Abstract
The development of a preventive vaccine to neutralize the highly variable and antigenically diverse human immunodeficiency virus type 1 (HIV-1) has been an indomitable goal. The recent discovery of a number of cross-neutralizing and potent monoclonal antibodies from elite neutralizers has provided important insights in this field. Neutralizing antibodies (NAbs) are useful in identifying neutralizing epitopes of vaccine utility and for understanding the mechanism of potent and broad cross-neutralization thus providing a modality of preventive and therapeutic value. In this article we review the current understanding on the potential use of broadly neutralizing antibodies (bNAbs) in their full-length IgG structure, engineered domain antibody or bispecific versions towards preventive and therapeutic applications. The potential implications of NAbs are discussed in the light of the recent developments as key components in vaccination against HIV-1. The development of a vaccine immunogen which elicits bNAbs and confers protective immunity remains a real challenge.
Keywords: HIV-1, neutralizing antibodies, vaccines
Introduction
Acquired immunodeficiency syndrome (AIDS) caused by the human immunodeficiency virus type 1 (HIV-1) is a major public health problem and warrants the urgent need for the development of a preventive vaccine. Evidence gathered from recent discoveries backed by basic scientific research and clinical trials indeed provides optimism about the possibility of developing a vaccine against HIV-1. Antibodies that block the virus entry are referred as neutralizing antibodies (NAbs) and these are thought to be a crucial correlate of protection in some of the successful viral vaccines and can also be effective against HIV-1 [Haynes and Montefiori, 2006; Mascola, 2003; Montefiori et al., 2007a; Plotkin, 2008]. However, for complete protection, both arms of the adaptive immune response, antibody response and T-cell response, seem to be necessary [Walker and Burton, 2008]. Both of these responses work in concert: the antibodies control the cell free virus entry into the target cells whereas the T-cell response controls the viral reservoirs and is required to check viral replication [McMichael and Hanke, 2002; Pantaleo and Koup, 2004]. Virus transmission after breaching the mucosal barrier into the submucosal environment leads to the infection of surrounding CD4+ T cells and thereafter disseminates into the systemic infection. The persistent viral replication leads to the destruction of CD4+ T cells culminating in AIDS [Letvin, 2006; McMichael, 2006]. Envelope protein (gp160) encoded by HIV env is the outermost protein expressed on HIV. It acts as a molecular machine that binds the virus to the target cell receptors, thus mediating the cell membrane fusion and virus entry [Wyatt et al. 1998]. Env is a crucial component of viral entry and represents an attractive target for vaccine-induced antibodies that has potential to bind with Env and block the entry of virus into the target cell [Burton et al. 2004; Haynes and Montefiori, 2006; Montefiori et al. 2007b]. In recent years substantial progress has been made on antibody discovery and highly potent and broad NAbs have been isolated from chronically infected HIV-positive patients with broadly neutralizing serum activity, also referred to as elite neutralizers. These antibodies upon passive immunization of animals conferred protection in nonhuman primates and humanized mice [Burke and Barnett, 2007; Klein et al. 2012b; Mascola, 2003, Moldt et al., 2012]. The in vivo expression of broadly neutralizing antibodies (bNAbs) by vector-mediated gene transfer also showed high efficacy in humanized mice [Balazs et al. 2012]. However, attempts to elicit such antibodies by immunization have not been very successful [Burton et al. 2004; Haynes and Montefiori, 2006]. The initial recombinant protein vaccine based on gp120 protein induced only immunogen-specific antibodies which could neutralize lab-adapted virus strains but not the primary isolates and thus showed no clinical relevance [Flynn et al. 2005; Gilbert et al. 2005; Graham and Mascola, 2005]. However, the recent RV144 HIV-1 vaccine trial of the canarypox vector (ALVAC-HIV) plus the gp120 AIDSVAX B/E vaccine demonstrated moderate efficacy and promise that the antibodies induced by vaccination can provide protective immunity against HIV-1 [Baden and Dolin, 2012; Rerks-Ngarm et al. 2009]. Intriguingly, the antibodies in RV144 trial were mostly non-neutralizing; however, it is the binding of IgG antibodies to the V1V2 region of the gp120 Env that likely was the correlate of protection in this trial [Haynes et al. 2012a]. Although this regimen failed to produce NAbs, the results of this trial may provide a valuable guide as to the immunogen improvement efforts and antibodies required for protection against HIV-1 infection. Efforts are being made to build improved immunogens based on the newer detailed structural insights in Env protein that exhibit a better antibody response [Kovacs et al. 2012; Phogat and Wyatt, 2007]. The improved knowledge of the Env structure and neutralization epitopes will help improve the rational immunogen design in order to elicit potent bNAbs [Dormitzer et al. 2008; Kwong and Wilson, 2009; Montefiori et al. 2007b; Phogat and Wyatt, 2007; Stamatatos et al. 2009]. The present paper reviews the current understanding about the progress in the discovery of broad and potent NAbs to HIV-1 as well as their potential in HIV-1 therapeutics and prophylactics.
Neutralizing epitopes on the HIV-1 envelope
Although antibodies are elicited against most of the viral proteins, those that bind to Env protein and prevent viral entry are referred to as NAbs [Mascola and Montefiori, 2010; Pantophlet and Burton, 2006; Zolla-Pazner, 2004]. The unique subunit architecture of HIV-1 Env trimer that induces NAbs is particularly challenging to achieve [Mao et al. 2012]. The antibodies in the early infection are generally strain specific but in some patients bNAbs develop in the chronic stage of infection. Around 20% of HIV patients with chronic infection develop NAbs with potential to neutralize diverse HIV-1 strains, and 2–4% of such subjects have even greater serum neutralizing activity that neutralize most HIV-1 strains from different clades [Simek et al. 2009]. Antigenically Env protein is highly variable and virus can quickly escape from the selective pressure from existing NAbs. Nevertheless, sera from certain chronically infected patients exhibit broader neutralizing activity which attributes to single, few or multiple specificities [Scheid et al. 2009; Walker et al. 2009; Walker et al. 2010; Wu et al. 2010]. The first broadly neutralizing human monoclonal antibody (mAb) b12 was isolated from a clade B infected patient and binds to gp120 at its CD4 binding site (CD4bs) [Burton et al. 1994]. b12 was found to neutralize more than 50% of clade B viral isolates and about 30% of non–clade B viruses [Binley et al. 2004; Kulkarni et al. 2009]. Recently, novel forms of broad and potent CD4bs antibodies have been isolated from elite neutralizer using reverse vaccinology approaches [Falkowska et al. 2012; Wu et al. 2011a]. These human mAbs were isolated by exploiting the ability of a resurfaced stabilized gp120 core protein mimicking the CD4 binding site to capture only broadly NAbs based on affinity and deep recognition (weakly binding non-NAbs were kept at bay). Using this modified gp120 core as a fluorescent-labeled probe to capture antigen-specific memory B cells, CD4bs-specific VRC antibodies were also isolated. VRC01 and VRC02 are the broadest, neutralizing 91% of primary Env pseudoviruses while VRC03 was found to neutralize 57% of the circulating viruses of the panel (Table 1) [Wu et al. 2010]. NIH45-46 is a more potent clonal variant of VRC01 and has a four-residue insertion in the heavy chain complementarity determining region 3 (CDR3) which enhances gp120 binding [Scheid et al. 2011]. 3BNC117 [Scheid et al. 2011] and VRC-PG04 [Wu et al. 2011b] are also CD4bs antibodies with neutralization breadth and potency similar to VRC01. Although all of these antibodies bind to CD4bs there are differences in the way they interact with this site. Structural analyses of the CD4bs interaction with mAbs b12 (prototype CD4bs NAb), F105 (non-neutralizing), and VRC01 (very broadly neutralizing) revealed the unusual binding into a narrow site [Schief et al. 2009; Zhou et al. 2010]. VRC01 approaches the conformationally invariant site following the initial CD4 attachment, escaping the hindrance by conformational masking which most CD4bs antibodies experience, thus diminishing their neutralization potency. Partial receptor mimicry and extensive affinity maturation thus facilitate effective neutralization of HIV-1 by natural human antibodies such as VRC01 [Zhou et al. 2010]. Two prototype membrane-proximal external region (MPER) gp41 NAbs are 2F5 and 4E10 [Cardoso et al. 2005; Ofek et al. 2004; Stiegler et al. 2001]. The gp41 subunit is far more conserved than gp120 but kinetic and steric constraints potentially protect its vulnerable regions from NAb attack. MAbs 2F5, 4E10 and Z13 were isolated from an HIV-positive patient and show considerable potency, with 4E10 being the most broadly neutralizing [D’Souza et al. 1997]. These MAbs bind to the intermediate conformation of gp41 during the fusion process [Frey et al. 2008]. The native gp41 seems to be inaccessible to antibodies and reveals epitopes during the fusion process. Therefore, either limited antibodies are produced against these epitopes, as their exposure is too short to be recognized by B-cell receptors to elicit antibodies against them, or the antibody has a little window of opportunity to bind to short-lived epitopes that are exposed only during fusion [Alam et al. 2011; Ringe and Bhattacharya, 2012; Shen et al. 2010]. 2F5 binds to a core target at ELDKWA in the heptad repeat-2 region of gp41, whereas 4E10 binds to the NWFDIT sequence in the MPER [Cardoso et al. 2005; Ofek et al. 2004]. These antibodies bind to both lipid–MPER peptide complexes and to HIV virions [Alam et al. 2009] in a two-step conformation change model wherein they bind first to virion lipids, then surf the viral membrane while awaiting transient exposure of their neutralizing epitopes during fusion with a target cell. These antibodies are autoreactive because of their reactivity with lipids [Alam et al. 2009]. Recently, a very potent and broadly cross-neutralizing gp41 MPER-specific antibody, named 10E8, has been reported by Huang and colleagues [Huang et al. 2012]. 10E8 neutralizes 98% of tested viruses (Table 1). Moreover, unlike 2F5 or 4E10, 10E8 does not bind phospholipids, is not autoreactive and binds to the cell-surface envelope, suggesting that the highly conserved epitope in the gp41 ectodomain is directly accessible to 10E8 and can potentially increase the window of opportunity to access the epitope. The study also showed that 8% of the sera from healthy HIV-1-positive individuals contained 10E8-like specificities suggesting that the corresponding epitope is immunogenic in nature and 10E8-like antibodies are not deleted from the B-cell repertoire because of autoreactivity. The structure of 10E8 in complex with the complete MPER and the mutagenesis studies revealed a binding site comprising a narrow stretch of highly conserved gp41-hydrophobic residues and a critical arginine or lysine just before the transmembrane region [Huang et al. 2012]. The highly conserved MPER is a target for potent, non-self-reactive NAbs. The frequent generation of 10E8-like antibodies in HIV-1-infected individuals suggests that this specificity can be induced by vaccination in a larger fraction of HIV-negative or HIV-positive individuals than other gp41 specific antibodies. Another immunodominant region in Env is the V3 loop on gp120 and antibodies are often made against the epitopes at the tip of this loop. V3 specific antibodies have generally a narrow reactivity and can neutralize only tier 1 or T-cell line adapted strains [Davis et al. 2009]. Many V3-specific antibodies with some broad reactivity from chronically infected HIV-1 subjects have been isolated that recognize a strain-specific quaternary epitope involving gp120 V2 and V3 loops in the context of Env trimer [Zolla-Pazner and Cardozo, 2010]. The new technical advances in single memory B-cell stimulation and high-throughput microneutralization assay facilitated the antibody screening to higher levels. The isolation of potent and broadly neutralizing mAbs PG9 and PG16 (closely related somatic mutants) from a patient was carried out by Walker and colleagues using this methodology [Walker et al. 2009]. PG9 and PG16 recognize a novel epitope that is composed of V1V2 and V3 loops on the Env trimer and exhibit remarkable neutralization breadth and potency [McLellan et al. 2011; Walker et al. 2011]. More recently, by using the same technology, many additional monoclonal antibodies, referred to as PGT antibodies, have been isolated from four elite neutralizers which target a glycan-dependent epitope and exhibit about 10 times more potency than VRC01, PG9 and PG16 and 100 times more than old-generation prototypic antibodies. Among these PGT antibodies, PGT141-145 targets glycan-dependent quaternary epitopes on gp120 whereas PGT 125-128 and PGT130-131 interact specifically with the Man8/9 glycans on gp120. These antibodies are very potent, with some neutralizing >70% of HIV-1 env pseudotyped virus panel of 162 envelopes (Table 1) [Pancera et al. 2010; Walker et al. 2009, 2011]. The crystal structure of PGT128, the most potent and broad PGT, complexed with a fully glycosylated gp120 outer domain, reveals that the antibody penetrates the glycan shield and recognizes two conserved glycans as well as a short β-strand segment of the gp120 V3 loop, accounting for its high binding affinity and broad specificity [Pejchal et al. 2011]. The 2G12, prototypic carbohydrate specific mAb, is unusual in its structure and binding specificity. Unlike PG9 or PGT mAbs, it is a canonical glycan-binding bNAb with a unique domain-swap structure. Its Fab region is composed of a heavy chain and a light chain which are shared by the other arm of the 2G12 antibody [Calarese et al. 2003; Scanlan et al. 2002]. The glycans on gp120 are the result of host cell posttranslational modifying glycosidases and therefore resemble host carbohydrates, possibly reducing the immunogenicity of gp120 [Scanlan et al. 2002, 2003]. The glycans recognized by 2G12 comprise a unique conformational epitope of oligomannose glycans in the outer domain of gp120 that is poorly immunogenic [Astronomo et al. 2008]. However, the newer glycan-binding antibodies recognize these domains far more strongly and neutralize many primary isolates [Walker et al. 2011]. This suggests that the immune response in HIV infection takes a complex pathway to evolve and recognize such complex and difficult epitopes [Mouquet et al. 2012]. Thus, the HIV-1 envelope has at least five conserved regions, each with overlapping epitopes that can be targets for bNAbs (Figure 1).
Table 1.
Characteristics of broad and potent neutralizing antibodies to HIV-1
| Antibody Name | Site of Contact | Study | Antibody Type | Glycan dependence | Quaternary Structure dependence | Neutralization Breadth (%) | Infecting Subtype | Therapeutic Potential |
|---|---|---|---|---|---|---|---|---|
| IgG1b12 | CD4bs | Burton et al. (1991) Proc. Natl. Acad. Sci. USA; 88: 10134-10137 | Whole Antibody | No | No | 35 (n=190) | B | Effective against SHIV vaginal challenge and protects macaques. |
| VRC01,02,03 | CD4bs | Wu et al. (2010) Science329: 856-861 | Whole Antibody | No | No | 91, 91, 57 (n=190) | B | Effective against SHIV mucosal challenge and protects macaques. |
| NIH45-46 | CD4bs | Scheid et al. (2011) Science, 333: 1633-1637 | Whole Antibody | No | No | 96 (n=118) | B | Not tested |
| VRC-PG04 | CD4bs | Wu et al. (2011) Science; 333:1593-602 | Whole Antibody | No | No | 76 (n=178) | A-D recombinant | Not tested |
| 3BNC117 | CD4bs | Scheid et al. (2011) Science, 333: 1633-1637 | Whole Antibody | No | No | 96 (n=118) | B | Not tested |
| VHH J3 | CoRbs | McCoy, L. and Weiss, R. (2013). J Exp Med 210: 209-223. | Domain Antibody | NA | NA | 96 (n=100) | - | Not tested |
| m36 | CoRbs | Chen (2008) Proc Natl Acad Sci USA | Domain Antibody | No | No | 91 (n=11) | - | Not tested |
| 2008; 105: 17121-17126 | ||||||||
| 2G12 | Glycan | Trkola et al. (1996) J. Virol. 70: 1100-1108) | Whole Antibody | Yes | No | 32 (n=162) | B | Reduces viral load and increases CD4 T cell count in combination with 2F5, 4E10 |
| PGT121-123 | Glycan | Walker et al. (2011) Nature; 477:466-470) | Whole Antibody | Yes | No | 65-70 (n=162) | A | Not tested |
| PGT125-128,130-131 | Glycan | Walker et al. (2011) Nature; 477:466-470) | Whole Antibody | Yes | No | 40-72 (n=162) | CRF02_AG | Not tested |
| PGT135 | Glycan | Walker et al. (2011) Nature; 477:466-470) | Whole Antibody | Yes | No | 33 (n=162) | C | Not tested |
| PGT141-145 | Glycan | Walker et al. (2011) Nature; 477:466-470) | Whole Antibody | Yes | 38-78 (n=162) | A or D | Not tested | |
| PG9, PG16 | Quaternary structure including V1V2,V3 | Walker et al. (2009) Science 326: 285-289 | Whole Antibody | Yes | Yes | 79 and 73 (n=190) | A | Not tested |
| 2F5 | MPER | Purtscher et al. (1994) AIDS Res Human Retroviruses 10: 1651-1658 | Whole Antibody | No | No | 57 (n=177) | B | Tested in combination with 2G12. Help reduce viral load and increase CD4+ T cell count in HIV infected individuals. |
| 4E10 | MPER | Stiegler et al. (2001) AIDS Res. Hum. Retroviruses 17:1757-1765 | Whole Antibody | No | No | 98 (n=180) | B | Moderately suppresses viral load in combination with 2F5 |
| 10E8 | MPER | Huang et al (2012) Nature, 491: 406-412) | Whole Antibody | No | No | 98 (n=180) | B | Not tested |
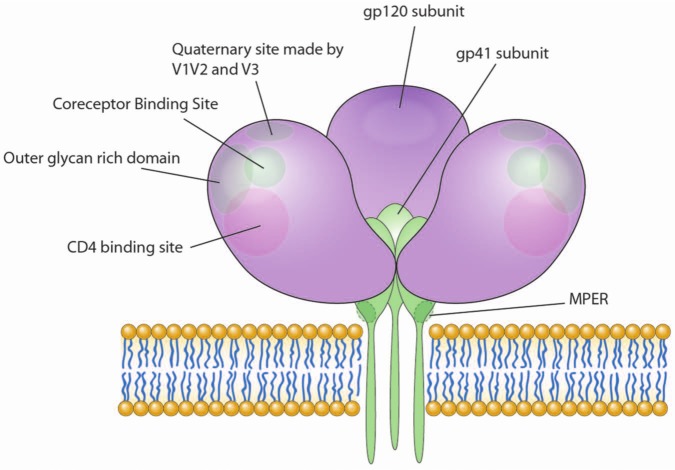
Figure 1.
Potential sites of antibody response on Env. The Envelope trimer is composed of three gp120 surface protein subunits (purple) and three transmembrane gp41 subunits (green) as shown. gp120 has multiple potential clusters (indicated by circles) against which neutralizing antibodies are elicited in HIV-infected individuals (many have been isolated from broadly neutralizing sera). The gp41 protein has so far shown one site in the ectodomain region (indicated by dotted circles) against which broadly neutralizing antibodies are isolated. The envelope trimer is shown in an open conformation (post-CD4 engagement) to reveal the coreceptor binding site cluster which is exposed after gp120 is engaged with CD4. MPER, membrane-proximal external region. Illustration courtesy of Alessandro Baliani. Copyright © 2013.
HIV-1 NAbs and antibody-based inhibitors
NAbs are very powerful agents against most of the animal viruses and in vaccine-induced immune responses [Casadevall, 2002; Klasse and Sattentau, 2002]. However, the treatment of HIV-1 with NAbs has not been successful because of the rapid mutability of the virus [Manrique et al. 2007; Wei et al. 2003]. Increasing the potency and breadth might overcome this daunting problem. Substantial efforts have been made in this direction and several antibody-based inhibitors have been made with desirable features. In addition to informing strategies to design vaccine immunogens and elicit similar antibody responses, therapeutic application of bNAbs in patients failing antiretroviral therapy (ART) or those who developed resistance represents a promising approach towards the eradication of virus. Thus, bNAbs can be used individually or in combination with ART for effective elimination of virus [Chen et al. 2013; Corti and Lanzavecchia, 2013]. In this review, we discuss how the antibody modifications are made to achieve the desirable characteristics and how monoclonal antibodies, in their native form or as engineered versions, can be used to confer protection in HIV infection.
Neutralization of HIV-1 by native forms of mAbs
As discussed earlier, few crucial neutralizing epitopes have been characterized against which bNAbs have been isolated. One of the best characterized broadly cross-reactive and potent human mAbs targeting the CD4bs of gp120 is b12. This was selected almost 20 years ago by phage display from an antibody library made from the bone marrow of an HIV-1-infected donor [Burton et al. 1994; Roben et al. 1994]. Intravenous transfusion of b12 was found to partially protect the macaques from vaginally challenged R5 virus SHIV162P4 and later it was found that vaginal administration of b12 can partially protect macaques from vaginal simian/human immunodeficiency virus (SHIV) transmission [Parren et al. 2001; Veazey et al. 2003]. These observations suggest that mAbs can be a potential modality to prevent the sexual transmission of HIV-1 to humans. The synergistic effect of multiple mAbs together can improve the protective effect and may compromise the virus to the level from which viral rebound is difficult [Klein et al. 2012a]. However, the mechanism by which NAbs confer protection in vivo is not very clear. In addition to a direct neutralization, antibody Fc receptor-mediated effector functions are also important for protection against HIV-1. Removal of Fc-receptor binding of b12 resulted in a loss of its protective function suggesting that the antibody has both cell-free and cell-associated virus neutralizing abilities [Hessell et al. 2007]. Moldt and colleagues [Moldt et al. 2012] demonstrated that an increase of the b12 interaction with FcgammaRIIIa enhanced the antiviral activity in vitro but could not exert the same effect in vivo. Thus, the exact antiviral mode of action of NAbs in vivo remains unclear. The carbohydrate-specific antibody 2G12 has been tested intravenously in the animal models for its efficacy against vaginally challenged SHIV. Results showed a substantial protection of macaques or reduced viral load and delay in CD4+ T-cell loss [Chen and Dimitrov, 2012; Mascola et al. 1999, 2000]. Hessell and colleagues [Hessell et al. 2009] have also shown that low intravenous administration of 2G12 can protect the animals from vaginal challenge of SHIV, in contrast to the observation that high-dose b12 was required to confer protective immunity. In these studies the challenge virus used was highly sensitive to the NAb tested. In vitro studies of 2F5 and 4E10 demonstrated that the neutralizing activity of these mAbs is enhanced when tested in combination [Mascola et al. 1997] and therefore they were evaluated for their antiviral activity in vivo mainly in combination in animal models and in HIV-infected persons. Alone, neither 2F5 nor 2G12 completely protected macaques from intravenous challenge but treated animals showed a less profound drop in CD4+ T cells suggesting that these antibodies compromise virus replication in vivo [Mascola et al. 1999]. These initial studies in animal models documented some success in protecting against infection or delaying the viral rebound/CD4+ T-cell loss upon the vaginal challenge of SHIV. Later the 2F5/2G12 combination was also evaluated in a phase I clinical trial in seven HIV-1-infected patients [Armbruster et al. 2002] which confirmed the safety of these mAbs. In addition, transient reductions in viral loads were observed in five of the seven subjects while improvement in CD4+ T-cell counts and CD4+/CD8+ ratios were observed in all individuals. However, the HIV virus can develop resistance to 2G12 making this approach ineffective. For this reason, a combination of three mAbs 4E10, 2F5 and 2G12 [Armbruster et al. 2004] was also evaluated in seven individuals. The study confirmed the safety of this combination and some protection. In a phase II clinical trial [Trkola et al. 2005] the capacity of these antibodies to suppress or delay the viral rebound caused by the ART was evaluated. A substantial delay in viral rebound was observed in four of six acutely infected individuals and only in two of eight chronically infected patients. This suggests that the mAbs can prevent disease progression when HIV infection is at early stage and viral diversity is limited [Bar et al. 2012]. In chronic infection viral diversity and viral reservoirs may be too high to be controlled by mAbs. Resistance to 2G12 but not to 2F5 or 4E10 was observed in 12 of 14 individuals, suggesting that the glycosylation pattern can be readily changed without compromising the gp120 function to resist the binding to 2G12 whereas MPER is crucial in the fusion process and the acquisition of resistance to the corresponding antibodies may require a higher fitness cost [Manrique et al. 2007]. Subsequent studies indicated that the antiviral effect of these three mAbs is the result of direct in vivo neutralization in addition to antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis mechanisms [Huber et al. 2008].
Antibody modifications to enhance antiviral activity
A whole antibody is a large molecule which generally binds to a larger portion of an antigenic protein to enhance its avidity. The problem associated with these large molecules is that they may not access the epitopes when these are sterically occluded. This is especially common for many epitopes of the HIV-1 envelope proteins resulting in resistance against the antibodies induced by HIV infection [Burton et al. 2005; Mascola and Montefiori, 2010]. For such changing epitopes small fragments of antibody could be more effective and more able to control virus replication. The binding site for such entities is smaller and therefore steric occlusion can be avoided [Labrijn et al. 2003]. Labrijn and colleagues [Labrijn et al. 2003] showed that the neutralization potency of CD4-induced (CD4i) epitope binding antibody is inversely correlated with the size of the antibody fragments scFv (~25 kDa). It appears that smaller antibody formats can access the binding site more easily than the whole antibody as the access for the latter is sterically restricted due to the small space between the viral envelope and cell membranes after or before the engagement of Env trimer with the CD4 receptor [Labrijn et al. 2003]. Few CD4i NAbs have been isolated from HIV patients are represented by 17b (IgG), X5 [Chen and Dimitrov, 2009; Moulard et al. 2002], m9 and m16 [Zhang et al. 2004]. These CD4i antibodies have strong neutralizing activities and therapeutic relevance as their target site is mostly conserved on the Env structure. A newer class of antibodies are called domain antibodies (dAbs) which are represented by engineered antibody fragments that are fairly potent and smaller in size compared with Fab or scFv [Chen et al. 2008a, 2008b; Holt et al. 2003; Vanlandschoot et al. 2011]. These small antibody fragments, ranging from 11 to 15 kDa in size, lack either the VL or VH domain and are highly directed against conserved domains of the epitope. This is a particularly important characteristic as most of the immunogenic conserved epitopes of the Env are guarded by conformational masking by variable loops or the glycan shield and escape from immune recognition [Kwong et al. 1998; Pantophlet and Burton, 2006; Wyatt et al. 1998]. Epitopes which are beyond the reach of a full-size antibody or a Fab/scFv because of steric constraints are easily accessed by dAbs [Chen and Dimitrov, 2009; Chen et al. 2008a; Gong et al. 2012]. The coreceptor binding site (CD4i) is the most sterically occluded immunogenic structure in gp120 as its formation is induced only following gp120 binding to CD4. It was therefore hypothesized that small antibody fragments (derived from whole antibody domains) targeting such epitopes could neutralize the virus with breadth and potency [Chen et al. 2010]. The highly potent dAb m36 reported by Chen and colleagues is directed at a conserved structure of the coreceptor binding site (CD4i) [Chen et al. 2008a]. This antibody is broadly cross-neutralizing, potent in its neutralizing activity and showed higher potency than scFv antibody m9 [Chen and Dimitrov, 2009]. Such dAbs can be particularly effective against kinetic signatures such as CD4i and MPER epitopes. Some dAbs have also been isolated recently from recombinant gp120-based immunization of llamas and exhibited potent neutralizing activity against HIV-1 [Forsman et al. 2008]. Some variable domains derived from heavy-chain antibodies (VHH) such as A12, D7 and C8 were able to neutralize HIV-1 clade B and C isolates [Forsman et al. 2008; Strokappe et al. 2012]. Using the more stringent criteria of selection, VHH was able to neutralize 42% of the strains tested in vitro with IC50 in the range of <0.2–2533 nM although they have in general a higher specificity towards the immunizing strain or clade. These dAbs block the binding of CD4 to gp120 and can compete with the binding of CD4bs mAbs to gp120 [Forsman et al. 2008]. The crystal structure of a llama heavy-chain antibody fragment VHH D7, revealed the presence of two canonical CDR1 and CDR2 but a longer and highly mobile CDR3 which is probably required for recognizing and conferring more binding energy for the interaction with gp120 CD4bs and virus neutralization [Hinz et al. 2010; Koh et al. 2010; McCoy and Weiss, 2013]. Using a family-specific approach, Koh and colleagues have recently isolated the largest possible diversity of related VHH antibodies that compete with soluble CD4 for binding to the HIV-1 envelope glycoprotein [Koh et al. 2010; Strokappe et al. 2012]. The dAb list was then expanded by Matz and colleagues with the immunization of llamas using trimeric gp140, free or bound to a CD4 mimic, in order to isolate CD4bs and CoRbs dAbs [Matz et al. 2013]. The single-domain antibodies (sdAbs) isolated in this study potently neutralized subtype B viruses but also showed neutralizing activity against viruses carrying envelopes from A, C, G, CRF01_AE and CRF02_AG, subtypes including tier 3 viruses. A new modified screening process able to distinguish between neutralizing and non-NAbs, allowed the isolation of the extremely broad and potent VHH J3 by screening of a phagemid VHH library generated from a llama immunized with two recombinant HIV gp140 proteins. This VHH dAb neutralized 96% of the large panel of HIV-1 strains and represents a potential therapeutic candidate [McCoy and Weiss, 2013]. This work demonstrated that broad and potent smaller format antibodies can be obtained upon immunization and considered for several applications such as anti-HIV-1 microbicide and for the rational HIV immunogen design of HIV to define vulnerable epitopes on the Env protein. However, few limitations are associated with these antibodies. They have smaller half-life and low retention in vivo. In addition, the antiviral action of the antibody fragment lies in competitive binding and neutralization. However, other IgG functions such as the Fc-mediated ADCC or phagocytic effects are absent which may reduce the antiviral effect of these antibodies. Finally, the development of resistance to a single mode of antiviral action can be less difficult than that observed in antibodies with multiple antiviral mechanisms.
Bispecific antibodies
Dealing with efficacy issues at the clinical level and viral resistance to bNAbs has prompted the need to enhance the potency and breadth of the NAbs. Few studies in recent years have described the generation of antibody conjugation products with two different specificities on the Env protein. The soluble form of CD4 is a virus-neutralizing protein and has been used to conjugate with the antibodies that target the epitopes that are induced or exposed upon CD4 binding to Env [Chamow et al. 1992; Chen et al. 2013; Traunecker et al. 1992; West et al. 2010]. External domains of CD4 receptor D1D2 conjugated with the antibody specific to CD4i epitope (CD4-CD4i antibody) showed enhanced neutralization potency and breadth [Chen and Dimitrov, 2009; West et al. 2010]. The bivalent reagent that fuses CD4 to the heavy chain of the CD4i antibody E51 showed similar or higher neutralizing potency than that of the well-known bNAbs [West et al. 2010]. Similarly, immunoadhesin–antibody hybrids in which scFv is conjugated with the Fc domain has been explored and their neutralization potencies compared to those of the parent IgG. Immunoadhesins made from PG9, PG16 and VRC01 showed reduced potency likely because of the reduced affinity of the cognate epitope. However, the attachment of the VRC01 scFv to PG16 IgG yielded a bispecific reagent whose neutralization activity combined activities from both parent antibodies and also fewer strains escaped neutralization [West et al. 2012]. Such approach of combining antigen-binding sites together in a single antibody enhances the avidity of the antibody that likely translates into increased potency [Cavacini et al. 1994; Kausmally et al. 2004]. In addition to the valency, improved flexibility and size of antigen binding sites can have an impact on the neutralization potency and breadth. The efficiency of b12 to neutralize a panel of clade B envelopes was seen to increase with valency and flexibility between antigen binding sites. Engineered b12 displayed the ability to bind bivalently and cross-link envelope spikes on the virion surface. This was not observed with similarly engineered 4E10 antigen-binding sites probably because the 4E10 epitope is difficult to access in a native trimer and such design may be of least relevance [Klein et al. 2009]. Recent studies have established the fact that most bNAbs are polyreactive and hypermutated [Mouquet et al. 2010]. The bNAbs are generated in the chronic stage of HIV infection and are affinity matured by virtue of somatic hypermutations in mostly but not limited to the antigen-combining sites [Klein et al. 2013; Mouquet and Nussenzweig, 2012; Mouquet et al. 2010; Scheid et al. 2011; Wu et al. 2011b]. The affinity maturation and polyreactivity is explained to corroborate the binding to high-affinity anti-Env-combining site and a second low-affinity site on another molecular structure on HIV-1 [Mouquet et al. 2010]. Antibody engineering methods aimed at enhancing the apparent affinity and neutralization potency were reviewed recently by Mouquet and Nussenzweig [Mouquet and Nussenzweig, 2012]. Bispecific anti-HIV-1 antibodies (BiAbs) that can bind bivalently by virtue of one scFv arm that binds to gp120 and a second arm to the gp41 subunit of gp160 showed enhanced neutralization [Mouquet et al. 2012]. Thus, antibodies engineered to contain different combining sites could be potential neutralizers and therapeutic candidates against HIV-1. The actual in vivo therapeutic or prophylactic value could also be measured in efficacy trials in the future.
Summary
A major goal towards finding an effective intervention to HIV/AIDS is to develop an effective vaccine which can check the new acquisitions and the therapy for already infected individuals. Antiretroviral therapy based on small-molecule drugs against reverse transcriptase has paid rich dividends and increased the life expectancy of infected people; however, several limitations need to be considered. The circulating virus can develop resistance to therapy and rebounds after cessation of therapy. In addition, side effects associated with antiviral therapy translate into an increasing economic burden. While an HIV vaccine has been sought after for some time, the development of a suitable immunogen has been hampered by the extreme genetic diversity, mutability of envelope protein, and immunological constraints such as affinity maturation. In the recent years several potent bNAbs have been isolated from HIV-positive patients which showed a great potential to neutralize primary viruses and their combination has yielded promising results as a therapeutic intervention [Burton et al., 2012b; Klein et al. 2012b]. In addition, many new dAbs have also been isolated which showed enhanced antiviral activity. While designing appropriate immunogens towards eliciting NAb response is of high priority [Burton et al., 2012a; Haynes et al. 2012b], passive immunity mediated by the combination of antibodies can be an effective prophylactic control against HIV in high-risk people and at the same time represents an alternative to combination therapy in infected individuals. The alteration of properties in engineered antibodies such as improved accessibility of recessed epitopes and enhanced delivery to lymphoid and mucosal tissues can improve their efficacy. The inclusion of such antibodies in the mainstream repertoire of full-size antibodies may enhance the overall antiviral effect by combining effector and neutralization functions. No vaccine has provided complete protection against HIV but substantial progress and understanding has been made as to how potent antibodies neutralize the virus and what are the vulnerable epitopes on the HIV-1 envelope. However, it is less clear how these bNAbs are eventually developed in HIV-positive patients due to the extremely complex antibody repertoire activation and maturation pathways. A deeper understanding of crucial specificities and the isolation of more broad and potent antibodies can coalesce to pave the way to the design of an effective therapy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Contributor Information
Rajesh Ringe, Weill Medical College of Cornell University, New York, NY, USA.
Jayanta Bhattacharya, International AIDS Vaccine Initiative (IAVI), THSTI-IAVI HVTR Laboratory, Translational Health Science and Technology Institute (THSTI), Gurgaon-122016, Haryana, India..
References
- Alam S., Liao H., Dennison S., Jaeger F., Parks R., Anasti K., et al. (2011) Differential reactivity of germ line allelic variants of a broadly neutralizing HIV-1 antibody to a gp41 fusion intermediate conformation. J Virol 85: 11725–11731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S., Morelli M., Dennison S., Liao H., Zhang R., Xia S., et al. (2009) Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A 106: 20234–20239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster C., Stiegler G., Vcelar B., Jager W., Koller U., Jilch R., et al. (2004) Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother 54: 915–920 [DOI] [PubMed] [Google Scholar]
- Armbruster C., Stiegler G., Vcelar B., Jager W., Michael N., Vetter N., et al. (2002) A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. Aids 16: 227–233 [DOI] [PubMed] [Google Scholar]
- Astronomo R., Lee H., Scanlan C., Pantophlet R., Huang C., Wilson I., et al. (2008) A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol 82: 6359–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L., Dolin R. (2012) The Road to an Effective HIV Vaccine. N Engl J Med 366: 1343–1344 [DOI] [PubMed] [Google Scholar]
- Balazs A., Chen J., Hong C., Rao D., Yang L., Baltimore D. (2012) Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481: 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar K., Tsao C., Iyer S., Decker J., Yang Y., Bonsignori M., et al. (2012) Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog 8(5): e1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley J., Wrin T., Korber B., Zwick M., Wang M., Chappey C., et al. (2004) Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 78: 13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Barnett S. (2007) Broadening our view of protective antibody responses against HIV. Curr HIV Res 5: 625–641 [DOI] [PubMed] [Google Scholar]
- Burton D., Ahmed R., Barouch D., Butera S., Crotty S., Godzik A., et al. (2012a) A Blueprint for HIV Vaccine Discovery. Cell Host Microbe 12: 396–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D., Desrosiers R., Doms R., Koff W., Kwong P., Moore J., et al. (2004) HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5: 233–236 [DOI] [PubMed] [Google Scholar]
- Burton D., Poignard P., Stanfield R., Wilson I. (2012b) Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337: 183–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D., Pyati J., Koduri R., Sharp S., Thornton G., Parren P., et al. (1994) Efficient neutralization of primary isolates of HIV- 1 by a recombinant human monoclonal antibody. Science 266: 1024–1027 [DOI] [PubMed] [Google Scholar]
- Burton D., Stanfield R., Wilson I. (2005) Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A 102: 14943–14948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarese D., Scanlan C., Zwick M., Deechongkit S., Mimura Y., Kunert R., et al. (2003) Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300: 2065–2071 [DOI] [PubMed] [Google Scholar]
- Cardoso R., Zwick M., Stanfield R., Kunert R., Binley J., Katinger H., et al. (2005) Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22: 163–173 [DOI] [PubMed] [Google Scholar]
- Casadevall A. (2002) Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis 8: 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L., Emes C., Power J., Duval M., Posner M. (1994) Effect of antibody valency on interaction with cell-surface expressed HIV-1 and viral neutralization. J Immunol 152: 2538–2545 [PubMed] [Google Scholar]
- Chamow S., Duliege A., Ammann A., Kahn J., Allen J., Eichberg J., et al. (1992) CD4 immunoadhesins in anti-HIV therapy: new developments. Int J Cancer Suppl 7: 69–72 [PubMed] [Google Scholar]
- Chen W., Dimitrov D. (2009) Human monoclonal antibodies and engineered antibody domains as HIV-1 entry inhibitors. Curr Opin HIV AIDS 4: 112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Dimitrov D. (2012) Monoclonal antibody-based candidate therapeutics against HIV type 1. AIDS Res Hum Retroviruses 28: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Ying T., Dimitrov D. (2013) Antibody-based candidate therapeutics against HIV-1: implications for virus eradication and vaccine design. Expert Opin Biol Ther 13: 657–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhu Z., Feng Y., Dimitrov D. (2008a) Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc Natl Acad Sci U S A 105: 17121–17126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhu Z., Feng Y., Dimitrov D. (2010) A large human domain antibody library combining heavy and light chain CDR3 diversity. Mol Immunol 47: 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhu Z., Feng Y., Xiao X., Dimitrov D. (2008b) Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J Mol Biol 382: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Lanzavecchia A. (2013) Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31: 705–742 [DOI] [PubMed] [Google Scholar]
- D’Souza M., Livnat D., Bradac J., Bridges S. (1997) Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. AIDS Clinical Trials Group Antibody Selection Working Group. J Infect Dis 175: 1056–1062 [DOI] [PubMed] [Google Scholar]
- Davis K., Gray E., Moore P., Decker J., Salomon A., Montefiori D., et al. (2009) High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology 387: 414–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormitzer P., Ulmer J., Rappuoli R. (2008) Structure-based antigen design: a strategy for next generation vaccines. Trends Biotechnol 26: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E., Ramos A., Feng Y., Zhou T., Moquin S., Walker L., et al. (2012) PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J Virol 86: 4394–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn N., Forthal D., Harro C., Judson F., Mayer K., Para M. (2005) Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 191: 654–665 [DOI] [PubMed] [Google Scholar]
- Forsman A., Beirnaert E., Aasa-Chapman M., Hoorelbeke B., Hijazi K., Koh W., et al. (2008) Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J Virol 82: 12069–12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G., Peng H., Rits-Volloch S., Morelli M., Cheng Y., Chen B. (2008) A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A 105: 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Peterson M., Follmann D., Hudgens M., Francis D., Gurwith M., et al. (2005) Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis 191: 666–677 [DOI] [PubMed] [Google Scholar]
- Gong R., Chen W., Dimitrov D. (2012) Candidate antibody-based therapeutics against HIV-1. BioDrugs 26: 143–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B., Mascola J. (2005) Lessons from failure—preparing for future HIV-1 vaccine efficacy trials. J Infect Dis 191: 647–649 [DOI] [PubMed] [Google Scholar]
- Haynes B., Gilbert P., McElrath M., Zolla-Pazner S., Tomaras G., Alam S., et al. (2012a) Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366: 1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B., Kelsoe G., Harrison S., Kepler T. (2012b) B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 30: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B., Montefiori D. (2006) Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines 5: 579–595 [DOI] [PubMed] [Google Scholar]
- Hessell A., Hangartner L., Hunter M., Havenith C., Beurskens F., Bakker J., et al. (2007) Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449: 101–104 [DOI] [PubMed] [Google Scholar]
- Hessell A., Rakasz E., Poignard P., Hangartner L., Landucci G., Forthal D., et al. (2009) Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5(5): e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A., Lutje Hulsik D., Forsman A., Koh W., Belrhali H., Gorlani A., et al. (2010) Crystal structure of the neutralizing Llama V(HH) D7 and its mode of HIV-1 gp120 interaction. PLoS One 5(5): e10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L., Herring C., Jespers L., Woolven B., Tomlinson I. (2003) Domain antibodies: proteins for therapy. Trends Biotechnol 21: 484–490 [DOI] [PubMed] [Google Scholar]
- Huang J., Ofek G., Laub L., Louder M., Doria-Rose N., Longo N., et al. (2012) Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491: 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., von Wyl V., Ammann C., Kuster H., Stiegler G., Katinger H., et al. (2008) Potent human immunodeficiency virus-neutralizing and complement lysis activities of antibodies are not obligatorily linked. J Virol 82: 3834–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausmally L., Waalen K., Lobersli I., Hvattum E., Berntsen G., Michaelsen T., et al. (2004) Neutralizing human antibodies to varicella-zoster virus (VZV) derived from a VZV patient recombinant antibody library. J Gen Virol 85: 3493–3500 [DOI] [PubMed] [Google Scholar]
- Klasse P., Sattentau Q. (2002) Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 83: 2091–2108 [DOI] [PubMed] [Google Scholar]
- Klein F., Diskin R., Scheid J., Gaebler C., Mouquet H., Georgiev I., et al. (2013) Somatic Mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153: 126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Gaebler C., Mouquet H., Sather D., Lehmann C., Scheid J., et al. (2012a) Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med 209: 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Gnanapragasam P., Galimidi R., Foglesong C., West A., Jr, Bjorkman P. (2009) Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc Natl Acad Sci U S A 106: 7385–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Halper-Stromberg A., Horwitz J., Gruell H., Scheid J., Bournazos S., et al. (2012b) HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492: 118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W., Steffensen S., Gonzalez-Pajuelo M., Hoorelbeke B., Gorlani A., Szynol A., et al. (2010) Generation of a family-specific phage library of llama single chain antibody fragments that neutralize HIV-1. J Biol Chem 285: 19116–19124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J., Nkolola J., Peng H., Cheung A., Perry J., Miller C., et al. (2012) HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci U S A 109: 12111–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Lapedes A., Tang H., Gnanakaran S., Daniels M., Zhang M., et al. (2009) Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C HIV-1 Env clones from India. Virology 385: 505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P., Wilson I. (2009) HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol 1: 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P., Wyatt R., Robinson J., Sweet R., Sodroski J., Hendrickson W. (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393: 648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn A., Poignard P., Raja A., Zwick M., Delgado K., Franti M., et al. (2003) Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77: 10557–10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. (2006) Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol 6: 930–939 [DOI] [PubMed] [Google Scholar]
- Manrique A., Rusert P., Joos B., Fischer M., Kuster H., Leemann C., et al. (2007) In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J Virol 81: 8793–8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Wang L., Gu C., Herschhorn A., Xiang S., Haim H., et al. (2012) Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat Struct Mol Biol 19: 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J. (2003) Defining the protective antibody response for HIV-1. Curr Mol Med 3: 209–216 [DOI] [PubMed] [Google Scholar]
- Mascola J., Lewis M., Stiegler G., Harris D., VanCott T., Hayes D., et al. (1999) Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73: 4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J., Louder M., VanCott T., Sapan C., Lambert J., Muenz L., et al. (1997) Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol 71: 7198–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J., Montefiori D. (2010) The role of antibodies in HIV vaccines. Annu Rev Immunol 28: 413–444 [DOI] [PubMed] [Google Scholar]
- Mascola J., Stiegler G., VanCott T., Katinger H., Carpenter C., Hanson C., et al. (2000) Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6: 207–210 [DOI] [PubMed] [Google Scholar]
- Matz J., Kessler P., Bouchet J., Combes O., Ramos O., Barin F., et al. (2013) Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved CD4 and coreceptor binding sites of HIV-1 gp120. J Virol 87: 1137–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy L., Weiss R. (2013) Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med 210: 209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J., Pancera M., Carrico C., Gorman J., Julien J., Khayat R., et al. (2011) Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480: 336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. (2006) HIV vaccines. Annu Rev Immunol 24: 227–255 [DOI] [PubMed] [Google Scholar]
- McMichael A., Hanke T. (2002) The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat Rev Immunol 2: 283–291 [DOI] [PubMed] [Google Scholar]
- Moldt B., Shibata-Koyama M., Rakasz E., Schultz N., Kanda Y., Dunlop D., et al. (2012) A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 86: 6189–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.Y., Swiderek K., Weisgrau K.L., et al. (2012) Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci (USA) 109: 18921–18925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D., Morris L., Ferrari G., Mascola J. (2007a) Neutralizing and other antiviral antibodies in HIV-1 infection and vaccination. Curr Opin HIV AIDS 2: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D., Sattentau Q., Flores J., Esparza J., Mascola J. (2007b) Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS Med 4(12): e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M., Phogat S., Shu Y., Labrijn A., Xiao X., Binley J., et al. (2002) Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci U S A 99: 6913–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Nussenzweig M. (2012) Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol Life Sci 69: 1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Scharf L., Euler Z., Liu Y., Eden C., Scheid J., et al. (2012) Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109: E3268–E3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Scheid J., Zoller M., Krogsgaard M., Ott R., Shukair S., et al. (2010) Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 467: 591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek G., Tang M., Sambor A., Katinger H., Mascola J., Wyatt R., et al. (2004) Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol 78: 10724–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M., McLellan J., Wu X., Zhu J., Changela A., Schmidt S., et al. (2010) Crystal structure of PG16 and chimeric dissection with somatically related PG9: Structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol 84: 8098–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Koup R. (2004) Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med 10: 806–810 [DOI] [PubMed] [Google Scholar]
- Pantophlet R., Burton D. (2006) GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24: 739–769 [DOI] [PubMed] [Google Scholar]
- Parren P., Marx P., Hessell A., Luckay A., Harouse J., Cheng-Mayer C., et al. (2001) Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75: 8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R., Doores K., Walker L., Khayat R., Huang P., Wang S., et al. (2011) A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334: 1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phogat S., Wyatt R. (2007) Rational modifications of HIV-1 envelope glycoproteins for immunogen design. Curr Pharm Des 13: 213–227 [DOI] [PubMed] [Google Scholar]
- Plotkin S. (2008) Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47: 401–409 [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R., et al. (2009) Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209–2220 [DOI] [PubMed] [Google Scholar]
- Ringe R., Bhattacharya J. (2012) Association of enhanced HIV-1 neutralization by a single Y681H substitution in gp41 with increased gp120-CD4 interaction and macrophage Infectivity. PLoS One 7(5): e37157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roben P., Moore J., Thali M., Sodroski J., Barbas C., III, Burton D. (1994) Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol 68: 4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan C., Pantophlet R., Wormald M., Ollmann Saphire E., Stanfield R., Wilson I., et al. (2002) The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1–>2 mannose residues on the outer face of gp120. J Virol 76: 7306–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan C., Pantophlet R., Wormald M., Saphire E., Calarese D., Stanfield R., et al. (2003) The carbohydrate epitope of the neutralizing anti-HIV-1 antibody 2G12. Adv Exp Med Biol 535: 205–218 [DOI] [PubMed] [Google Scholar]
- Scheid J., Mouquet H., Feldhahn N., Seaman M., Velinzon K., Pietzsch J., et al. (2009) Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458: 636–640 [DOI] [PubMed] [Google Scholar]
- Scheid J., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T., et al. (2011) Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333: 1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schief W., Ban Y., Stamatatos L. (2009) Challenges for structure-based HIV vaccine design. Curr Opin HIV AIDS 4: 431–440 [DOI] [PubMed] [Google Scholar]
- Shen X., Dennison S., Liu P., Gao F., Jaeger F., Montefiori D., et al. (2010) Prolonged exposure of the HIV-1 gp41 membrane proximal region with L669S substitution. Proc Natl Acad Sci U S A 107: 5972–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek M., Rida W., Priddy F., Pung P., Carrow E., Laufer D., et al. (2009) Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83: 7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L., Morris L., Burton D., Mascola J. (2009) Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15: 866–870 [DOI] [PubMed] [Google Scholar]
- Stiegler G., Kunert R., Purtscher M., Wolbank S., Voglauer R., Steindl F., et al. (2001) A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 17: 1757–1765 [DOI] [PubMed] [Google Scholar]
- Strokappe N., Szynol A., Aasa-Chapman M., Gorlani A., Forsman Quigley A., Hulsik D., et al. (2012) Llama antibody fragments recognizing various epitopes of the CD4bs neutralize a broad range of HIV-1 subtypes A, B and C. PLoS One 7(3): e33298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Lanzavecchia A., Karjalainen K. (1992) Janusin: new molecular design for bispecific reagents. Int J Cancer Suppl 7: 51–52 [PubMed] [Google Scholar]
- Trkola A., Kuster H., Rusert P., Joos B., Fischer M., Leemann C., et al. (2005) Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11: 615–622 [DOI] [PubMed] [Google Scholar]
- Vanlandschoot P., Stortelers C., Beirnaert E., Ibanez L., Schepens B., Depla E., et al. (2011) Nanobodies(R): new ammunition to battle viruses. Antiviral Res 92: 389–407 [DOI] [PubMed] [Google Scholar]
- Veazey R., Shattock R., Pope M., Kirijan J., Jones J., Hu Q., et al. (2003) Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9: 343–346 [DOI] [PubMed] [Google Scholar]
- Walker B., Burton D. (2008) Toward an AIDS vaccine. Science 320: 760–764 [DOI] [PubMed] [Google Scholar]
- Walker L., Huber M., Doores K., Falkowska E., Pejchal R., Julien J., et al. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477: 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L., Phogat S., Chan-Hui P., Wagner D., Phung P., Goss J., et al. (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326: 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L., Simek M., Priddy F., Gach J., Wagner D., Zwick M., et al. (2010) A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog 6(8): e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Decker J., Wang S., Hui H., Kappes J., Wu X., et al. (2003) Antibody neutralization and escape by HIV-1. Nature 422: 307–312 [DOI] [PubMed] [Google Scholar]
- West A., Jr, Galimidi R., Foglesong C., Gnanapragasam P., Klein J., Bjorkman P. (2010) Evaluation of CD4-CD4i antibody architectures yields potent, broadly cross-reactive anti-human immunodeficiency virus reagents. J Virol 84: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A., Jr, Galimidi R., Gnanapragasam P., Bjorkman P. (2012) Single-chain Fv-based anti-HIV proteins: potential and limitations. J Virol 86: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Changela A., O’Dell S., Schmidt S., Pancera M., Yang Y., et al. (2011a) Immunotypes of a quaternary site of HIV-1 vulnerability and their recognition by antibodies. J Virol 85: 4578–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Yang Z., Li Y., Hogerkorp C., Schief W., Seaman M., et al. (2010) Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329: 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhou T., Zhu J., Zhang B., Georgiev I., Wang C., et al. (2011b) Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333: 1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R., Kwong P., Desjardins E., Sweet R., Robinson J., Hendrickson W., et al. (1998) The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393: 705–711 [DOI] [PubMed] [Google Scholar]
- Zhang M., Shu Y., Sidorov I., Dimitrov D. (2004) Identification of a novel CD4i human monoclonal antibody Fab that neutralizes HIV-1 primary isolates from different clades. Antivir Res 61: 161–164 [DOI] [PubMed] [Google Scholar]
- Zhou T., Georgiev I., Wu X., Yang Z., Dai K., Finzi A., et al. (2010) Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329: 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S. (2004) Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol 4: 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S., Cardozo T. (2010) Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat Rev Immunol 10: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]