Abstract
Human umbilical cord mesenchymal stem cells (hUCMSCs) are considered to be an ideal replacement for bone marrow MSCs. However, up to date, there is no convenient and efficient method for hUCMSC isolation and culture. The present study was carried out to explore the modified enzyme digestion for hUCMSC in vitro. Conventional enzyme digestion, modified enzyme digestion, and tissue explant were used on hUCMSCs to compare their efficiencies of isolation and culture, to observe primary cell growth and cell subculture. The results show that the cells cultured using the tissue explant method had a longer culture cycle (P < 0.01) and lower yield of primary cells per centimetre of umbilical cord (P < 0.01) compared with the two enzyme digestion methods. Subculture adherence and cell doubling took significantly less time with the tissue explant method (P < 0.05) than with the conventional enzyme digestion method; however, there was no significant difference between the tissue explant method and the modified enzyme digestion method (P > 0.05). Comparing two enzyme digestion methods, the modified method yielded more cells than did the conventional method (P < 0.01), and primary cell adherence took significantly less time with the modified method than with the conventional method (P < 0.05). Cell cycle analysis of the third-generation hUCMSCs cultured by modified enzyme digestion method indicated that most cells were quiescent. Immunofluorescence staining showed that these cells expressed MSC markers CD44 and CD90. And Von Kossa and oil red O staining detection showed that they could be differentiated into osteoblasts and adipocytes with induction medium in vitro. This study suggests that hUCMSC isolation and culture using 0.2 % collagenase II at 37 °C for digestion of 16–20 h is an effective and simple modified enzyme digestion method.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-012-9528-0) contains supplementary material, which is available to authorized users.
Keywords: Mesenchymal stem cell, Umbilical cord, Culture, Isolation, Modified enzyme digestion
Introduction
Mesenchymal stem cells (MSCs) are a type of adult stem cells with significant self-renewal ability and multilineage differentiation capacity. Under certain induction conditions, they can differentiate into various cell lineages, such as osteoblasts, chondrocytes, muscle cells, and adipocytes (Pittenger et al. 1999). MSCs were first discovered in bone marrow and were later widely studied and used in tissue engineering and cell therapy (Kortesidis et al. 2005). However, the isolation of MSCs from bone marrow is an invasive procedure that is painful for patients; additionally, the ability of cells to differentiate declines with increasing patient age (Baxter et al. 2004). These problems have limited the clinical applications of bone marrow MSCs (Lavik and Langer 2004). Compared to bone marrow MSCs, human umbilical cord mesenchymal stem cells (hUCMSCs) have many advantages, such as a wide variety of sources, easy acquisition, high proliferation ability, low immunogenicity, and fewer bioethics issues involved in their use (Baksh et al. 2007). Therefore, hUCMSCs are considered to be an ideal replacement for bone marrow MSCs. The optimization of the in vitro isolation and culture of hUCMSCs and the examination of their biological properties are important prerequisites for their application. Currently, there are many methods of hUCMSC isolation and culture, but none of them are stable, highly efficient, or convenient; they all are associated with problems, such as a low yield of primary cells or a long culture cycle, and thus, they are insufficient for supporting large-scale clinical applications. The purpose of this study is to establish a convenient and efficient method for hUCMSC isolation and culture. Conventional enzyme digestion, a modified enzyme digestion, and tissue explant were used on hUCMSCs to compare their efficiencies of isolation and culture, to observe primary cell growth, and to optimize the enzyme digestion protocol, providing an experimental foundation for further hUCMSC applications.
Materials and methods
Reagents and samples
Fresh umbilical cords were obtained from healthy full-term neonates after cesarean section in the First Hospital Affiliated to the General Hospital of PLA: about 15–20 cm per umbilical cord. Informed consent was obtained from women delivering full-term infants before collecting umbilical cord. Type II and type IV collagenase (Worthington, Lakewood, NJ, USA), Trypsin–EDTA and FBS (Gibco - Life Technologies, Carlsbad, CA, USA), and an inverted microscope and photo acquisition system (Leica, DMI6000, Wetzlar, German) were used in the present study. Primary antibodies to CD44, CD90, CK31 and CD45, and secondary antibodies conjugated to fluorescein isothiocyanate (FITC) were purchased from Abcam (Cambridge, UK).
Primary MSC isolation and culture
Umbilical cord tissue was removed from a sterile 0.9 % sodium chloride solution with Penicillin (200 units/ml)–Streptomycin (200 μg/ml) in a laminar flow clean bench and washed twice with PBS. A 10 cm umbilical cord was cut into three equal pieces. Each piece was cut into small pieces of about 1 cm in length, the pieces were washed thoroughly to remove blood and blood clots, and the umbilical arteries, veins, and umbilical cord adventitia were removed to obtain Wharton’s jelly. Then, Wharton’s jelly was dissected into small pieces of approximate 1 mm3 and used to isolate and culture primary hUCMSCs by three methods, including the tissue explant method, the conventional enzyme digestion method, and a modified enzyme digestion method. In this study, six independent human umbilical cords were processed using three isolation methods. The detailed protocols are as follows:
Conventional enzyme digestion method: pieces of tissue of approximately 1 mm3 in size were transferred into 0.1 % collagenase IV and digested at 37 °C for 16–18 h until Wharton’s jelly was completely digested. This was followed by centrifugation at 2,500 rpm for 5 min. After the supernatant was removed, the pellet was resuspended with 0.1 % trypsin and further digested for 30 min at 37 °C. The digestion reaction was stopped by adding DMEM with 10 % FBS. After thorough re-suspension, the mixture was filtered through a 74 μm cell strainer. The filtrate was centrifuged at 1,500 rpm for 10 min, washed with PBS, and centrifuged twice. Cells were then completely resuspended and transferred into a 10 cm culture dish to be incubated at 37 °C under 5 % CO2 and saturated humidity.
Modified enzyme digestion method: the Wharton’s jelly was transferred to a 0.2 % collagenase II solution and digested at 37 °C for 16–20 h until it was completely digested. The enzyme digestion was terminated as above, and the reaction solution was filtered and centrifuged for primary cell culturing (the data on optimization of collagenase II concentration and digestion time for the modified enzyme digestion method are provided in supplementary material).
Tissue explant method: Properly cut tissue pieces were inserted into 10 cm tissue culture dishes at 0.5 cm intervals. Complete medium (Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100μg /ml)) was added, and tissues were cultured under 5 % CO2 and saturated humidity at 37 °C. Five milliliters of complete medium was added 4 h later for a prolonged period of culture, and the media was changed every 3–4 days.
Primary cell adherence and growth were observed under an inverted phase contrast microscope. The timing of cell confluence for all methods of enzyme digestion, and cell adherence for the two enzyme digestion methods was recorded. Trypan blue staining was used to calculate the amount of primary cells cultured and the amount of cells collected from each 1 cm-long umbilical cord fragment. A total cell count was accomplished using a haemocytometer chamber after staining the cells with 0.4 % trypan blue. The cells that were not stained with trypan blue were considered viable cells.
Cell Subculture
When primary cells that had been cultured using the above three methods reached 90 % confluence, they were treated with 0.25 % trypsin (with 0.02 % EDTA), were collected, and were resuspended to make the 1 × 105 cells/ml cell suspension. Ten millilitres of cell suspension were cultured in a 75 cm flask and then sub-cultured. The time frame for cell adherence and the doubling time was observed and recorded for all three methods.
Cell cycle analysis and immunofluorescence staining
Third-generation UCMSCs that were cultured by an improved enzyme digestion method were selected and treated with 0.25 % trypsin (with 0.02 % EDTA). Then, cells were collected and resuspended to make a 1 × 106 cells/ml cell suspension, which was subjected to flow cytometry for cell cycle analysis. The cell suspension was placed on a coverslip in a cell culture dish and maintained in culture. Two days later, after the cells on the coverslip were fixed, FITC-labelled anti-CD31, CD44, CD45, or CD90 antibodies were added followed by an incubation at 37 °C for 1h. Cells were then washed with PBS and counterstained with DAPI. The antigen expressions of CD31, CD44, CD45 and CD90 were observed under a fluorescence microscope.
Differentiation capability studies
The differentiation of third-generation hUCMSCs that were cultured by modified enzyme digestion method was assessed. The cells were cultured in a complete medium which contained either adipogenic (0.5 μM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM insulin, and 200 μM indomethacin) or osteogenic (0.1 μM dexamethasone, 10 μM β-glycerophosphate, and 50 μM ascorbate-phosphate) reagents. As a negative control, cells were cultured in a complete medium deprived of the differentiation factors. All regents were from Sigma-Aldrich (St. Louis, MO, USA). Two weeks later, intracellular lipid accumulation was visualised using Oil-Red-O staining, and osteogenic differentiation was assessed by the examination of Von Kossa staining (Karahuseyinoglu et al. 2007).
Statistical analysis
Statistical analysis was performed with SPSS 10.0 statistics software (SPSS Inc. Chicago, IL, USA). Data are presented as mean ± SD and Student t test was used for statistical analysis. A value of P < 0.05 was considered statistically significant.
Results
Cell morphology observation
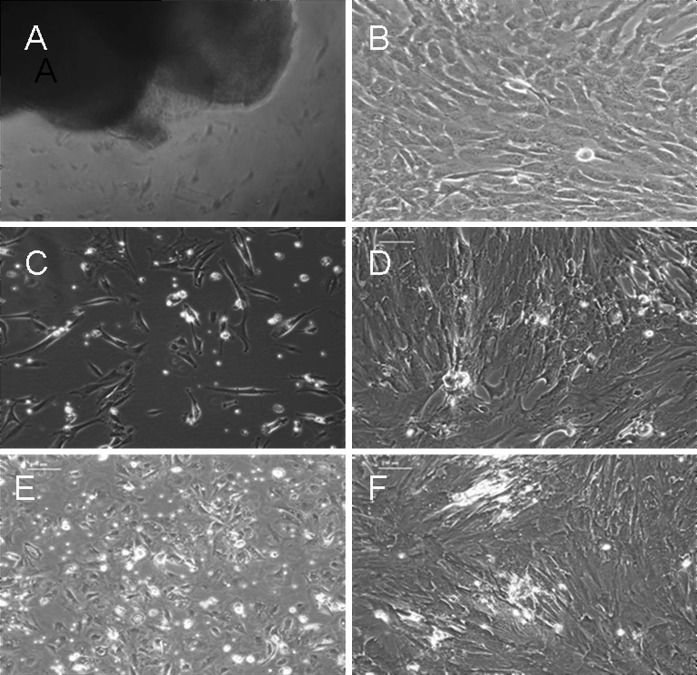
After 4–5 days, cultures that had been treated by the tissue explant method showed rod-like and irregularly shaped cells growing out of the tissue in a dispersed pattern. After around 15 days in culture, fibroblast-like cells could be found around the tissue explants, with the cell density decreasing with increasing distance from the centre of the explants. Tissue explants were then removed, the medium was changed, and the cells were kept in culture. After 22–28 days, cells reached 90 % confluence, and the cell morphology was relatively homogeneous (Fig. 1a–b). For the cells that had been isolated by the conventional enzyme digestion method, spindle-like or polygonal fibroblast-like cells could be observed, growing in a dispersed pattern after 3 days in culture. After 14–18 days in culture, the cells were approximately 90 % confluent and had large morphological differences (Fig. 1c–d). For the cells that had been isolated by the modified enzyme digestion method, short rod-like and polygonal fibroblast-like cells could be observed after 1 day in culture. Cells were 90 % confluent after 9–12 days in culture and were morphologically diverse (Fig. 1e–f). At the third subculture, cells from both enzyme digestion methods and the tissue explant methods were spindle-like and showed no significant differences in morphology (Fig. 2).
Fig. 1.
Morphological characteristics of hUCMSCs isolated and cultured with both enzyme digestion methods and the tissue explant method under an inverted phase contrast microscope (×100). a primary cells isolated with the tissue explant method at 5 days of culture. b primary cells isolated with the tissue explant method at 24 days of culture. c primary cells isolated with the conventional enzyme digestion method at 4 days of culture. d primary cells isolated with the conventional enzyme digestion method at 16 days of culture. e primary cells isolated with the modified enzyme digestion method at 2 days of culture. f primary cells isolated with the modified enzyme digestion method at 9 days of culture
Fig. 2.
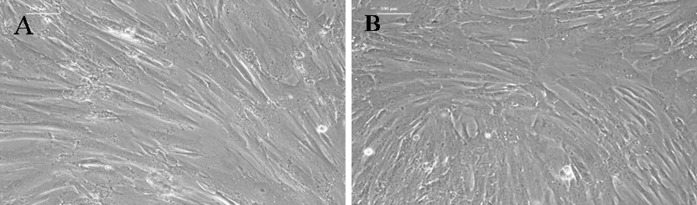
Morphological characteristics of the third-generation hUCMSCs under inverted phase contrast microscope (×100). a primary cells isolated with the tissue explant method at 5 days of culture. b primary cells isolated with the modified enzyme digestion method at 5 days of culture
Cell culture efficiency analysis
Cell confluence and adherence were observed under an inverted phase contrast microscope for the timing of these events and for cell counting. Comparing the efficiencies of the three isolation methods, we found that the cells isolated using the tissue explant method had a significantly longer culture cycle (P < 0.01) and lower yield of primary cells per centimetre of umbilical cord (P < 0.01) than did cells isolated using the two enzyme digestion methods (Table 1). Subculture adherence and cell doubling took significantly less time with the tissue explant method (P < 0.05) and the modified enzyme digestion method (P < 0.05) than with the conventional enzyme digestion method; however, there was no significant difference between the tissue explant method and the modified enzyme digestion method (P > 0.05) (Table 1). Comparing the two enzyme digestion methods, the modified method yielded significantly more cells than did the conventional method (P < 0.01), and primary cell adherence took significantly less time with the modified method than with the conventional method (P < 0.05) (Table 2).
Table 1.
Comparison of hUCMSCs isolation and culture with tissue explant and both enzyme digestion methods
| Groups | PCCT (days) | PCN (105/cm) | SAT (h) | SCDT (h) |
|---|---|---|---|---|
| TE | 24.8 ± 4.0 | 6.3 ± 1.7 | 3.0 ± 1.0 | 39.0 ± 7.8 |
| CED | 16.3 ± 1.7b | 11.0 ± 1.8b | 7.3 ± 1.5a | 69.5 ± 8.5b |
| MED | 10.3 ± 1.3b,d | 12.1 ± 2.0b | 4.2 ± 1.7c | 41.3 ± 7.5d |
TE tissue explant, CED conventional enzyme digestion, MED modified enzyme digestion, PCCT primary cell confluency time, PCN primary cell number, SAT subculture adherence time, SCDT subculture cell doubling time
Data are expressed as mean ± SEM (n = 6), a P<0.05 versus TE, b P < 0.01 versus TE, c P < 0.05 versus CED, d P<0.01 versus CED
Table 2.
Comparison of hUCMSCs isolation and culture with both enzyme digestion methods
| Groups | Enzyme digestion time (h) | Collected nuclear cells (106/cm) | Primary cell adherence time (h) |
|---|---|---|---|
| CED | 17.0 ± 1.0 | 26.5 ± 3.1 | 35.5 ± 11.7 |
| MED | 18.7 ± 2.1 | 52.3 ± 6.3a | 18.8 ± 3.8b |
TE tissue explant, CED conventional enzyme digestion, MED modified enzyme digestion
Data are expressed as mean ± SEM (n = 6), a P < 0.01 versus CED, b P<0.05 versus CED
Cell cycle analysis and immunofluorescence staining
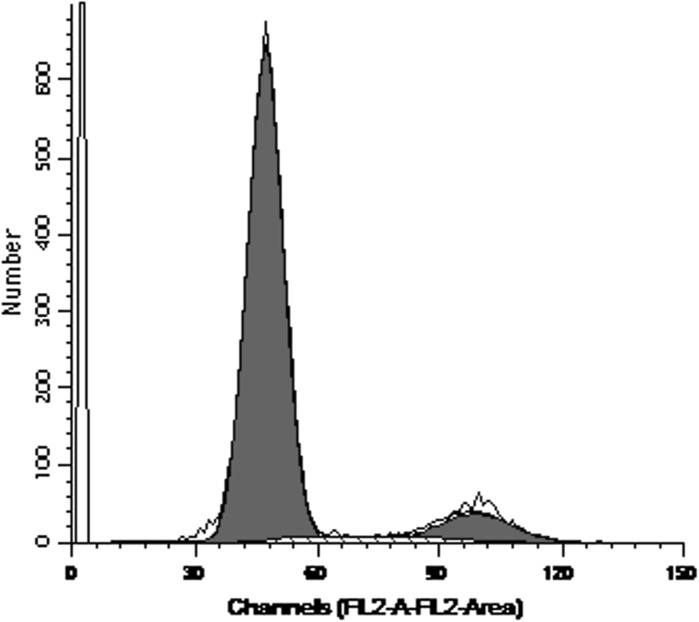
Cell cycle analysis of the third-generation hUCMSCs showed that 85.99 % cells were in the G0-G1 phase and 14.01 % were in the S + G2 + M phase, indicating that most cells were quiescent, matching the self-renewal property of stem cells (Fig. 3).
Fig. 3.
Cell cycle analysis of the third-generation of confluent hUCMSCs isolated with the modified enzyme digestion method
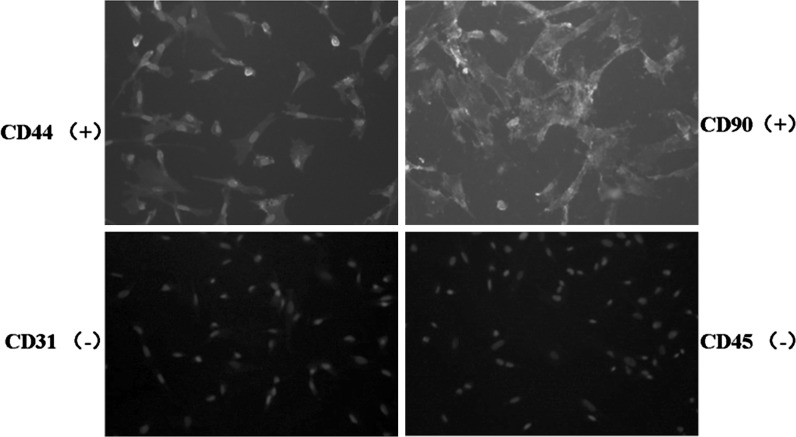
Immunofluorescence staining showed that the fibroblast-like cells obtained from cord tissue expressed mesenchymal stem cell markers CD44 and CD90 but not the endothelial cell antigen CD31 or the hematopoietic cell antigen CD45 (Fig. 4).
Fig. 4.
Identification of cell antigen markers of the third-generation of hUCMSCs isolated by the modified enzyme digestion method (×100). Immunofluorescence staining showed positive expression for CD44 and CD90, negative expression for CD45 and CD31. The nucleus was stained blue with DAPI
Differentiation of hUCMSCs into adipocytes and osteocytes
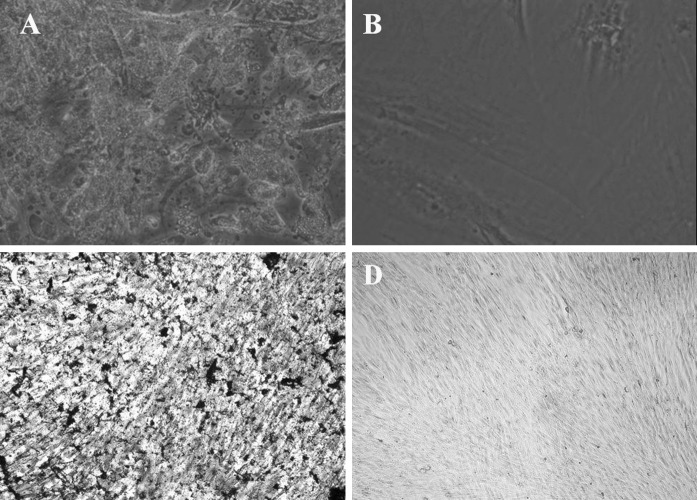
With adipogenic supplementation, part of the MSCs contained numerous Oil-Red-O-positive lipid droplets at the end of the second week, (Fig. 5a). Similarly, the part of the cells became Von Kossa positive which were induced with osteogenic medium (Fig. 5c). Non-treated control cultures did not show spontaneous adipocyte or osteoblast formation at the end of the second week (Fig. 5b, d).
Fig. 5.
Multi-lineage differentiation potential of third-generation hUCMSCs isolated by the modified enzyme digestion method (×100). a Results of Oil-Red-O staining in cell cultures grown for 2 weeks in adipogenic medium. Part of the cells contained numerous Oil-Red-O-positive lipid droplets. b Control group cells grown in the regular medium, no cells contained Oil-Red-O-positive lipid droplets. c Result of Von Kossa staining in cell cultures grown for 2 weeks in osteogenic medium. Part of the hUCMSCs became Von Kossa positive. d Control group cells grown in the regular medium, hUCMSCs were Von Kossa negative
Discussion
MSCs have a distinct self-renewal ability and differentiation potential. Whether they are cultured in vitro or in vivo, they can differentiate into osteoblasts, chondrocytes, adipocytes, myoblasts, and neuronal cells, suggesting their extensive clinical application potential. MSCs exist in a variety of tissues, such as bone marrow, periosteum, thymus, skin, adipose, muscle, umbilical cord, and umbilical cord blood (Schneider et al. 2010; Chamberlain et al. 2007). From the perspective of embryo development, the umbilical cord is the structure in which stem cells develop and migrate, and umbilical cord stromal cells have been found among populations of embryonic stem cells (Thomson and Odorico 2000). The gelatinous connective tissue around the umbilical cord, called Wharton’s jelly, is a continuous skeleton formed by interwoven collagen and small fibres (Vizza et al. 1995), and it contains a large number of myofibroblast-like mesenchymal cells (Kobayashi et al. 1998). However, in the cord blood, the number of MSCs was very small, and thus, their growth rate in culture was slow and the success in culture was very low (Lee et al. 2004). These studies suggest that umbilical cord tissue may become a new source of MSCs. In addition, hUCMSCs are considered to be the ideal alternative to bone marrow mesenchymal stem cells (Baksh et al. 2007; Karahuseyinoglu et al. 2007).
Currently, there are two types of methods for hUCMSC isolation and culture: tissue explant and enzyme digestion. However, the tissue explant method has low separation efficiency, and the enzyme digestion method has many varying protocols, none of which are stable and efficient. In this study, we have compared the conventional tissue explant method (Ma et al. 2005; Qiao et al. 2008), the conventional enzyme digestion method (Wang et al. 2004; Seshareddy et al. 2008; Tong et al. 2011) and a modified enzyme digestion method. Our results showed that the tissue explant method led to a significantly longer culture cycle and lower yield of primary cells than did the enzyme digestion method (P < 0.01). However, this method was characterized by a significantly shorter adherence and doubling times in culture than observed for the conventional enzyme digestion method (P < 0.05); there is no significant difference in these factors between the tissue explant method and the modified enzyme digestion method. Comparing the two enzyme digestion methods, significantly more cells were collected using the modified method than using the conventional method (P < 0.01). The modified method was also characterized by significantly shorter adherence and incubation times in primary culture and subculture than observed for the conventional method (P < 0.05). An analysis of these results showed that enzyme digestion was more efficient for cell isolation and culturing, while adherence and proliferation after subculturing were more efficient with the tissue explant method, indicating that cell viability was better with this method than with the conventional enzyme digestion method. Therefore, the advantages of tissue explant are that there is no need of digestive enzymes, no damage of the vitality of cells (Conconi et al. 2006), and a low cost of cultivation. Enzymes may degrade the cell membrane, which makes the cells lose their ability to adhere stably or at all, resulting in further damage to these cells. Therefore, it is important to control the use of enzymes and their dosages and digestion times. The results of this study show that primary cells cultured using the modified enzyme digestion method showed no difference in subculture adherence or doubling times compared to the tissue explant method; this indicates that using the modified enzyme digestion method does not affect cell viability but results in a higher culture efficiency than the conventional enzyme digestion and tissue explant methods.
In this study the enzyme digestion method was modified by replacing the conventional 0.1 % collagenase IV plus trypsin with a single 0.2 % collagenase II digestion for 18 h, which significantly improved the culture efficiency. After 10 days, primary cells could already be collected. This indicates that the concentration and type of the enzyme are very critical, especially when collagenase and other enzymes are used in combination, which may result in the destruction of cell membranes and subsequently reduce the adhesion ability and culture efficiency of isolated hUCMSCs. In view of type IV collagen as main component of collagen in Wharton’s jelly of human umbilical cord, Collagenase IV may play a role in digestion of Wharton’s jelly (Can and Karahuseyinoglu 2007). However, Wharton’s jelly of human umbilical cord is known to contain hyaluronic acid and sulphated glycosaminoglycans immobilized in an insoluble microfibril network. Collagenase II is stronger for its clostripain activity, which is more efficient in solubilizing the UC microfibrils than other types of collagenase (Meyer et al. 1983). Previous study suggest that degradation of the extracellular matrix and disintegration of cell membranes may have caused cellular damage through the use of trypsin alone for a long period, because some cells are sensitive to exposure to trypsin but not to collagenase (Oyama et al. 1990). Therefore, in this study, we only applied collagenase II for digestion of Wharton’s jelly of human umbilical cord in the modified enzyme digestion method.
In preliminary experiments, we isolated, cultured, and identified hUCMSCs by the tissue explant method (Han et al. 2011). In these experiments, we cultured primary hUCMSCs obtained by the tissue explant and modified enzyme digestion methods, subcultured to the third generation until confluent state, and found no difference in cell morphology for these two methods. Flow cytometry and immunofluorescence staining of third-generation cells isolated by the modified enzyme digestion method showed 85.99 % of the cells in the quiescent stage with the self-renewal characteristics of stem cells. These cells were positive for cell surface antigens CD90 and CD44 and negative for antigens CD45 and CD31, and they could be differentiated into osteoblasts and adipocytes in vitro, therefore, multi-lineage differentiation capacity was confirmed by induced adipogenesis and osteogenesis. These results indicate that cells isolated by the modified enzyme digestion method have the characteristics of mesenchymal stem cells (Dominici et al. 2006).
In summary, in this study we have isolated hUCMSCs from Wharton’s jelly using the enzyme digestion methods and the tissue explant method and examined their isolation and culture efficiencies and effectiveness. Results showed that the umbilical cord is rich in MSCs and that the cells are highly proliferative. By comparing the efficiency and cell growth of cells derived with three isolation methods of hUCMSCs, we optimized an effective and simple modified enzyme digestion method—that is, 0.2 % collagenase II at 37 °C for digestion of 16–20 h. Therefore, this study laid the foundation for the next step in the research and large-scale clinical applications of hUCMSCs.
Electronic supplementary material
Acknowledgments
This study was supported by grants from the National Health Public Welfare Special Scientific Research Foundation of China (200802066), China Postdoctoral Science Foundation special fund project (201104777), Capital Medical University basic-clinical medical research cooperation project (12JL81), National Natural Science Foundation of China (81101423), and Military Medical Science and Technology Research Project of “Twelfth Five-Year Plan” of China (CWS11J111).
Footnotes
Yan-Fu Han, Ran Tao, Tian-Jun Sun have been contributed equally to this work.
References
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells, following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Conconi MT, Burra P, Di Liddo R, Calore C, Turetta M, Bellini S, Bo P, Nussdorfer GG, Parnigotto PP. CD105 (+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089–1096. [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Han Y, Chai J, Sun T, Li D, Tao R. Differentiation of human umbilical cord mesenchymal stem cells into fibroblasts in vitro. Biochem Biophys Res Commun. 2011;413:561–565. doi: 10.1016/j.bbrc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kubota T, Aso T. Study on myofibroblast diferentiation in the stromal cells of Wharton’s jelly: expression and localization of alpha-smooth muscle actin. Early Hum Dev. 1998;51:223–233. doi: 10.1016/S0378-3782(97)00123-0. [DOI] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Lavik E, Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J. 2005;118:1987–1993. [PubMed] [Google Scholar]
- Meyer FA, Laver-Rudich Z, Tanenbaum R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton’s jelly. Biochim Biophys Acta. 1983;755:376–387. doi: 10.1016/0304-4165(83)90241-6. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Hori N, Allen CN, Carpenter DO. Influences of trypsin and collagenase on acetylcholine responses of physically isolated single neurons of Aplysia califonica. Cell Mol Neurobiol. 1990;10:193–205. doi: 10.1007/BF00734573. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H, Wang X, Chen Y. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Schneider RK, Püllen A, Kramann R, Bornemann J, Knüchel R, Neuss S, Perez-Bouza A. Long-term survival and characterisation of human umbilical cord-derived mesenchymal stem cells on dermal equivalents. Differentiation. 2010;79:182–193. doi: 10.1016/j.diff.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord. Methods Cell Biol. 2008;86:101–119. doi: 10.1016/S0091-679X(08)00006-X. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Odorico JS. Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol. 2000;18:53–57. doi: 10.1016/S0167-7799(99)01410-9. [DOI] [PubMed] [Google Scholar]
- Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]
- Vizza E, Correr S, Goranova V, Heyn R, Muglia U, Papagianni V. The collagen fibrils arrangement in the Wharton’s jelly of full-term human umbilical cord. Ital J Anat Embryol. 1995;100:495–501. [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s Jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.