Waldschmidt TJ, et al. Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity. 2004;21(2):255–66. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Panagopoulos D, Victoratos P, Alexiou M, Kollias G, Mosialos G. Comparative analysis of signal transduction by CD40 and the Epstein-Barr virus oncoprotein LMP1 in vivo. J Virol. 2004;78(23):13253–61. doi: 10.1128/JVI.78.23.13253-13261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimber-Strobl U, Kempkes B, Marschall G, Zeidler R, Van Kooten C, Banchereau J, et al. Epstein-Barr virus latent membrane protein (LMP1) is not sufficient to maintain proliferation of B cells but both it and activated CD40 can prolong their survival. EMBO J. 1996;15(24):7070–8. [PMC free article] [PubMed] [Google Scholar]
- 55.Lam N, Sugden B. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 2003;15(1):9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 56.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, et al. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271(46):28745–8. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 57.Schultheiss U, Puschner S, Kremmer E, Mak TW, Engelmann H, Hammerschmidt W, et al. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 2001;20(20):5678–91. doi: 10.1093/emboj/20.20.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izumi KM, Cahir McFarland ED, Ting AT, Riley EA, Seed B, Kieff ED. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-kappaB activation. Mol Cell Biol. 1999;19(8):5759–67. doi: 10.1128/mcb.19.8.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Termini JM, Niu L, Kanagavelu SK, Schmidtmayerova H, Snarsky V, et al. EBV LMP1, a viral mimic of CD40, activates dendritic cells and functions as a molecular adjuvant when incorporated into an HIV vaccine. J Leukoc Biol. 2011;90(2):389–98. doi: 10.1189/jlb.0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci USA. 2004;101(20):7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta S, Termini JM, Niu L, Kanagavelu SK, Rahmberg AR, Kornbluth RS, et al. Latent membrane protein 1 as a molecular adjuvant for single-cycle lentiviral vaccines. Retrovirology. 2011;8:39. doi: 10.1186/1742-4690-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izumi KM, Kieff ED. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci USA. 1997;94(23):12592–7. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 64.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4(1):1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- 65.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–92. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopp J, Schwede T. The SWISS-MODEL repository: new features and functionalities. Nucleic Acids Res. 2006;34:D315–8. doi: 10.1093/nar/gkj056. [DOI] [PMC free article] [PubMed] [Google Scholar]


