Abstract
One of the prototype mammalian kinases is PKA and various roles have been defined for PKA in malaria pathogenesis. The recently described phospho-proteomes of Plasmodium falciparum introduced a great volume of phospho-peptide data for both basic research and identification of new anti-malaria therapeutic targets. We discuss the importance of phosphorylations detected in vivo at different sites in the parasite R and C subunits of PKA and highlight the inhibitor sites in the parasite R subunit. The N-terminus of the parasite R subunit is predicted to be very flexible and we propose that phosphorylation at multiple sites in this region likely represent docking sites for interactions with other proteins, such as 14-3-3. The most significant observation when the P. falciparum C subunit is compared to mammalian C isoforms is lack of phosphorylation at a key site tail implying that parasite kinase activity is not regulated so tightly as mammalian PKA. Phosphorylation at sites in the activation loop could be mediating a number of processes from regulating parasite kinase activity, to mediating docking of other proteins. The important differences between Plasmodium and mammalian PKA isoforms that indicate the parasite kinase is a valid anti-malaria therapeutic target.
Keywords: Plasmodium, Malaria, Kinase, cAMP-dependent protein kinase, Phosphorylation, Therapeutic target
1. Introduction
Of the nearly 2000 new drugs brought to market in the twenty-year period between the mid 1970s and 1990s, only 3 of these were anti-malarial therapies [1]. The emergence of drug-resistant malaria has further intensified the need for novel therapies. Commonly used treatments such as chloroquine and sulphadoxine/pyrimethamine are now less useful [2]. Today, the most effective treatment is artemisinin combination therapy (ACT) [3], combining arteminisin, a natural-product peroxide-containing compound, with a second anti-malarial therapy of a different class. Although ACTs have been shown to be effective [3], resistance has been recently observed [4].
1.1. Importance of phosphorylation and cAMP signaling in Plasmodium falciparum pathogenesis
A mechanism for the ability of P. falciparum to invade, adapt to and significantly modify host cells is through controlling protein phosphorylation [5,6]. Strict regulation of cellular events during various life cycle stages relies heavily on phosphorylation [6–10]. The malarial protein kinome has been previously described and different bioinformatics analyses have identified between 60 and 90 putative protein kinases in the Plasmodium genome [11–20].
1.2. The importance of cyclic-nucleotide signaling in malarial pathogenesis
Cyclic nucleotides like cyclic-adenosine monophosphate (cAMP) are intracellular signaling molecules that allow for cellular communication. These molecules are commonly referred to as “ancient signaling molecules.” In fact, cAMP is a signal for stress organisms from humans to bacteria [21]. In Plasmodium cyclic nucleotides like cAMP and cGMP can manage many functions, for example, inducing chemotaxis and sexual commitment [22]. A genome-encoded adenylate cyclase generates cAMP in P. falciparum [23] and cAMP-dependent protein kinase has been implicated in a number of processes in malarial pathogenesis. The parasite can modify the movement of sugars, peptides, amino acids, nucleotides, waste, and cation/anions across the red blood cell membrane for survival in the host, establishing intracellular anion channels and “new permeation pathways” (NPPs) [24–26].
1.3. P. falciparum PKA R-subunit (PfPKA-R) in malaria disease
Tight regulation of cAMP signaling is critical, as over expression of the regulatory subunit led to decreased PKA activity and reduced parasite growth [26]. An evaluation of the importance of apical membrane antigen-1 (AMA-1) found that its function in parasite invasion of red blood cells depends on phosphorylation by PKA [27]. Specifically, Ser610 on the C-terminal cytoplasmic tail of AMA-1 is phosphorylated by PfPKA, and both mutation of Ser610Ala, or pharmacological inhibition of PKA phosphorylation (using H89 inhibition), elicits a dramatic reduction in the parasite’s ability to invade the RBC [27]. A recent phospho-proteome study identified 919 P. falciparum schizont phospho-proteins 425 (46%) of which share an extended PKA phosphorylation motif [5]. It indicated the potential involvement of PKA-mediated signaling in regulating phosphatase and kinase activities, membrane lipid metabolism, chromatin remodeling, actin cytoskeleton organization, and locomotion/entry into the host cell. These data highlight the importance of PfPKA-R in the study of malarial pathogenesis, and thus establish a key platform for investigating protein specific features and considering it as a valid therapeutic target.
1.4. The catalytic-subunit of PKA
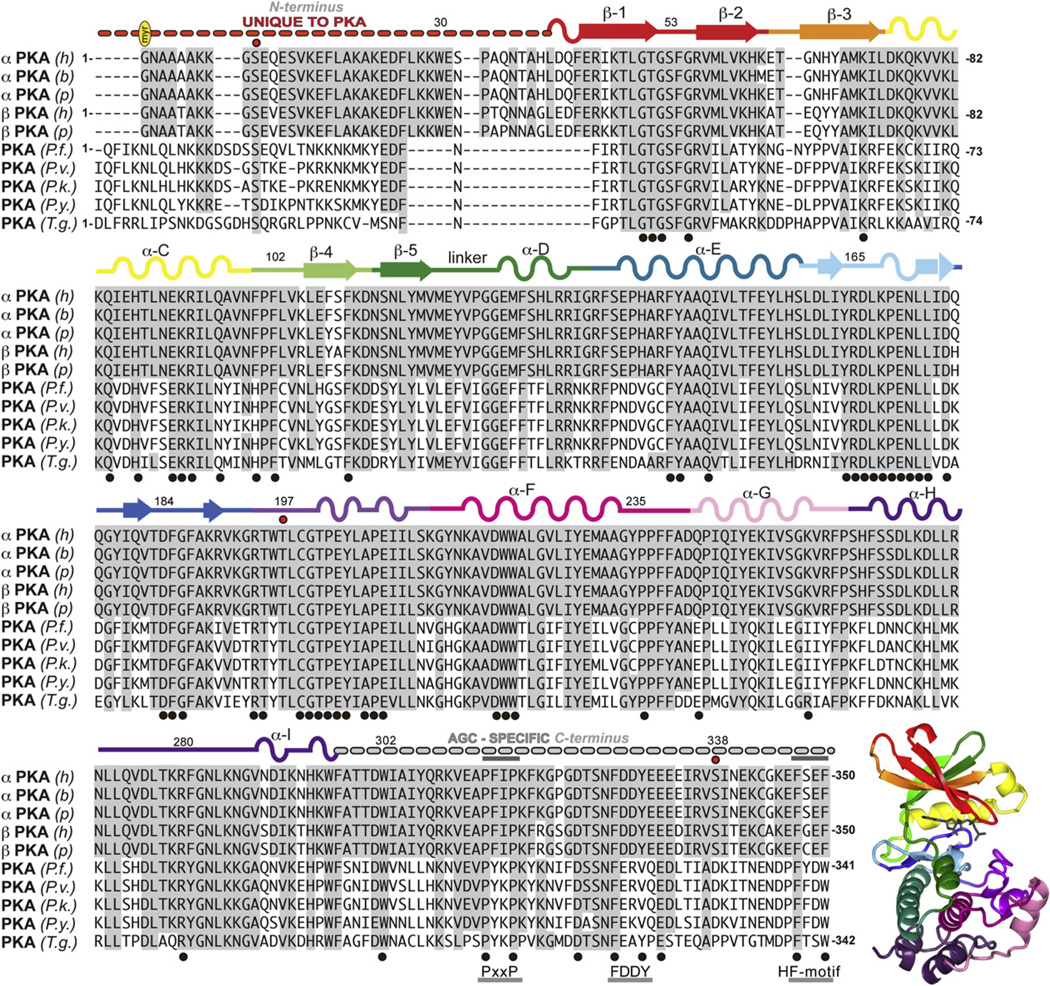
The mammalian PKA-C subunit is a member of the AGC kinase family. Members of this family, including Protein kinase C, Akt (Protein Kinase B), cGMP-dependent protein kinase (PKG), S6 Ribosomal Kinase, and others share similar structural and sequence motifs [28]. The kinase core, residues 40–300 in PKA (Fig. 1), is conserved in every eukaryotic protein kinase and harbors many conserved sequence motifs [29]. If one looks more closely at features that are uniquely conserved within the AGC subfamily, however, one finds that there are AGC-specific residues in the core and these interact very specifically with the C-terminal tail, which is now recognized to be a unique feature of every AGC kinase (Figs. 1 and 2) [28]. Although the N- and C-terminal tails (N-Tail and C-Tail) of PKA are both highly dynamic and play roles in binding and recognizing protein substrates, the N-Tail is unique to PKA (Figs. 1 and 2A) and even more specifically to the mammalian PKAs.
Fig. 1.
Sequence alignment of C and C mammalian cAMP-dependent protein kinase C-subunits (PKA) with Plasmodium and Toxoplasma gondii isoforms. Sequences are labeled according to species and isoform: (h), Homo sapiens, PKA (P17612 KAPCA_HUMAN), PKA (P22694 KAPCB_HUMAN); (b) = bovine, (Bos taurus) PKA (P00517 KAPCA_BOVIN); (p), pig (Sus scrofa) PKA (P36887 KAPCA_PIG), PKA (P05383 KAPCB_PIG); (P.f.), Plasmodium falciparum isolate 3D7 (gene PfPKAc, uniprot Q7K6A0_PLAF7); (P.v.), Plasmodium vivax (gene PVX_086975, uniprot A5KE97_PLAVI); (P.k.), Plasmodium knowlesi strain H (gene PKH_073290, uniprot B3L322_PLAKH); (P.y.), Plasmodium yoelii yoelli (gene PY052325, uniprot Q7RE33_PLAYO); (T.g.) Toxoplasma gondii VEG (gene TGVEG_066990, uniprot B9Q7X8_TOXGO). The structure of PKA (1ATP) is shown colored by subdomains as annotated on the sequence.
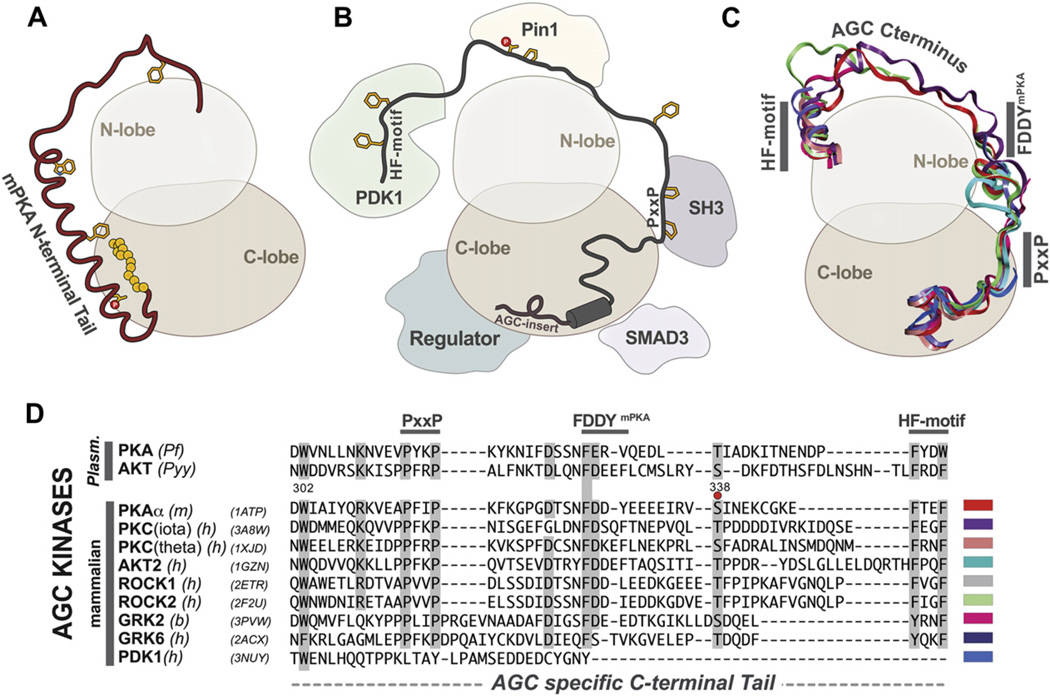
Fig. 2.
Unique N and C terminal tails of AGC type kinases. (A) Cartoon illustrating the distinctive N-terminal tail of PKA kinases highlighting the myristylation site (yellow spheres), the N-terminal Ser10 phosphorylation site and the Trp30 residue on the A-helix that are conserved in mammalian isoforms. (B) Illustrates the protein:protein interaction sites of the conserved AGC-type C-terminal tail. (C) AGC kinases share a distinct C-terminal tail consisting of putative protein-binding sites and the conserved PxxP and HF-motif. PDB IDs and colors of each C-tail are indicated in the alignment in panel D. (D) Alignment of representative AGC C-terminal tails indicate conservation between two representative plasmodia forms (PfPKA and PyyAKT2) with a variety of mammalian AGC isoforms. Sequences are labeled according to species and isoform: PfPKA (O15906, Pf), PyyAKT/PKB (Q7RSF6, Pyy), PKA (P05132, mouse), PKC-iota (P41743, human), PKC-theta (Q04759, human), AKT2 (P31751, human), ROCK1 (Q13464, human), ROCK2 (Q28021, bovine), PDK1 (O15530, human), GRK2 (P21146, bovine), GRK6 (P43250, human). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The C-Tail (residues 301–350) (Figs. 1, 2C and 2D) is conserved in all members of the AGC subfamily, where it functions as an essential cis-regulatory element [28]. The enzyme is not active without this C-Tail even though it is not part of the core. Several motifs are harbored within this C-Tail. The C-terminal PxxP motif, for example, provides a unique protein-binding site for other proteins such as Hsp60 [30], while the hydrophobic capping motif (HF-motif) at the C-terminus (Fig. 2B–D), consisting of a consensus FXXF, interacts with resides in the N-lobe of the core [31,32]. The HF-motif is thought to be a docking site for PDK1, the activating kinase for most AGC kinases (Fig. 2B). The FDDY motif (residues 327–330 of mammalian PKA (mPKA)) (Fig. 2B–D) is an essential part of the ATP binding site [33]. The C-Tail also has an essential phosphorylation site that is highly regulated. In PKA this phosphate is added co-translationally and primes the enzyme for post-translational phosphorylation of the activation loop [34]. This activation loop phosphate is essential for activity [35]. The other AGC kinases also have a “turn motif” phosphorylation site in this region, which is somewhat different from the PKA site. This site is also highly regulated and in the case of PKC is a site for regulation by the proline isomerase, Pin1 [36]. Most other AGC kinases, with the exception of PKG and PKA, have an additional and highly regulated phosphorylation site that lies just beyond the HF motif. PKA and PKG, however, end with the HF motif [28].
The N-Tail of mammalian PKA (residues 1–39) (Figs. 1 and 2A) is also a cis-regulatory element that is essential for activity [37], but it is unique to PKA and is not even conserved in different PKAs such as in yeast. In addition to playing a dynamic role in mediating cross talk between the different domains in the core, the N-Tail carries a glycine that serves as a myristylation site that anchors to a hydrophobic pocket in C-lobe in the dissociated C-subunit. However, when the C-subunit associates with RII subunits, but not RI subunits, the myristyl moiety becomes flexible and promotes association of the holoenzyme with membranes. The N-Tail is thus a dynamic switch that mediates isoform-specific interactions with lipid membranes [38]. A conserved Trp30 is seen in all mammalian PKA N-termini and functions to stabilize the small and large lobes of the C-subunit [37]. In addition, Ser10 is a conserved phosphorylation site that also mobilizes the myristyl moiety [37,39]. Asn2 can be deaminated in cells, and this promotes localization of the C-subunit to the nucleus [40]. The A helix is a dominant motif that flanks both the N-lobe and the C-lobe. It is also a docking site for the A Kinase Interacting Protein, AKIP1, which is important for PKA localization to the nucleus [41,42].
1.5. Comparison of mammalian and Plasmodium PKA-C
In comparing mammalian C-subunits to the Plasmodium counterpart (Fig. 1) a high degree of conservation is noted. On further assessment, specific differences can be noted that may specifically impact on the activity, regulation, and/or targeting of the parasite C-subunit. Many of the key differences are localized to the tails. As discussed above, the N-Tail is variable in most PKAs (Figs. 1 and 2A), as evidenced by the PKA subunits expressed in yeast (Tpk1, 2 and 3) (not shown), whereas the C-Tail is highly conserved in all PKA-C-subunits. Even the different mammalian C-subunit isoforms vary at the N-terminus, and there are a number of splice variants in the first exons that correspond to residues 1–4 [43]. The N-terminus is the region where the mammalian and PfPKA-C-subunits diverge the most. The N-Tail myristylation site conserved in mammalian forms is absent in the parasite C-subunit (Figs. 1 and 2A), and the A-helix, including Trp30, noted above for its important interactions in the hinge region of the kinase core and as a docking site for AKIPa (Fig. 2A) [41,42], is also missing. The region that interfaces with the hinge region in the PfPKA-C-subunit will be completely different just as it is different for other AGC kinases. Phe100 (PKA) in mammals and other species such as worms and flies is conserved in this position, whereas PfPKA-C differs from mammalian PKA-C by having a histidine like mammalian Akt, PKC and RSK [28]. Phe108, conserved in mammalian C-subunits, is glycine in Plasmodium spp, an observation also seen in PKN; however, the biological significance of this mutation is not clear.
Overall the C-Tail is conserved in mammalian and PfPKA-C-subunits; however, the phosphorylated Ser338 in PKA is mutated to an acidic Asp in Plasmodium (Fig. 2C,D). The phosphorylation of the tails in AGC kinases is highly regulated and coordinated with the activation loop phosphorylation, and the absence of this site is thus likely to be significant. This C-Tail phosphorylation site is also missing in PKG and in PRKX, a distant relative of PKA [44]. The FDDYmPKA motif is also different (Fig. 2D). The Phe, which interacts with the adenine ring of ATP in the closed conformation, is present, but the Tyr that interacts with the ribose ring is replaced with a Val. An Arg replaces one of the aspartic acids. Two residues that flank Trp30 in the hinge region, Arg93 in the C Helix and R190 in β strand 9, are also changed emphasizing that this interface is different when the N-terminal tail is altered.
1.6. Activation loop phosphorylation sites
Thr197 in the activation loop is a major regulatory site for the mammalian PKA catalytic (C) subunit and this is true for all of the AGC kinases. Thr197 is autophosphorylated in trans when the C-subunit is expressed in bacteria and it can be phosphorylated in trans by PDK1, which is the master activator for AGC kinases in mammalian cells [45]. Phosphorylation of this site creates a very stable enzyme that has full catalytic activity. A recent structure of the C-subunit that lacks this phosphate shows the activation loop and the C-terminal tail to be highly disordered [35]. Phosphorylation of this site requires Arg194, which defines a classic PKA recognition site. An equivalent Arg is present in the PfPKA-C subunit, so Thr197 (mammalian PKA-C numbering, see Fig. 1) is likely to be an essential phosphorylation site in the parasite enzyme [8]. Phosphorylation of Thr195 that is found in vivo for PfPKA-C has not been observed in mammalian C subunits, but phosphorylation of this residue could be easily accommodated, since in resolved structures this hydroxyl moiety binds to one of the phosphate oxygens of phospho-Thr186 [35]. Thr201 is phosphorylated in PfPKA-C in vivo, but in mammalian C subunits phosphorylation of Thr201 would inactivate the enzyme, as it is a key residue at the active site. The role of Thr201 phosphorylation in malaria parasite C subunits must therefore remain speculative.
1.7. The N-terminus of PfPKA regulatory subunit is unique
There are also significant differences when the mammalian and Plasmodium spp. subunits are compared (Fig. 3). Mammalian R-subunits all have a highly conserved domain organization (Figs. 3A and 4). At the C-terminus are two tandem Cyclic Nucleotide Binding (CNB) domains, CNB-A and CNB-B (Fig. 3B, turquoise (CNB-A) and blue (CNB-B)). These are joined by a flexible linker to a stable N-terminal dimerization domain (Fig. 3A and B (pink D/D)), which also serves as a docking site for A Kinase Anchoring Proteins (AKAPs) that target PKA to specific sites in the cell [46]. Within the linker is an Inhibitor Site (IS) that docks to the active site cleft in the holoenzyme (Fig. 3A). While the IS through to the C-terminus is highly conserved, just as the kinase core is conserved in the catalytic subunits, the N-terminus of PfPKA-R is highly divergent from mammalian isoforms (Fig. 3A,B and C). Mammalian R-subunits have a stable N-terminal domain of approximately 50 residues that forms an anti-parallel X-type helical bundle (Fig. 3C). This interaction forms a stable dimer composed of two R-subunits (Fig. 3C). The hydrophobic surface formed by this helix bundle forms a docking motif for binding to AKAPs. The domain is thus referred to as a dimerization/docking (D/D) domain (Fig. 3C). As shown in Figs. 3 and 4, there are four mammalian isoforms. They have tissue-specific expression, are functionally non-redundant, they interact with different proteins and they are localized to different subcellular compartments.
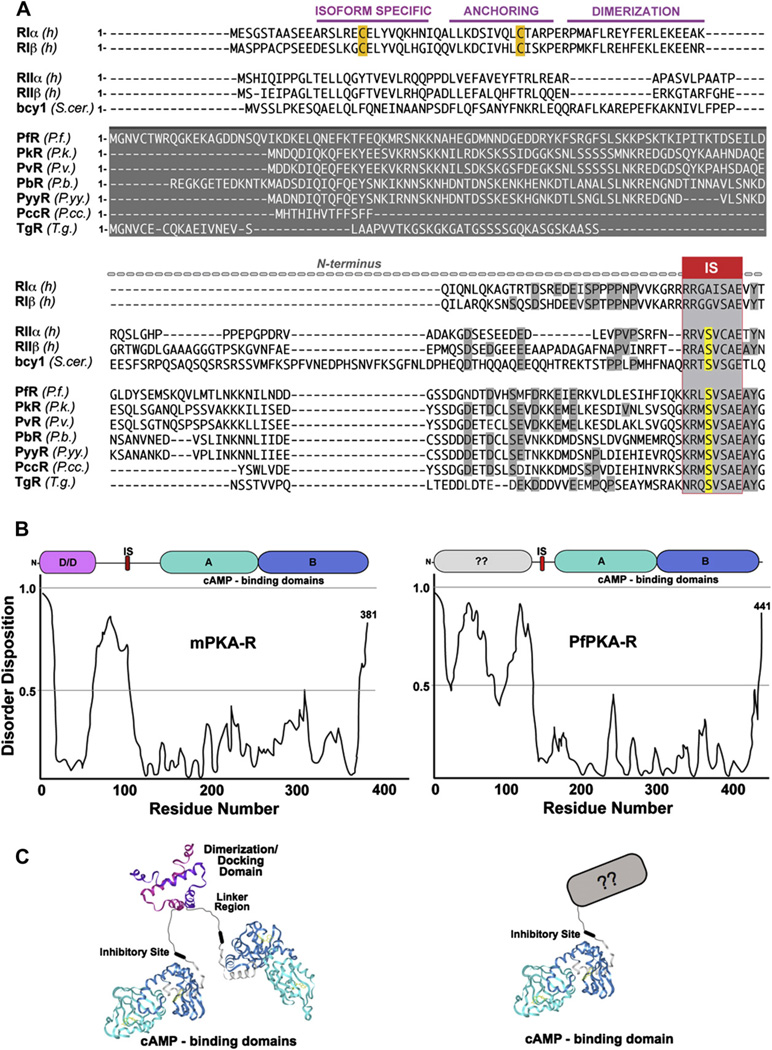
Fig. 3.
The P. falciparum PKA regulatory subunit N-terminus is highly divergent from mammalian isoforms. (A) Sequence alignment of various isoforms. (B) Disorder prediction by disprot-Database of protein disorder (http://www.disprot.org/) for PfPKA-R and mammalian PKA-R shows significant differences in the N-terminal region. (C) Structural schematics of mammalian and Plasmodia R-subunits.
Fig. 4.
Core domain alignment of cAMP-dependent protein kinase (PKA) R-subunits of mammalian, yeast, Plasmodia and Toxoplasma isoforms. Structure shown is of RI (1RGS) colored according to the representative sequence. Sequences are labeled according to species and isoform: RI (P10644, human), RIβ (P31321, human), RII (P13861, human), RIIβ (P31323, human), bcy1 (P07278, yeast), PfR (Q8T323, P. falciparum), PkR (B3LCL9, P. knowlesi), PvR (gi 156103253, P. vivax), PbR (gi68068779, P. berghei), PyyR (gi 83318121, P. yoelii yoelli), PccR (gi 70947112, P. chabaudi chabaudi), TgR (gi 12698442, T. gondii).
In contrast to the mammalian R-subunits, the N-terminal portion of Plasmodium R-subunits is very different. It is much longer and does not share the prototypical sequence for forming a helical bundle (Fig. 3A,C). Instead, we hypothesize, based on the primary sequence, that the PfPKA-R-subunit is a monomer. Since targeting is one of the principle functions of the N-terminus, this divergence suggests that the Plasmodium PKA will use a different mechanism for subcellular targeting. Even within just the Plasmodium spp. one can observe great variability in the length and sequence characteristics of the N-terminal regions (Fig. 3A). The N-terminus of PfPKA-R is at least 10 or more residues longer than any of the other compared Plasmodia. In addition, when analyzed for the inherent disorder using DisProt (Database of Protein Disorder), the N-terminal region of PfPKA-R is highly disordered compared to that of the mammalian RIα (Fig. 3B).
1.8. Putative N-terminal localization motifs in PfPKA-R
Sequence analyses yielded a key observation regarding the N-terminus of PfPKA-R that may impact on its localization and function within the cell. The N-terminus contains a motif, MGxxC, that is a putative myristylation (Gly) site and palmitylation (Cys) site [47] (Fig. 5). It is only present in P. falciparum, as the other Plasmodium species do not possess this N-terminal MGxxC motif. BLAST searches with other Apicomplexa, however, indicated that the regulatory subunit of Toxoplasma gondii also contains a similar N-terminal putative myristylation and palmitylation motif (Fig. 5). Some of the PKGs also contain this motif. Myristylation itself is a weak membrane anchor, but the combination of both acylations is a strong membrane-targeting motif that is found, for example, in many of the non-receptor tyrosine kinases such as Lck and Hck [48,49]. This motif will almost certainly localize PfPKA-R to plasma membranes, either its own or the host’s, where it will be strategically positioned in close proximity to transporters, which are major targets for PKA regulation [25,26], as well as other signaling proteins.
Fig. 5.
Predicted myristylation and palmitylation sites in the P. falciparum R-subunit and P. falciparum encoded acyltransferases.
P. falciparum encodes for both an N-myristyltransferase and for a palmityl transferase. The N-myristyltransferase in P. falciparum is expressed in asexual blood-stage forms [50], and sequence alignments with the human counterpart indicate high sequence identity (Fig. 5). Furthermore, the P. falciparum genome encodes for 13 distinct proteins with a conserved DHHC-motif (Fig. 5) indicative of an active palmitylation system [51]. We hypothesize that the N-terminal motifs found in P. falciparum and T. gondii PKA-R subunits (Fig. 5) are substrates for both N-myristyltransferase and for palmitylation and that these modifications provide a strong mechanism for parasite R-subunit localization to membranes.
1.9. Phosphorylations sites in PfPKA-R
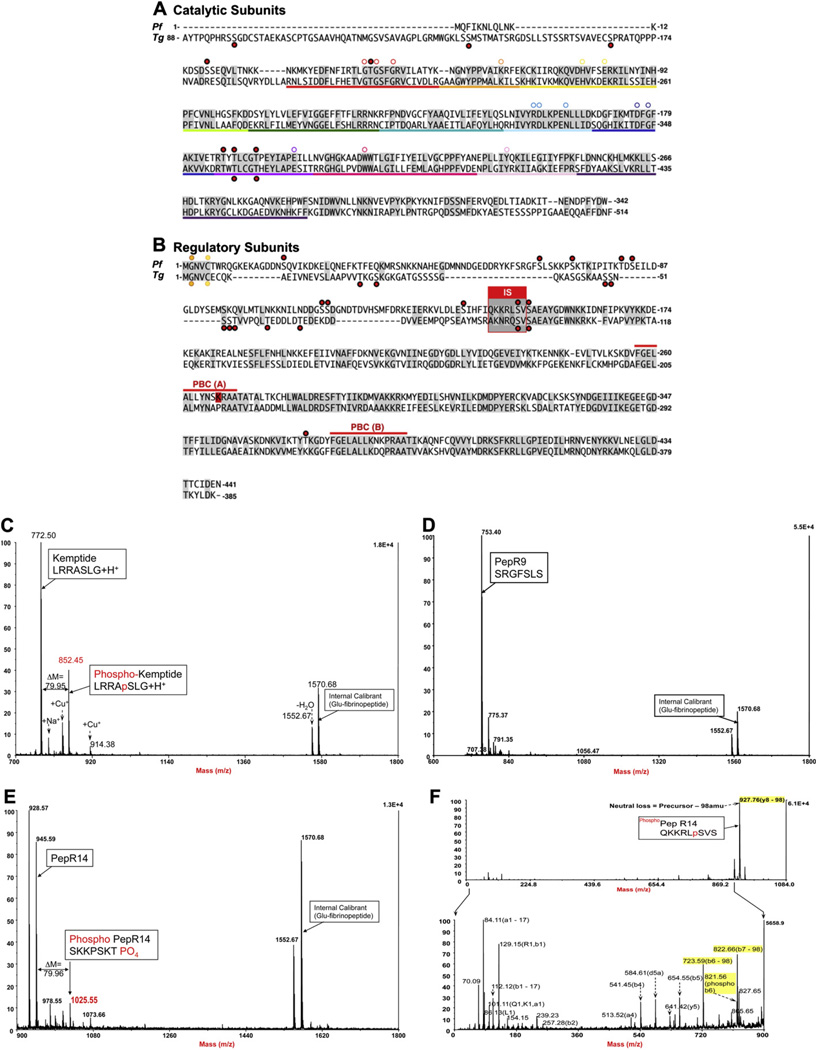
Eleven different phosphorylation sites have been recently identified in P. falciparum PfPKA-R, and most of these are localized to the N-terminal segment that is divergent from the mammalian R-subunits (Fig. 6) [5,9]. Analyses of PfPKA-R for ordered regions predict that the N-terminus is highly disordered making it accessible to many modifications. Some of these sites may introduce a docking site for another protein such as parasite 14-3-3 that was recently shown to be a substrate for PKA [52], or for Rab7 that has been shown to bind PfPKA-C [53]. Clearly, N-terminal binding of non-AKAP proteins could introduce an alternative mechanism for R subunit dimerization. According to a recent phosphoproteomic analyses, Ser19 in PfPKA-R was identified as a phosphorylated residue [5,9], and this has the potential to weaken interactions with membranes. Many myristylated proteins, for example, such as Src have a phosphorylation site close to the N-terminus, and this inhibits docking to membranes [54]. Thus, in Plasmodium parasites myristylation-mediated membrane association of PfPKA-R could be regulated by the phosphorylation status of Ser19. In comparing the given R-subunit alignment, Ser19 of P. falciparum aligns with a threonine residue in Plasmodium berghei (Thr12). This suggests that P. berghei may also be phosphorylated at the same position (Fig. 6). Sequence homology between the two proteins further supports this hypothesis with homology at Arg8PfR/Arg1PbR, Gly10PfR/Gly3PbR, Lys11PfR/Lys4PbR, Asp16PfR/Asp9PbR and Asn18PfR/Asn11PbR.
Fig. 6.
Putative phosphorylation sites of the catalytic and regulatory subunits of PfPKA and TgPKA, as detected by Treeck et al, 2011. (A) Sequences of PfPKA-C and TgPKA-C and (B) PfPKA-R and TgPKA-R with key conserved residues highlighted by a white circle and phosphorylation sites indicated by red dots. S149 in PfPKA-R is a bona fide PKA substrate. Spectra of PfPKA-R peptides, PepR149 (QKKRLSVS) harboring S149, PepR68 (SRGFSLS) harboring S68 and Kemptide (LRRASLG) following in vitro phosphorylation by bovine PKA. (C) MS trace of Kemptide used as positive control. The presented spectrum is a composite of two distinct spectra, since the phosho Kemptide (m/z = 852.5) was eluted around 1 min before its non-phosphorylated counterpart (m/z = 772.5). (D) MS trace of pepR68 used as negative control. PepR68 was visible in MS on a few consecutive fractions at around 28min elution. The presented spectrum shows the non-phosphorylated pepR68 (m/z = 753.4), while the phosphorylated form was not detected in any of the fractions. (E) MS trace of pepR149. Non-phosphorylated PepR149 was visible in MS on a few consecutive fractions at 20min elution. The presented spectrum is an intermediary fraction between the two elution peaks. Phosphorylated PepR149 (m/z = 1025.55) was eluted 30 s before its non-phosphorylated counterpart (m/z = 945.59). (D) MS/MS trace of CID-fragmented phosphoPepR149 (m/z = 1025.55); (F1) full spectrum showing an intense signal corresponding to H2PO4 (98amu) loss; (F2) zoomed spectrum below neutral loss m/z showing fragmented ions’ assignation of b and y ions according to Roepstorff and Fohlman [62], nomenclature correlating to PepR14. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In recent structures of the PKA holoenzymes one finds that the linker region that joins the D/D domain to the IS is quite disordered even though the C-terminal region from the IS to the C-terminus binds tightly to the C-subunit and forms a beautiful symmetry related tetramer [55]. In addition to Ser19 discussed above, PfPKA-R has 10 additional residues phosphorylated in vivo [5,6,9]. Several of these sites are in the linker region, although their functional significance is not yet known. In general, many phosphoryaltion sites tend to cluster in intrinsically disordered regions of signaling proteins and clearly this is true for the PfPKA-R-subunit.
1.10. The inhibitory sequence of PfPKA-R is a hybrid between “type I” and “type II” mammalian R-subunits
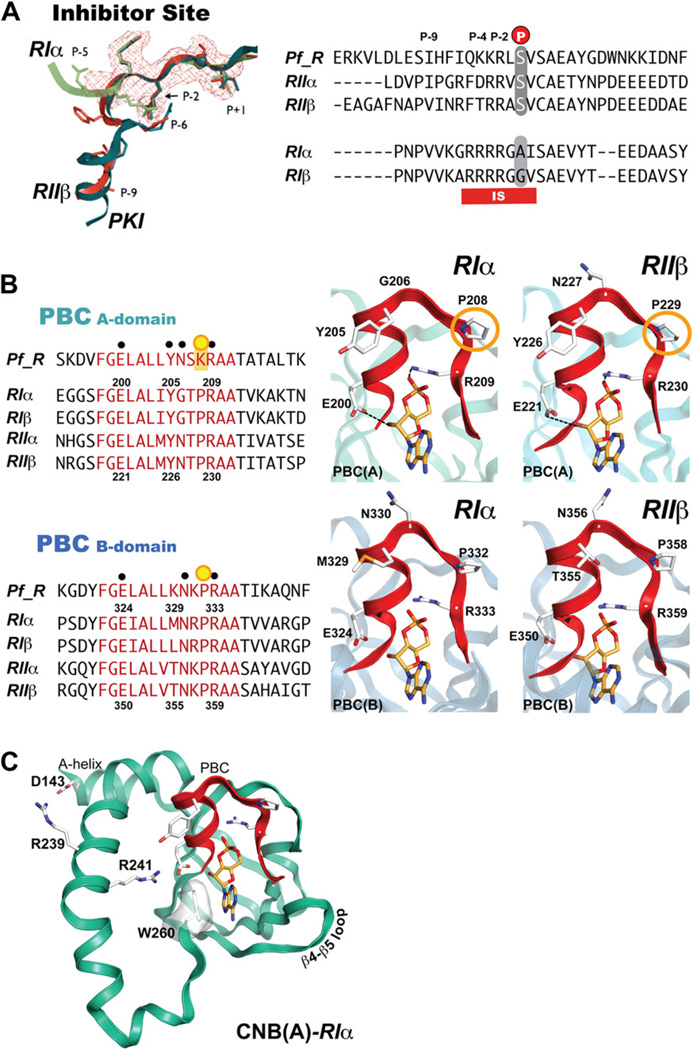
Mammalian PKA-R subunits are multifunctional and have highly dynamic modular domains. They exist in two general forms, RI and RII that are distinct in their sequence, capacity to be autophosphorylated, sensitivity to activation by cAMP and localization within the cell. RI and RII each have α and β isoforms, and each of these have different expression profiles in cells and tissues. R-subunits act as competitive inhibitors of PKA. The IS is located in the linker region between the N-terminus and the two cAMP binding domains, and in the holoenzyme the IS occupies the active site cleft of the C-subunit and thereby blocks its activity. RI and RII isoforms have distinct inhibitor sites. RII-subunits always have a serine in the phosphorylation site (P-site) that is autophosphorylated by the C-subunit. RI isoforms, however, are distinct in that they contain a pseudosubstrate inhibitor site, with an alanine, or glycine in the P-site (Fig. 7A). PfPKA-R has a serine in the P-site and thus appears to be more RII-like, and indeed this serine is phosphorylated by PKA in vitro (Fig. 7). Just beyond the P-site Ser is another serine at the P + 2 position. This is actually a characteristic feature of the RI subunits, and in all RII subunits this site is a Cys. This Ser is not an autophosphorylation site, but instead is a PKG specific site [56]. This site is also found phosphorylated in vivo in PfPKA-R. Phosphorylation of this site in mammalian RIα prevents its reassociation with the C-subunit and provides a mechanism for cross talk between PKA and PKG (unpublished results). Since Plasmodium also encodes for a PKG [57], this may be a significant mechanism for cross talk between these two kinases. The conservation of both a P-site Ser and a P + 2 Ser also suggests that PfPKA-R is a hybrid between RI and RII.
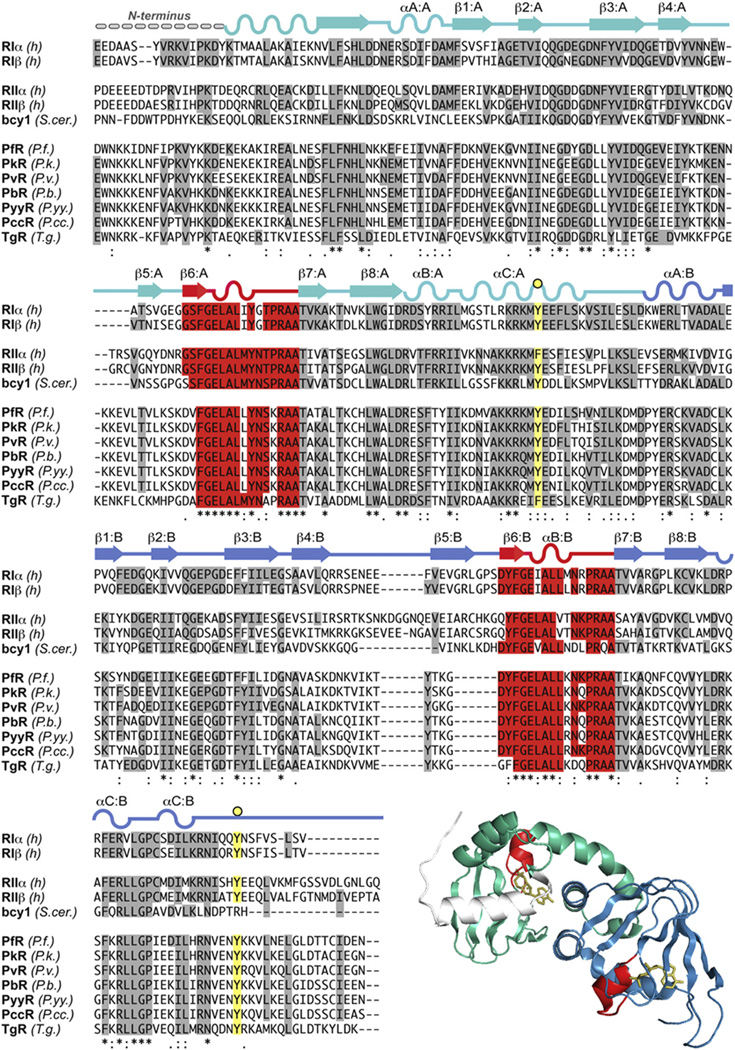
Fig. 7.
The inhibitor-site and phosphate (PBC) cassettes of the P. falciparum R-subunit compared to mammalian isoforms (A) Comparison of mammalian and PfPKA-R inhibitor site. (B) Alignment of isoform-specific phosphate-binding cassettes. (C) Cyclic-nucleotide bindign domain A (CNB-A) of RIα and critical residues involved in protein:protein interactions and the capping of cAMP.
1.11. Features of the PfPKA-R cAMP binding domains
The cAMP-binding domain is an ancient signaling motif and is conserved throughout all genomes [21,58]. In mammalian PKA, cAMP binding to the holoenzyme promotes dissociation of the R- and C-subunits and consequent activation of the C-subunit. Each cAMP-binding domain is comprised of an A-helix followed by an eight-stranded β-barrel and two additional helices that form a helical sub-domain with the A Helix [28,59]. Between β strands 6 and 7 is the conserved cAMP-binding motif, the Phosphate-Binding Cassette (PBC), which is the hallmark signature motif for this domain (Fig. 7B). The PBC is the docking site for cAMP. The three-dimensional structure of the PBC is quite conserved and serves to dock cAMP and protect the phosphate molecule from break down by cellular phosphodiesterases. As expected, comparison of Plasmodium and mammalian R-subunits indicate that the PBCs are highly conserved. Within the mammalian PBC are two residues essential for binding cAMP. The arginine (R209RIa-PBC(A)/R230RIIb-PBC(A), R333RIa-PBC(B)/R359RIIb-PBC(B) in Fig. 7B) and glutamic acid (E200RIa-PBC(A)/E221RIIb-PBC(A), E324RIa-PBC(B)/E250RIIb-PBC(B) in Fig. 7B) serve as crucial residues for binding to cAMP. These residues are conserved in PfPKA-R (Fig. 7B) and moreover, the tyrosine residue that is known to interact with the C-subunit in holoenzyme formations of RIα (Tyr205RIa, Fig. 7B) is also conserved. One key difference in the A domain PBC, however, is the absence of a conserved proline. In PfPKA-R, the conserved CNB-A proline (P208RIa/P229RIIb in Fig. 7B) is a lysine (site indicated by an orange circle in Fig. 7B). BLAST searches indicate that there are numerous species and CNB domains that contain a similar basic residue in this position.
In addition to the PBC, the cAMP-binding site in each CNB domain has a conserved hydrophobic shield that surrounds the cyclic nucleotide [21]. The residues that form this hydrophobic shield are made up of residues from the PBC and allosteric residues that in the three-dimensional structure form a hydrophobic pocket. A hydrophobic W260RIa (not shown in Fig. 7B) at the C-terminal end of CNB-A serves as a capping residue for the adenine ring cAMP. A similar hydrophobic Y371RIa is serves as a CNB-B domain capping residue (Fig. 4, yellow background). Both capping residues are conserved as hydrophobic tyrosines in the Plasmodium isoforms. The capping of cAMP bound to PBC-A by Trp260RIa is a conserved and unique feature of RI-subunits. The capping residue and the interface between the PBC-A and PBC-B domains are different in RIIa and also in Bcy1, the yeast homolog of the PKA-R subunit. The conformation of the cAMP bound PfPKA-R is therefore most likely to resemble RIα. Two other residues crucial to the molecular dynamics of the R-subunit helix are also conserved in Plasmodia. Arg239RIa is important for holoenzyme formation. This basic residue forms ionic bonds with an aspartic residue of the A-helix, Asp143RIa (Fig. 7C), which in turn interfaces with Lys213 in the C-subunit (not shown). In PfPKA-R, Arg239RIa is a basic lysine, thus probably conserving the dynamics of the C-helix. Lys213mPKA is also replaced with a Val in the PfPKA-C, so this interface will differ somewhat. A second arginine, Arg 241RIa, is important for C-subunit activation (Fig. 7C). It forms ionic bonds with the PBC Glu200RIa residue that coordinates cAMP binding. The 241-positionRIa is conserved in PfPKA-R, thus conserving the electrostatic switch with Glu200RIa. In general, the core cAMP-binding domains of PfPKA-R are highly conserved among mammalian isoforms although key residues such at the conservation of Trp260RIa indicate that the PfPKA-R will fold up similar to RI in its cAMP bound conformation (Fig. 7C). The major differences between the R-subunits lie mainly in the N-terminus and linker region of PfPKA-R, and this region is important for localization and is targeted for phosphorylation at several sites.
The β4–β5 loop in the CNB-A domain of mammalian RIα [60] and RIIβ [55] is a critical docking site for the assembly of the full-length holoenzymes (Fig. 7C), and these structures predict that this β4–β5 loop in the CNB-B domain could also be an important binding site for another protein. In PfPKA-R, a putative phosphorylation site, Thr370 sits within the β4–β5 loop and close to PBC-B (indicated by black dot in Fig. 6B in the PfPKA-R sequence) could either disrupt or promote holoenzyme formation or binding of another unknown protein.
The cAMP bound states of each PBC domain are likely to be highly conserved; however, when the R-subunit releases cAMP and binds to C there is a major conformational change in the cAMP-binding pocket. The capping residue in PBC-A is moved over 40 Å away, and there is a new pocket that is formed by both the R-subunit and the C-subunit. This pocket is likely to be different in the Plasmodium and mammalian holoenzymes, and this could be an excellent target for drug discovery. There are cAMP analogs that are selective for RI versus RII mammalian C-subunits and the PfPKA holoenzyme also shows specificity for different analogs of cAMP. There is an excellent high throughput assay for identifying compounds that can either activate or inhibit PKA [61] and this has recently been adapted to discriminate between RI and RII isoforms. With purified PfPKA-R and PfPKA-C subunits, a similar high throughput assay could be used to identify Plasmodium specific activators and inhibitors of PfPKA.
2. Conclusions
PKA plays a crucial role in a variety of Plasmodia cellular events, including its involvement in parasite development, as well as merozoite invasion of and survival within RBCs. The C-subunit shares high sequence conservation between species, although it does not contain a putative myristylation site at its N-terminus, and the N-terminal Tail is very different. Since this region flanks both the N-lobe and C-lobe of the kinase core, it is a parasite-specific region to target. P. falciparum has only one R-subunit isoform that shares both similarities and contrasting differences between the four mammalian R subunit isoforms. The core cAMP-binding (PBC) domains of PfPKA-R share features of all isoforms with the exception of a lysine residue in place of the conserved Pro208 (RIα) in the phosphate-binding cassette. In contrast, the N-terminus is longer than any mammalian R and does not share the similar dimerization/docking X-type helix bundle motif that is common to mammalian subunits. Therefore, we hypothesize that PfPKA-R exists as a monomer, rather than a dimer, and this implies that if it binds to an AKAP, binding, must involve novel mechanism. The N-terminal sequence is further unique from mammalian isoforms in that it contains an N-terminal glycine and cysteine, which are putative myristylation and palmitylation sites. Thus, it may not need a classical mammalian AKAP for membrane targeting. Such a motif is conserved in the T. gondii PKA R-subunit, as well as in T. gondii and E. tenella PKG, where it mediates membrane localization, perhaps localizing it to caveoli and lipid rafts. With great need for new therapies to treat drug-resistant malaria, PfPKA-R poses a valid therapeutic target, and by targeting the R-subunit, therapies could possibly have multifold inhibitory effects on invasion, intra-erythrocyte development, and new permeation pathway activity.
Acknowledgments
We thank Ganapathy Sarma, Mira Sastri, Manjula Darshi, Michael Deal, and Jessica Bruystens for helpful discussion. N.M.H was supported by the Ruth L. Kirschstein National Research Service Award (NRSA) from National Institutes of Health Grants (5F31 GM090658-02). SST is supported by grants from the national Institutes of Health (GM19301 and GM34921). HT, AM and GL thank INSERM, CNRS and FP7 MALSIG for support and the 3P5 proteomics facility of the Université Paris Descartes, Sorbonne Paris Cité for mass spectrometry profile analyses.
References
- 1.Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 2.Wells TN. Microbiology. Is the tide turning for new malaria medicines? Science. 2010;329:1153–1154. doi: 10.1126/science.1194923. [DOI] [PubMed] [Google Scholar]
- 3.Lin JT, Juliano JJ, Wongsrichanalai C. Drug-resistant malaria: the era of ACT. Curr. Infect. Dis. Rep. 2010;12:165–173. doi: 10.1007/s11908-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia–Thailand border. Emerg. Infect. Dis. 2008;14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasonder E, Green JL, Camarda G, Talabani H, Holder AA, Langsley G, Alano P. The phosphoproteome of Plasmodium falciparum reveals extensive phosphatidylinositol and cAMP-protein kinase A signalling during blood stage development. Mol. Cell. Prot. doi: 10.1021/pr300557m. (submitted for publication). [DOI] [PubMed] [Google Scholar]
- 6.Lasonder E, Treeck M, Alam MM, Tobin AB. Insights in to the P. falciparumschizont phospho-proteome. Microbes Infect. 2012;14:811–819. doi: 10.1016/j.micinf.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe. 2011;10:410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun. 2011;2:565. doi: 10.1038/ncomms1558. [DOI] [PubMed] [Google Scholar]
- 11.Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim. Biophys. Acta. 2004;1697:155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Doerig C, Abdi A, Bland N, Eschenlauer S, Dorin-Semblat D, Fennell C, Halbert J, Holland Z, Nivez MP, Semblat JP, Sicard A, Reininger L. Malaria: targeting parasite and host cell kinomes. Biochim. Biophys. Acta. 2010;1804:604–612. doi: 10.1016/j.bbapap.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC. Protein kinases of malaria parasites: an update. Trends Parasitol. 2008;24:570–577. doi: 10.1016/j.pt.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Doerig C, Billker O, Pratt D, Endicott J. Protein kinases as targets for antimalarial intervention: kinomics, structure-based design, transmission-blockade, and targeting host cell enzymes. Biochim. Biophys. Acta. 2005;1754:132–150. doi: 10.1016/j.bbapap.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Doerig C, Meijer L. Antimalarial drug discovery: targeting protein kinases. Expert Opin. Ther. Targets. 2007;11:279–290. doi: 10.1517/14728222.11.3.279. [DOI] [PubMed] [Google Scholar]
- 16.Leroy D, Doerig C. Drugging the Plasmodium kinome: the benefits of academia-industry synergy. Trends Pharmacol. Sci. 2008;29:241–249. doi: 10.1016/j.tips.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Anamika, Srinivasan N, Krupa A. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins. 2005;58:180–189. doi: 10.1002/prot.20278. [DOI] [PubMed] [Google Scholar]
- 18.Anamika K, Srinivasan N. Comparative kinomics of Plasmodium organisms: unity in diversity. Protein Pept. Lett. 2007;14:509–517. doi: 10.2174/092986607780989949. [DOI] [PubMed] [Google Scholar]
- 19.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Miranda-Saavedra D, Luebbering N, Singh A, Sibbet G, Ferguson MA, Cleghon V. Deep evolutionary conservation of an intra-molecular protein kinase activation mechanism. PLoS One. 2012;7:e29702. doi: 10.1371/journal.pone.0029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman HM, Ten Eyck LF, Goodsell DS, Haste NM, Kornev A, Taylor SS. The cAMP binding domain: an ancient signaling module. Proc. Natl. Acad. Sci. USA. 2005;102:45–50. doi: 10.1073/pnas.0408579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DA. Cyclic nucleotide signalling in malaria parasites. Cell Microbiol. 2011;13:331–339. doi: 10.1111/j.1462-5822.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 23.Read LK, Mikkelsen RB. Plasmodium falciparum-infected erythrocytes contain an adenylate cyclase with properties which differ from those of the host enzyme. Mol. Biochem. Parasitol. 1991;45:109–119. doi: 10.1016/0166-6851(91)90032-2. [DOI] [PubMed] [Google Scholar]
- 24.Martin RE, Ginsburg H, Kirk K. Membrane transport proteins of the malaria parasite. Mol. Microbiol. 2009;74:519–528. doi: 10.1111/j.1365-2958.2009.06863.x. [DOI] [PubMed] [Google Scholar]
- 25.Merckx A, Bouyer G, Thomas SL, Langsley G, Egee S. Anion channels in Plasmodium falciparum-infected erythrocytes and protein kinase A. Trends Parasitol. 2009;25:139–144. doi: 10.1016/j.pt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Merckx A, Nivez MP, Bouyer G, Alano P, Langsley G, Deitsch K, Thomas S, Doerig C, Egee S. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathog. 2008;4:e19. doi: 10.1371/journal.ppat.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leykauf K, Treeck M, Gilson PR, Nebl T, Braulke T, Cowman AF, Gilberger TW, Crabb BS. Protein kinase a dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog. 2010;6:e1000941. doi: 10.1371/journal.ppat.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan N, Haste N, Taylor SS, Neuwald AF. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc. Natl. Acad. Sci. USA. 2007;104:1272–1277. doi: 10.1073/pnas.0610251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. Faseb J. 1995;9:576–596. [PubMed] [Google Scholar]
- 30.Gould CM, Kannan N, Taylor SS, Newton AC. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J. Biol. Chem. 2009;284:4921–4935. doi: 10.1074/jbc.M808436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano RA, Kannan N, Kornev AP, Allison CJ, Taylor SS. A chimeric mechanism for polyvalent trans-phosphorylation of PKA by PDK1. Protein Sci. 2009;18:1486–1497. doi: 10.1002/pro.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. Embo J. 2000;19:979–988. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Kennedy EJ, Wu J, Deal MS, Pennypacker J, Ghosh G, Taylor SS. Contribution of non-catalytic core residues to activity and regulation in protein kinase A. J. Biol. Chem. 2009;284:6241–6248. doi: 10.1074/jbc.M805862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keshwani MM, Klammt C, von Daake S, Ma Y, Kornev A, Choe S, Insel PA, Taylor SS. Co-translational cis-phosphorylation of the COOH-terminal tail is a key priming step in the maturation of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 2012;109:E1221–E1229. doi: 10.1073/pnas.1202741109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steichen JM, Kuchinskas M, Keshwani MM, Yang J, Adams JA, Taylor SS. Structural basis for the regulation of protein kinase A by activation loop phosphorylation. J. Biol. Chem. 2012;287:14672–14680. doi: 10.1074/jbc.M111.335091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahamsen H, O’Neill AK, Kannan N, Taylor SS, Jennings PA, Newton AC. The peptidyl–prolyl isomerase Pin1 controls the down-regulation of conventional protein kinase C isozymes. J. Biol. Chem. 2012;287:13262–13278. doi: 10.1074/jbc.M112.349753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herberg FW, Zimmermann B, McGlone M, Taylor SS. Importance of the A-helix of the catalytic subunit of cAMP-dependent protein kinase for stability and for orienting subdomains at the cleft interface. Protein Sci. 1997;6:569–579. doi: 10.1002/pro.5560060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gangal M, Clifford T, Deich J, Cheng X, Taylor SS, Johnson DA. Mobilization of the A-kinase N-myristate through an isoform-specific intermolecular switch. Proc. Natl. Acad. Sci. USA. 1999;96:12394–12399. doi: 10.1073/pnas.96.22.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaffarogullari EC, Masterson LR, Metcalfe EE, Traaseth NJ, Balatri E, Musa MM, Mullen D, Distefano MD, Veglia G. A myristoyl/phosphoserine switch controls cAMP-dependent protein kinase association to membranes. J. Mol. Biol. 2011;411:823–836. doi: 10.1016/j.jmb.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jedrzejewski PT, Girod A, Tholey A, Konig N, Thullner S, Kinzel V, Bossemeyer D. A conserved deamidation site at Asn 2 in the catalytic subunit of mammalian cAMP-dependent protein kinase detected by capillary LC-MS and tandem mass spectrometry. Protein Sci. 1998;7:457–469. doi: 10.1002/pro.5560070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King CC, Sastri M, Chang P, Pennypacker J, Taylor SS. The rate of NF-kappaB nuclear translocation is regulated by PKA and A kinase interacting protein 1. PLoS One. 2011;6:e18713. doi: 10.1371/journal.pone.0018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sastri M, Barraclough DM, Carmichael PT, Taylor SS. A-kinase-interacting protein localizes protein kinase A in the nucleus. Proc. Natl. Acad. Sci. U S A. 2005;102:349–354. doi: 10.1073/pnas.0408608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orstavik S, Reinton N, Frengen E, Langeland BT, Jahnsen T, Skalhegg BS. Identification of novel splice variants of the human catalytic subunit Cbeta of cAMP-dependent protein kinase. Eur. J. Biochem. 2001;268:5066–5073. doi: 10.1046/j.0014-2956.2001.02429.x. [DOI] [PubMed] [Google Scholar]
- 44.Diskar M, Zenn HM, Kaupisch A, Kaufholz M, Brockmeyer S, Sohmen D, Berrera M, Zaccolo M, Boshart M, Herberg FW, Prinz A. Regulation of cAMP-dependent protein kinases: the human protein kinase X (PrKX) reveals the role of the catalytic subunit alphaH–alphaI loop. J. Biol. Chem. 2010;285:35910–35918. doi: 10.1074/jbc.M110.155150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng X, Ma Y, Moore M, Hemmings BA, Taylor SS. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc. Natl. Acad. Sci. U S A. 1998;95:9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, Spraggon G, Xuong NH, Jennings PA, Taylor SS. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol. Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donald RG, Liberator PA. Molecular characterization of a coccidian parasite cGMP dependent protein kinase. Mol. Biochem. Parasitol. 2002;120:165–175. doi: 10.1016/s0166-6851(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 48.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. Embo J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaman SN, Resek ME, Robbins SM. Dual acylation and lipid raft association of Src-family protein tyrosine kinases are required for SDF-1/CXCL12-mediated chemotaxis in the Jurkat human T cell lymphoma cell line. J. Leukoc. Biol. 2008;84:1082–1091. doi: 10.1189/jlb.1007698. [DOI] [PubMed] [Google Scholar]
- 50.Gunaratne RS, Sajid M, Ling IT, Tripathi R, Pachebat JA, Holder AA. Characterization of N-myristoyltransferase from Plasmodium falciparum. Biochem. J. 2000;348(2):459–463. [PMC free article] [PubMed] [Google Scholar]
- 51.Seydel KB, Gaur D, Aravind L, Subramanian G, Miller LH. Plasmodium falciparum: characterization of a late asexual stage golgi protein containing both ankyrin and DHHC domains. Exp. Parasitol. 2005;110:389–393. doi: 10.1016/j.exppara.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 52.Lalle M, Curra C, Ciccarone F, Pace T, Cecchetti S, Fantozzi L, Ay B, Breton CB, Ponzi M. Dematin, a component of the erythrocyte membrane skeleton, is internalized by the malaria parasite and associates with Plasmodium 14-3-3. J. Biol. Chem. 2011;286:1227–1236. doi: 10.1074/jbc.M110.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rached FB, Ndjembo-Ezougou C, Chandran S, Talabani H, Yera H, Dandavate V, Bourdoncle P, Meissner M, Tatu U, Langsley G. Construction of a Plasmodium falciparum Rab-interactome identifies CK1 and PKA as Rab-effector kinases in malaria parasites. Biol. Cell. 2012;104:34–47. doi: 10.1111/boc.201100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang X, Lu Y, Wilkes M, Neubert TA, Resh MD. The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J. Biol. Chem. 2004;279:8133–8139. doi: 10.1074/jbc.M311180200. [DOI] [PubMed] [Google Scholar]
- 55.Zhang P, Smith-Nguyen EV, Keshwani MM, Deal MS, Kornev AP, Taylor SS. Structure and allostery of the PKA RIIbeta tetrameric holoenzyme. Science. 2012;335:712–716. doi: 10.1126/science.1213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geahlen RL, Krebs EG. Regulatory subunit of the type I cAMP-dependent protein kinase as an inhibitor and substrate of the cGMP-dependent protein kinase. J. Biol. Chem. 1980;255:1164–1169. [PubMed] [Google Scholar]
- 57.Hopp CS, Bowyer PW, Baker DA. The role of cGMP signalling in regulating life cycle progression of Plasmodium. Microbes Infect. 2012;14:831–837. doi: 10.1016/j.micinf.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canaves JM, Taylor SS. Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J. Mol. Evol. 2002;54:17–29. doi: 10.1007/s00239-001-0013-1. [DOI] [PubMed] [Google Scholar]
- 59.Kornev AP, Taylor SS, Ten Eyck LF. A generalized allosteric mechanism for cis-regulated cyclic nucleotide binding domains. PLoS Comput. Biol. 2008;4:e1000056. doi: 10.1371/journal.pcbi.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boettcher AJ, Wu J, Kim C, Yang J, Bruystens J, Cheung N, Pennypacker JK, Blumenthal DA, Kornev AP, Taylor SS. Realizing the allosteric potential of the tetrameric protein kinase A RIalpha holoenzyme. Structure. 2011;19:265–276. doi: 10.1016/j.str.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saldanha SA, Kaler G, Cottam HB, Abagyan R, Taylor SS. Assay principle for modulators of protein–protein interactions and its application to non-ATP-competitive ligands targeting protein kinase A. Anal. Chem. 2006;78:8265–8272. doi: 10.1021/ac061104g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]