Abstract
Hematopoiesis is the process by which blood cells (hemocytes) mature and subsequently enter the circulation and we have developed a new technique to culture the hematopoietic progenitor cells in vitro. The reason for the successful culture was the isolation of a plasma protein that turned out to be a novel cytokine, astakine 1 (Ast1) containing a domain present in several vertebrates, so-called prokineticins. Now we have detected several astakines from other invertebrate species. Depending on our discovery of the cytokine Ast1 we have an opportunity to study in detail the differentiation of cells in the hematopoietic tissue of a crustacean, a tissue of evolutionary interest for studies of the connection between the vascular system and the nervous system. We have been able to isolate the entire hematopoietic tissue and for the first time detected a link between this tissue and the brain. We have further localized a proliferation center in the tissue and characterized its different parts. We have also used this system to isolate a new hematopoietic factor CHF that is important in the crossroad between apoptosis and hemocyte differentiation. Our technique for culture of crayfish hematopoietic stem cells provides a simple tool for studying the mechanism of hematopoiesis, but also enables detailed studies of immune defense reactions. Further, the culture system has been used for studies of viral defense and the system is suitable for gene silencing which allows functional characterization of different molecules involved in host defense as well as in hemocyte differentiation.
Keywords: Hematopoietic stem cell culture, Invertebrate immunity, Innate immunity, Astakine
The hematopoietic tissue in crayfish
The freshwater crayfish Pacifastacus leniusculus has been used for several decades to study innate immunity and mainly the melanization reaction in crustaceans (Cerenius et al. 2010). During all these years we have observed that the hemocyte number in the circulation of crayfish varies a lot. Various internal and external factors influence the number of circulating hemocytes over time in different ways, e.g. during a severe infection the number often falls at the beginning and then rises again if the animal survives (Söderhäll et al. 2003). In order to understand how the regulation of hemocyte number operates, and especially how an infection might trigger this increase in hemocyte synthesis we started to investigate the hematopoietic process in P. leniusculus.
There are three major hemocyte types in this animal: the hyaline cells (HC, phagocytes); the semi-granular cells (SGC, involved in encapsulation and phagocytosis) and the granular cells (GC, main actors in melanization and antimicrobial defense). The SGC and GC both contain the proPO activating system (Lin and Söderhäll 2011). These hemocytes can be easily separated by Percoll gradient centrifugation, which allows functional studies of the individual cell types (Söderhäll and Smith 1983).
Earlier investigators have identified a tissue in the crayfish located at the dorsal side of the stomach as a site for hemocyte synthesis, and detailed TEM studies of this hematopoietic tissue (HPT) and the progenitor cells have been reported (Chaga et al. 1995). Now we have got more detailed knowledge about how the HPT is organized and we have recently published a paper about how the HPT is connected to the brain. We then localized a certain area, named as APC, anterior proliferation center that is responsible for high cell division and seems to be an important center for regulation of stem cell activity. Most important is also that we can isolate the whole tissue in one piece (Noonin et al. 2012).
In order to be able to study the differentiation of hemocytes in the HPT, an in vitro culture system seemed logical to use, as this tissue is likely to have proliferative ability (Söderhäll et al. 2003). To test this, we first injected BrdU into the crayfish and then looked for proliferating cells in the HPT. As expected, the tissue was found to be loaded with proliferating cells, which was also confirmed by staining for mitosis with anti-phosphohistone antibodies (Söderhäll et al. 2003). Thus, we started to pick out the HPT and released the individual cells by treating the tissue with a mixture of collagenases, since they are loosely held together in lobules of collagenous material. We further reasoned that the cells may need some components from the crayfish in order to survive and proliferate so we tried plasma, and as a result we could establish a cell culture system for these HPT cells (Söderhäll et al. 2005).
Astakine a hematopoietic growth factor
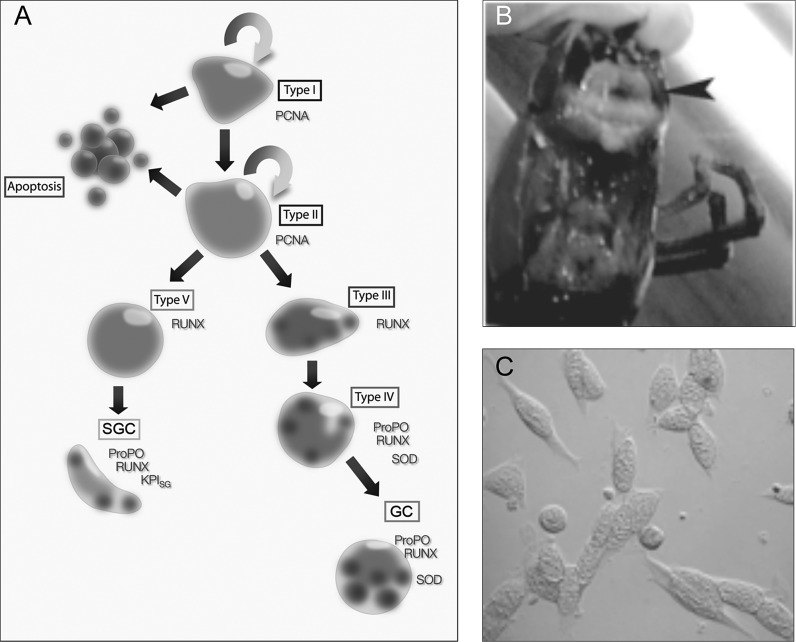
Our first aim was to find any possible molecule or molecules that were of importance to the differentiation process. The HPT consists of lobules with densely packed precursor cells, and these cell types can be categorized into 5 types. Type 1, which is a common type in the APC, is the stem cell with large nuclei surrounded by a very thin layer of cytoplasm. Type 2 is also a proliferating cell and a SGC and GC precursor. Type 3 and 4 are GC precursors and finally type 5 is a SGC precursor. The HC are very rare in P. leniusculus and we are not really sure about their origin and differentiation pathway. A model for the differentiation process in HPT is shown in Fig. 1.
Fig. 1.
a A hypothetical model for differentiation of the SGC and GC lineage, respectively, in the HPT of freshwater crayfish. Type I cells represent a pluripotent stem cell, Type II cells are SGC and GC precursors while Type III and Type IV represent GC precursors, and finally Type V is a precursor of SGC. The specific marker proteins are indicated at the side of the different cell types. b The location of the HPT in freshwater crayfish is indicated by an arrow and is located at the dorsal part of the stomach underneath the carapace. c Isolated HPT cells cultured for 3 days in culture medium containing Ast1. This figure was originally published in Lin and Söderhäll (2011). © by the American Society of Hematology
We noticed that apoptosis was regularly occurring in the HPT and if we injected LPS or laminarin into the crayfish, apoptosis decreased and more cells were after an initial fall entering into the hemolymph. So stimulating hemocyte synthesis turned the cells away from apoptosis, and into the pathways to SGC and GC. Further, we could identify a RUNX transcription factor that was needed for the differentiation into these two cell types (Söderhäll et al. 2003). However, prophenoloxidase (proPO) an important component of the innate immune system was found only in a very small population of the HPT cells (type 4) and is mainly a marker for mature hemocytes. For the culture we realized that a component of plasma was needed for proliferation but also to induce RUNX transcription in the cultured cells. We found that plasma from LPS-injected animals was far more effective in the culture while LPS itself did not have any effect showing that a factor was produced in the hemolymph by LPS stimulation, and specifically induced proliferation of the HPT cells. We could assay for this activity by measuring proliferation as BrdU incorporation but also more easily by looking at cell spreading.
Using a protein purification scheme we were able to isolate a responsible protein, purify it to homogeneity and identify its amino acid sequence. We named this new cytokine as astakine, and could show that this purified astakine was responsible for inducing proliferation. In addition, astakine could induce the differentiation process in vitro measured as induction of the RUNX transcription by in situ hybridization and RT-PCR. When we later analyzed plasma of LPS injected crayfish, we could confirm that this plasma contained far higher amounts of astakine when compared to plasma from untreated animals. Further, we went to live animals to confirm the effect of astakine on hemocyte synthesis, and showed that injection of purified or recombinant astakine into animals indeed resulted in a dramatic increase in total hemocyte count (THC). And more importantly if astakine was silenced by RNA interference the recovery of hemocytes after an LPS injection was not found and finally the animals would dye by lack of hemocytes (Söderhäll et al. 2005).
What is astakine like? Sequence analysis of astakine shows that it contains a conserved PROK domain (pfam 06607) with 10 cysteine residues in the mature protein. It shows some homology with the PROK domain of the Bv8 peptides from frog and mammals. Later, we did clone a similar protein from shrimp (P. monodon), with an insert of 13 amino acids in the middle, and further we did find a similar protein in crayfish, and now these two proteins are named as Ast1 (for the first isolated form) and Ast2. Interestingly, the N-terminal sequence of all known vertebrate PROKs contain seven amino acids that are completely conserved and these are critical for receptor binding in vertebrates. This sequence is not present in the invertebrate astakines, and when we compare the structure of the astakines with the known gene structure of human PROK2 we can easily identify that human exon 2 and 4 share similarity with the astakine sequences. We have searched available databases and detected astakine-like sequences in a vast number of invertebrates, mainly in arthropods such as spiders, tics, crustaceans, scorpions and some insects. It is interesting to note that these molecules seem to be absent in diptera, coleoptera and lepidoptera (Lin et al. 2010).
Hemocyte lineage marker proteins
Next we asked, how does astakine induce the release of new hemocytes into the circulation. One first step then was to be able to distinguish the different pathways leading to the different hemocyte types. By a proteomic approach we could identify specific marker proteins for the SGC and GC. Proliferating cell nuclear antigen was detected as a marker for the dividing cells and for the SGC one Kazal type proteinase inhibitor was found to be specific, while an extracellular superoxide dismutase was specifically expressed by the G cell lineage (Wu et al. 2008). Using these markers we could then show that Ast1 mainly was involved in stimulation of the SGC lineage since KPI was induced and also in vivo injection of Ast1 mainly induced increase in the number of SGCs. Ast2 on the other hand was shown to be involved in some way in regulating the G cell lineage, and more importantly be an important molecule involved in the light regulation of the hematopoietic process (Watthanasurorot et al. 2011, 2013b).
There are unfortunately no such things as crayfish mutants. To be able to perform real functional studies in the HPT cell culture we needed a method for gene silencing. Therefore we started to develop a method for RNA interference in the stem cell culture and it turned out to be very successful. We first tried several commercially available transfection agents such as Ca-phosphate, Lipofectin and Effectene and all these agents seemed to be useful in the sense of viability of the cells. However, they were all differently efficient when it came to gene silencing. The method that we came up with was to use Histone 2A as transfection agent a method that is very efficient in silencing, harmless to the cells and also very cheap (Liu and Söderhäll 2007). By using this method we did find that one of the most common proteins present in the HPT, transglutaminase (TGase) a cross-linking enzyme that among other things is involved in the clotting reaction, has an important function in the hematopoietic process. By using the gene silencing technique we could demonstrate that TGase silenced cells started to spread and migrate. This phenotype reminded on the effect we see when Ast1 is added to the cells, so we went further to analyze TGase activity in Ast1 treated and control cell cultures. Thereby we could see that one clear effect of Ast1 is a decrease in extracellular TGase activity. Our conclusion is, that a decrease in TGase activity may be important in enabling a release of cells into the circulation, since one substrate for TGase is collagen, which is a main component of the extracellular matrix in the HPT. Interestingly we could also show that the TGase gene is regulated by GATA factors, which are known to be very important in hematopoietic regulation (Lin et al. 2008).
Next, another strategy was applied to find out the mechanism by which Ast1 induces HPT cell proliferation. By using SSH, suppression subtractive hybridization, with or without Ast1 in the culture medium we could identify some transcripts that were highly upregulated by the presence of Ast1. One novel factor, named as the crustacean hematopoietic factor (CHF), was identified from the SSH library as dependent of Ast1 for its expression. CHF is a small cysteine-rich protein (∼9 kDa) with high similarity to the N-terminal region of vertebrate CRIM1, and contains an insulin growth factor binding protein motif with unknown function. Silencing of CHF did not affect the renewal of HPT cells, but induced apoptosis. CHF is exclusively expressed in the blood cell lineage, and in vivo RNA interference experiments showed that knockdown of this gene results in severe loss of blood cells and a higher apoptotic rate in the HPT. In summary, CHF acts by blocking the road to apoptosis, and thereby Ast1 will turn the differentiation pathway into the SGC lineage.
HPT culture as a tool in immunology research
A second aim for the use of the HPT stem cell culture is to use it for studies of the innate immune defense against bacteria and virus. In our viral experiments we have used the WSSV (white spot syndrome virus) a large enveloped DNA virus with broad host range among arthropods as a model. First, Jiravanichpaisal et al. (2006) have established a reproducible model for viral replication in the HPT culture. We could also show that the morphology of the cells were severely affected by viral infection and that the virus really replicated by showing expression of structural genes such as the envelope protein VP28. We could further identify viral particles inside the cells by TEM (Jiravanichpaisal et al. 2006). Moreover, the replication was dependent on temperature so that at 4 °C no replication occurs, while 16 and 25 °C are optimal, but if the temperature increases to 32 °C the cells are not dividing and so the virus can not replicate either.
We have used this culture system to identify some factors involved in viral infection and antiviral defense, and some brief examples follow below. First we used SSH and could identify an ALF-like protein, an antilipopolysaccharide factor as was first found in horseshoe crab (Tanaka et al. 1982). We used the cultured cells to knock down ALF and showed its importance for cell resistance to WSSV (Liu et al. 2006). Further we have revealed the function of gC1qR also known as p32 or mitochondrial matrix protein, and identified as the globular head of the C1q binding protein. Silencing of gC1qR makes the virus replicate more, and the addition of recombinant gC1qR blocks viral replication. We have now shown that this is somehow related to the apoptotic process. So the viral strategy is in some way to enable enough cells to use for their proliferation and the virus has to initiate this pathway. Here is gC1qR an important player (Watthanasurorot et al. 2010, Watthanasurorot et al. 2013a).
In conclusion, we have used a highly proliferative tissue such as the HPT that contains hematopoietic stem cells in order to establish a cell culture system to investigate several physiological processes in the animal. This culture system has enabled us to discover unknown regulators of differentiation as well as of new proteins involved in innate immunity.
Acknowledgments
This work was funded by the Swedish Research Council VR (621-2011-4797).
References
- Cerenius L, Kawabata S, Lee BL, Nonaka M, Söderhäll K. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci. 2010;35:575–583. doi: 10.1016/j.tibs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chaga O, Lignell M, Söderhäll K. The haemopoietic cells of the freshwater crayfish, Pacifastacus leniusculus. Anim Biol. 1995;4:59–70. [Google Scholar]
- Jiravanichpaisal P, Söderhäll K, Söderhäll I. Characterization of white spot syndrome virus replication in in vitro-cultured haematopoietic stem cells of freshwater crayfish, Pacifastacus leniusculus. J Gen Virol. 2006;87:847–854. doi: 10.1099/vir.0.81758-0. [DOI] [PubMed] [Google Scholar]
- Lin X, Söderhäll I. Crustacean hematopoiesis and the astakine cytokines. Blood. 2011;117:6417–6424. doi: 10.1182/blood-2010-11-320614. [DOI] [PubMed] [Google Scholar]
- Lin X, Söderhäll K, Söderhäll I (2008) Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol 9:58 [DOI] [PMC free article] [PubMed]
- Lin X, Novotny M, Söderhäll K, Söderhäll I. Ancient cytokines, the role of astakines as hematopoietic growth factors. J Biol Chem. 2010;285:28577–28586. doi: 10.1074/jbc.M110.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Söderhäll I. Histone H2A as a transfection agent in crayfish hematopoietic tissue cells. Dev Comp Immunol. 2007;31:340–346. doi: 10.1016/j.dci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Liu HP, Jiravanichpaisal P, Söderhäll I, Cerenius L, Söderhäll K (2006) Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the crayfish Pcifastacus leniusculus. J Virol 80:10365–10371 [DOI] [PMC free article] [PubMed]
- Noonin C, Lin X, Jiravanichpaisal P, Söderhäll K, Söderhäll I. Invertebrate hematopoisis: an anterior proliferation centre as a link between the hematopoietic tissue and the brain. Stem Cells Dev. 2012;21:3173–3186. doi: 10.1089/scd.2012.0077. [DOI] [PubMed] [Google Scholar]
- Söderhäll K, Smith VJ. Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev Comp Immunol. 1983;7:229–239. doi: 10.1016/0145-305X(83)90004-6. [DOI] [PubMed] [Google Scholar]
- Söderhäll I, Bangyeekhun E, Mayo S, Söderhäll K. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev Comp Immunol. 2003;27:661–672. doi: 10.1016/S0145-305X(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Söderhäll I, Kim YA, Jiravanichpaisal P, Lee SY, Söderhäll K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J Immunol. 2005;174:6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Nakamura T, Morita T, Iwanaga S. Limulus anti-LPS factor: an anticoagulant which inhibits the endotoxin mediated activation of Limulus coagulation system. Biochem Biophys Res Commun. 1982;105:717–723. doi: 10.1016/0006-291X(82)91493-0. [DOI] [PubMed] [Google Scholar]
- Watthanasurorot A, Jiravanichpaisal P, Söderhäll I, Söderhäll K. A gC1qR prevents white spot syndrome virus replication in the freshwater crayfish Pacifastacus leniusculus. J Virol. 2010;84:10844–10851. doi: 10.1128/JVI.01045-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Watthanasurorot A, Söderhäll K, Jiravanichpaisal P, Söderhäll I. An ancient cytokine, astakine mediates circadian regulation of invertebrate hematopoiesis. Cell Mol Life Sci. 2011;68:315–323. doi: 10.1007/s00018-010-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watthanasurorot A, Jiravanichpaisal P, Söderhäll K, Söderhäll I. A calreticulin/gC1qR complex prevents cells from dying: a conserved mechanism from arthropods to humans. J Mol Cell Biol. 2013;5:120–131. doi: 10.1093/jmcb/mjt005. [DOI] [PubMed] [Google Scholar]
- Watthanasurorot A, Saelee N, Phongdara A, Roytrakul S, Jiravanichpaisal P, Söderhäll K, Söderhäll I. Astakine 2—the dark knight linking melatonin to circadian regulation in crustaceans. PLoS Genet. 2013;9:e1003361. doi: 10.1371/journal.pgen.1003361. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu C, Kim YA, Liu HP, Söderhäll I, Söderhäll K. Hemocyte lineage marker proteins in a crustacean, the freshwater crayfish Pacifastacus leniusculus. Proteomics. 2008;8:4226–4235. doi: 10.1002/pmic.200800177. [DOI] [PubMed] [Google Scholar]