Abstract
The eradication of poliovirus from the majority of the world has been achieved through the use of two vaccines: the inactivated poliovirus vaccine (IPV) and the live-attenuated oral poliovirus vaccine (OPV). Both vaccines are effective at preventing paralytic poliomyelitis, however, they also have significant differences. Most importantly for this work is the risk of revertant virus from OPV, the greater cost of IPV, and the low mucosal immunity induced by IPV. We and others have previously described the use of an alphavirus-based adjuvant that can induce a mucosal immune response to a co-administered antigen even when delivered at a non-mucosal site. In this report, we describe the use of an alphavirus-based adjuvant (GVI3000) with IPV. The IPV-GVI3000 vaccine significantly increased systemic IgG, mucosal IgG and mucosal IgA antibody responses to all three poliovirus serotypes in mice even when administered intramuscularly. Furthermore, GVI3000 significantly increased the potency of IPV in rat potency tests as measured by poliovirus neutralizing antibodies in serum. Thus, an IPV-GVI3000 vaccine would reduce the dose of IPV needed and provide significantly improved mucosal immunity. This vaccine could be an effective tool to use in the poliovirus eradication campaign without risking the re-introduction of revertant poliovirus derived from OPV.
Keywords: Inactivated poliovirus vaccine, adjuvant, mucosal immunity, alphavirus
1. Introduction
The Global Poliovirus Eradication Initiative (GPEI) has reduced poliovirus cases by more than 99% worldwide since it was initiated in 1988 by the World Health Organization (WHO) [1]. To highlight a recent milestone by GPEI, wildtype poliovirus cases in India have not been reported for over two years [2]. Currently, however, the risk of wildtype poliovirus spreading from the endemic countries of Afghanistan, Pakistan, and Nigeria to polio-free countries continues to require vaccination coverage worldwide.
Poliovirus infects the gut and is transmitted primarily through shedding in feces by the fecal-oral route, but can also be transmitted by the oral-oral route [3]. In <1% of cases [4], acute flaccid paralysis occurs when the virus spreads to the central nervous system (CNS) [3]. Two vaccines are in use to protect against poliovirus: the inactivated poliovirus vaccine (IPV) and the live-attenuated oral poliovirus vaccine (OPV), with each containing the three poliovirus serotypes. Both IPV and OPV induce serum antibodies that prevent poliovirus spread to the CNS, but OPV is superior at inducing mucosal immunity, shortening the period of poliovirus replication in the gut and subsequent duration of shedding (after ≥ 2 doses OPV) [5–7]. OPV is also thought to reduce transmission in this manner, but the induction of mucosal immunity can be incomplete and the relationship between the level of mucosal immunity and likelihood of transmission is unknown [8–10]. Nevertheless, OPV use has led to the eradication of poliovirus in several countries.
One significant disadvantage of OPV, however, is that in rare cases (about 1 in 0.9 million vaccinees, [11]), an attenuated strain in OPV can revert to virulence and cause vaccine-associated paralytic poliomyelitis (VAPP). The use of OPV may also lead to vaccine-derived polioviruses (VDPVs) capable of spread between individuals [12–16]. Another disadvantage of OPV, is that in its trivalent form the three vaccine strains compete with one another to infect the gut, resulting in a stronger immune response to type 2 versus types 1 and 3 [17]. More recently, the use of monovalent and bivalent OPV has helped to overcome this issue, but still relies on infection of the gut which can lower vaccine efficacy when there are intercurrent infections [18]. Use of IPV avoids these issues since it lacks replicating virus and uses a different route of administration (intramuscular). OPV was selected over IPV as the vaccine for worldwide eradication due to its ability to induce mucosal immunity, its lower production cost, and ease of administration [1, 19]. If a new IPV vaccine formulation had a lower cost and induced mucosal immunity this would be a significant asset to the GPEI. Such a vaccine could be used after cessation of OPV use in the post-eradication era or in mop-up campaigns where wildtype poliovirus has been introduced into a polio-free country [20].
Currently, IPV is not used with an adjuvant and an adjuvant that induces a mucosal immune response by a non-mucosal intramuscular route like that used for IPV would be advantageous. Without inducing mucosal immunity, IPV can prevent symptomatic poliomyelitis but may not reduce infection and asymptomatic excretion of wildtype poliovirus [21]. Previously, the adjuvant 1,25 dihydroxyvitamin D3 was shown to enhance the mucosal IgA immune response to IPV in mice, but the fold increase was very small [22]. An IPV adjuvant that allows for dose-sparing to lower cost and improves the mucosal immune response would greatly improve this vaccine.
A promising mucosal adjuvant for IPV is a novel alphavirus-based adjuvant. This adjuvant enhances humoral, cellular and mucosal immunity to antigens, even when delivered at a non-mucosal site [23–25]. The alphavirus-based adjuvant is a disarmed RNA virus particle which targets inflammatory dendritic cells in the draining lymph node and mimics the earliest stages of viral infection [26]. The disarmed virus cannot propagate as the RNA genome lacks the structural genes of the virus. Inside the cell, replication of the RNA genome induces an antiviral innate immune response. When this adjuvant is co-administered with an antigen, the adaptive immune system sees this antigen as if it was the product of a viral infection. Accordingly, the resulting humoral, cellular and mucosal immune responses are significantly improved relative to antigen alone. An IPV vaccine that includes an alphavirus-based adjuvant could allow for dose-sparing to reduce cost and also induce mucosal immunity that would increase protection against poliovirus replication in the gut, reduce poliovirus excretion into the environment and induce serum antibodies that would prevent spread of poliovirus to the CNS. Such a vaccine could help break transmission cycles during a poliovirus outbreak in a previously poliovirus-free country.
A previous study demonstrated that GVI3000, an adjuvant derived from the alphavirus Venezuelan equine encephalitis virus (VEE), increased the potency of IPV made from inactivated Sabin strains (sIPV), as measured by neutralizing antibody titers in rats [27]. Since the protective efficacy of sIPV in humans has not been evaluated, in the work described herein we further investigated the ability of GVI3000 to increase the potency of IPV in rats and mice, and to determine whether GVI3000 can induce a mucosal immune response to poliovirus antigens. The ability of GVI3000 to allow dose-sparing and to enhance mucosal immune responses to IPV is important for its utility as an adjuvant and whether an IPV-GVI3000 vaccine would be useful to the GPEI after global use of OPV has ceased in the post-eradication era.
2. Materials and methods
2.1. GVI3000 replicon particles
Production of GVI3000 has been described [28, 29]. Briefly, GVI3000 replicon RNA and two helper RNAs expressing either capsid or glycoproteins are electroporated into BHK-21 cells. The helper RNAs produce the structural proteins in trans but lack the cis-acting packaging sequence, so that only the replicon RNA containing the packaging sequence is incorporated into the adjuvant particles. GVI3000 replicon particles are packaged in the wild-type (V3000) VEE envelope [30]. After purification, the absence of detectable propagation-competent virus is confirmed by cytopathic effect assay which can detect 1 PFU VEE. GVI3000 is titered by immunofluorescent staining of VEE non-structural proteins in infected BHK-21 cells.
2.2. Mice and immunizations
6–8 week old female BALB/c mice were purchased from Charles River and housed at the University of North Carolina Division of Laboratory Animal Medicine animal facility according to protocols approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee. 6–10 mice per group were used in these studies. Mice were injected intramuscularly (i.m.) in the gastrocnemius at weeks 0 and 4 with IPV from Intravacc. All animals received 0.8/0.2/0.6 D antigen units IPV, types 1, 2, and 3, respectively with or without 105 infectious units (IU) of GVI3000. This IPV dose was 50-fold lower than the dose used in humans (40/8/32 DU). The dose was chosen based on a previous experiment that showed GVI3000 has its maximum adjuvant effect and antibody titers with IPV at this dose and further increases in dose did not increase titers (data not shown). This dose was a 1:5 dilution of 10 uL IPV stock (80/16/64 D antigen units per mL) into 40 uL PBS. See Supplementary Materials and Methods for analysis of poliovirus-specific IgG and IgA by ELISA.
2.3. Potency test in rats
In collaboration with Intravacc in the Netherlands, we evaluated the efficacy of the GVI3000 adjuvant combined with IPV in a potency test in outbred Rivm:TOXrats [27]. On day 0 and 28, 15 rats per group were immunized by i.m. injection with 1.5/0.3/1.2 D antigen units of IPV with or without 105 IU of GVI3000. All rats were bled on day 21 (3 weeks post-prime) and day 49 (3 weeks post-boost) and sera were analyzed for neutralizing antibodies to all three poliovirus serotypes [31]. The rat immunogenicity tests were approved by an Animal Welfare Committee.
2.4. Statistical analysis
For mouse experiments, the means of reciprocal endpoint dilutions between groups with and without GVI3000 were compared by Student’s t-test as indicated by horizontal bars in the figures. The same test was performed on the endpoint dilutions of the rat neutralization titers. Statistical analyses were performed using GraphPad Prism 5® software.
3. Results
3.1. GVI3000 potentiated humoral and mucosal immune responses to IPV
Previous reports have shown that peripherally inoculated GVI3000 induced a significant increase in humoral and mucosal antibodies to co-delivered antigens [23, 32]. To determine if GVI3000 could potentiate immune responses to IPV, groups of six mice were immunized i.m. with IPV alone (0.8/0.2/0.6 DU) or in combination with GVI3000. Serotype-specific antibody levels in vaccinated animals were detected 3 weeks post-boost by ELISA. GVI3000 significantly increased the serum IgG titers to all three PV serotypes compared to IPV alone (Fig. 1A). Within IgG subclasses, GVI3000 induced 6.5–30 times more IgG2a than IPV alone (Fig. 1B) and comparable IgG1 levels (Fig. 1C). IgG2a and IgG1 are markers that correlate with the induction of Th1 and Th2 responses, respectively [33–35]. An IgG2a/IgG1 ratio close to 1 (Fig. 1D) indicated that GVI3000 increased the Th1 response leading to a more balanced Th1/Th2 response compared to IPV alone.
Figure 1. Humoral immune response to IPV and GVI3000 in mice.

BALB/c mice (6 per group) were primed and boosted i.m. with IPV alone or co-administered with GVI3000. The levels of (A) IgG, (B) IgG2a and (C) IgG1 serotype-specific antibodies to PV in the serum were determined at 3 weeks post-boost by ELISA. Values represent the reciprocal of the mean endpoint dilution ± SEM. A ratio between the endpoint dilutions of IgG2a and IgG1 is shown in (D) and the dashed line represents a ratio of 1. Statistical significance, ns= not significant, *=p<0.05, **=p<0.01. Similar significant differences in serum IgG titers with and without adjuvant were achieved in two separate experiments using BALB/c mice.
To determine whether non-mucosal GVI3000 delivery resulted in the induction of mucosal immunity to IPV, fecal pellets were collected at 10 days post-boost, and poliovirus-specific IgA was detected in fecal extracts by ELISA (S1). Mice immunized with IPV alone had levels of IgA close to the limit of detection; however, in GVI3000 adjuvanted groups, the levels of poliovirus-specific IgA to all three poliovirus serotypes were significantly increased (Fig. 2), indicating that i.m. delivery of GVI3000 effectively potentiated systemic IgG and mucosal IgA antibody responses to IPV.
Figure 2. Mucosal immune response to IPV and GVI3000 in mice.
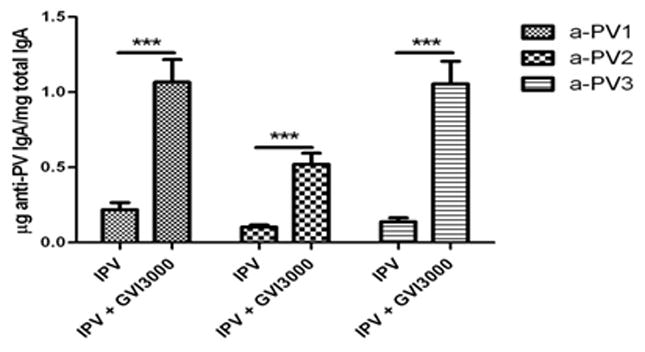
BALB/c mice (6 per group) were primed and boosted i.m. with IPV alone or co-administered with GVI3000. The levels of serotype-specific IgA in fecal extracts at 10 days post-boost were determined by ELISA. Values represent the mean endpoint dilution ± SEM. Statistical significance, ***=p<0.001. Similar significant differences in mucosal IgA titers with and without adjuvant were achieved in two separate experiments using BALB/c mice.
3.2. I.m. delivery of IPV-GVI3000 resulted in the induction of locally produced mucosal immunity
To determine whether non-mucosal delivery of IPV-GVI3000 resulted in the induction of locally produced IgG and IgA in lymphoid organs and the mucosa, two groups of ten female BALB/c mice each were immunized i.m. with IPV alone or IPV combined with 105 IU of GVI3000. At 4 weeks post-prime, all mice were boosted and at 14 days post-boost, the spleen, popliteal lymph node and small intestine from each mouse were harvested for analysis in a lymphoid culture assay originally developed by Cebra and colleagues (S2 [36]). Poliovirus-specific IgG and IgA in supernatants from these ex vivo organ cultures was detected by ELISA (S1). This assay has not been used previously for the detection of poliovirus-specific antibodies, but was useful for isolating organs that produced IgG and IgA after vaccination with IPV.
IPV adjuvanted with GVI3000 induced significantly increased IgG levels to all poliovirus serotypes in each organ culture compared with responses induced by IPV alone (Fig. 3A). IPV alone induced very low IgA production in ex vivo cultures (Fig. 3B). In contrast, mice vaccinated with IPV combined with GVI3000 had IgA levels about 30 times higher in the spleen and about 10 times higher in the draining lymph node and intestine than IPV alone. The increased secretory IgA found in the intestine was consistent with the increased IgA found in fecal samples (Fig. 2). Previous experiments have demonstrated that like IgA found at mucosal surfaces, antigen specific IgA induced by GVI3000 in the draining popliteal lymph node was multimeric and J-chain linked [24].
Figure 3. Antibody response to IPV and GVI3000 in different lymphoid organs.

BALB/c mice (10 per group) were primed and boosted i.m. with IPV alone or co-administered with GVI3000. 14 days post-boost, the spleen, popliteal lymph nodes (PLN), and intestine were harvested and cultured. On day 7 the levels of serotype-specific IgG (A) and IgA (B) in culture supernatants were determined. Values represent the mean endpoint dilution ± SEM. Statistical significance, *=p<0.05, **=p<0.01, ***=p<0.001.
3.3. GVI3000 enhanced IPV potency in rats
To determine the potency of new IPV lots, the World Health Organization recommends a standardized IPV potency test in rats that correlates with vaccine potency in humans [31]. The ability of GVI3000 to enhance the potency of IPV after prime and boost was tested in collaboration with Intravacc. Groups of 15 rats each were given either IPV, IPV + 105 IU GVI3000, or an IPV reference standard PU01-91 in a prime and boost schedule. The IPV dose was 1.5/0.30/1.2 D antigen units per rat and the inoculation route was i.m. in the hind limb. This dose was chosen because it was in the low linear range of a dose response curve conducted previously for IPV (data not shown).
As can be seen in Figure 4, the IPV lot used in this experiment was consistent with the PU01-91 reference standard in terms of its ability to induce neutralizing antibody to all three serotypes. The inclusion of GVI3000 adjuvant with IPV significantly increased IPV potency. After just one inoculation, the magnitude of neutralizing antibodies to all three poliovirus serotypes 3 weeks post-prime increased by 4, 4, and 9-fold, for poliovirus serotypes 1, 2, and 3, respectively (Fig. 4A). Additionally, 3 weeks post-boost the titer for antibodies to all three serotypes were further increased, with the potency to poliovirus being increased 23, 4, and 11-fold for poliovirus serotypes 1, 2, and 3, respectively (Fig. 4B). Several rats tested at the upper limit of detection for the assay, suggesting that some titers may have been even higher than what was measured (Fig. 4, dashed line).
Figure 4. IPV and GVI3000 in a rat IPV potency model.

Outbred rats (n=15/group) were primed and boosted i.m. with IPV alone or with GVI3000. Neutralizing antibody titers against PV types 1, 2 and 3 were determined at 21 days post-prime (A) and 21 days post-boost (B). Each symbol represents an individual titer and the line indicates the group mean. The dashed line is the last dilution tested for neutralizing antibody and represents the upper limit of detection. Statistical significance, *=p<0.05, **=p<0.01, ***=p<0.001.
4. Discussion
GVI3000 functioned as an effective adjuvant for IPV. It increased both the quality and magnitude of the immune response, enhancing systemic, serotype-specific IgG and mucosal IgA, even when administered at a non-mucosal site. By strongly potentiating the production of IgG2a, the GVI3000 adjuvant also induced a more balanced Th1/Th2 immune response compared to vaccine alone. The shift toward Th1 responses promoted by GVI3000 suggests that induction of T cell immunity also occurred. Although cellular immunity was not measured here, we have demonstrated the adjuvanted induction of T cells in systemic and mucosal compartments with other antigens [25, 32]. While the antibody titers for mice were measured by ELISA, the ability of these antibodies to neutralize poliovirus was not tested and needs to be evaluated in future experiments.
Additionally, GVI3000 significantly increased the potency of IPV in rats. After a prime inoculation, GVI3000 increased the potency of IPV for all three serotypes with all neutralizing titers in adjuvanted groups being at or above 8 (Fig. 4A) [37]. Post-boost, GVI3000 further increased neutralizing titers to 1024 (10 on a log2 scale) or greater for the three poliovirus serotypes, between 5 and 20-fold higher than IPV alone (Fig. 4B). Neutralizing titers in rats in response to IPV strongly correlate with that in humans, but in general are about 10-fold higher per D antigen unit given [31]. While the results in Figure 4 suggest that it may also be possible for GVI3000 to increase the potency of IPV in humans, the exact formulation and long term protection of such a vaccine needs to be investigated in a clinical trial.
Cost is a significant factor that prevents large scale vaccination with IPV in developing countries, which is one reason why OPV has been the GPEI’s vaccine of choice for eradication. By increasing IPV potency, GVI3000 could substantially lower the cost of IPV by reducing the amount of D antigen per dose. Based on estimates of effective dose from unpublished results in primates, we estimate that GVI3000 would add about $0.05/dose to the cost-of-goods for IPV(over $2/dose) [38]. Additionally, in previous experiments with other antigens, we have seen up to a 100-fold induction of antibodies in experimental groups receiving the adjuvant compared with antigen alone [39]. Therefore, it is possible that an even greater immune response could be induced by GVI3000 when the dose and formulation of IPV is further optimized, providing additional dose-sparing.
GVI3000 increased the immune response to all three poliovirus serotypes and has also been shown to increase the immune response to several other antigens in monovalent and multivalent formulations [23–25, 40]. In relation to licensed vaccines, GVI3000 has been shown to adjuvant an immune response when used with inactivated flu vaccine, but has not yet been tested with other existing vaccines such as DTP [23]. Therefore, it may also be possible to include GVI3000 with IPV (or sIPV) and other antigens or existing vaccines, without reducing the adjuvant effect and possibly allowing further cost savings.
Since IPV induces significantly less mucosal immunity than OPV, an improvement in the mucosal immune response to IPV would represent a significant advance toward switching from OPV to IPV while retaining the advantages of mucosal immunity. An IPV-GVI3000 vaccine could be useful to the GPEI as a replacement for OPV in mop-up immunizations to stop a poliovirus outbreak within a previously polio-free country without risking the introduction of VDPVs [41]. Similarly, an IPV-GVI3000 vaccine could serve as a post-eradication vaccine that would reduce long-term shedding of revertant OPV and interdict the spread of VDPVs, either of which could threaten the integrity of the eradication campaign [42].
A disadvantage of OPV is that many vaccinees in developing countries do not develop protective immunity, requiring a large number of doses (>10) per vaccinee to stop transmission of wildtype poliovirus, such as was achieved in India [9, 43, 44]. This is possibly due to competition among the three poliovirus serotypes in OPV during infection of the gut [45], chronic activation of an antiviral state due to intercurrent infections and/or waning mucosal immunity post-vaccination [10, 45]. Parenteral administration of an IPV-GVI3000 vaccine could induce mucosal immunity while avoiding the associated problems of relying on infection of the gut. Inclusion of GVI3000 with IPV boosted the immune response to all three poliovirus serotypes, demonstrating that there is no significant interference between the antigens of the individual poliovirus serotypes.
While the experiments demonstrating the induction of mucosal immunity to IPV were performed in mice, there is evidence that GVI3000 functions as an effective mucosal adjuvant in macaques [46]. Co-administration of Fluzone® with GVI3000 raised influenza-specific IgG antibodies in broncho-alveolar lavage (BAL) and showed anamnestic IgA responses in BAL following intranasal influenza challenge. Influenza virus replication post-challenge was significantly reduced in groups that received GVI3000. The safety profile of GVI3000 in humans is unknown though it has not caused adverse events in mice, rats, guinea pigs or macaques. The ability of GVI3000 to induce a mucosal immune response in humans is also unknown, but efforts to advance GVI3000 into cGMP production and a Phase I clinical trial are ongoing.
Supplementary Material
Highlights.
GVI 3000 adjuvant increased the potency of IPV in mice.
Systemic and mucosal immune responses to IPV were increased in mice.
IPV+GVI3000 delivered at a non-mucosal site still induced mucosal immunity.
GVI3000 increased the potency of IPV in rats by up to 23-fold.
Acknowledgments
The authors are grateful to Debbie Brugmans (Intravacc) for technical assistance. IPV was a kind gift from Bilthoven Biologicals. Funding was from the NIH U01-AI70976, R01AI088250, and the WHO HQPOL0801925.
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathanson N, Kew OM. From Emergence to Eradication: The Epidemiology of Poliomyelitis Deconstructed. American Journal of Epidemiology. 2010 Dec 1;172(11):1213–29. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mdodo RM, Wassilak S. Progress Toward Interruption of Wild Poliovirus Transmission--Worldwide, January 2011--March 2012. MMWR. 2012;61(19):353–7. [PubMed] [Google Scholar]
- 3.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Okayasu H, Sutter RW, Czerkinsky C, Ogra PL. Mucosal immunity and poliovirus vaccines: Impact on wild poliovirus infection and transmission. Vaccine. 2011;29(46):8205–14. doi: 10.1016/j.vaccine.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal Immunity Induced by Enhanced-Potency Inactivated and Oral Polio Vaccines. Journal of Infectious Diseases. 1991 Jan 1;163(1):1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Ghendon Y, Robertson SE. Interrupting the transmission of wild polioviruses with vaccines: immunological considerations. Bulletin of the World Health Organization. 1994;72(6):973–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Hird T, Grassly N. Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge. PLoS Pathog. 2012;8(4) doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, et al. Expert Review on Poliovirus Immunity and Transmission. Risk Analysis. 2012;33(4):544–605. doi: 10.1111/j.1539-6924.2012.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, et al. Asymptomatic Wild-Type Poliovirus Infection in India among Children with Previous Oral Poliovirus Vaccination. Journal of Infectious Diseases. 2010;201(10):1535–43. doi: 10.1086/651952. [DOI] [PubMed] [Google Scholar]
- 10.Grassly NC, Jafari H, Bahl S, Sethi R, Deshpande JM, Wolff C, et al. Waning Intestinal Immunity After Vaccination With Oral Poliovirus Vaccines in India. Journal of Infectious Diseases. 2012;205(10):1554–61. doi: 10.1093/infdis/jis241. [DOI] [PubMed] [Google Scholar]
- 11.Alexander L, Seward JF, Santibanez TA, et al. Vaccine policy changes and epidemiology of poliomyelitis in the united states. JAMA. 2004;292(14):1696–701. doi: 10.1001/jama.292.14.1696. [DOI] [PubMed] [Google Scholar]
- 12.Ogra PL. Comparative Evaluation of Immunization with Live Attenuated and Inactivated Poliovirus Vaccines. Annals of the New York Academy of Sciences. 1995;754(1):97–105. doi: 10.1111/j.1749-6632.1995.tb44442.x. [DOI] [PubMed] [Google Scholar]
- 13.Chumakov K, Ehrenfeld E, Plotkin S. New Generation of Inactivated Poliovirus Vaccines for Universal Immunization after Eradication of Poliomyelitis. Clinical Infectious Diseases. 2008 Dec 15;47(12):1587–92. doi: 10.1086/593310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, et al. Transmission of Imported Vaccine-Derived Poliovirus in an Undervaccinated Community in Minnesota. Journal of Infectious Diseases. 2009 Feb 1;199(3):391–7. doi: 10.1086/596052. [DOI] [PubMed] [Google Scholar]
- 15.Kohler KA, Banerjee K, Gary Hlady W, Andrus JK, Sutter RW. Vaccine-associated paralytic poliomyelitis in India during 1999: decreased risk despite massive use of oral polio vaccine. Bulletin of the World Health Organization. 2002;80:210–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-Derived Polioviruses and The Endgame Strategy for Global Polio Eradication. Annual Review of Microbiology. 2005;59(1):587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 17.Krugman S, Warren J, Eiger MS, Berman PH, Michaels RM, Sabin AB. Immunization with live attenuated poliovirus vaccine. American Journal of Diseases of Children. 1961;101(1):23–9. doi: 10.1001/archpedi.1961.04020020025005. [DOI] [PubMed] [Google Scholar]
- 18.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, et al. Mucosal Immunity after Vaccination with Monovalent and Trivalent Oral Poliovirus Vaccine in India. Journal of Infectious Diseases. 2009 Sep 1;200(5):794–801. doi: 10.1086/605330. [DOI] [PubMed] [Google Scholar]
- 19.Plotkin SA, Vidor E. Poliovirus vaccine-inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Elsevier Inc; 2008. pp. 605–29. [Google Scholar]
- 20.Ehrenfeld E, Modlin J, Chumakov K. Future of polio vaccines. Expert Rev Vaccines. 2009;8(7):899–905. doi: 10.1586/erv.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, et al. Review and Assessment of Poliovirus Immunity and Transmission: Synthesis of Knowledge Gaps and Identification of Research Needs. Risk Analysis. 2012;33(4):606–46. doi: 10.1111/risa.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-Dihydroxyvitamin D3 Enhances Systemic and Mucosal Immune Responses to Inactivated Poliovirus Vaccine in Mice. Journal of Infectious Diseases. 2006 Feb 15;193(4):598–600. doi: 10.1086/499970. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EMB, Davis NL, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proceedings of the National Academy of Sciences of the United States of America. 2006 Mar 7;103(10):3722–7. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson JM, Nicholson MG, Whitmore AC, Zamora M, West A, Iwasaki A, et al. Nonmucosal Alphavirus Vaccination Stimulates a Mucosal Inductive Environment in the Peripheral Draining Lymph Node. The Journal of Immunology. 2008 Jul 1;181(1):574–85. doi: 10.4049/jimmunol.181.1.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson JM, Whitmore AC, Staats HF, Johnston RE. Alphavirus replicon particles acting as adjuvants promote CD8+ T cell responses to co-delivered antigen. Vaccine. 2008;26(33):4267–75. doi: 10.1016/j.vaccine.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonkin DR, Whitmore A, Johnston RE, Barro M. Infected dendritic cells are sufficient to mediate the adjuvant activity generated by Venezuelan equine encephalitis virus replicon particles. Vaccine. 2012;30(30):4532–42. doi: 10.1016/j.vaccine.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westdijk J, Koedam P, Barro M, Steil BP, Collin N, Vedvick TS, et al. Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains. Vaccine. 2013;31(9):1298–304. doi: 10.1016/j.vaccine.2012.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis NL, Caley IJ, Brown KW, Betts MR, Irlbeck DM, McGrath KM, et al. Vaccination of Macaques against Pathogenic Simian Immunodeficiency Virus with Venezuelan Equine Encephalitis Virus Replicon Particles. Journal of Virology. 2000 Jan 1;74(1):371–8. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-Helper Systems from Attenuated Venezuelan Equine Encephalitis Virus: Expression of Heterologous Genesin Vitroand Immunization against Heterologous Pathogensin Vivo. Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 30.Davis NL, Powell N, Greenwald GF, Willis LV, Johnson BJB, Smith JF, et al. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: Construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183(1):20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 31.van Steenis G, van Wezel A, Sekhuis V. Potency testing of killed polio vaccine in rats. Dev Biol Stand. 1981;47:119–28. [PubMed] [Google Scholar]
- 32.Tonkin DR, Jorquera P, Todd T, Beard CW, Johnston RE, Barro M. Alphavirus replicon-based enhancement of mucosal and systemic immunity is linked to the innate response generated by primary immunization. Vaccine. 2010;28(18):3238–46. doi: 10.1016/j.vaccine.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boom WH, Liano D, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. The Journal of Experimental Medicine. 1988 Apr 1;167(4):1350–63. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J-H, Sohn H-J, Lee J, Yang H-J, Chwae Y-J, Kim K, et al. Vaccination with Lentiviral Vector Expressing the nfa1 Gene Confers a Protective Immune Response to Mice Infected with Naegleria fowleri. Clinical and Vaccine Immunology. 2013 Jul 1;20(7):1055–60. doi: 10.1128/CVI.00210-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimoto T, Mizuno D, Takei T, Kunimi T, Ono S, Sakai S, et al. Intranasal influenza vaccination using a new synthetic mucosal adjuvant SF-10: induction of potent local and systemic immunity with balanced Th1 and Th2 responses. Influenza and Other Respiratory Viruses. 2013 May 26; doi: 10.1111/irv.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cebra JJ, Logan AC, Weinstein PD. The preference for switching to expression of the IgA isotype of antibody exhibited by B lymphocytes in Peyer’s patches is likely due to intrinsic properties of their microenvironment. Immunologic Research. 1991;10(3–4):393–5. doi: 10.1007/BF02919728. [DOI] [PubMed] [Google Scholar]
- 37.Plotkin SA. Correlates of Protection Induced by Vaccination. Clinical and Vaccine Immunology. 2010 Jul 1;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangrujee N, Cáceres VM, Cochi SL. Cost analysis of post-polio certification immunization policies. Bulletin of the World Health Organization. 2004;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson JM, Whitmore AC, Staats HF, Johnston R. The contribution of type I interferon signaling to immunity induced by alphavirus replicon vaccines. Vaccine. 2008;26(39):4998–5003. doi: 10.1016/j.vaccine.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LoBue AD, Thompson JM, Lindesmith L, Johnston RE, Baric RS. Alphavirus-Adjuvanted Norovirus-Like Particle Vaccines: Heterologous, Humoral, and Mucosal Immune Responses Protect against Murine Norovirus Challenge. Journal of Virology. 2009 Apr 1;83(7):3212–27. doi: 10.1128/JVI.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassilak S, Pate MA, Wannemuehler K, Jenks J, Burns C, Chenoweth P, et al. Outbreak of Type 2 Vaccine-Derived Poliovirus in Nigeria: Emergence and Widespread Circulation in an Underimmunized Population. Journal of Infectious Diseases. 2011 Apr 1;203(7):898–909. doi: 10.1093/infdis/jiq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLennan C, Dunn G, Huissoon AP, Kumararatne DS, Martin J, O’Leary P, et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. The Lancet. 2004;363(9420):1509–13. doi: 10.1016/S0140-6736(04)16150-3. [DOI] [PubMed] [Google Scholar]
- 43.John T, Vashishtha V. Eradicating poliomyelitis: India’s journey from hyperendemic to polio-free status. 2013;137:881–94. [PMC free article] [PubMed] [Google Scholar]
- 44.Polio Global Eradication Initiative. [cited 2013 10-1-13]; Available from: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx.
- 45.Patriarca PA, Wright PF, John TJ. Factors Affecting the Immunogenicity of Oral Poliovirus Vaccine in Developing Countries: Review. Reviews of Infectious Diseases. 1991;13(5):926–39. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 46.Carroll TD, Matzinger SR, Barro M, Fritts L, McChesney MB, Miller CJ, et al. Alphavirus replicon-based adjuvants enhance the immunogenicity and effectiveness of Fluzone® in rhesus macaques. Vaccine. 2011;29(5):931–40. doi: 10.1016/j.vaccine.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


