Abstract
The decline of European abalone Haliotis tuberculata populations has been associated with various pathogens including bacteria of the genus Vibrio. Following the summer mortality outbreaks reported in France between 1998 and 2000, Vibrio harveyi strains were isolated from moribund abalones, allowing in vivo and in vitro studies on the interactions between abalone H. tuberculata and V. harveyi. This work reports the development of primary cell cultures from abalone gill tissue, a target tissue for bacterial colonisation, and their use for in vitro study of host cell—V. harveyi interactions. Gill cells originated from four-day-old explant primary cultures were successfully sub-cultured in multi-well plates and maintained in vitro for up to 24 days. Cytological parameters, cell morphology and viability were monitored over time using flow cytometry analysis and semi-quantitative assay (XTT). Then, gill cell cultures were used to investigate in vitro the interactions with V. harveyi. The effects of two bacterial strains were evaluated on gill cells: a pathogenic bacterial strain ORM4 which is responsible for abalone mortalities and LMG7890 which is a non-pathogenic strain. Cellular responses of gill cells exposed to increasing concentrations of bacteria were evaluated by measuring mitochondrial activity (XTT assay) and phenoloxidase activity, an enzyme which is strongly involved in immune response. The ability of gill cells to phagocyte GFP-tagged V. harveyi was evaluated by flow cytometry and gill cells-V. harveyi interactions were characterized using fluorescence microscopy and transmission electron microscopy. During phagocytosis process we evidenced that V. harveyi bacteria induced significant changes in gill cells metabolism and immune response. Together, the results showed that primary cell cultures from abalone gills are suitable for in vitro study of host-pathogen interactions, providing complementary assays to in vivo experiments.
Keywords: Haliotistuberculata, Vibrioharveyi, Gill cell culture, Pathogenicity, Phenoloxidase, Phagocytosis
Introduction
Diseases affecting cultured molluscs are caused by protozoans, fungi, bacteria or viruses, leading to severe damages at any stage of production from larvae to adults (Paillard et al. 2004). Bacterial diseases like Brown Ring disease (BRD) and Juvenile oyster disease (JOD) caused high mortalities in hatcheries and were commonly described in larval and adult bivalves (Lauckner 1983; Paillard et al. 2004; Paillard and Maes 1989; Boettcher et al. 1999, 2000). Summer mortalities caused by Vibrio bacterial strains have recently been described in various cultured mollusc as the Pacific oyster Crassostrea gigas (Le Roux et al. 2002; Saulnier et al. 2010) and the European abalone Haliotis tuberculata (Nicolas et al. 2002). European abalone stocks are currently threatened by several pathogens causing severe mass mortalities in wild or farmed populations, among them Xenohaliotis californiensis, Haplosporidium sp. and Vibrio harveyi (Nicolas et al. 2002; Azevedo et al. 2006; Balseiro et al. 2006; Huchette and Clavier 2004).
Vibrio harveyi (Syn. V. carchariae) is a marine bacterium common in the ocean (Gauger and Gomez-Chiarri 2002), and known to be pathogenic in a large range of vertebrates and invertebrates including molluscs (Austin and Zhang 2006). Abalone diseases due to the pathogen V.harveyi have been already described for Haliotis diversicolor (Nishimori et al. 1998), for Haliotis laegivata (Handlinger et al. 2005; Vakalia and Benkendorff 2005; Dang et al. 2011a) and for H. tuberculata (Nicolas et al. 2002), in tropical or temperate seawater, causing septicaemia. Since 1998, summer mass mortality outbreaks of the European abalone H. tuberculata reported along the north coast of Britanny (France) have been associated with Vibrio bacteria (Nicolas et al. 2002). Bacterial strains were isolated from both farmed and wild abalones, allowing in vivo and in vitro studies on the interactions between abalone H. tuberculata and V. harveyi (Nicolas et al. 2002; Travers et al. 2008b, 2009). Vibrio harveyi is an opportunistic bacterium becoming pathogenic if immunity is compromised due to summer heat stress, poor water quality or reproduction period (Cheng et al. 2004a, b; Vakalia and Benkendorff 2005; Hooper et al. 2007; Travers et al. 2008b; Mottin et al. 2010; Dang et al. 2011a, b; Latire et al. 2012). Considering the development of aquaculture in Europe (Huchette and Clavier 2004), the evolution of reliable diagnostic tools is of major importance for the control and prevention of bacterial diseases (Paillard et al. 2004). Cell cultures provide alternative and controlled experimental models for basic research and for various applications in pathology, ecotoxicology and natural compounds production (Rinkevich 1999, 2011; Auzoux-Bordenave and Domart-Coulon 2010). Although there is currently no cell lineage in marine invertebrates, primary cell cultures provide suitable tools for complementary in vivo studies. Invertebrate immune defence is mainly mediated by circulating hemocytes (Smith 1991), including phagocytosis, encapsulation, production of antimicrobial compounds as well as the phenoloxidase cascade that is an important reaction of melanisation involved in immunity (Cheng 1981; Bachère et al. 1995; Hooper et al. 2007; Travers et al. 2008c).
Antibacterial activity of abalone hemolymph has been demonstrated against Vibrio using in vitro assays (Vakalia and Benkendorff 2005; Travers et al. 2009; Dang et al. 2011a, b) such as phagocytosis, ROS activity or phenoloxidase activity, and secretion of antibacterial peptides or lipophilic antibacterial compounds. The immune response of abalone hemocytes has been previously shown to change according to the pathogenicity of bacterial strains (Travers et al. 2009). Thus pathogen strains would be able to proliferate in contact with hemolymph avoiding phagocytosis and preventing immune response. Moreover, only few host responses were investigated based on cell morphology or using cultured hemocytes (Nicolas et al. 2002; Travers et al. 2008c). While primary culture of hemocytes have been previously used for studying host-pathogen interactions at a cellular level (Boulo et al. 1991; Ford and Ashton-Alcox 1993) there are only few assays using other target tissues such as mantle, digestive gland or gills (Dang et al. 2011b; Faucet et al. 2003).
The present work aimed at studying abalone H.tuberculata–V.harveyi interactions in gill primary cultures. Explant cultures were developed from the gills, a target tissue for bacterial infection, and subcultured to perform in vitro pathogenicity assays. The characterization of cell morphology, viability and the physiological status of the cells were monitored over culture time using flow cytometry and semi-automated assay (XTT). Gill cell cultures were used to investigate in vitro the mode of action of two bacterial strains of V. harveyi. The responses of gill cells to V. harveyi bacteria, including phagocytosis, were evaluated by using a combination of semi-quantitative enzymatic in vitro assays (XTT, phenoloxidase), flow cytometry, as well as fluorescence and transmission electron microscopy analysis.
Materials and methods
Animals
European adult abalone (H.tuberculata), 40–60 mm shell length, were collected from the France-Haliotis farm (Plouguerneau, Brittany). The animals were maintained at the laboratory in an 80 L tank supplied with aerated natural seawater. Abalones were fed once a week with red algae Palmaria palmata. Two days before experiments, abalones were starved and seawater was UV treated to prevent contamination.
Bacterial strains
Two V. harveyi strains were used for in vitro pathogenicity assays: a virulent strain ORM4 isolated from a moribund abalone in 1998 (Nicolas et al. 2002) and a non pathogenic strain LMG7890 (former V. carchariae type strain) isolated from a dead brown shark Carcharinus plumbeus (Colwell 1982). For microscopic observations and bacterial phagocytosis assays, GFP-tagged V. harveyi strains were used (Travers et al. 2008a).
The two bacterial strains were cultured on Luria–Bertani Agar (Sigma, St. Louis, MO, USA) supplemented in salinity (to 20 g L−1) and incubated at 28 °C for 24 h. For in vitro contact, bacterial strains were washed in sterilized seawater. Bacterial concentrations were determined by optical density measurements at 490 nm. For all tests controls consisted of cells without bacteria, and cells with heat inactivated bacteria.
Gill cell primary culture
Primary cultures were performed according to the explant method previously developed for mollusc mantle culture (Auzoux-Bordenave et al. 2007) and recently adapted to the gills by Gaume et al. (2012). The gills were aseptically dissected and placed for 2 days in an antiseptic solution (= natural filtered sea water containing 200 U/mL penicillin–200 μg/mL streptomycin (Sigma), 250 μg/mL gentamycin (Sigma) and 2 μg/mL amphotericin B (Sigma)). Tissues were then minced into 2–3 mm3 explants which were placed to adhere onto the bottom of plastic 6-well plates (Dutscher, Brumath, France). After 30 min of adhesion, explants were covered with 1 mL modified Leibovitz L-15 medium (Sigma) adjusted to 1,100 mosm L−1 by addition of mineral salts (20.2g/L NaCl (Sigma) 0.54g/L KCl (Sigma) 0.6g/L CaCl2 (Sigma) 1g/L MgSO4.7H2O (Fluka) 3.9g/L MgCl2.6H2O (Sigma)) and supplemented with 100 U mL−1 penicillin–streptomycin (Sigma), 200 μg mL−1 gentamycin (Sigma) and 1 μg mL−1 amphotericin B (Sigma) to limit external contamination. The pH was adjusted to 7.4. Explant primary cell cultures were incubated at 18 °C in a humidified incubator and observed daily with an inverted phase contrast microscope (Telaval 3, Zeiss, Jena, Germany).
After 4–5 days of primary culture, a large quantity of gill cells was generated from the explants and the cells were collected. Cell suspension was strained on a 70 μm mesh filter (Cell strainer, VWR International, Fontenay-sous-Bois, France), resuspended in modified L-15 medium and assessed for cell density using a hemocytometer. Cells were subcultured in multi-well microplates to perform in vitro assays. For optimal cellular response, cell density was adjusted to 500,000 cells/well in 24-well microplates (Dutscher) and 150,000 cells/well in 96-well microplates (Dutscher).
Cell population distribution
Analyses were performed using FACS Calibur flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA) equipped with a 488 nm laser. 1,000 events were counted for each sample. Results are expressed as cell cytogram indicating the size (FSC value), the complexity (SSC value), and the level of fluorescence. Cells were incubated for 30 min with SYBR Green fluorescent dye (Molecular Probes, Eugene, OR, USA) at 10−3 dilution of the commercial stock solution. Gill cells were gated to estimate the population distribution in terms of forward scattering (FSC, size) and side scattering (SSC, granularity and volume).
Phagocytosis assay
Phagocytosis assays were performed by flow cytometry according to the protocol for hemocytes adapted from Allam et al. (2002). After 4 days explant culture, gill cells were collected and diluted in L-15 culture medium. Phagocytosis index was defined by the percentage of cells ingesting three or more fluorescent beads, and was measured by flow cytometry. 10 μL of bead solution (Fluorospher, Invitrogen, Carlsbad, CA, USA) was added to 200 μL of cell suspension (700 beads per cells), and were incubated for 1 h at room temperature in the dark. Flow cytometry analysis was performed as described previously. Results are expressed as cell cytogram indicating the size (FSC value), the complexity (SSC value), and the level of fluorescence.
To measure the ability of gill cells to phagocyte the two V. harveyi strains, the same protocol was used. GFP-tagged bacterial strains were added to the culture and incubated at room temperature in the dark for 1, 2 and 20 h. Fluorescence was measured by flow cytometry. Data obtained were the mean ± SE of three values expressed as percentage of fluorescence, and statistical analysis was performed using a two-tailed t test, with significance at p < 0.05.
XTT reduction assay
Cellular activity was measured by the XTT assay (Roche Laboratories, Meylan, France) based on the reduction of a tetrazolium salt (XTT-sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) into yellow formazan salt by active mitochondria (Mosmann 1983). The XTT assay, previously adapted to mollusc cells, provides a global evaluation of the number of viable cells through the measurement of their mitochondrial activity (Domart-Coulon et al. 2000). Assays were performed in 96-well microplates. For this, 50 μL of a mixture of XTT/PMS (N-methyl dibenzopyrazine methyl sulfate) was added to 100 μL of cells/well. Plates were incubated for 20 h at 18 °C, and the intensity of the coloration was measured by spectrophotometry at 490 nm with a 655 nm reference wave-length (BioTek plate reader, Winooski, VT, USA). For in vitro assays, bacterial concentration was adjusted in seawater, and 50 μL of this mixture were added to the cell culture for 1, 5 and 20 h. After the required incubation time, XTT assay was performed as described before.
Data obtained were the mean ± SE of six values expressed as optical density (OD). The effects of bacteria on cell viability were expressed as percentage of the control (cells without contaminant). Statistical analysis was performed using a two-tailed t test, with significance at p < 0.05.
Phenoloxidase activity
Since the phenoloxidase enzyme is strongly involved in immune response, the assessment of its activity is frequently used to evaluate the cell response to external contaminants (Bachère et al. 1995; Cerenius et al. 2008; Hellio et al. 2007). Phenoloxidase activity assays were performed in 96-well microplates. 50 μL of TRIS/HCl buffer (0.2 M, pH 8.0) and 10 μL l-DOPA (l-3,4-dihydrophenyl-alanine, 20 mM, Sigma) were added to the supernatant of 200,000 cells. The microplate was mixed gently for 10 s prior measurement. Detection of phenoloxidase activity was carried out by the measurement of l-3-4-dihydroxyphenylalanine (l-DOPA) transformation in dopachromes. The colorimetric reaction was measured in triplicate at room temperature every 10 min for 100 min at 492 nm, and expressed as changes in optical density per mg of protein. Controls consisted of TRIS/HCl and l-DOPA without cells or bacteria, and were performed in parallel assays. Protein amount in each test, cellular extract and controls, were determined by the Bradford method (Bradford 1976), with serum albumin as standard. Data obtained were the mean ± SE of six values expressed as OD. The impact of bacteria on the phenoloxidase activity was expressed as percentage of control (cells without contaminant). Statistical analysis was performed using a two-tailed t test, with a significant at p < 0.05.
Fluorescence microscopy
Gill cells from four-day-old subcultures, were incubated in the presence of the two GFP-tagged bacterial strains for one or 2 h and immediately observed with a direct fluorescence microscope (Olympus).
Transmission electron microscopy
Four days old subcultured gill cells were incubated for 2 h with V. harveyi bacterial strains. For ultrasructural analysis, gill cells were fixed with 2 % glutaraldehyde in 0.1 M Sörensen phosphate buffer containing 0.6 M sucrose at pH 7.4. Cell samples were post-fixed in 2 % OsO4 in Sörensen phosphate buffer (0.18M = 5.33g/L NaH2PO4.2H2O (Sigma) and 20,7 g/L Na2HPO4 (Sigma) in distilled water (pH 7.4)) for 1 h at room temperature, then rinsed three times in Sörensen buffer, dehydrated in ethanol and embedded in Spürr’s resin (Electron Microscopy Sciences, Aygnesvives, France). Ultra-thin sections (about 50 nm) were cut on an Ultracut Microtome (Ultramictomes Reichert-Jung Ultratome E) with a diatome Ultra 45° diamond, and mounted on formavar-coated Cu-grids (Electron Microscopy Sciences), stained with uranyl acetate 2 % in 50 % ethanol and observed at 75 kV with Hitachi H-7100 transmission electron microscope (Tokyo, Japan) equipped with a digital CCD Hamamatsu Camera.
Results
Cell culture from abalone gills
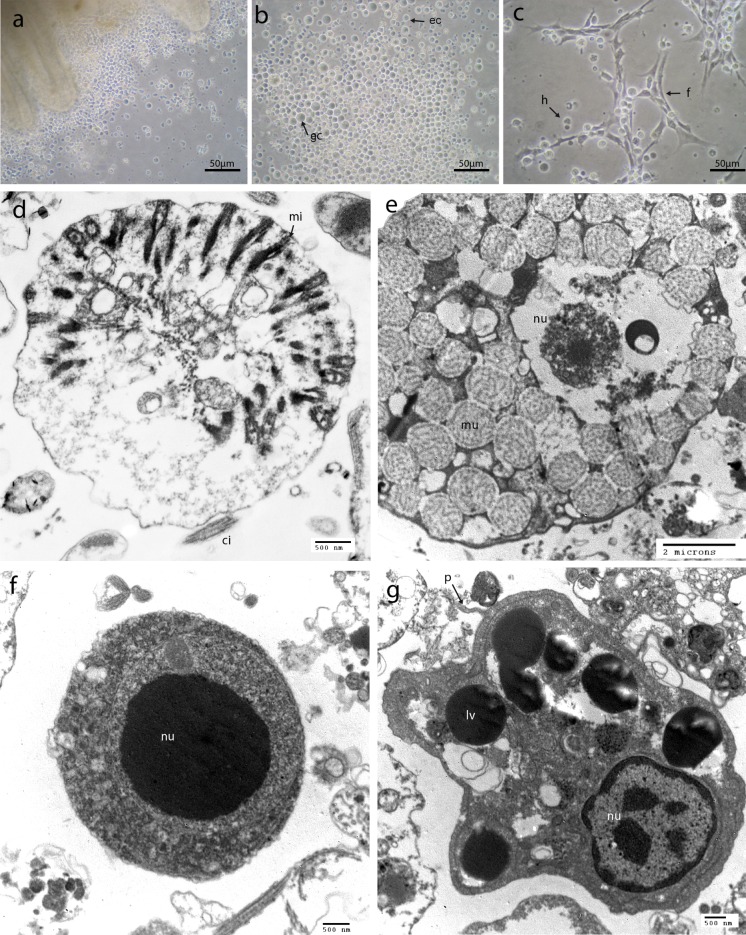
After 1 day of primary culture, 80 % of the explants adhered to the flask bottom and gill cells started to migrate out of the explant (Fig. 1a). During the first 3 days of culture, a large quantity of gill cells was generated from the explants and the cells were spread out onto the bottom of the flask. Gill cell population consisted of a majority of rounded epithelial cells (Fig. 1a), glandular cells (Fig. 1b) and hemocytes (Fig. 1c). The ciliary activity of epithelial cells within the gill filaments demonstrated the vitality of explant primary cultures, according to previous observations from Gaume et al. (2012). Typical hemocyte morphological cell types were recognized in vitro according to previous studies on abalone hemocytes (Auzoux-Bordenave et al. 2007; Travers et al. 2008c): the majority appeared small round and brightly coloured, amoeboid-like, and fiboblastic-like (Fig. 1c), most of them adhering strongly to the plastic flask bottom.
Fig. 1.
Abalone gill cell primary cultures observed under inverted phase contrast microscope (a, b, c) and transmission electron microscopy (d, e, f, g). a One-day-old gill explant primary gill cells culture from H. tuberculata showing the outgrowth of cells from an explant in a 6-well plate, b three day-old explant primary culture showing cell spreading around the flask bottom. Cell population consisted of rounded epithelial cells (ec), and glandular cells (gc) c three day-old explant culture showing hemocytes represented by small hyalinocytes (b) and fibroblastic like cells (f). Transmission electron micrograph of four-day-old cell culture d epithelial cell, e mucous cell, f hyalinocyte, g granulocyte. ci: cilia, nu: nucleus, mi: microvilli, mu: mucous vesicle, p: pseudopodia of the phagocytosis vesicle in formation, lv: lipofuschin vesicles
Ultrastructural observations of cultured cells by TEM allowed the characterization of different cell morphotypes: gill cells consisted of ciliated epithelial cell with cilia, microvilli and numerous mitochondria (Fig. 1d) while glandular cells contained mucopolysaccharide vesicles (Fig. 1e). Hyalinocytes exhibited a large round nucleus containing electron dense chromatin nucleoli (Fig. 1f) and granulocytes contained electron dense granules and exhibited pseudopodia (Fig. 1g).
After 4–5 days of primary culture, cells originated from the explants were subcultured in multi-well plates for in vitro assays. Subcultured cells exhibited the same morphotypes all along culture time, fibroblastic cells being less numerous compared to the explant cultures.
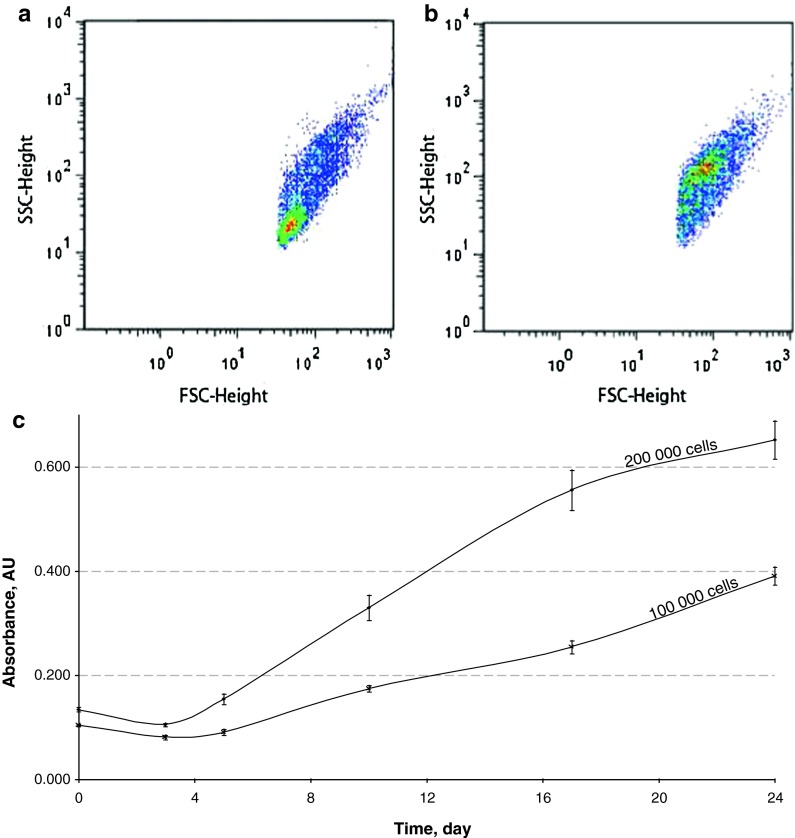
Flow cytometry analysis allowed the comparison of freshly isolated gill cells (Fig. 2a) and 3-day-old subcultured gill cells (Fig. 2b). Results showed that freshly dissociated gill cells were mainly composed of small cells with a relative low intracellular complexity. In contrast, subcultures were mainly composed of larger cells harbouring relatively high intracellular complexity, showing the selection of cells following the transfer in subculture.
Fig. 2.
Flow cytometry analysis and mitochondrial activity of gill cells of H. tuberculata. Characteristics of cell populations of freshly mechanically dissociated gill (a) and gill cells after 3 days in subculture (b), size (FSC) against internal complexity (SSC) density plot representation, after 30 min of incubation with SYBR Green. (c) Evolution of the XTT response of gill cells in subculture at two densities: 100,000 and 200,000 cells/well in 96-well microplates. Data are the mean ± SE of ten values expressed as OD 490/655 nm
Gill cells were successfully subcultured in multi-well plates and cell viability was evaluated by the XTT assay. A linear relationship between mitochondrial activity and cell number was previously evidenced at any time of cell culture (data not shown). Figure 2c shows the XTT response of subcultured gill cells as a function of cell density, over a 24 days period. The evolution of XTT response (absorbance) showed a slight decrease during the first 3 days, and an increase in global cell metabolism with cell density overtime. The two concentrations tested, 100,000 and 200,000 cells per well, allowed to adjust the optimal cell density for further in vitro assays to 500,000 cells per well in 24 well-plates and to 150,000 cells per well in 96 well-plates, respectively.
Phagocytosis
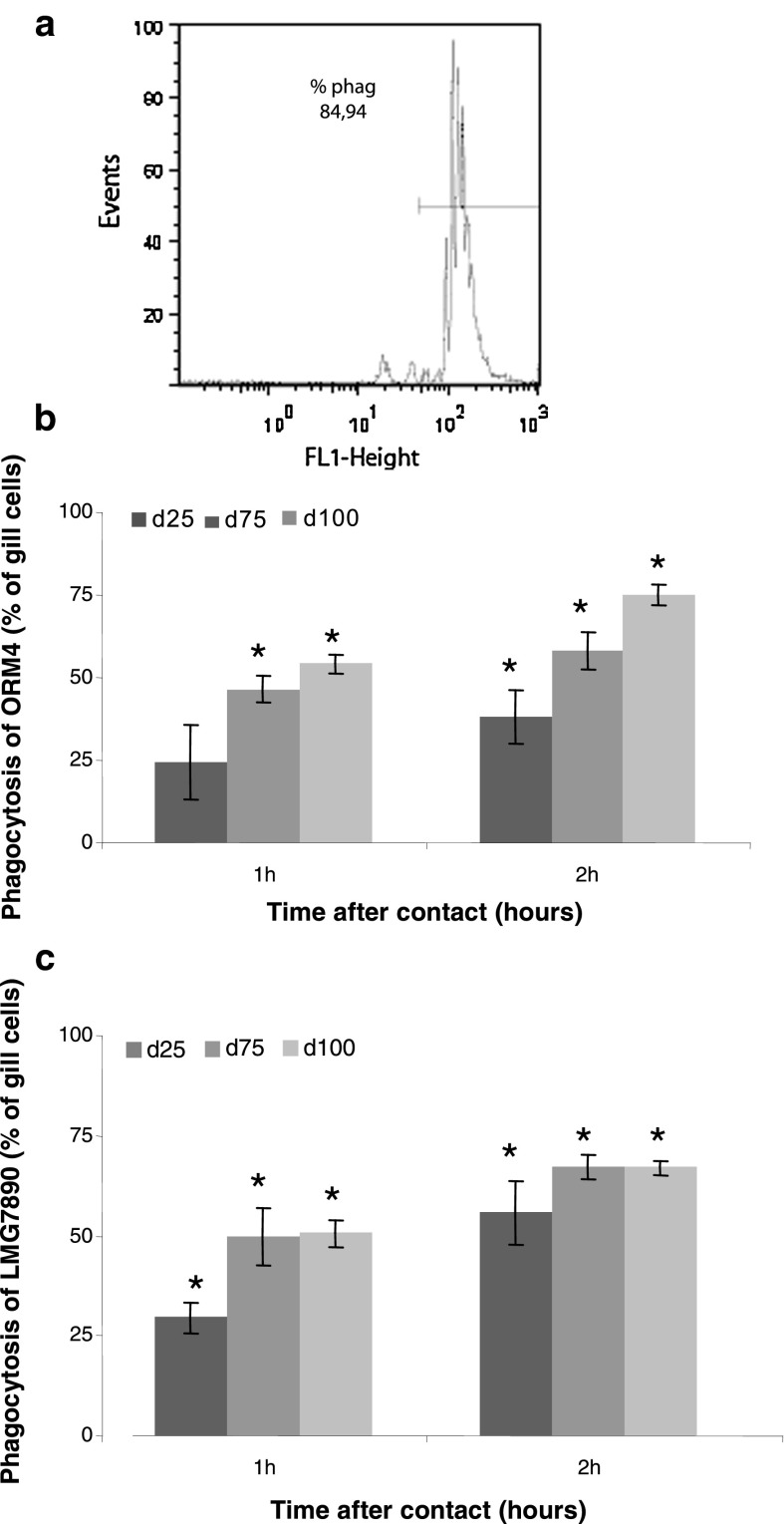
After 3–4 days of explant cell cultures, cells were collected, and were brought into contact with fluorescent beads or with pathogenic and non pathogenic V. harveyi at different concentrations to evaluate their phagocytosis capacity. Cell population distribution was estimated according to the size (FSC, forward scattering ) against complexity (SSC, side scattering) of gill cells stained with SYBR Green after 1 h of contact with fluorescent beads (Fig. 3a). Gill cells which ingested three or more beads displayed a bigger size and a higher complexity than cells which did not ingest beads. Smaller cells did not absorb fluorescent beads. Gill cells were able to phagocyte more than 80 % of fluorescent beads as estimated by the phagocytosis index after 1 h of incubation (Fig. 3a), and up to 70 % of pathogenic and non pathogenic bacterial strains after 2 h of contact (Fig. 3b, c). For concentrations of 25 or 75 bacteria per cell, the percentage of phagocytosis was significantly increased between one and 2 h, with a higher phagocytosis for LMG7890 than for ORM4. When bacteria were placed into saturation relative to gill cell density (100 bacteria per cell) the same phagocytosis ability was observed for the two bacterial strains.
Fig. 3.
Phagocytosis by H. tuberculata gill cells estimated by flow cytometry. Phagocytosis index was expressed as percentage of gill cells containing three or more beads after 1 h of contact (a). Percentage of phagocytosis of gill cells in presence of the pathogenic ORM4 (b) and the non-pathogenic LMG7890 (c) bacterial strain after one and 2 h of contact with three densities of bacteria: 25, 75 and 100 bacteria/cell. Data are the mean ± SE of triplicate experiment. Significant differences (p < 0.05) from absorbances measured in control cells are indicated by an asterisk
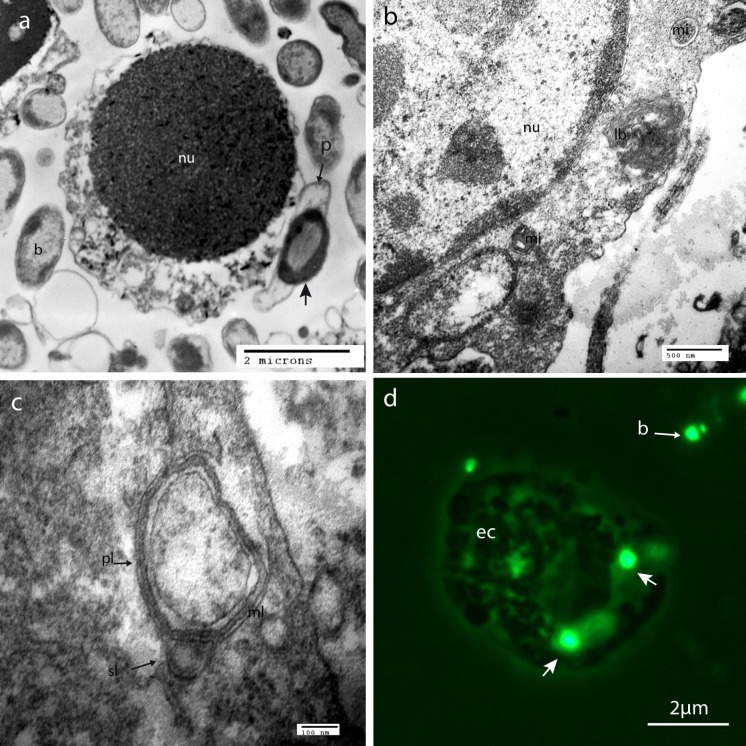
Bacterial phagocytosis by gill cells was investigated by TEM and fluorescent microscopy using GFP-tagged bacteria. Control cells did not display any bacteria, either on the outside or the inside of cells. After 2 h of contact with both bacterial strains, many bacteria were present around the cells while some of them appeared internalized within the host cells (Fig. 4). Some cells appeared to be damaged. Two cell morphotypes were able to phagocytose Vibrio: the hemocytes (Fig. 4a) and the epithelial cells (Fig. 4b). Figure 4b shows an encapsulated bacterium in the cytoplasm, residual body after digestion, and condensed mitochondria in an hyperphosphorylation oxydative state. Details of endocytosed bacteria were observed in epithelial cells, with lysosome I digesting bacteria and lysosome II dispensing their enzymatic content (Fig. 4c). Fluorescence microscopy observations with GFP-tagged bacteria allowed to localize V. harveyi bacteria very close to the host epithelial cells and potentially inside the host cell cytoplasm after 2 h of contact (Fig. 4d). Similar observations were made for the pathogenic ORM4 and the non-pathogenic LMG7890 bacterial strains.
Fig. 4.
Phagocytosis by Haliotis tubeculata gill cells after 2 h of contact observed by TEM and fluorescent microscopy. a Internalization of ORM4 bacteria by hemocytes, b cytoplasm of an epithelial cell showing internalized bacteria, lysosomal body and condensed mitochondria, c detail of lysosome digesting bacteria, d fluorescent micrograph showing ORM4-GFP tagged bacteria in close association with gill cell cytoplasm. b: bacteria around cells, ec: epithelial cell, lb: lysosomal body, pl: primary lysosome, sl: secondary lysosome, nu: nucleus, m: mitochondria, ml: lysosomal multilamellar membrane, p: pseudopodia of the phagocytosis vesicle, arrows: internal bacteria
Effect of V. harveyi on gill cell viability
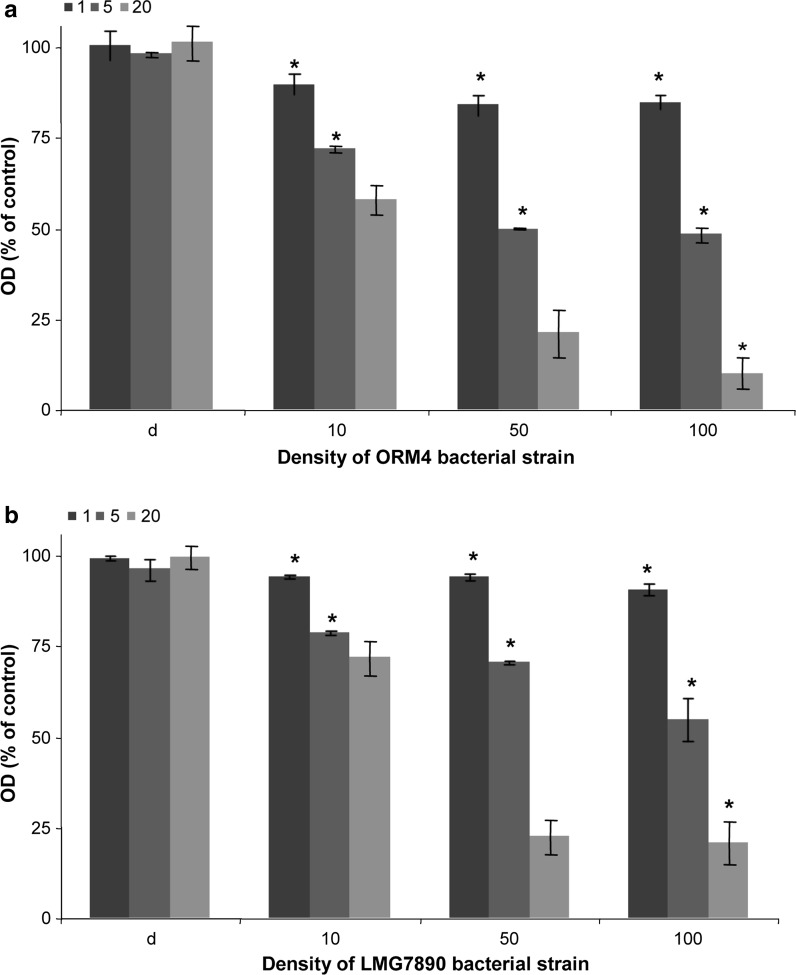
The effects of V. harveyi bacterial strain on gill cell metabolism were evaluated by the XTT assay (Fig. 5). Gill cells from 4-day-old subcultures in 96-well plates were exposed to increased concentrations of bacteria (heat inactivated bacteria and 10, 50 and 100 live bacteria per cell), and their XTT responses were measured up to 20 h, and compared to the metabolism activity of gill cell control. Figure 5 shows that the metabolic activity of gill cells decreased in the presence of the two bacterial strains from the first hour, whereas it remained constant for 20 h for control cells and cells supplemented with dead bacteria. The metabolic activity of gill cells was shown to significantly decrease as a function of bacterial concentration. At a concentration of 100 bacteria per cell XTT response of gill cells decreased to 90 % after 20 h of contact with the pathogen V. harveyi ORM4 and 80 % with the non pathogen LMG7890. A decrease of 50 % of viability was observed at a density of 50 bacteria per cell after 5 h of contact with ORM4, while this level was reached only after 20 h with LMG7890, even with a density of 100 bacteria per cell.
Fig. 5.
Effect of the pathogenic strain ORM4 (a) and the non-pathogenic LMG7890 strain (b) of V. harveyi on the metabolic activity of gill cells. XTT response from 4-day-old subcultures after 1, 5 and 20 h of contact with dead bacteria (D), and for ratio of 10, 50 and 100 bacteria/cell. Data are the mean ± SE of six values of two independent experiments. Significant differences (p < 0.05) from absorbances measured in control cells are indicated by an asterisk
Effect of V. harveyi on phenoloxidase activity
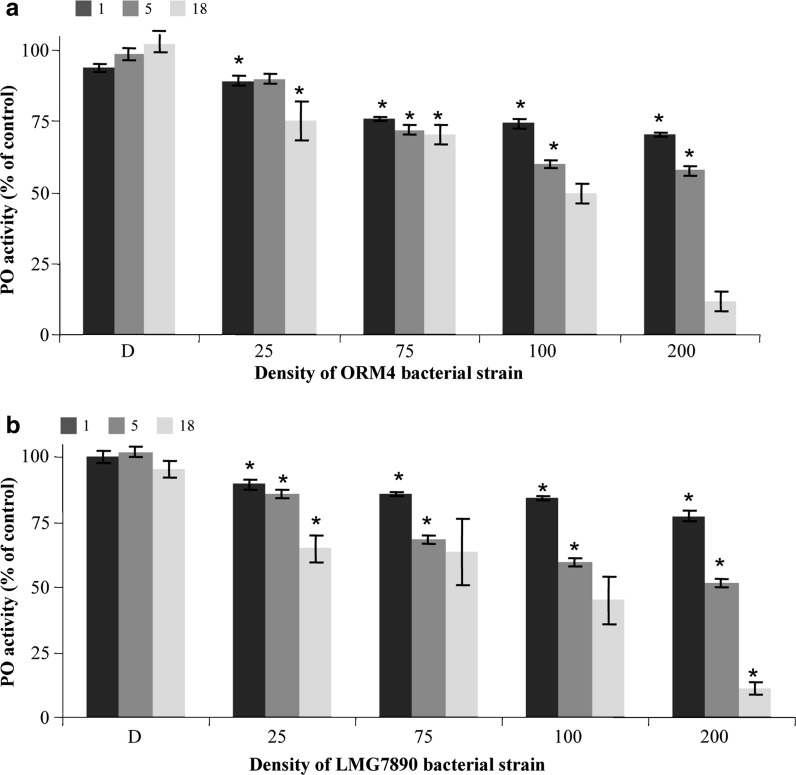
The immune response of gill cells exposed to bacteria was measured by the phenoloxidase activity, an enzyme strongly involved in the immune response. Phenoloxidase activity of gill cells remained stable for 18 h without bacterial strains or in presence of dead bacteria, but strongly decreased in a dose dependant manner in presence of the pathogenic and the non pathogenic V. harveyi, as shown in Fig. 6. For low concentrations (density of 25 bacteria per cell) phenoloxidase activity was slightly reduced during the first 5 h, but a significant decrease of phenoloxidase activity was shown after 18 h of contact. Phenoloxidase activity decreased after 5 h of contact, with a reduction of 40–60 % of phenoloxidase activity with a concentration of 100 and 200 bacteria per cell, respectively, for both strains compared to the control. Similar results were obtained for the pathogenic and the non pathogenic V. harveyi.
Fig. 6.
Effect of the pathogenic strain ORM4 (a) and the non-pathogenic LMG7890 strain (b) of V. harveyi on immune response of gill cells, measured by the phenoloxidase activity (PO) after 1, 5 and 18 h of contact with bacteria killed by boiling (D), and for ratio of 25, 75, 100 and 200 bacteria per cell. Data are the mean ± SE of three values of independent triplicate experiment. Significant differences (p < 0.05) from control cells are indicated by an asterisk
Discussion
In this work, we developed primary cell cultures from H. tuberculata gills, which are a target tissue for bacterial infection in marine mollusks. As previously observed in primary cultures of mollusc tissues, the explant method provided a suitable in vitro model that contained all the cell types present in the tissue of origin (Kleinschuster et al. 1996; Auzoux-Bordenave and Domart-Coulon 2010). Gill cells were successfully sub-cultured for up to 24 days with a persistent metabolic activity as demonstrated by the XTT assay. The decrease of mitochondrial activity over the first 4 days following subculture initiation may be attributed to the adaptation of cells to in vitro conditions. Abalone gill cell primary culture was first performed by Suwattana et al. (2010) on Haliotis asinina. These authors reported viable cell cultures for up to 28 days but observed many contaminations due to the initial presence of bacteria in the gills before culture. Contaminations by bacteria or fungi remain a real problem in primary cell culture, especially for tissues that are directly exposed to seawater such as the gills and the mantle (Rinkevich 1999; Van der Merwe et al. 2010). In the present work, the addition of antimicrobial components to the culture medium allowed to prevent bacterial and fungal proliferation in vitro without damaging gill cell viability.
Gill cell cultures exhibited the main morphotypes previously described in mollusc gills and hemocytes in vivo, i.e. epithelial cells, including glandular cells, and the two categories of hemocytes, i.e. hyalinocytes and granulocytes (Auzoux-Bordenave et al. 2007; Travers et al. 2008c; De Oliveira et al. 2008; Manganaro et al. 2012).
Because circulating hemocytes are the first target cells involved in the immune response of marine molluscs, previous studies mainly focused on the interactions between abalone hemocytes and Vibrio bacteria. In vitro immunity assays based on hemocyte cultures allowed to evidence the antibacterial activity of abalone hemocytes exposed to Vibrio species (Vakalia and Benkendorff 2005; Dang et al. 2011b; Travers et al. 2009). Significant variations of antibacterial activity of hemocytes were observed depending on environmental conditions such as temperature, quality of seawater, diet, or spawning period (Travers et al. 2008b; Mottin et al. 2010; Dang et al. 2011a, b). Furthermore, immune depression and antibacterial activity were reported in abalones affected by viruses or bacteria in hemolymph but also in the digestive gland (Travers et al. 2009; Dang et al. 2011a).
Since the gills are in direct contact with seawater, they represent a target tissue for the entrance and adhesion of bacteria. Moreover the mucus covering the gill filaments may attract or serve as nutrient for various microbes (Rosenberg and Falkovitz 2004; Sharon and Rosenberg 2008). Although the entrance routes of V. harveyi bacteria in abalone have not yet been identified, recent studies evidenced that the gills might be directly infected by bacteria (Travers et al. 2008a). In decapod crustacea, the gills have been shown as the main site of bacterial accumulation, suggesting a role of this tissue in the interactions with pathogens (Martin et al. 1993; Alday-Sanz 2002; Burgents et al. 2005).
Using cell cultures from gill tissues, the present work aimed at studying the interactions between V.harveyi bacteria and gill cells from the abalone (Haliotistuberculata). Flow cytometry analysis combined with microscopic observations evidenced that cultured gill cells were able to phagocyte both pathogenic and non pathogenic V. harveyi strains. Vibrios are known to produce compounds that inhibit host hemocyte responses and especially phagocytosis (Labreuche et al. 2006; Allam and Ford 2006). Furthermore, Vibrio bacteria develop rapidly in the presence of hemocytes and have been shown to interact directly with the hemocytes, preventing immunity and phagocytosis (Travers et al. 2009). Phagocytosis is one of the first lines of defence in molluscs and is mainly performed by hemocytes. Fluorescent microscopy and TEM analysis allowed the identification of internalized bacteria both within hemocytes and epithelial cells. The presence in infected cells of condensed mitochondria in hyperphosphorylation oxydative state demonstrated a high activity induced by bacterial phagocytosis. Furthermore, the identification of lysosomes within the host cells, provided further evidence of bacterial endocytosis in vitro. Lysosomes, which are involved in the degradation of the bacterial cell wall, play a crucial role during phagocytosis by secreting hydrolytic enzymes. The use of lysosomes as biomakers to assess the cytotoxicity of external contaminants has been previously reported on abalone hemocytes (Latire et al. 2012).
The gill cell capacity of phagocytosis, measured by flow cytometry was beyond 80 %. Only after 2 h of contact with the two bacterial strains, gill cells were able to phagocyte 70 % of bacteria. Travers et al. (2009) reported an increase in the capacity of hemocytes to phagocyte fluorescent beads in the presence of the non-pathogenic LMG7890 bacterial strain. In the same way, we observed that the phagocytic ability of gill cells was higher in the presence of the non-pathogenic strain compared to the pathogenic one. Despite the ability of abalone cells to phagocyte V. harveyi, our study evidenced that, both, viability and immune response of gill cells were directly affected by the two bacterial strains. Although the LMG7890 strain is not pathogenic for abalones in vivo, Travers et al. (2009) reported that a direct injection could induce mortalities, but to a lower extent compared to the ORM4 strain. In this study, the presence of pathogenic and non-pathogenic bacteria significantly decreased the cell viability in a concentration dependent manner, as measured by the metabolic activity assay (XTT). The sharp decrease of cell viability observed in the presence of ORM4 bacteria confirmed the virulence of this strain against abalone gill cells. These results differed from those obtained in abalone hemocytes, showing that ORM4 bacterial strain had no significant effect on hemocytes viability, even after 28 h of contact (Travers et al. 2009).
In addition to the effects on global cell metabolism, our results demonstrated that the two bacterial V. harveyi strains induced a significant decrease of the phenoloxidase activity in vitro. Phenoloxidase has been previously detected in the hemolymph of several invertebrates and its activity has been shown to be directly involved in the immune response (Coles and Pipe 1994; Lacoue-Labarthe et al. 2009). Recent studies reported the use of phenoloxidase activity to measure the immune response of abalone hemocytes exposed to various environmental stressors such as temperature, salinity, trace metals and pathogens (Cheng et al. 2004a, b; Travers et al. 2008b; Mottin et al. 2010; Dang et al. 2011b). Since hemocytes are very abundant in gill tissues, the phenoloxidase activity measured in vitro may be partly due to the hemocytes. Nevertheless, our results are consistent with previous studies using phenoloxidase activity as a marker to measure the immune response of mollusc to external contaminants (Bachère et al. 1995; Hellio et al. 2007).
In this study, we evidenced that primary cultures of abalone gills provide suitable models for in vitro pathogenicity assays. This work demonstrates for the first time that V. harveyi bacteria induced significant alterations on gill cells viability and immune responses, although important phagocytic activity was detected in these target cells. Further studies using other immune indicators such as ROS activity, combined with lysosome quantification in target cells will help specify the mode of action of V. harveyi on abalone cells.
Acknowledgments
This work was supported by ECC (SUDEVAB N°222156 “Sustainable development of European SMEs engaged in abalone aquaculture”) and the Brittany region (Programme “Ormeaux”, Pôle Mer Bretagne). We thank Marion Cardinaud for the construction of the strain LMG7890-GFP tagged that she kindly provided for in vitro biotests on gill cells.
References
- Alday-Sanz V. Clearing mechanisms on Vibrio vulnificus biotype I in the black tiger shrimp Penaeus monodon. Dis Aquat Org. 2002;48:91–99. doi: 10.3354/dao048091. [DOI] [PubMed] [Google Scholar]
- Allam B, Ford SE. Effects of the pathogenic Vibrio tapetis on defence factors of susceptible an non-susceptible bivalve species. 1. Haemocyte changes following in vitro challenge. Fish Shellfish Immunol. 2006;20:374–383. doi: 10.1016/j.fsi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Allam B, Paillard C, Ford SE. Pathogenicity of Vibrio tapetis, the etiologic agent of browning disease in clams. Dis Aquat Org. 2002;48:221–231. doi: 10.3354/dao048221. [DOI] [PubMed] [Google Scholar]
- Austin B, Zhang ZH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol. 2006;43:119–124. doi: 10.1111/j.1472-765X.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Auzoux-Bordenave S, Domart-Coulon I (2010) Marine invertebrate cell cultures as tools for biomineralization studies. J Sci Hal Aquat 2:42–47
- Auzoux-Bordenave S, Fouchereau-Peron M, Helleouet M-N, Doumenc D. CGRP regulates the activity of mantle cells and hemocytes in abalone primary cell cultures (Haliotis tuberculata) J Shellfish Res. 2007;26:887–894. doi: 10.2983/0730-8000(2007)26[887:CRTAOM]2.0.CO;2. [DOI] [Google Scholar]
- Azevedo C, Balseiro P, Casal G, Gestal C, Aranguren R, Stokes NA, Carnegie RB, Novoa B, Burreson EM, Figueras A. Ultrastructural and molecular characterization of Haplosporidium montfortin. sp., parasite of the European abalone Haliotis tuberculata. J Invertebr Pathol. 2006;92:23–32. doi: 10.1016/j.jip.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bachère E, Mialhe E, Noël D, Boulo V, Morvan A, Rodriguez J. Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture. 1995;132:17–32. doi: 10.1016/0044-8486(94)00389-6. [DOI] [Google Scholar]
- Balseiro P, Aranguren R, Gestal C, Novoa B, Figueras A. Candidatus Xenohaliotis californiensis and Haplosporidium montforti associated with mortalities of abalone Haliotis tuberculata cultured in Europe. Aquaculture. 2006;258:63–72. doi: 10.1016/j.aquaculture.2006.03.046. [DOI] [Google Scholar]
- Boettcher KJ, Barber BJ, Singer JT. Use of antibacterial agents to elucidate the etiology of Juvenile oyster disease (JOD) in Crassostrea virginica and numerical dominance of an a-proteobacterium in JOD-affected animals. Appl Environ Microbiol. 1999;65:2534–2539. doi: 10.1128/aem.65.6.2534-2539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Barber BJ, Singer JT. Additional evidence that juvenile oyster disease is caused by a member of the Roseobacter group and colonization of nonaffected animals by Stappiastellulata_like strains. Appl Environ Microbiol. 2000;66:3924–3930. doi: 10.1128/AEM.66.9.3924-3930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulo V, Hervio D, Morvan A, Bachere E, Mialhe E (1991) In vitro culture of mollusc hemocytes. Functional study of burst respiratory activity and analysis of interactions with protozoan and procaryotic pathogens. In Vitro 27:56–64
- Bradford MM. A refined and sensitive method for quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgents JE, Burnett LE, Stabb EV, Burnett KG. Localization and bacteriostasis of Vibrio introduced into the Pacific white shrimp, Litopenaus vannamei. Dev Comp Immunol. 2005;29:681–691. doi: 10.1016/j.dci.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Lee B, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Cheng TC. Bivalves. In: Ratcliffe NA, Rowley AF, editors. Invertebrate blood cells. London: Acadameic Press; 1981. pp. 233–299. [Google Scholar]
- Cheng W, Hsiao IS, Hsu CH, Chen JC. Change in water temperature on the immune response of Taiwan abalone Haliotisdiversicolorsupersexta and its susceptibility to Vibrioparahaemolyticus. Fish Shellfish Immunol. 2004;17:235–243. doi: 10.1016/j.fsi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Cheng W, Juang FM, Chen JC. The immune response of Taiwan abalone Haliotisdiversicolorsupersexta and its susceptibility to Vibrioparahaemolyticus at different salinity levels. Fish Shellfish Immunol. 2004;16:295–306. doi: 10.1016/S1050-4648(03)00111-6. [DOI] [PubMed] [Google Scholar]
- Coles JA, Pipe RK. Phenoloxidase activity in the hemolymph and hemocytes of the marine mussel Mytilus edulis. Fish Shellfish Immunol. 1994;4:337–352. doi: 10.1006/fsim.1994.1030. [DOI] [Google Scholar]
- Colwell RR (1982) http://bccm.belspo.be/about/lmg.php
- Dang VT, Speck P, Doroudi M, Bekendorff K. Variation in the antiviral and antibacterial activity of abalone Haliotis laevigata, H. rubra and their hybrid in South Australia. Aquaculture. 2011;315:242–249. doi: 10.1016/j.aquaculture.2011.03.005. [DOI] [Google Scholar]
- Dang VT, Li Y, Speck P, Benkendorff K. Effects of micro and macroalgal diet supplementations on growth and immunity of greenlip abalon, Haliotis laevigata. Aquaculture. 2011;320:91–98. doi: 10.1016/j.aquaculture.2011.08.009. [DOI] [Google Scholar]
- De Oliveira DJA, Salaroli RB, Fontanetti CS. Fine structure of Mytella falcata (Bivalvia) gill filaments. Micron. 2008;39:329–336. doi: 10.1016/j.micron.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Domart-Coulon I, Auzoux-Bordenave S, Doumenc D, Khalanski M. Cytotoxicity assessment of antibiofouling compounds and by-products in marine bivalve cell cultures. Toxicol In Vitro. 2000;14:245–251. doi: 10.1016/S0887-2333(00)00011-4. [DOI] [PubMed] [Google Scholar]
- Faucet J, Maurice E, Gagnaire B, Renault T, Burgeot T. Isolation and primary culture of gill and digestive gland cells from the common mussels Mytilus edulis. Methods Cell Sci. 2003;23:177–184. doi: 10.1007/s11022-004-8227-4. [DOI] [PubMed] [Google Scholar]
- Ford SE, Ashton-Alcox SA. In vitro interactions between bivalve hemocytes and the oyster pathogen Haplosporidium nelsoni (MSX) J Parasitol. 1993;79:255–265. doi: 10.2307/3283516. [DOI] [Google Scholar]
- Gauger EJ, Gomez-Chiarri M. 16S ribosomal DNA sequencing confirms the synonymy of Vibrioharveyi and V.carchariae. Dis Aquat Organ. 2002;52:39–46. doi: 10.3354/dao052039. [DOI] [PubMed] [Google Scholar]
- Gaume B, Bourgougnon N, Auzoux-Bordenave S, Roig B, Le Bot B, Bedoux G. In vitro effects of triclosan and methyl-triclosan on the marine gastropod Haliotis tuberculata. Comp Biochem Physiol C. 2012;156:87–94. doi: 10.1016/j.cbpc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Handlinger J, Carson J, Donachie L, Gabor L, Taylor D (2005) Bacterial infection in Tasmanian farmed abalone: causes pathology, farm factors and control options. In: Walker P, Lester R, Bondad Reantaso MG (eds) Proceedings of the 5th symposium on diseases in Asian aquaculture, Australia, pp 289–300
- Hellio C, Bado-Nilles A, Gagnaire B, Renault T, Thomas-Guyon H. Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in pacific oyster, Crassostreagigas (Thunberg) in vitro. Fish Shellfish Immunol. 2007;22:433–440. doi: 10.1016/j.fsi.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Hooper C, Day R, Slocombe R, Handlinger J, Benkendorff K. Stress and immune responses in abalone: limitations in current knowledge and investigative methods based on other models. Fish Shellfish Immunol. 2007;22:363–379. doi: 10.1016/j.fsi.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huchette SMH, Clavier J. Status of the ormer (Haliotis tuberculata L.) industry in Europe. J Shellfish Res. 2004;23:951–955. [Google Scholar]
- Kleinschuster SJ, Parent J, Walter CW, Farley CA. A cardiac cell line from Mya arenaria (Linneaus, 1759) J Shellfish Res. 1996;15:695–707. [Google Scholar]
- Labreuche Y, Soudant P, Goncalves M, Lambert C, Nicolas JL. Effects of extracellular products from the pathogenic Vibrio aestuarianus strain 01/32 on lethality and cellular immune responses of the oyster Crassostrea gigas. Dev Comp Immunol. 2006;30:367–379. doi: 10.1016/j.dci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lacoue-Labarthe T, Bustamante P, Horlin E, Luna-Acosta A, Bado-Nilles A, Thomas-Guyon H. Phenoloxidase activation in the embryo of the common cuttlefish Sepia officinalis and responses to the Ag and Cu exposure. Fish Shellfish Immunol. 2009;27:516–521. doi: 10.1016/j.fsi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Latire T, Le Pabic C, Mottin E, Mottier A, Costil K, Koueta N, Lebel JM, Serpentini A. Responses of primary cultured haemocytes from the marine gastropod Haliotis tuberculata under 10-day exposure to cadmium chloride. Aquatic Toxicol. 2012;109:213–221. doi: 10.1016/j.aquatox.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Lauckner G (1983) Diseases of Mollusca: Bivalvia. In: Kinne O (ed) Diseases of Marine animals. Biologische Anstalt Helgoland, Hamburg, pp 477–963
- Le Roux F, Gay M, Lambert C, Waechter M, Poubalanne S, Chollet B, Nicolas JL, Berthe F. Comparative analysis of Vibrio splendidus-related strains isolated during Crassostrea gigas mortality events. Aquat Living Resour. 2002;15:251–258. doi: 10.1016/S0990-7440(02)01176-2. [DOI] [Google Scholar]
- Manganaro M, Laura R, Guerrera MC, Lanteri G, Zaccone D, Marino F. The morphology of gills of Haliotis tuberculata (Linnaeus, 1758) Acta Zoologica. 2012;93:436–443. doi: 10.1111/j.1463-6395.2011.00518.x. [DOI] [Google Scholar]
- Martin GG, Poole D, Poole C, Hose JE, Arias M, Reynolds L, McKrell N, Whang A. Clearance of bacteria injected into the haemolymph of the penaeid shrimp, Syconia ingentis. J Invert Pathol. 1993;62:308–315. doi: 10.1006/jipa.1993.1118. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mottin E, Caplat C, Mahaut ML, Costil K, Barillier D, Lebel JM, Serpentini A. Effect of in vitro exposure to zinc on immunological parameters of haemocytes from the marine gastropod Haliotis tuberculata. Fish Shellfish Immunol. 2010;29:846–853. doi: 10.1016/j.fsi.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Nicolas JL, Basuyaux O, Mazurie J, Thebault A. Vibrio carchariae, a pathogen of the abalone Haliotis tuberculata. Dis Aquat Org. 2002;50:35–43. doi: 10.3354/dao050035. [DOI] [PubMed] [Google Scholar]
- Nishimori E, Hasegawa O, Numata T, Wakabayashi H. Vibrio carchariae causes mass mortalities in Japanes abalone, Sulculus diversicolor supratexta. Fish Pathol. 1998;33:495–502. doi: 10.3147/jsfp.33.495. [DOI] [Google Scholar]
- Paillard C, Maes P. Origine pathogène de l’”anneau brun” chez Tapes philippinarum (Mollusque, bivalve) C R Acad Sci Paris Ser III. 1989;309:235–241. [Google Scholar]
- Paillard C, Leroux F, Borrego JJ. Bacterial diseases in marine bivalves: review of recent studies. Trends Evol Aquat Liv Resour. 2004;17:477–498. doi: 10.1051/alr:2004054. [DOI] [Google Scholar]
- Rinkevich B. Cell cultures from marine invertebrates: obstacles, new approaches and recent improvements. J Biotechnol. 1999;70:133–153. doi: 10.1016/S0168-1656(99)00067-X. [DOI] [Google Scholar]
- Rinkevich B. Cell cultures from marine invertebrates: new insights for capturing endless stemness. Mar Biotechnol. 2011;13:345–354. doi: 10.1007/s10126-010-9354-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Falkovitz L. The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annu Rev Microbiol. 2004;58:143–159. doi: 10.1146/annurev.micro.58.030603.123610. [DOI] [PubMed] [Google Scholar]
- Saulnier D, De Decker S, Haffner P, Cobret L, Robert M, Garcia C (2010) A large-Sscale epidemiological study to identify bacteria pathogenic to pacific oyster Crassostrea gigas and correlation between virulence and etalloprotease-like activity. Microb Ecol 59:787–798 [DOI] [PubMed]
- Sharon G, Rosenberg E. Bacterial growth on coral mucus. Curr Microbiol. 2008;56:481–488. doi: 10.1007/s00284-008-9100-5. [DOI] [PubMed] [Google Scholar]
- Smith VJ. Invertebrate immunology: phylogenetic, ecotoxicological and biomedical implications. Comp Haematol Int. 1991;1:60–76. doi: 10.1007/BF00422876. [DOI] [Google Scholar]
- Suwattana D, Jirasupphachok J, Jarayabhand P, Koykul W. In vitro culture of gill and heart tissues of the abalone Haliotis asinina. J Shellfish Res. 2010;29:651–653. doi: 10.2983/035.029.0314. [DOI] [Google Scholar]
- Travers MA, Barbou A, Le Goic N, Huchette S, Paillard C, Koken M. Construction of a stable GFP-tagged Vibrioharveyi strain for bacterial dynamics analysis of abalone infection. FEMS Microbiol Lett. 2008;289:34–40. doi: 10.1111/j.1574-6968.2008.01367.x. [DOI] [PubMed] [Google Scholar]
- Travers MA, Le Goïc N, Huchette Skoken M, Paillard C. Summer immune depression associated with increased susceptibility of the European abalone Haliotistuberculata to Vibrioharveyi infection. Fish Shellfish Immunol. 2008;25:800–808. doi: 10.1016/j.fsi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Travers MA, Da Silva PM, Le Goïc N, Marie D, Donval A, Huchette SMH, Koken M, Paillard C. Morphologic, cytometric and functional characterization of abalone (Haliotis tuberculata) hemocytes. Fish Shell Immunol. 2008;24:400–411. doi: 10.1016/j.fsi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Travers MA, Le Bouffant R, Friedman CS, Buzin F, Cougard B, Huchette S, Koken M, Paillard C. Pathogenic Vibrio harveyi, in contrast to non pathogenic strains, intervenes with the p38 MAPK pathway to avoid an abalone haemocyte immune response. J Cell Biochem. 2009;106:152–160. doi: 10.1002/jcb.21990. [DOI] [PubMed] [Google Scholar]
- Vakalia S, Benkendorff K (2005) Antimicrobial activity in the haemolymph of Haliotis laevigata and the effects of a dietary immunostimulant. In: Fleming AE (ed) Proceedings of the 12th annual Abalone Aquaculture Workshop. McLarren Vale, Adelaide, Australia. Abalone Aquaculture Subprogram, Fisheries Research and Development Corporation, Canberra, Australia, pp. 29–36
- Van der Merwe M, Auzoux-Bordenave S, Niesler C, Roodt-Wilding R. Investigating the establishment of primary cell culture from different abalone (Haliotis midae) tissues. Cytotechnol. 2010;62:265–277. doi: 10.1007/s10616-010-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]