Abstract
Reversible inactivation was used to examine the conditioned stimulus (CS) pathway for visual eyeblink conditioning (EBC). Previous research has shown that the ventral lateral geniculate (LGNv) and nucleus of the optic tract (NOT) could play a role in visual EBC through ipsilateral projections to the medial pontine nuclei. Rats were given visual EBC followed by inactivation of the ventral lateral geniculate (LGNv), nucleus of the optic tract (NOT), or both nuclei contralateral to the conditioned eye. Muscimol infusions into the NOT or LGNv impaired retention. Combined inactivation of LGNv/NOT produced the most severe impairment. Rats given inactivation of these visual nuclei after training with a vibration CS showed no impairment. The findings indicate that a parallel pathway of visual CS information projects from the LGNv and NOT to the medial pontine nuclei.
Keywords: ventral lateral geniculate, nucleus of the optic tract, eyeblink conditioning, visual CS, CS pathway
Eyeblink conditioning (EBC) is established by pairing a conditioned stimulus (CS; e.g. tone or light) with an unconditioned stimulus (US; e.g. shock), which elicits a blink unconditioned response (UR). After repeated pairings of the CS and US an adaptive eyelid closure conditioned response (CR) emerges prior to US onset. This paradigm has been extensively used to study the neural correlates and mechanisms of learning. The essential neural circuitry underlying delay EBC includes the cerebellar cortex and anterior interpositus nucleus (Thompson & Steinmetz, 2009; Freeman & Steinmetz, 2011). Recent studies have been conducted to examine the neural pathways for CS inputs to the cerebellum that are necessary for EBC. Most of this work has been conducted for the neural pathway necessary for an auditory CS. The medial auditory thalamic nuclei are necessary for acquisition and retention and receive parallel inputs from the cochlear nucleus, superior olive, nucleus of the lateral lemniscus, and inferior colliculus (Campolattaro, Halverson, & Freeman, 2007; Freeman et al., 2007; Halverson & Freeman, 2006; Halverson et al., 2008; 2010a,b). The medial auditory thalamic nuclei projects to the lateral pontine nuclei, which project to the anterior interpositus nucleus and the cerebellar cortex. The neural CS pathway for visual EBC is less well delineated.
Lesions of visual cortex or decortication do not prevent acquisition or retention of visual eyeblink conditioning (Hilgard & Marquis, 1935, 1936; Oakley & Russell, 1975, 1976, 1977). Koutalidis et al. (1988) made lesions of the superior colliculus, lateral geniculate, visual cortex, and pretectal nuclei during acquisition to a visual CS. Lesions of any of the regions alone produced either no impairment or a minor impairment; whereas combined lesions of two of the structures produced a more severe impairment. A combined lesion of all of these areas blocked acquisition of eyeblink conditioning with a visual CS (Koutalidis et al., 1988). These results are supported by more recent findings that electrical stimulation of either lateral geniculate, superior colliculus or visual cortex as a CS is sufficient for acquisition of EBC (Halverson et al 2009). In this stimulation study EBC was acquired faster with lateral geniculate or visual cortex stimulation than superior colliculus stimulation (Halverson et al., 2009). Halverson and Freeman (2010a) inactivated the medial pontine nuclei and found impairments in EBC using stimulation of the ventral lateral geniculate nucleus (LGNv) as a CS or a visual CS, but only a minor deficit with an auditory CS, indicating that the primary visual CS input is projected to the medial pontine nuclei. This study also used a retrograde tracer in the medial pontine nuclei and found labeled cells in the ipsilateral LGNv and nucleus of the optic tract (NOT). The LGNv and NOT were then hypothesized to be the critical visual CS input to the medial pontine nuclei.
As mentioned above, lesions of the lateral geniculate nucleus alone did not abolish acquisition of eyeblink conditioning (Koutalidis et al., 1988). It is possible that other areas that process visual information in parallel could compensate for the LGN lesion. Thus, inactivation of this structure following EBC provides a method for examining the role of the LGNv in EBC without lesion-related compensation or reorganization. Additionally, the role of the NOT in EBC has not been investigated. The current study used muscimol, a GABA agonist, to inactivate the LGNv, NOT or both nuclei during retention of eyeblink conditioning using a light CS. Rats were then trained using a vibration CS, as a control condition, to examine the specificity of the inactivation effects for visual EBC.
Method
Subjects
Subjects were 29 male Long-Evans rats weighing 250–350 g at the beginning of the experiment. One week prior to training, the rats were given surgery to implant differential electromyography (EMG) electrodes in the upper-left orbicularis oculi muscle. A bipolar stimulating electrode for delivering the US was implanted subdermally caudal to the left eye.
Surgery
Cannulae were implanted into the right LGN, the right NOT, or both right LGN and NOT. The stereotaxic coordinates were taken from a stereotaxic brain atlas (Paxinos & Watson, 2007); 4.6 mm posterior, 4.0 mm lateral, and 5.4 mm ventral to the skull surface for LGNv. Coordinates for the NOT were 5.3 mm posterior, 2.0 mm lateral, and 4.1 mm ventral and 5.8 mm posterior, 2.8 mm lateral, and 5.4 mm ventral to the skull surface.
Apparatus
The conditioning apparatus consisted of four small-animal sound attenuation chambers (BRS/LVE, Laurel, MD). Within each sound- attenuation chamber was a small animal operant chamber (BRS/LVE, Laurel, MD) where the rats were located during conditioning. One wall of the operant chamber was fitted with two speakers in which the CS was presented. The electrode leads from the rat's headstage were connected to peripheral equipment. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). The shock US (2–3 mA, DC constant current) was delivered through a stimulus isolator (Model number 365A, World Precision Instruments, Sarasota FL). EMG activity was recorded differentially, filtered (500–5000 Hz) and integrated by equipment (JSA Designs, Raleigh, NC).
Conditioning procedures
All rats completed 14 consecutive daily sessions of training. Sessions 1–14 of the experiment had 10 blocks of 9 paired CS-US presentations and 1 CS alone probe trial. The CS for sessions 1–7 was a 400 ms incandescent light (100 lux). The CS for sessions 8–14 was a vibrating grid floor (150 Hz; .45 m/s2, .002 mm displacement). Muscimol (.2 μL in the NOT, .3 μL in the LGNv, 10.0 mM) or PBS (.2 μL in the NOT, .3 μL in the LGNv) was infused 30 min prior to retention tests (sessions 6, 7, 13 and 14). The vibration CS was used as a control condition rather than an auditory CS due to the proximity of the medial auditory thalamus to the LGNv. It would have been nearly impossible to completely inactivate the LGNv without, at least partially, inactivating the medial auditory thalamus, which impairs auditory conditioning (Halverson et al., 2008). Both CSs terminated with a 25 ms periorbital stimulation US. The US intensity was adjusted in each rat to elicit a blink and slight head movement (2.5 – 3.5 mA). CRs were defined as EMG activity that exceeded a threshold of 0.4 units (amplified and integrated units) above the baseline mean during the CS period after 80 ms. Measures of CR amplitude, onset latency, and peak latency were taken from CS-alone probe trials. URs were defined as responses that exceeded the threshold after the onset of the US.
Results
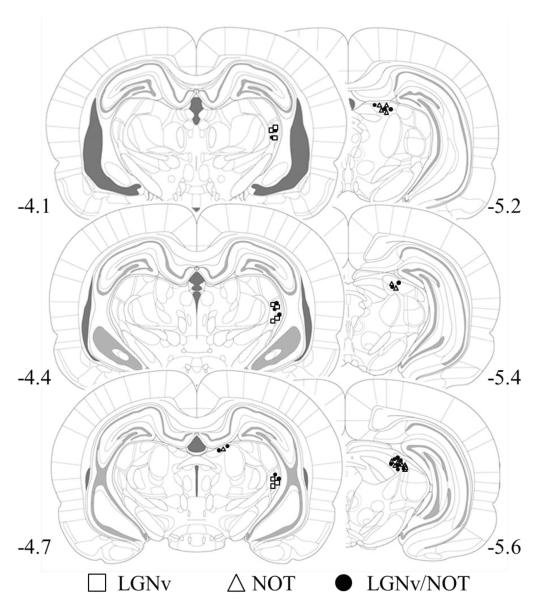
Cannula placements in the LGNv and NOT were verified by examining a series of coronal sections (Figure 1). Twenty four rats had cannula placements in either the medial division of the ventral division of the lateral geniculate (LGNv; n = 10), nucleus of the optic tract (NOT; n = 7), or in both the LGNv and NOT (LGNv/NOT; n = 7). Five rats had missed cannula placements and their data were not used in the following analyses.
Figure 1.
Drawings of coronal sections depicting cannula placements in the nucleus of the optic tract (NOT) and ventral lateral geniculate nucleus (LGNv) for the NOT-only (white triangles), LGNv-only (white square), and combined LGNv/NOT groups (black circles). Numbers represent distance from bregma. The coronal sections are taken and adapted with permission from The Rat Brain in Stereotaxic Coordinates (5th ed., pp. 67–80), by G. Paxinos & C. Watson, 2005, New York, NY: Academic Press. Copyright 2005 by Elsevier Academic Press. The numbers refer to the position of the section relative to bregma.
A repeated measures analysis of variance (ANOVA) was conducted for the acquisition phases for both the light and vibration CSs. No significant differences in CR acquisition rate were observed during either the light or vibration CS between rats with cannula placements in LGNv, NOT, or LGNv/NOT (Figure 2; Sessions 1–5, 8–12). Further analyses were conducted on the muscimol retention tests in order to examine effects of the NOT, vLGN, and combined VLGN/NOT inactivation during both the light and vibration CSs (Figure 2). A repeated measures ANOVA on CR percentage during the muscimol retention tests, the session prior to the muscimol tests, and the session following the muscimol tests for each CS (sessions 5–7 and 12–14) revealed a Session x Cannula Location x CS interaction (F(4,42) = 16.60 P < 0.001). Post-hoc tests (Tukey-Kramer) revealed that rats exhibited significantly fewer CRs during the light CS with the muscimol retention test when compared to both the last acquisition session and saline recovery session (P < 0.05; Fig 2). Post-hoc tests also revealed that combined LGNv/NOT group exhibited fewer CRs than both the LGNv and NOT groups (P < .05). Additionally, the LGNv group exhibited significantly fewer CRs than the NOT group (P<.05). There were no significant differences when the LGNv, NOT, or LGNv/NOT were infused with muscimol during testing with the vibration CS.
Figure 2.
Eyelid conditioned response (CR) percentage (+ SEM) for rats receiving muscimol (Mus) and phosphate buffered saline (Sal) infusions into the NOT-only group (white triangle), LGNv-only (white diamond), or combined LGNv/NOT infusions (white square) during separate testing sessions with a light CS (Sessions 1–5) and a vibration CS (Sessions 8–12). Rats with combined inactivation of the LGNv and NOT were the most impaired during retention of visual eyeblink conditioning. There were no significant impairments in retention of somatosensory eyeblink conditioning with Mus infusions into the visual nuclei. Data from Session 8 are shown in the insert in 10-trial blocks.
Analyses were also conducted to examine the effect of muscimol on CR amplitude. A repeated measures ANOVA for CR amplitude revealed significant CS × Session (F(4,42) = 7.651 P = 0.003) and CS × Cannula Location (F(4,42) = 2.701 P = 0.034) interactions but no CS × Cannula Location × Session interaction. Post-hoc tests indicated that there was a significant decrease in CR amplitude during the muscimol infusion with the light CS compared to the control sessions; there were no group differences in CR amplitude with the vibration CS. The CR amplitude means during the light CS were: LGNv saline = 5.69; LGNv muscimol = 2.68; NOT saline = 4.59; NOT muscimol = 3.86; LGNv/NOT saline = 4.79; and LGNv/NOT muscimol = 1.95.
Analyses of the CR onset latency and peak latency data resulted in a CS × Session interaction for CR onset latency (F(4,42) = 21.318 P < 0.001) and CR peak latency (F(4,42) = 10.125 P = 0.001). Post-hoc tests for the CR onset latency data indicated that CR onset was earlier during the vibration CS (175 ms) than during the visual CS (305 ms) and later during the muscimol session with the light CS (374 ms) than the other sessions (all comparisons, P < .05). The CR onset latency means during the light CS were: LGNv saline = 239.63 ms; LGNv muscimol = 350.69 ms; NOT saline = 305.53 ms; NOT muscimol = 365.61 ms; LGNv/NOT saline = 287.09 ms; and LGNv/NOT muscimol = 416.54 ms. Additionally, post-hoc tests for CR peak latency data indicated that the peak occurred earlier during the vibration CS (386 ms) than during the visual CS (427 ms), and later during the muscimol session with the light CS (473 ms) than the other sessions (all comparisons, P < .05). The CR peak latency means were: LGNv saline = 394.47 ms; LGNv muscimol = 460.28 ms; NOT saline = 416.23 ms; NOT muscimol = 468.71 ms; LGNv/NOT saline = 405.71 ms; and LGNv/NOT muscimol = 497.51 ms.
Discussion
The current study examined the effects of inactivating the LGNv, NOT, and both nuclei on retention of EBC with visual and somatosensory CSs. Rats were impaired with muscimol inactivation of the LGNv, NOT, or LGNv and NOT. The largest decrements in EBC were seen when infusions were made into both the LGNv and NOT. Additionally, LGNv inactivation produced a greater decrease in CR percentage than NOT inactivation. Rats did not show a decrement in CR percentage when muscimol was infused during the vibration CS, indicating that the inactivation effects were selective for the visual modality.
Koutalidis et al. (1988) first examined the visual CS inputs to the cerebellum by lesioning the LGN, pretectal nuclei, superior colliculus, and visual cortex prior to acquisition. It was reported that lesions of each area alone produced small decrements whereas combined lesions produced larger impairments; lesions of all areas blocked acquisition completely. Thus, the authors concluded that the visual system projects to the pontine nucleus through parallel pathways. The CS inputs into the pontine nucleus are segregated with the medial pontine nuclei necessary for a visual CS, whereas the lateral pontine nuclei are necessary for an auditory CS (Steinmetz et al., 1987; Halverson & Freeman, 2010a,b). Retrograde tracing from the medial pontine nuclei labeled cells in the LGNv, NOT, and anterior pretectal nucleus (APN). However, APN has been shown to not be critical for EBC since stimulation of APN as a CS does not result in conditioning (Campolattaro, Halverson, & Freeman, 2007). Stimulation of the visual cortex as a CS does support learning; however, it projects to the lateral pontine nucleus (Halverson et al., 2009; Wells et al., 1989). Thus, it was proposed that the LGNv and NOT are the necessary pathway for visual CS inputs to the medial pontine nuclei (Halverson et al., 2010).
The current study supports the hypothesis that the LGNv and NOT are the necessary pathway for visual EBC. Inactivation of both the LGNv and NOT combined abolished CRs to the light CS. Interestingly, the effects of inactivation of either LGNv or NOT were significantly different from the combined inactivation. This finding suggests that the LGNv and NOT provide parallel input to the medial pontine nucleus during EBC. The LGNv inactivation resulted in a larger decrement in learning than NOT inactivation, suggesting that although parallel pathways exist there are differences in the efficacy of the visual inputs to the pontine nuclei. In contrast, the auditory CS pathway involves a thalamo-pontine projection without parallel projections from other auditory nuclei (Halverson et al., 2008; Halverson & Freeman, 2006, 2010).
Acknowledgment
This study was supported by NIMH grant MH80005 to J.H.F.
References
- Campolattaro MM, Halverson HE, Freeman JH. Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learning and Memory. 2007;14:152–159. doi: 10.1101/lm.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Halverson HE, Hubbard EM. Inferior colliculus lesions impair eyeblink conditioning in rats. Learning and Memory. 2007;14:842–846. doi: 10.1101/lm.716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning and Memory. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behavioral Neuroscience. 2006;120:880–887. doi: 10.1037/0735-7044.120.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Poremba A, Freeman JH. Medial auditory thalamus inactivation prevents acquisition and retention of eyeblink conditioning. Learning and Memory. 2008;15:532–538. doi: 10.1101/lm.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Hubbard EM, Freeman JH. Stimulation of the lateral geniculate, superior colliculus, or visual cortex is sufficient for eyeblink conditioning in rats. Learning and Memory. 2009;16:300–307. doi: 10.1101/lm.1340909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiology of Learning and Memory. 2010a;93:92–98. doi: 10.1016/j.nlm.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Lee I, Freeman JH. Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. The Journal of Neuroscience. 2010b;30:8787–8796. doi: 10.1523/JNEUROSCI.0208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard ER, Marquis DG. Acquisition, extinction, and retention of conditioned lid responses to light in dogs. Journal of Comparative Psychology. 1935;19:29–58. [Google Scholar]
- Koutalidis O, Foster A, Weisz DJ. Parallel pathways can conduct visual CS information during classical conditioning of the NM response. The Journal of Neuroscience. 1988;8:417–427. doi: 10.1523/JNEUROSCI.08-02-00417.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis DG, Hilgard ER. Conditioned lid responses to light in dogs after removal of the visual cortex. Journal of Comparative Psychology. 1936;22:157–178. [Google Scholar]
- Nicholson DA, Freeman JH., Jr Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116:22–36. [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Role of cortex in Pavlovian discrimination learning. Physiology and Behavior. 1975;15:315–321. doi: 10.1016/0031-9384(75)90099-2. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Subcortical nature of Pavlovian differentiation in the rabbit. Physiology and Behavior. 1976;17:947–954. doi: 10.1016/0031-9384(76)90013-5. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Subcortical storage of Pavlovian conditioning in the rabbit. Physiology and Behavior. 1977;18:931–937. doi: 10.1016/0031-9384(77)90203-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Academic press; San Diego, CA: 2007. pp. 67–80. [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences USA. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Wells GR, Hardiman MJ, Yeo CH. Visual projections to the pontine nuclei in the rabbit: Orthograde and retrograde tracing studies with WGA HRP. Journal of Comparative Neurology. 1989;279:629–652. doi: 10.1002/cne.902790410. [DOI] [PubMed] [Google Scholar]