Abstract
Microcell-mediated chromosome transfer (MMCT) technology enables a single and intact mammalian chromosome or megabase-sized chromosome fragments to be transferred from donor to recipient cells. The conventional MMCT method is performed immediately after the purification of microcells. The timing of the isolation of microcells and the preparation of recipient cells is very important. Thus, ready-made microcells can improve and simplify the process of MMCT. Here, we established a cryopreservation method to store microcells at −80 °C, and compared these cells with conventionally- (immediately-) prepared cells with respect to the efficiency of MMCT and the stability of a human artificial chromosome (HAC) transferred to human HT1080 cells. The HAC transfer in microcell hybrids was confirmed by FISH analysis. There was no significant difference between the two methods regarding chromosome transfer efficiency and the retention rate of HAC. Thus, cryopreservation of ready-to-use microcells provides an improved and simplified protocol for MMCT.
Keywords: Chromosome, Human artificial chromosome, Microcell-mediated chromosome transfer, HAC, Cancer, Synthetic biology, Gene delivery
Introduction
Conventional gene transfer techniques using plasmids, viral vectors, P1 phage-derived artificial chromosomes (PACs), bacterial artificial chromosomes (BACs) and yeast artificial chromosomes (YACs) are limited to a loading capacity of under several hundreds kbp. In order to achieve physiological regulation, a genomic fragment of greater than several hundreds kbp that includes a gene and its promoter region is generally required (O’Connor and Crystal 2006). Human artificial chromosomes (HACs) are exogenous mini-chromosomes artificially created by chromosome engineering technology in DT40 cells (Kazuki and Oshimura 2011). Various HACs exhibit certain characteristics of an ideal gene delivery vector, including stable episomal maintenance and the capacity to carry large genomic loci with their regulatory elements, thus allowing the physiological regulation of the introduced gene in a manner similar to that of native chromosomes (Kazuki and Oshimura 2011). We have previously reported the construction of DYS-HAC, which contained a complete 2.4 Mbp genomic dystrophin region including the region that regulates physiological functions, for use in gene therapy (Kazuki et al. 2010; Hoshiya et al. 2009). The DYS-HAC was transferred to fibroblasts derived from a patient with Duchenne Muscular Dystrophy, and the cells were induced to become induced pluripotent stem cells (iPSCs). Thus, the iPS cells were completely corrected for the defect, as expected from the properties of the HAC. In addition, multiple acceptor sites with different integrases were introduced into the HAC for the simultaneous delivery of multiple genes (Yamaguchi et al. 2011). Thus, HACs can be applied to basic and translational research, i.e., gene function (Kim et al. 2011), protein expression (Kurosaki et al. 2011; Tomizuka et al. 1997), iPS production (Hiratsuka et al. 2011; Kakeda et al. 2011), animal transgenesis (Shinohara et al. 2001; Tomizuka et al. 2000) and research into centromeric function (Kouprina et al. 2013).
Human artificial chromosomes for genetic correction or modification have been transferred to various cell types, e.g., iPSCs and Mesenchemal stem cells (MSCs), by fusing microcells with the recipient cells. Polyethylene glycol (PEG) has conventionally been used as a fusogen, but it is not suited to all types of cells because it is cytotoxic to some cell types. The colony efficiency of fusion between microcells and recipient cells is about 1 × 10−6 to 5 × 10−5 (Kazuki and Oshimura 2011). Measles virus fusogen envelope proteins, i.e., hemaglutinin (H) and fusion (F) proteins were expressed on the surface of microcells (Katoh et al. 2010). When these proteins were used to mediate the fusion of microcells with recipient cells, the cytotoxicity was reduced and the efficiency of MMCT was improved to 1 × 10−4.
The conventional MMCT method is performed immediately after the purification of microcells. The timing of the isolation of microcells and the preparation of recipient cells is very important. Thus, ready-to-use microcells can improve the process and the efficiency of MMCT. Microcell hybrids can be obtained by using measles viral fusogen or PEG because they have a lipid bilayer membrane, and the microcells may be manipulated and stored in the same way as normal cells.
In this study, we tested the feasibility of storing microcells in the deep freezer at −80 °C for convenience of use.
Materials and methods
Cell culture
HT1080 and A9 cells were grown in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO, USA) supplemented with 10 % fetal bovine serum (BioWest, Nuaillé, France). CHO cells were grown in Ham’s F-12 medium (Sigma) supplemented with 10 % fetal bovine serum.
Microcell-mediated chromosome transfer
The day before microcell fusion, recipient HT1080 cells were trypsinized and counted. A given number of recipient cells (1 × 106) was plated in 60 mm diameter culture dishes. Donor CHO cells grown in T-25 flasks (Thermo, Waltham, MA, USA) were treated with 0.1 μg/ml colcemid (Gibco, Carlsbad, CA, USA) for 72 h to induce micronuclei formation. Flasks were filled with medium containing 10 μg/ml cytochalasin B (Sigma) and centrifuged for 1 h at 8,000 rpm (11,899×g) in a JLA-10.5 rotor (Beckman, Brea, CA, USA). Pellets containing a crude microcell preparation were recovered and passed through membranes of 8-, 5-, and 3-μm pore size (Whatman, Springfield Mill, UK). Collected microcell preparations were used for fusion with recipient cells. For microcell fusion by measles virus fusogens, an aliquot of microcells prepared from CHO4H6.1M was overlaid on recipient cells and left for 24 h. The cells were then trypsinized, sparsely replated, and cultured for 14 days in medium containing 8 μg/ml.
Microcell cryopreservation and thawing condition
An aliquot of the prepared microcells was cryopreserved in a cell stock solution of 40 % fetal bovine serum, 10 % dimethyl sulfoxide (Sigma) in Dulbecco’s modified Eagle’s medium, which we normally use for the cryopreservation of whole cells.
Upon thawing, cryopreserved microcells were incubated at 37 °C for 1 min in water bath. Next, these microcells were resuspended in 10 mL serum-free Dulbecco’s modified Eagle’s medium and centrifuged at 2,000 rpm for 10 min. This washing process was repeated one more time. Then, these microcells were resuspended in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum to co-culture with recipient cells.
FISH analysis
Metaphase chromosomes were prepared from colcemid-treated cell cultures by hypotonic treatment with 0.075 M KCl and methanol/acetate (3:1) fixation. Fluorescence in situ hybridization was carried out using the p11-4 alphoid DNA probe labeled with digoxigenin (Roche, Basel, Switzerland) (Ikeno et al. 1994). Hybridization of the probe and immunochemical staining were performed using a Ventana XT-Discovery system (Roche). The digoxigenin signal was detected with an anti-digoxigenin-rhodamine complex (Roche). The chromosomes were counterstained with DAPI (Sigma).
Photographs were taken using a CCD camera mounted on a fluorescence microscope (Zweiss, Oberkochen, Germany). Images were processed using the software provided with the microscope.
Giemsa staining
Drug-resistant colonies were fixed in methanol for 5 min. Giemsa staining solution was prepared at a concentration of 5 % Giemsa (MERCK, Darmstadt). The fixed samples were soaked in the Giemsa staining solution for 15 min. After staining, the samples were washed with tap water.
Results
Comparing the efficiency of MV-MMCT with cryopreserved MMCT
The strategy is outlined in Fig. 1. Previously, we reported a new method using the measles virus fusogen (MV-MMCT) (Katoh et al. 2010), which is more effective than PEG fusion for MMCT. Thus, in this study, the MV-MMCT method was applied instead of PEG fusion. A CHO cell line containing the introduced MV-H and F protein genes and 21HAC2 (Kazuki et al. 2011), namely CHO 4H6.1M, was used. 21HAC2 contains the enhanced green fluorescent protein (EGFP) and the blasticidin (Bsd) genes. When the CHO 4H6.1M cells were treated with colcemid, micronuclei were formed in CHO cells (Koi et al. 1989). The microcells were isolated from the treated CHO 4H6.1M cells by centrifugation. These microcells were fused with HT1080 cells in order to examine the effect of cryopreservation on MMCT.
Fig. 1.
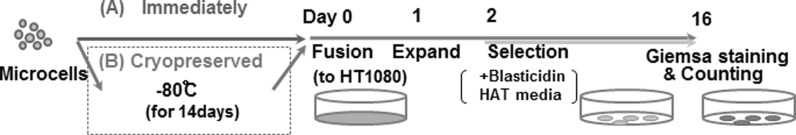
Schematic time schedule for comparing conventional and cryopreserved MMCT. Microcells were isolated from CHO cells in which measles virus envelope protein was expressed. The isolated microcells were divided into two aliquots to use with each method. For conventional MMCT, the microcells were co-cultured with HT1080 cells immediately. For cryopreserved MMCT, the microcells were frozen as a stock solution at −80 °C for 2 weeks, following which they were thawed and co-cultured with HT1080 cells. Microcells and HT1080 cells fused spontaneously via the MV envelope. The time at which culture of the microcells and HT1080 cells was started was designated as Day 0. Microcell hybrid clones were selected in medium containing BS and HAT for 14 days, following which they were isolated and analyzed by FISH. The number of GFP-positive clone was counted for the calculation of transfer efficiency
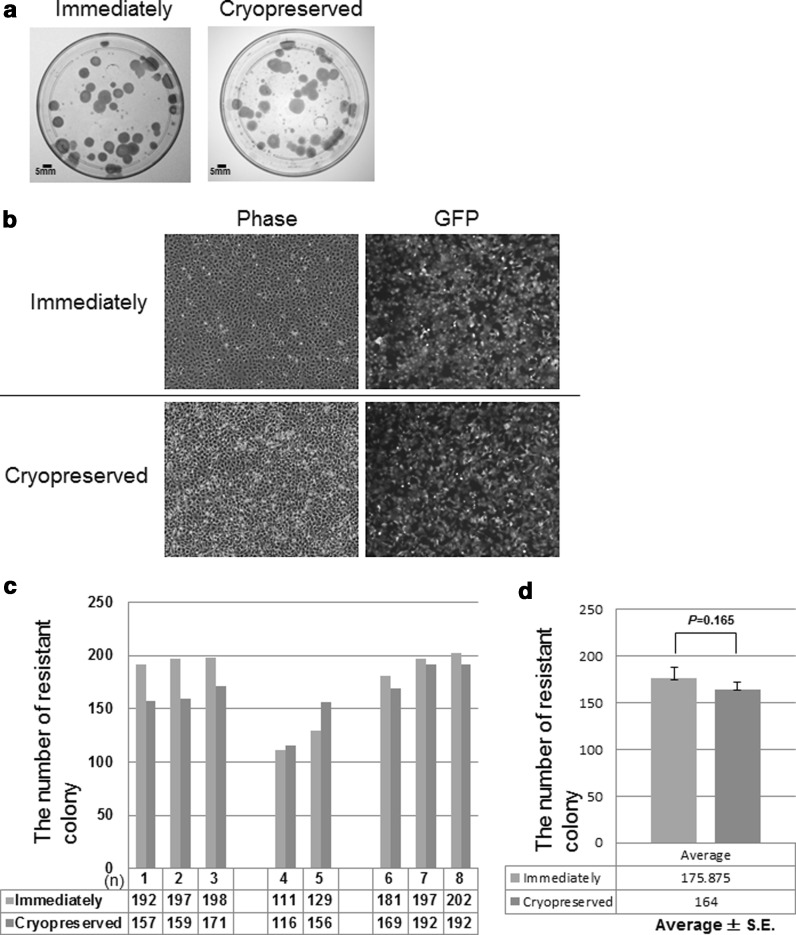
As a first step, we collected microcells from 7.2 × 107 CHO 4H6.1M cells. The collected microcells was divided equally. Half of the cells was fused with HT1080 cells immediately after collection, i.e., using the conventional immediate method. The other half was cryopreserved for 2 weeks at −80 °C in medium containing 50 % FCS and 10 % DMSO. After 2 weeks of storage, the microcells were thawed and co-cultured with 2 × 106 HT1080 cells. The cells were co-cultured and selected in DMEM containing blasticidin S and HAT for 2 weeks. Because the transferred 21HAC2 was tagged with Bsd and EGFP, microcell hybrids containing 21HAC2 were expected to be positive for EGFP. Fifteen and seventeen colonies from each condition were randomly picked and propagated for the following analysis. The remaining colonies were stained with Giemsa dye (Fig. 2a). These established clones were grown in the presence of blasticidin and stably expressed EGFP (Fig. 2b).
Fig. 2.
The efficiency of MMCT was similar using the conventional and cryopreservation methods. a Following selection in culture for 14 days, drug-resistant colonies were stained with Giemsa and photographed. Each technique produced about 40 colonies in a 100 mm dish. The scale bar shows 5 mm. b The frequency of GFP-positive cells obtained by each method. The conventional method (upper row) and the cryopreserved method (lower row) are shown. Phase-contrast (left), fluorescence (right) are shown. c The efficiency of MMCT was determined by counting the GFP-positive colonies formed in each experiment (n). The bars represent the number of colonies from cell fusion according to the conventional method (gray) or the cryopreservation method (blue). d The average number of EGFP–positive colonies obtained by immediate and cryopreserved methods from Fig 2c and expressed as the mean ± S.E. Student’s t test was used to determine the statistical difference. No significant difference was observed (P = 0.165). (Color figure online)
Karyotypes of the obtained clones by both MMCT methods are similar
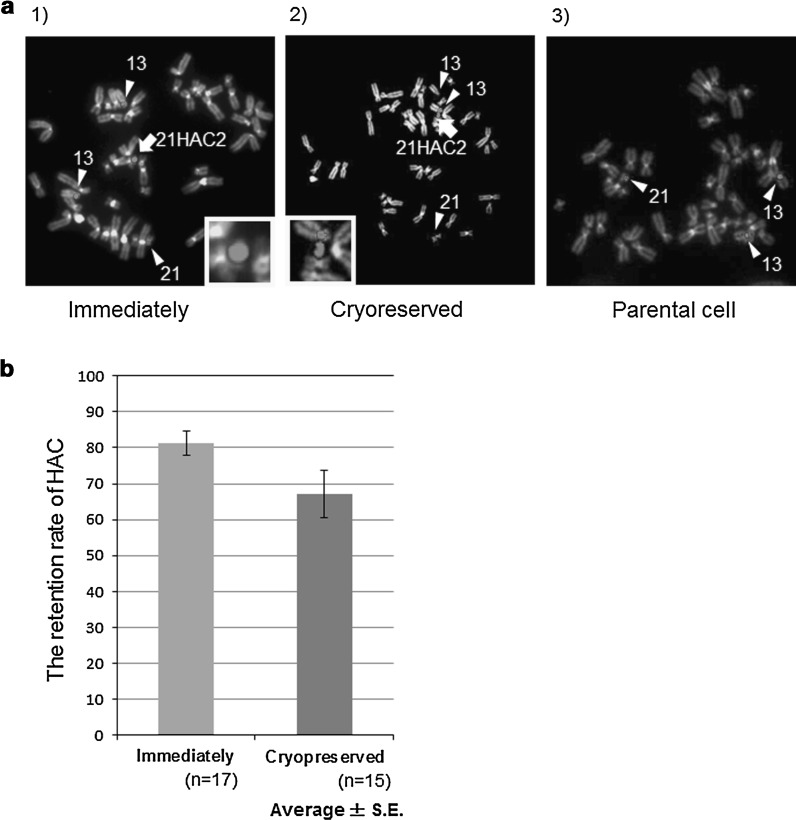
In the MV-MMCT experiment, the chromosome transfer efficiency was compared between the two microcell preparation methods, i.e., the immediate preparation method and the cryopreservation method. Giemsa stained positive colonies were counted (Fig. 2c). In total, when the average efficiency for each method was compared, no significant difference in fusion efficiency was observed. Thus, the results indicated that transfer of the HAC vector from the cryopreserved CHO microcells to HT1080 cells was as efficient as from CHO microcells prepared by the direct method (Fig. 2d). Next, in order to confirm the presence of the HAC in the drug-resistant and EGFP-positive cells, we examined clones from immediately (n = 17) or cryopreserved (n = 15) condition by FISH analysis (Fig. 3a). In addition to endogenous chromosomes 21 and 13, the alphoid satellite probe hybridized with a minichromosome not present in the parental HT1080 cells (Fig. 3a 1–3). In comparison to endogenous chromosome 21, the minichromosome matched the predicted size of the HAC (Kazuki et al. 2011) (Fig. 3a 1, 2). In parental HT1080 cells, the alphoid probe detected a pair of endogenous chromosome 13′s and a single chromosome 21 (Fig. 3a 3). The HT1080 cells are immortalized and possess an aberrant chromosome number and undergo frequent polyploidy (Katoh et al. 2010). FISH analysis showed that 21HAC2 was present as an individual chromosome in all of the analyzed HT1080 clones (Fig 3a 1, 2). Apparent and gross chromosome aberrations, such as translocations and insertions of host chromosomes or the HAC vector, were not observed with either the conventional or cryopreservation methods (Fig. 3a 1–3). Insertion of 21HAC2 into a host HT1080 chromosome occurred in only one in 600 cells. Next, we determined the retention rate of the HAC vector in these clones from immediately (n = 17) or cryopreserved (n = 15) condition. Twenty cells in metaphase were analyzed, and the retention rate of the HAC vector was calculated. A considerable number of analyzed clones showed a high retention rate of the HAC vector in both the methods (Fig. 3b). These data suggested that the cryopreservation method did not significantly affect the stability of the HAC vector.
Fig. 3.
Identical karyotypes were observed in microcell hybrid clones generated with both methods. (a 1–3) Fluorescence in situ hybridization (FISH) analysis of the microcell hybrids generated by the conventional and cryopreservation methods. Arrows indicate 21HAC2 during metaphase. Arrowheads indicate chromosome 13 or 21. Cells were probed with human alphoid satellite DNA (red). Insets exhibit enlarged 21HAC2. b Mitotic stability of 21HAC2 in microcell hybrid clones generated by the conventional (gray) and cryopreservation (blue) methods. Mann–Whitney test was used to determine the statistical difference. No significant difference was observed (P > 0.05). (Color figure online)
Additional experiments showed that the cryopreservation method was also suitable for MMCT using another donor cell (A9; mouse fibro sarcoma cell). Seventy-four colonies of HAC transferred EGFP-positive clone were obtained with 2 × 106 cells of HT1080 as recipient cells using the PEG MMCT method.
Discussion
We aimed to determine whether microcell fusion could be achieved using a cryopreservation method. We showed that storage of microcells at −80 °C enables microcells to be preserved prior to use. This method has several advantages over the immediate method for chromosome transfer. First, a large number of microcells can be collected from one experiment, thus improving the efficiency and convenience of MMCT. Second, cryopreserved microcells can be transported to other laboratories, even in other countries. Somatic stem cells, in particular, are very difficult to establish. Therefore, cryopreserved microcells can be transported to the appropriate laboratory for subsequent manipulation. This is important because HACs are powerful tools for gene delivery in a variety of applications.
Another issue to consider when using the cryopreservation method for MMCT is whether or not gene expression is altered by mutations or silencing. As models of transgenes, the blasticidin-resistance and EGFP genes were introduced and expressed correctly. Thus, the cryopreservation method does not result in genomic disruption, meaning that the method can be widely used. In comparison with a previous report, the efficiency of chromosome transfer was confirmed in 2 × 106 recipient cells, with a transfer efficiency of 0.01–0.005 % (Katoh et al. 2010). MMCT using MV membrane proteins provided reproducible results. Therefore, this MMCT method resolved the practical difficulties and transfer efficiency disparities of the PEG fusion method.
In this study, we examined the stability of randomly selected EGFP-positive clones to determine the retention rate. The retention rates of HACs were variable in each clone, and also confirmed to be constant in each clone at initial point and in long-term culture (data was not shown).These observations were similar to our previous experience, although different HACs were used in each experiment (Ren et al. 2005). At this point, the reason for the variable retention rate in each clone cannot be explained. It is well-known that cells are more or less damaged by cryopreservation. A similar damage may occur in cryopreserved microcells, and affect the efficiency of chromosome transfer as well as the retention rate of the HAC vector. However, the cryopreservation at least does not significantly affect the retention rate and stability of HAC (P > 0.05 using Mann–Whitney test). If any, the damage is minimal.
Acknowledgments
This study was supported by Regional Innovation Strategy Support Program from The Ministry of Education, Culture, Sports, Science, and Technology of Japan (M. O.), Japan Science and Technology Agency, CREST (M. O.).
Author contributions
N. U. designed and performed most of the experiments. K. U., S. Z., K. U. and H. M. performed the experiments and analyzed the data. M. O. designed the experiments and supervised the entire project. N. U. and M. O. wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Abbreviations
- HAC
Human artificial chromosome
- PAC
P1 phage-derived artificial chromosome
- BAC
Bacterial artificial chromosome
- YAC
Yeast artificial chromosome
- iPSC
Induced pluripotent stem cell
- MSC
Mesenchemal stem cell
- PEG
Polyethylene glycol
- H
Hemaglutinin
- F
Fusion
- MMCT
Microcell mediated chromosome transfer
- MV-MMCT
Microcell mediated chromosome transfer using Measles virus fusogen
- EGFP
Enhanced green fluorescent protein
- Bsd
Blasticidin
Contributor Information
Narumi Uno, Phone: +81-859-386412.
Katsuhiro Uno, Phone: +81-859-38-6412.
Susi Zatti, Phone: +39-049-8275472.
Masaharu Hiratsuka, Phone: +81-859-386412.
Mitsuo Oshimura, Phone: +81-859-386211, FAX: +81-859-386210, Email: oshimura@med.tottori-u.ac.jp.
References
- Hiratsuka M, Uno N, Ueda K, Kurosaki H, Imaoka N, Kazuki K, Ueno E, Akakura Y, Katoh M, Osaki M, Kazuki Y, Nakagawa M, Yamanaka S, Oshimura M. Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS ONE. 2011;6:e25961. doi: 10.1371/journal.pone.0025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiya H, Kazuki Y, Abe S, Takiguchi M, Kajitani N, Watanabe Y, Yoshino T, Shirayoshi Y, Higaki K, Messina G, Cossu G, Oshimura M. A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mol Ther. 2009;17:309–317. doi: 10.1038/mt.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M, Masumoto H, Okazaki T. Distribution of CENP-B boxes reflected in crest centromere antigenic sites on long-range alpha-satellite DNA arrays of human-chromosome-21. Hum Mol Genet. 1994;3:1245–1257. doi: 10.1093/hmg/3.8.1245. [DOI] [PubMed] [Google Scholar]
- Kakeda M, Nagata K, Osawa K, Matsuno H, Hiratsuka M, Sano A, Okazaki A, Shitara S, Nishikawa S, Masuya A, Hata T, Wako S, Osaki M, Kazuki Y, Oshimura M, Tomizuka K. A new chromosome 14-based human artificial chromosome (HAC) vector system for efficient transgene expression in human primary cells. Biochem Biophys Res Commun. 2011;415:439–444. doi: 10.1016/j.bbrc.2011.10.088. [DOI] [PubMed] [Google Scholar]
- Katoh M, Kazuki Y, Kazuki K, Kajitani N, Takiguchi M, Nakayama Y, Nakamura T, Oshimura M. Exploitation of the interaction of measles virus fusogenic envelope proteins with the surface receptor CD46 on human cells for microcell-mediated chromosome transfer. BMC Biotechnol. 2010;10:37. doi: 10.1186/1472-6750-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuki Y, Oshimura M. Human artificial chromosomes for gene delivery and the development of animal models. Mol Ther. 2011;19:1591–1601. doi: 10.1038/mt.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H, Hiramatsu K, Yoshino T, Kazuki K, Ishihara C, Takehara S, Higaki K, Nakagawa M, Takahashi K, Yamanaka S, Oshimura M. Complete genetic correction of iPS cells from duchenne muscular dystrophy. Mol Ther. 2010;18:386–393. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuki Y, Hoshiya H, Takiguchi M, Abe S, Iida Y, Osaki M, Katoh M, Hiratsuka M, Shirayoshi Y, Hiramatsu K, Ueno E, Kajitani N, Yoshino T, Kazuki K, Ishihara C, Takehara S, Tsuji S, Ejima F, Toyoda A, Sakaki Y, Larionov V, Kouprina N, Oshimura M. Refined human artificial chromosome vectors for gene therapy and animal transgenesis. Gene Ther. 2011;18:384–393. doi: 10.1038/gt.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kononenko A, Erliandri I, Kim TA, Nakano M, Iida Y, Barrett JC, Oshimura M, Masumoto H, Earnshaw WC, Larionov V, Kouprina N. Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells. Proc Natl Acad Sci USA. 2011;108:20048–20053. doi: 10.1073/pnas.1114483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M, Shimizu M, Morita H, Yamada H, Oshimura M. Construction of mouse A9 clones containing a single human-chromosome tagged with neomycin-resistance gene via microcell fusion. Jpn J Cancer Res. 1989;80:413–418. doi: 10.1111/j.1349-7006.1989.tb02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouprina N, Earnshaw WC, Masumoto H, Larionov V (2013) A new generation of human artificial chromosomes for functional genomics and gene therapy. Cell Mol Life Sci 70:1135–1148 [DOI] [PMC free article] [PubMed]
- Kurosaki H, Hiratsuka M, Imaoka N, Iida Y, Uno N, Kazuki Y, Ishihara C, Yakura Y, Mimuro J, Sakata Y, Takeya H, Oshimura M. Integration-free and stable expression of FVIII using a human artificial chromosome. J Hum Genet. 2011;56:727–733. doi: 10.1038/jhg.2011.88. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Crystal RG. Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet. 2006;7:261–276. doi: 10.1038/nrg1829. [DOI] [PubMed] [Google Scholar]
- Ren X, Katoh M, Hoshiya H, Kurimasa A, Inoue T, Ayabe F, Shibata K, Toguchida J, Oshimura M. A novel human artificial chromosome vector provides effective cell lineage-specific transgene expression in human mesenchymal stem cells. Stem Cells. 2005;23:1608–1616. doi: 10.1634/stemcells.2005-0021. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Tomizuka K, Miyabara S, Takehara S, Kazuki Y, Inoue J, Katoh M, Nakane H, Iino A, Ohguma A, Ikegami S, Inokuchi K, Ishida I, Reeves RH, Oshimura M. Mice containing a human chromosome 21 model behavioral impairment and cardiac anomalies of Down’s syndrome. Hum Mol Genet. 2001;10:1163–1175. doi: 10.1093/hmg/10.11.1163. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Yoshida H, Uejima H, Kugoh H, Sato K, Ohguma A, Hayasaka M, Hanaoka K, Oshimura M, Ishida I. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat Genet. 1997;16:133–143. doi: 10.1038/ng0697-133. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Shinohara T, Yoshida H, Uejima H, Ohguma A, Tanaka S, Sato K, Oshimura M, Ishida I. Double trans-chromosomic mice: maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc Natl Acad Sci USA. 2000;97:722–727. doi: 10.1073/pnas.97.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kazuki Y, Nakayama Y, Nanba E, Oshimura M, Ohbayashi T. A method for producing transgenic cells using a multi-integrase system on a human artificial chromosome vector. PLoS ONE. 2011;6:e17267. doi: 10.1371/journal.pone.0017267. [DOI] [PMC free article] [PubMed] [Google Scholar]