Abstract
A human immunodeficiency virus (HIV) vaccine remains a central component in the quest to control the worldwide epidemic. To examine the status of the development of HIV vaccines, we review the results of the efficacy trials carried out to date and the immunologic principles that guided them. Four vaccine concepts have been evaluated in HIV-1 vaccine efficacy trials, and the results of these trials have provided significant information for future vaccine development. While one of these trials demonstrated that a safe and effective HIV vaccine is possible, many questions remain regarding the basis for the observed protection and the most efficient way to stimulate it. Novel HIV vaccine strategies including induction of highly potent broadly neutralizing antibodies, use of novel homologous and heterologous vector systems, and vectored immunoprophylaxis seek to expand and build upon the knowledge gained from these trials.
Keywords: HIV vaccine, HIV vaccine clinical trials, HIV vaccine review, AIDSVAX, Step, RV144, HVTN 505
Introduction
Despite almost 30 years of intensive research, a human immunodeficiency virus (HIV) vaccine remains elusive. Effective vaccines generally stimulate protective immunity similar to that which occurs during natural infection. However, naturally acquired immunity against HIV infection may not exist, which presents an unprecedented challenge for vaccine development. The mechanism by which an HIV vaccine might confer protection therefore remains uncertain, and an effective vaccine may require induction of an immune response that is significantly different from that seen during natural infection [Johnston and Fauci, 2011]. The extreme diversity of HIV presents another challenge to vaccine design. Considering its relatively recent origin, the diversity of HIV, and HIV-1 in particular, is extraordinary. Within the main HIV-1 subgroup, Group M, there are nine clades as well as dozens of recombinant forms, and clades can vary up to 42% at the amino acid level [Hemelaar, 2012]. A vaccine immunogen derived from a particular clade may therefore be ineffective against other clades, posing a significant obstacle to the creation of a global HIV vaccine.
In this review, we examine the current status of HIV vaccine research by reviewing the results of candidate HIV vaccine efficacy trials and the immunologic principles that guided them. We also review novel approaches that seek to build upon the strategies used in those trials. Four vaccine concepts have been evaluated in efficacy trials to date. The VAX004 and VAX003 trials evaluated the first concept, a protein subunit vaccine. The second concept, a recombinant adenovirus vector, was evaluated in the Step and HIV Vaccine Trials Network (HVTN) 503/Phambili trials. The third concept, a canarypox vector prime followed by a protein subunit boost, was evaluated in the RV144 trial. The fourth concept, a DNA prime followed by a recombinant adenovirus vector boost, was recently evaluated in the HVTN 505 trial. These trials are presented in chronological order, and their results are summarized in Table 1. We conclude this review with a discussion of additional novel strategies that have the potential to significantly advance HIV vaccine development.
Table 1.
HIV vaccine efficacy trials.
| Efficacy trial | Vaccine | Population | N | Efficacy | Other significant results | Immune response | Immune correlates of risk |
|---|---|---|---|---|---|---|---|
| VAX004 | AIDSVAX B/B (rgp120 immunogens) | Primarily high-risk MSM | 5403 | None [Flynn et al. 2005] | N/A | Weak nAb response [Gilbert et al. 2010] | N/A |
| VAX003 | AIDSVAX B/E (rgp120 immunogens) | Injection drug users | 2546 | None [Pitisuttithum et al. 2006] | N/A | Weak nAb response [Montefiori et al. 2012] | N/A |
| Step | MRKAd5 HIV-1 (rAd5 vector expressing Gag, Pol, and Nef) | Primarily high-risk MSM | 3000 | None; trial halted after meeting prespecified futility boundaries [Buchbinder et al. 2008] | Significantly increased risk of HIV infection in men who were both Ad5-seropositive and uncircumcised, which waned with time since vaccination [Duerr et al. 2012] | CD8+ T-cell response detected in the majority of vaccinees, although weak and of narrow breadth [McElrath et al. 2008] | N/A |
| HVTN 503/Phambili | Same as Step | Primarily heterosexuals | 801 | None; enrollment halted after lack of efficacy seen in Step [Gray et al. 2011b] | N/A | Similar to Step [Gray et al. 2011b] | N/A |
| RV144 | ALVAC (canarypox vector expressing Env, Gag, and Pol) prime followed by AIDSVAX B/E boost | Primarily low-risk heterosexuals | 16,402 | 31.2% overall efficacy for prevention of HIV-1 infection in the modified intention-to-treat analysis [Rerks-Ngarm et al. 2009]; no subsequent effect on viremia or CD4 count in vaccinees who were infected [Rerks-Ngarm et al. 2012] | 68% efficacy for low or medium risk participants, no efficacy in the high-risk group; efficacy was highest over the first 12 months and then fell rapidly [Robb et al. 2012] | Weak nAb response [Montefiori et al. 2012]. Moderate CD8+ and CD4+ T- cell response; the CD4+ T-cell response was directed against the V2 region of Env [de Souza et al. 2012] | Binding of IgG to the V1 and V2 regions of Env correlated with protection; protection was mitigated by the presence of plasma IgA directed against Env [Haynes et al. 2012a] |
| HVTN 505 | VRC-HIVDNA016-00-VP (DNA expressing Gag, Pol, Nef, and Env) prime followed by VRC-HIVADV014-00-VP (rAd5 expressing Gag, Pol, and Env) boost | High-risk MSM | 2504 | None; trial halted after meeting prespecified futility boundaries | Awaiting final trial results | Awaiting final trial results | N/A |
HIV, human immunodeficiency virus; HVTN, HIV Vaccine Trials Network; IgG, immunoglobulin G; MSM, men who have sex with men; nAb, neutralizing antibody; rgp, recombinant glycoprotein; rAd5, recombinant adenovirus serotype 5.
Protein subunit vaccines: the VAX trials and the search for neutralizing antibodies
The first HIV vaccine candidates to enter clinical trials were protein subunit vaccines. Prior to the development of protein subunit vaccines, both attenuated and inactivated vaccines had been tested in nonhuman primates (NHPs), but neither concept advanced to human trials [Girard et al. 2011]. Compared with other vaccine approaches such as viral vectors and DNA plasmids, protein subunits offer the advantage of considerable experience related to vaccine design and production, since an effective protein subunit vaccine has been developed for influenza A and B infections. Protein subunit vaccines for HIV are based on the HIV envelope. The HIV envelope is composed of glycoproteins, gp120 and gp41, which are cleaved from a gp160 precursor. The mature envelope spike forms as a trimer, composed of three gp120/gp41 complexes. Both recombinant gp160 (rgp160) and recombinant gp120 (rgp120) monomers were studied as immunogens in early HIV vaccine clinical trials. An rgp160 vaccine induced neutralizing antibodies against the homologous vaccine strain but not against heterologous strains, and stimulated limited antibody responses in general [Dolin et al. 1991; Keefer et al. 1994]. An rgp120 vaccine demonstrated somewhat improved immunogenicity in a phase I trial, including some neutralizing activity against a heterologous strain [Schwartz et al. 1993]. A similar rgp120 immunogen derived from a different HIV-1 strain (MN) conferred protection against heterologous strains in chimpanzees [Berman et al. 1996], and was found to be safe and immunogenic in humans [Migasena et al. 2000]. This immunogen served as the basis for the AIDSVAX vaccines used in the VAX004 and VAX003 trials.
VAX004 was the first HIV vaccine efficacy trial and began enrollment in 1998. It was conducted mostly in high-risk men who have sex with men (MSM) in North America and the Netherlands, and evaluated the AIDSVAX B/B vaccine. AIDSVAX B/B contained rgp120 immunogens from strains MN and GNE8. VAX003, which began enrollment in 1999, was conducted in injection drug users in Thailand. It evaluated AIDSVAX B/E, which contained rgp120 immunogens from strains MN and A244. MN was a laboratory-adapted strain, while GNE8 and A244 were primary isolates. The primary isolates were added as a result of evidence demonstrating that laboratory-adapted viruses differ from primary isolates in a number of respects, including the use of the CXCR4 coreceptor rather than CCR5, and increased sensitivity to neutralization. Efficacy against acquisition of infection was not demonstrated in either trial (Figures 1 and 2) [Flynn et al. 2005; Pitisuttithum et al. 2006]. The vaccines used in both trials elicited antibodies capable of neutralizing tier 1 viruses, which are sensitive to neutralization, but had little activity against tier 2 viruses, which are more resistant to neutralization [Gilbert et al. 2010; Montefiori et al. 2012]. These trials demonstrated that rgp120 monomers elicited limited neutralizing antibody responses and failed to protect against HIV infection in a high-risk population.
Figure 1.
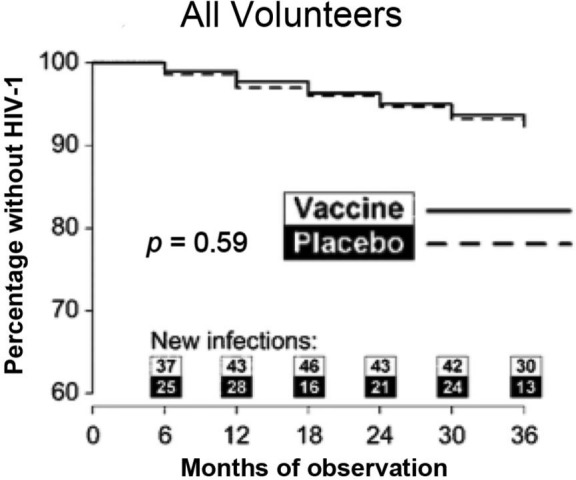
Kaplan–Meier curve from VAX004 showing time to human immunodeficiency virus type 1 (HIV-1) infection. (Reproduced from Flynn et al. [2005] with permission from Oxford University Press.)
Figure 2.

Kaplan–Meier curve from VAX003 showing time to human immunodeficiency virus type 1 (HIV-1) infection, p = 0.99. (Reproduced from Pitisuttithum et al. [2006] with permission from Oxford University Press.)
Current HIV protein subunit immunogens aim to elicit improved neutralizing antibody responses by more accurately representing the native viral envelope. The rgp120 monomers used in the VAX trials had the advantage of being relatively easy to produce, but lacked conformational structures of the native envelope trimer. Many of the critical epitopes that are targets of neutralizing antibodies, including the CD4-binding site, are conformational in nature and highly dependent on the three-dimensional structure of the envelope trimer [Gorny et al. 2005; Pantophlet and Burton, 2006; McElrath and Haynes, 2010; Moore et al. 2011]. A number of methods have been used in an attempt to stabilize recombinant trimers and thus make them suitable for production on a large scale [Phogat and Wyatt, 2007]. However, most recombinant trimers tested to date have demonstrated only marginally better immunogenicity compared with monomers [Grunder et al. 2005; Kim et al. 2005; Beddows et al. 2007], likely as a result of an inability to accurately represent the characteristics of the native envelope trimer. Another feature of the native viral envelope that may play an important role in the induction of neutralizing antibodies is glycosylation. While HIV envelope glycans have long been thought to play a role in shielding underlying epitopes from neutralizing antibodies, it has been appreciated that these glycans can serve as targets of neutralization as well [Kwong and Mascola, 2012; Moore et al. 2012; Lavine et al. 2012]. For proper glycosylation of protein subunits, immunogens need to be produced in specific human cell lines [Raska et al. 2010]; these cell lines were not utilized to produce the AIDSVAX vaccines. Recently, a stable envelope trimer has been developed that more closely represents antigenic properties of the native envelope trimer, including glycosylation. This trimer has been shown to elicit improved neutralizing antibody responses compared with monomers in guinea pigs [Kovacs et al. 2012]. The manufacturing of clinical grade material is under way, and the trimer will likely enter phase I trials in the next 1–2 years.
A recent major advance with important implications for vaccine design has been the detection of highly potent broadly neutralizing antibodies (bnAbs) against HIV-1. Examples include PG9 and PG16, with a breadth of around 80% [Walker et al. 2009], VRC-01, with a breadth of around 90% [Wu et al. 2010], and 10E8, which neutralizes 98% of tested viruses [Huang et al. 2012]. Other bnAbs have been described that do not attain the same degree of neutralization breadth but are almost 10-fold more potent than PG9, PG16, and VRC01 [Walker et al. 2011]. Structural modification techniques have also been employed to further enhance the potency and breadth of another bnAb, VRC07 [Kwon et al. 2012]. The bnAbs described to date share a number of characteristics. All recognize one of four different sites on the viral envelope spike: the CD4 binding site on gp120, the first and second variable regions (V1/V2) on gp120, the glycan-V3 site on gp120, or the membrane-proximal external region of gp41. In addition, all bnAbs have unusual features: either an uncommonly long complementarity-determining region, extensive somatic mutation, or both [Burton et al. 2012; Kwong and Mascola, 2012]. Passive immunization with bnAbs has been shown to confer robust protection against chimeric simian/human immunodeficiency virus (SHIV) infection in NHPs [Moldt et al. 2012; Hessell et al. 2009, 2010].
Novel strategies will likely be required to develop vaccines capable of stimulating bnAbs against HIV-1. The unusual features of bnAbs described above are highly atypical and only rarely occur in the course of ordinary antibody production. In addition, a number of bnAbs are polyreactive to host antigens, which serves as yet another obstacle to their development [McElrath and Haynes, 2010]. Even in the setting of chronic infection with HIV-1, bnAbs only arise in 10–30% of individuals after a period of 2–4 years [Gray et al. 2011a; Mikell et al. 2011; Moore et al. 2011]. The ability of traditional vaccine strategies to stimulate bnAbs is therefore uncertain, since the immune system may not generate such antibodies without extensive somatic mutation or may suppress the production of these antibodies. A new strategy termed B-cell-lineage vaccine design seeks to increase the elicitation of bnAbs by driving antibody responses along the desired B-cell maturation pathway [Haynes et al. 2012b]. B-cell-lineage vaccine design consists of identifying B cells that produce bnAbs and then inferring how those cells evolved from their naïve B-cell ancestor. Vaccine immunogens would then be designed to direct B-cell maturation accordingly. Interestingly, the antigen that stimulates the mature B cell may differ from the antigen that initially activated the naïve B cell. Similarly, different antigens may be required at each stage of B-cell development, resulting in a vaccination strategy consisting of a series of different immunogens. The evolution of the bnAb CH103 in a chronically infected individual has recently been detailed and may serve as a first step to guide the development of a vaccine based on the B-cell-lineage approach [Liao et al. 2013]. Another possible approach to increase the probability of eliciting bnAbs involves the use of adjuvants to activate enzymes that regulate somatic mutation. Activation-induced cytidine deaminase (AID) in particular has been extensively studied and may serve as a target for such an approach [Alt et al. 2013].
Viral vector vaccines: step and the cellular immune response
Cellular immunity has been shown to play an important role in the immune response against HIV infection. This was demonstrated in simian immunodeficiency virus (SIV) infection in rhesus macaques, where depletion of CD8+ T cells by administration of an anti-CD8 monoclonal antibody to chronically infected macaques resulted in a marked increase in viremia and disease progression [Schmitz et al. 1999]. Studies of elite controllers of HIV-1 infection have also shown the importance of cellular immunity in controlling viremia. Certain human leukocyte antigen (HLA) alleles, particularly HLA-B*57, HLA-B*27, and HLA-B*5701, have been correlated with the ability to control viral replication [Carrington and O’Brien, 2003], consistent with the importance of T cells in this effect. Indeed, differences in HLA-B*27-restricted CD8+ T cells between individuals who control disease and those who do not have recently been reported [Chen et al. 2012]. Of note, natural killer cells may also play a role in the HLA-mediated control of viral replication [Fadda et al. 2011], and it may be important to investigate the natural killer cell response as well in the assessment of immunogenicity of vaccine candidates. An approach to elicit a cellular immune response by vaccination is the use of recombinant viral vectors, in which a virus is engineered to express a gene of interest. Viral vectors tested as HIV vaccine candidates include viruses that are replication incompetent or poorly competent of replication in mammalian cells (canarypox and fowlpox), or viruses that have been made replication incompetent or poorly competent through the deletion of genes or through in vitro adaptation [adenoviruses, New York Vaccinia virus strain (NYVAC), and Modified Vaccinia Ankara (MVA)]. Of these vectors, recombinant adenovirus serotype 5 (rAd5) was found to be particularly immunogenic, and was selected as the vector for the Step and HVTN 503/Phambili trials, which were the first efficacy trials to evaluate an HIV vaccine designed to stimulate T-cell responses.
Both Step and HVTN 503/Phambili evaluated the same vaccine, an rAd5 vector expressing genes for Gag, Pol, and Nef from clade B viruses. Step was conducted primarily in high-risk MSM in North and South America, the Caribbean, and Australia. The trial was stopped at the first interim analysis after meeting prespecified futility boundaries for efficacy. In addition, a trend towards increased rates of HIV infection was found in male vaccinees who were Ad5 seropositive at baseline, uncircumcised, or both [Buchbinder et al. 2008]. In extended follow-up, men who were both uncircumcised and Ad5 seropositive were found to have a statistically significant increased risk of HIV infection, which waned with time from vaccination (Figure 3) [Duerr et al. 2012]. The mechanism behind the increased rates of infection in these subjects remains unclear [Barouch, 2010; Duerr et al. 2012]. While the rAd5 vaccine used in Step was found to induce CD8+ T-cell responses in a majority of vaccinees, the responses were weak and only directed against a limited number of epitopes [McElrath et al. 2008]. In vaccinees who became infected, no effect on viral load, CD4 count, or AIDS-free survival was demonstrated after 4 years. However, in a subset of infected subjects with protective HLA alleles, mean viral load was lower over time in vaccine recipients compared with placebo recipients [Fitzgerald et al. 2011]. The mechanism behind this effect is uncertain, but it raises the possibility that individuals expressing protective alleles may benefit from the vaccine. A second trial, HVTN 503/Phambili, was conducted in primarily low-risk heterosexuals in South Africa. Enrollment was halted after increased rates of HIV infection were observed in Step, and only 801 of a planned 3000 participants were enrolled [Gray et al. 2011b]. A higher rate of HIV infections was observed in vaccinees compared with placebo recipients, but the difference was not statistically significant.
Figure 3.
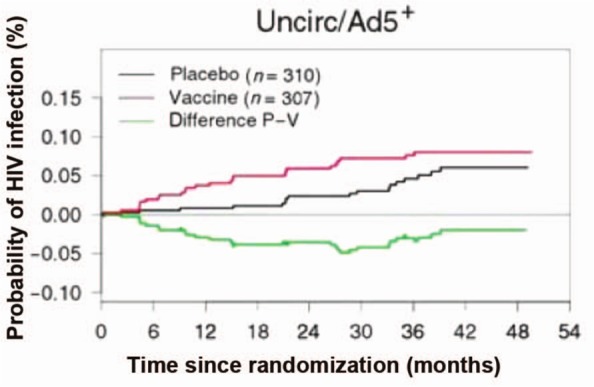
Kaplan–Meier curve from extended follow-up of the Step study, showing time to human immunodeficiency virus type 1 (HIV-1) infection in adenovirus serotype 5 (Ad5)-seropositive, uncircumcised (uncirc) men. p = 0.02 for the first 18 months of follow up. (Reproduced from Duerr et al. [2012] with permission from Oxford University Press.)
Following the results of Step, vectors based on other adenovirus serotypes with lower seroprevalence rates than Ad5 have been evaluated. Recombinant vectors using adenovirus serotype 26 (Ad26) and adenovirus serotype 35 (Ad35) were found to be immunogenic in phase I studies [Baden et al. 2012; Keefer et al. 2012]. In addition to adenovirus vectors, a number of poxvirus vectors have also been evaluated. A canarypox vector was found to have limited immunogenicity in phase I and II trials [Goepfert et al. 2005; Russell et al. 2007]. NYVAC and MVA appear to be more immunogenic than canarypox and are currently being evaluated in multiple early-phase trials [Gómez et al. 2012]. Studies in animal models using combinations of MVA, rAd26, and rAd35 have generally demonstrated improved protection against infection and superior cellular immune responses compared with homologous regimens [Barouch et al. 2012; Ratto-Kim et al. 2012]. Although only HIV vaccines based on viral vectors that are incapable or poorly capable of replication have been studied in clinical trials to date, replication-competent vectors may be more immunogenic and are also being investigated [Excler et al. 2010]. Another avenue of research into vaccines designed to elicit a CD8+ T-cell response has focused on the difference between effector memory and central memory CD8+ T cells. HIV vaccine candidates have generally elicited a central memory T-cell response, but it has been hypothesized that an effector memory T-cell response may be able to better suppress viremia following infection. Effector memory T-cell responses are primarily elicited through the presence of persistent infections, such as infection by cytomegalovirus (CMV). In a recent study, a CMV-vectored vaccine was administered to rhesus macaques that were then challenged with a highly pathogenic SIV strain, SIVmac239. Following repeated mucosal challenges, the majority of macaques that received the CMV-vectored vaccine demonstrated profound control of viremia, compared with none of the macaques that received a DNA/rAd5 vaccine [Hansen et al. 2012].
Heterologous prime-boost regimens: RV144 and a description of non-neutralizing antibodies
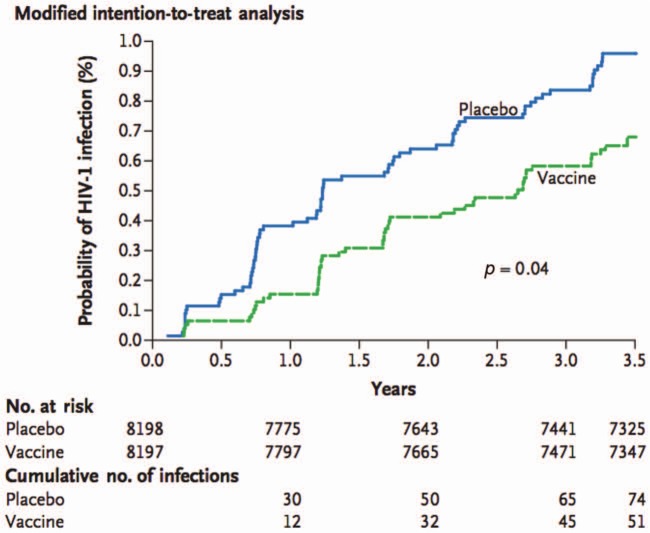
Heterologous prime-boost regimens seek to augment and broaden immune responses by combining different vaccine strategies. This approach was employed in RV144, in which a canarypox viral vector prime expressing Env, Gag, and Pol (ALVAC) was followed by an AIDSVAX B/E boost (the same protein subunit vaccine used in VAX003). The vaccines were administered in Thailand primarily to low-risk heterosexual men and women. Vaccine efficacy against acquisition of infection was found to be 31.2% in the modified intention-to-treat analysis (Figure 4) [Rerks-Ngarm et al. 2009]. In vaccinees who did become infected, no effect on CD4+ T-cell counts or viral load was observed [Rerks-Ngarm et al. 2012]. A subsequent analysis suggested that vaccine efficacy was significantly correlated with risk: efficacy was 68% for participants who maintained low or medium risk throughout the study, but only 5% in the high-risk group [Robb et al. 2012]. In addition, efficacy appeared to be highest during the first 12 months and then fell rapidly. In an analysis of immune correlates of risk, binding of immunoglobulin G (IgG) to V1/V2 was associated with a reduced risk of infection, while plasma IgA directed against Env abrogated this protection [Haynes et al. 2012a]. This latter effect may have been a result of the interference of IgA with IgG effector functions, which has previously been observed in the setting of both infection and cancer [Griffiss and Goroff, 1983; Jarvis and Griffiss, 1991; Mathew et al. 1981]. Indeed, it has recently been reported that some Env-directed IgA antibodies isolated from RV144 vaccinees are capable of blocking IgG-mediated antibody-dependent cellular cytotoxicity (ADCC) effector function [Tomaras et al. 2013]. In additional immunogenicity analyses, the RV144 regimen was found to elicit a weak neutralizing antibody response [Montefiori et al. 2012], and only a moderate T-cell response, although the CD4+ T-cell response that was elicited was directed against V2 [de Souza et al. 2012]. The possible effect of the immune response against V2 was also demonstrated by an analysis of breakthrough viral strains: vaccine efficacy against viruses matching the vaccine at amino acid position 169 was 48%, and vaccine efficacy against viruses mismatching the vaccine at position 181 was 78% [Rolland et al. 2012]. Both of these amino acids are located within the V2 region of Env.
Figure 4.
Kaplan–Meier curve from RV144 showing time to human immunodeficiency virus type 1 (HIV-1) infection in the modified intention-to-treat analysis. (Reproduced from Rerks-Ngarm et al. [2009] with permission of the Massachusetts Medical Society.)
The immune correlates analyses of RV144 did not identify neutralizing antibody or cellular immune responses that were correlated with reduction of risk, which raises the possibility that non-neutralizing antibodies against V1/V2 may play a role in protection. Following the results of RV144, non-neutralizing antibodies against V2 were also found to correlate with protection against a stringent SIV challenge in rhesus macaques vaccinated with DNA/MVA, rAd26/MVA, or MVA/rAd26 regimens [Barouch et al. 2012]. While the mechanism of protection in these trials remains unclear, non-neutralizing antibodies against V1/V2 may confer protection through effector functions such as ADCC, which occur in conjunction with cells of the innate immune system [Robinson, 2013]. The four IgG subclasses differ in their ability to mediate these effector functions, and IgG3 generally has the greatest activity [van de Winkel and Anderson, 1991]. Interestingly, while the antibody response in RV144 was weaker overall than VAX003, preliminary data suggest that the response in RV144 was skewed towards IgG3, which was not the case in VAX003 [Chung et al. 2012].
DNA vaccines and HVTN 505
DNA vaccines consist of a plasmid encoding a protein of interest. A DNA vaccine can deliver the same genes as a live-vectored vaccine without immunity developing against the vector, which may inhibit expression of the insert [Donnelly et al. 1997]. While DNA vaccines in general demonstrated promise in animal models, early candidate DNA vaccines were found to be poorly immunogenic when tested in humans for a number of viruses, including HIV [MacGregor et al. 1998; Ferraro et al. 2011]. Later generation DNA vaccines have incorporated various strategies to improve immunogenicity, including electroporation and the use of molecular adjuvants [Baden et al. 2011]. A number of DNA HIV vaccine candidates are currently being evaluated, mostly in combination with viral vectors in heterologous prime-boost regimens. In a phase I trial, a heterologous DNA/rAd5 regimen demonstrated improved CD4+ T-cell responses and increased CD8+ T-cell interleukin 2 production compared with a homologous rAd5/rAd5 regimen [Cox et al. 2008]. In another phase I trial, a DNA/rAd5 regimen elicited significantly improved T-cell and antibody responses compared with either DNA or rAd5 alone [Koup et al. 2010]. A DNA/MVA regimen was similarly found to improve the T-cell immune response compared with MVA alone, although interestingly the antibody response was superior when MVA was used without DNA priming [Goepfert et al. 2011].
The most recently conducted HIV vaccine efficacy trial, HVTN 505, is a phase IIb trial that evaluated a DNA prime followed by a rAd5-vectored boost in MSM in the United States. The DNA plasmid expressed Gag, Pol, Nef, and Env, and the rAd5 boost expressed Gag, Pol, and Env. This was in contrast to the rAd5 vaccine used in Step, which did not express Env. In an attempt to avoid the possibility of increased risk of HIV infection that occurred using the rAd5 vector in Step, only men who were circumcised and Ad5 seronegative were eligible for inclusion. The HVTN 505 trial enrolled 2504 subjects, but on 25 April 2013, the HVTN announced that the trial was halted for lack of efficacy because futility criteria were met for both primary endpoints: HIV acquisition and postacquisition viral load setpoint (the press release can be viewed at http://hvtn.org/505-announcement-25April2013.html). At the time the trial was halted, 27 HIV infections had occurred in vaccine recipients compared with 21 HIV infections in placebo recipients; however, the rates of HIV infections in vaccinees and placebo recipients were not statistically significantly different. Participants in the HVTN 505 trial will be followed closely for additional study endpoints. Further data and analyses should be forthcoming and may aid in the interpretation of the primary results.
Insights from the transmission event
The transmission of HIV-1 and the properties of transmitted viruses are being studied in detail to determine whether such information can inform development of an HIV vaccine. During chronic infection, a quasispecies of virus resides within a single individual [Korber et al. 2001]. However, studies of sexual transmission have found that in approximately 80% of cases only a single viral strain is transmitted [Keele et al. 2008; Abrahams et al. 2009]. Investigation of this ‘bottleneck’ may identify viral properties that affect the ability of a virus to establish infection. Indeed, a number of studies have found that transmitted viruses, also known as founder viruses, appear to have particular features. In an early study of three mother–infant pairs, the infants’ viral sequences were less diverse than the mothers’, and a conserved N-linked glycosylation site within the V3 region found in each of the mothers’ sequences was absent in the infants’ sequences [Wolinsky et al. 1992]. Transmitted clade C viruses were found to have shorter, less glycosylated envelope variable loops than viruses present during chronic infection, and were more sensitive to neutralization [Derdeyn et al. 2004]. In addition, the glycan at amino acid position 332 may be significantly underrepresented in transmitted clade C viruses compared with viruses present during chronic infection [Moore et al. 2012]. In a cohort of clade D and A transmission pairs, transmitted strains appeared to be more closely related to variants found early during the donor’s course of infection rather than to variants circulating in the donor near the time of transmission [Redd et al. 2012].
The above findings are complicated by the fact that both the mode of transmission and viral clade appear to play a role in the determination of the number of transmitted viruses and their particular features. While only a single variant is transmitted in approximately 80% of heterosexual transmission events, only 46% of transmitted viruses appeared to derive from a single variant in a cohort of MSM [Gottlieb et al. 2008]. A similar finding was also noted for MSM who were included in the study by Keele and colleagues cited above. In rhesus macaques, intravenous inoculation of SIV resulted in a more complex founder population than intravaginal inoculation [Greenier et al. 2001]. Even the bottleneck present during heterosexual transmission may not operate under certain conditions. A study conducted in a small heterosexual African cohort found that a single viral strain was transmitted in the vast majority of cases; however, more viral strains appeared to be transmitted in the presence of genital infection [Haaland et al. 2009]. This suggests that an intact mucosal barrier may be necessary to restrict the genetic diversity of the infecting virus. While the particular characteristics found in clade C transmitted viruses were also found in clade A viruses, the same characteristics were not noted in clade B or D viruses [Chohan et al. 2005; Frost et al. 2005; Sagar et al. 2009]. A specific viral signature that distinguishes transmitted viruses across all clades and modes of transmission has yet to be found. If transmitted viruses are in fact appreciably different from other HIV strains, future vaccines could be designed to particularly target these viruses.
The mosaic sequence insert
The diversity of HIV presents a significant challenge to the ultimate goal of creating a global vaccine. In an attempt to address this problem, mosaic sequences designed for insertion into viral vector vaccines have been developed. These genetic sequences were created using computer algorithms to maximize the coverage of potential T-cell epitopes from worldwide HIV-1 strains [Fischer et al. 2007]. T-cell epitopes are amino acid sequences presented on the surface of infected cells by HLA class 1 molecules and recognized by CD8+ T cells. By maximizing the representation of global viral strains, mosaic sequences may elicit immune responses capable of recognizing viruses from multiple clades. Studies in rhesus macaques have shown that mosaic sequences increase the breadth and depth of the T-cell response compared with consensus or natural sequences [Barouch et al. 2010; Santra et al. 2010]. However, mosaic sequences have not yet been evaluated in humans, and the immune response they will elicit remains unknown. Phase I clinical trials using mosaic sequence inserts in orthopox-vectored and adenovirus-vectored vaccines are planned in the coming year.
Vectored immunoprophylaxis
Vectored immunoprophylaxis is an approach that provides bnAbs by employing a viral vector containing inserts of immunoglobulin genes. This bypasses the difficult task of utilizing immunogens to elicit these unusual antibodies. The vector employed in a study of this approach was adeno-associated virus (AAV), into which genes were inserted that code for a bnAb against HIV-1. AAV has been extensively studied as a potential gene therapy vector because it is not known to cause disease and has been engineered so that it does not integrate into the human genome. Intramuscular administration of an AAV vector with human immunoglobulin genes into a humanized mouse resulted in prolonged expression of bnAbs and conferred protection against high-dose intravenous HIV challenges [Balazs et al. 2012]. The AAV vector is relatively inexpensive to produce and offers the possibility of long-lasting protection. However, this approach also presents concern about the ability to halt gene expression if toxicities related to immunoglobulin production were to occur. Methods to address this concern are currently being investigated.
Conclusion
Only four HIV vaccine concepts have undergone efficacy trials, and while only one has demonstrated efficacy, all four have generated important information. The VAX004/VAX003 trials indicated that a subunit vaccine composed of recombinant gp120 monomers was not effective in a high-risk population. An Ad5 vector vaccine failed to confer protection in the Step trial and appeared to be associated with an increased risk of HIV-1 infection in certain populations. The addition of a DNA prime and the inclusion of genes expressing Env to the Ad5 vector in HVTN 505 also failed to confer protection. A prime-boost regimen consisting of a canarypox vector prime and an rgp120 boost (RV144) demonstrated modest efficacy (31.2%), albeit for a short period of time. While the components of the immune response responsible for protection in RV144 are yet to be fully determined, initial studies have suggested that an antibody response to the envelope V1/V2 region may have played a role. Genetic sequences in the V2 region were also found to be associated with vaccine efficacy in an analysis of breakthrough viral strains. Interestingly, vaccination in both VAX003 and Step appeared to impact the sequences of breakthrough HIV strains as well [Shmelkov et al. 2011; Rolland et al. 2011]. This suggests that the vaccines used in those trials may have placed some selective pressure on transmitted viruses. The correlation between an HIV vaccine’s ability to exert genetic pressure on transmitted viral strains and its ability to confer protection remains to be determined.
RV144 demonstrated efficacy in a low-risk heterosexual population; however, no other trial to date has been completed in such a population (Table 1). The role of population risk factors in the ultimate success of the regimen used in RV144 is therefore unclear. As discussed above, a single viral strain is transmitted in the majority of heterosexual transmission events. Thus, a candidate vaccine administered to this population may only be required to inhibit a single viral strain, which could potentially enhance its effectiveness. The role of neutralizing antibodies in the protective effect observed in RV144 also remains unclear. Although the generation of neutralizing antibodies appeared to be weak, it is possible that a low level of neutralizing antibodies was able to confer protection in this particular setting [Bar et al. 2012].
Further studies are planned to confirm and extend the results of RV144. These studies will also evaluate viral-vector prime and protein-subunit boost regimens, and will incorporate novel vectors, inserts, and protein subunits. Vectors such as Ad26, MVA, and NYVAC have been found to be highly immunogenic, and future trials will evaluate these vectors both alone and in combination. The mosaic sequence insert, which may elicit a broader immune response than natural HIV-1 sequence inserts, will also be studied in upcoming trials. A novel recombinant glycoprotein trimer will be evaluated as a protein boost, and may elicit a more potent neutralizing antibody response than rgp120 monomers. Because the protection observed in RV144 was of limited duration, future studies evaluating similar regimens may incorporate extended immunization schedules, particularly to stimulate antibody responses, in an attempt to increase the duration of protection.
The discovery of highly potent bnAbs against HIV-1 is an observation of great importance for the development of an HIV vaccine. However, traditional vaccine strategies may be unable to elicit these antibodies. Novel strategies such as B-cell-lineage vaccine design and vectored immunoprophylaxis are being studied to elicit or provide these antibodies.
The HIV vaccine efficacy trials conducted to date have demonstrated that a safe and effective HIV vaccine is possible, and have made important contributions to our understanding of the path towards the development of such a vaccine. Future progress will depend on an iterative relationship between findings from preclinical studies and from properly designed, efficiently conducted clinical trials.
Footnotes
Funding: Generation of this review received no specific grant from any funding agency in the public, commercial, or not- for-profit sectors.
Conflict of interest statement: Dr Dolin is a consultant for Visterra, a biotechnology company.
Contributor Information
Yehuda Z. Cohen, Center for Virus and Vaccine Research, Beth Israel Deaconess Medical Center, E/CLS-1003, 330 Brookline Ave, Boston, 02215, USA
Raphael Dolin, Beth Israel Deaconess Medical Center, Boston, MA, USA.
References
- Abrahams M., Anderson J., Giorgi E., Seoighe C., Mlisana K., Ping L., et al. (2009) Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype c reveals a non-Poisson distribution of transmitted variants. J Virol 83: 3556–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F.W., Zhang Y., Meng F.L., Guo C., Schwer B. (2013) Mechanisms of Programmed DNA Lesions and Genomic Instability in the Immune System. Cell 152: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L., Blattner W., Morgan C., Huang Y., Defawe O., Sobieszczyk M., et al. (2011) Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J Infect Dis 204: 1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L., Walsh S., Seaman M., Tucker R., Krause K., Patel A., et al. (2012) First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207: 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs A., Chen J., Hong C., Rao D., Yang L., Baltimore D. (2012) Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481: 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar K., Tsao C., Iyer S., Decker J., Yang Y., Bonsignori M., et al. (2012) Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog 8: e1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D. (2010) Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS 5: 386–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D., Liu J., Li H., Maxfield L., Abbink P., Lynch D., et al. (2012) Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D., O’Brien K., Simmons N., King S., Abbink P., Maxfield L., et al. (2010) Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16: 319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddows S., Franti M., Dey A., Kirschner M., Iyer S., Fisch D., et al. (2007) A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360: 329–340 [DOI] [PubMed] [Google Scholar]
- Berman P., Murthy K., Wrin T., Vennari J., Cobb E., Eastman D., et al. (1996) Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis 173: 52–59 [DOI] [PubMed] [Google Scholar]
- Buchbinder S., Mehrotra D., Duerr A., Fitzgerald D., Mogg R., Li D., et al. (2008) Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D., Poignard P., Stanfield R., Wilson I. (2012) Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337: 183–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M., O’Brien S. (2003) The influence of HLA genotype on AIDS. Annu Rev Med 54: 535–551 [DOI] [PubMed] [Google Scholar]
- Chen H., Ndhlovu Z., Liu D., Porter L., Fang J., Darko S., et al. (2012) TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol 13: 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan B., Lang D., Sagar M., Korber B., Lavreys L., Richardson B., et al. (2005) Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1–V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol 79: 6528–6531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A., Robinson H., Ackerman M., Michael N., Kim J., Alter G. (2012) RV144 Vaccination induces a different antibody effector function profile in comparison to VAX003. Keystone Symposia, HIV Vaccines, Keystone, CO, USA, 24 March 2012 (oral presentation). [Google Scholar]
- Cox K., Clair J., Prokop M., Sykes K., Dubey S., Shiver J., et al. (2008) DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol 82: 8161–8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn C., Decker J., Bibollet-Ruche F., Mokili J. (2004) Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303: 2019–2022 [DOI] [PubMed] [Google Scholar]
- de Souza M., Ratto-Kim S., Chuenarom W., Schuetz A., Chantakulkij S., Nuntapinit B., et al. (2012) The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 188: 5166–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Graham B., Greenberg S., Tacket C., Belshe R., Midthun K., et al. (1991) The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med 114: 119–127 [DOI] [PubMed] [Google Scholar]
- Donnelly J., Ulmer J., Shiver J., Liu M. (1997) DNA vaccines. Annu Rev Immunol 15: 617–648 [DOI] [PubMed] [Google Scholar]
- Duerr A., Huang Y., Buchbinder S., Coombs R., Sanchez J., del Rio C., et al. (2012) Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step study). J Infect Dis 206: 258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excler J., Parks C., Ackland J., Rees H., Gust I., Koff W. (2010) Replicating viral vectors as HIV vaccines: summary report from the IAVI-sponsored satellite symposium at the AIDS vaccine 2009 conference. Biologicals 38: 511–521 [DOI] [PubMed] [Google Scholar]
- Fadda L., O’Connor G., Kumar S., Piechocka-Trocha A., Gardiner C., Carrington M., et al. (2011) Common HIV-1 peptide variants mediate differential binding of KIR3DL1 to HLA-Bw4 molecules. J Virol 85: 5970–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro B., Morrow M., Hutnick N., Shin T., Lucke C., Weiner D. (2011) Clinical applications of DNA vaccines: current progress. Clin Infect Dis 53: 296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Perkins S., Theiler J., Bhattacharya T., Yusim K., Funkhouser R., et al. (2007) Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 13: 100–106 [DOI] [PubMed] [Google Scholar]
- Fitzgerald D., Janes H., Robertson M., Coombs R., Frank I., Gilbert P., et al. (2011) An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step study). J Infect Dis 203: 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn N., Forthal D., Harro C., Judson F., Mayer K., Para M. (2005) Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 191: 654–665 [DOI] [PubMed] [Google Scholar]
- Frost S., Liu Y., Pond S., Chappey C., Wrin T., Petropoulos C., et al. (2005) Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J Virol 79: 6523–6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Wang M., Wrin T., Petropoulos C., Gurwith M., Sinangil F., et al. (2010) Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infec Dis 202: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Osmanov S., Assossou O., Kieny M. (2011) Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: a review. Vaccine 29: 6191–6218 [DOI] [PubMed] [Google Scholar]
- Goepfert P., Elizaga M., Sato A., Qin L., Cardinali M., Hay C., et al. (2011) Phase 1 safety and immunogenicity testing of DNA and recombinant modified Vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 203: 610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert P., Horton H., McElrath M., Gurunathan S., Ferrari G., Tomaras G., et al. (2005) High-dose recombinant Canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J Infect Dis 192: 1249–1259 [DOI] [PubMed] [Google Scholar]
- Gómez C., Perdiguero B., Garcia-Arriaza J., Esteban M. (2012) Poxvirus vectors as HIV/AIDS vaccines in humans. Hum Vaccin Immunother 8: 1192–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M., Stamatatos L., Volsky B., Revesz K., Williams C., Wang X., et al. (2005) Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J Virol 79: 5232–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G., Heath L., Nickle D., Wong K., Leach S., Jacobs B., et al. (2008) HIV-1 variation before seroconversion in men who have sex with men: analysis of acute/early HIV infection in the multicenter AIDS cohort study. J Infect Dis 197: 1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E., Madiga M., Hermanus T., Moore P., Wibmer C., Tumba N., et al. (2011a) The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 85: 4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G., Allen M., Moodie Z., Churchyard G., Bekker L., Nchabeleng M., et al. (2011b) Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 11: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenier J., Miller C., Lu D., Dailey P., Lu F., Kunstman K., et al. (2001) Route of Simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J Virol 75: 3753–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J., Goroff D. (1983) IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol 130: 2882–2885 [PubMed] [Google Scholar]
- Grundner C., Li Y., Louder M., Mascola J., Yang X., Sodroski J., et al. (2005) Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 33: 33–46 [DOI] [PubMed] [Google Scholar]
- Haaland R., Hawkins P., Salazar-Gonzalez J., Johnson A., Tichacek A., Karita E., et al. (2009) Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog 5: e1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S., Ford J., Lewis M., Ventura A., Hughes C., Coyne-Johnson L., et al. (2012) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B., Gilbert P., McElrath M., Zolla-Pazner S., Tomaras G., Alam S., et al. (2012a) Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366: 1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B., Kelsoe G., Harrison S., Kepler T. (2012b) B-cell–lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 30: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J. (2012) The origin and diversity of the HIV-1 pandemic. Trends Mol Med 18: 182–192 [DOI] [PubMed] [Google Scholar]
- Hessell A., Poignard P., Hunter M., Hangartner L., Tehrani D., Bleeker W., et al. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15: 951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A., Rakasz E., Tehrani D., Huber M., Weisgrau K., Landucci G., et al. (2010) Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 84: 1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ofek G., Laub L., Louder M., Doria-Rose N., Longo N., et al. (2012) Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491: 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis G., Griffiss J. (1991) Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J Immunol 147: 1962–1967 [PubMed] [Google Scholar]
- Johnston M., Fauci A. (2011) HIV vaccine development—improving on natural immunity. N Engl J Med 365: 873–875 [DOI] [PubMed] [Google Scholar]
- Keefer M., Gilmour J., Hayes P., Gill D., Kopycinski J., Cheeseman H., et al. (2012) A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS ONE 7: e41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer M., Graham B., Belshe R., Schwartz D., Corey L., Bolognesi D., et al. (1994) Studies of high doses of a human immunodeficiency virus type 1 recombinant glycoprotein 160 candidate vaccine in HIV type 1-seronegative humans. AIDS Res Hum Retroviruses 10: 1713–1723 [DOI] [PubMed] [Google Scholar]
- Keele B., Giorgi E., Salazar-Gonzalez J., Decker J., Pham K., Salazar M., et al. (2008) Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105: 7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Qiao Z., Montefiori D., Haynes B., Reinherz E., Liao H. (2005) Comparison of HIV Type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res Hum Retroviruses 21: 58–67 [DOI] [PubMed] [Google Scholar]
- Korber B., Gaschen B., Yusim K., Thakallapally R., Kesmir C., Detours V. (2001) Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull 58: 19–42 [DOI] [PubMed] [Google Scholar]
- Koup R., Roederer M., Lamoreaux L., Fischer J., Novik L., Nason M., et al. (2010) Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE 5: e9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J., Nkolola J., Peng H., Cheung A., Perry J., Miller C., et al. (2012) HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci U S A 109: 12111–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Georgiev I., O’Dell S., Shi W., Chuang G., Yang Y., et al. (2012) Structure-guided modification and optimization of antibody VRC07. Retrovirology 9(Suppl 2): O34 [Google Scholar]
- Kwong P., Mascola J. (2012) Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 37: 412–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine C.L., Lao S., Montefiori D.C., Haynes B.F., Sodroski J.G., Yang X., et al. (2012) High-Mannose Glycan-Dependent epitopes are frequently targeted in broad neutralizing antibody responses during Human Immunodeficiency Virus Type 1 Infection. J Virol 86: 2153–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Lynch R., Zhou T., Gao F., Alam S., Boyd S., et al. (2013) Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496: 469-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R., Boyer J., Ugen K., Lacy K., Gluckman S., Bagarazzi M., et al. (1998) First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis 178: 92–100 [DOI] [PubMed] [Google Scholar]
- Mathew G., Qualtiere L., Neel H., Pearson G. (1981) IgA antibody, antibody-dependent cellular cytotoxicity and prognosis in patients with nasopharyngeal carcinoma. Int J Cancer 27: 175–180 [DOI] [PubMed] [Google Scholar]
- McElrath M., De Rosa S., Moodie Z., Dubey S., Kierstead L., Janes H., et al. (2008) HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case–cohort analysis. Lancet 372: 1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M., Haynes B. (2010) Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33: 542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migasena S., Suntharasamai P., Pitisuttithum P., Kitayaporn D., Wasi C., Huang W., et al. (2000) AIDSVAX (MN) in Bangkok injecting drug users: a report on safety and immunogenicity, including macrophage-tropic virus neutralization. AIDS Res Hum Retroviruses 16: 655–663 [DOI] [PubMed] [Google Scholar]
- Mikell I., Sather D., Kalams S., Altfeld M., Alter G., Stamatatos L. (2011) Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 7: e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.Y., Swiderek K., Weisgrau K.L., et al. (2012) Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA 109: 18921–18925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D., Karnasuta C., Huang Y., Ahmed H., Gilbert P., de Souza M., et al. (2012) Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206: 431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Gray E., Sheward D., Madiga M., Ranchobe N., Lai Z., et al. (2011) Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J Virol 85: 3128–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Gray E., Wibmer C., Bhiman J., Nonyane M., Sheward D., et al. (2012) Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med 18: 1688–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R., Burton D. (2006) GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24: 739–769 [DOI] [PubMed] [Google Scholar]
- Phogat S., Wyatt R. (2007) Rational modifications of HIV-1 envelope glycoproteins for immunogen design. Curr Pharm Des 13: 213–227 [DOI] [PubMed] [Google Scholar]
- Pitisuttithum P., Gilbert P., Gurwith M., Heyward W., Martin M., van Griensven F., et al. (2006) Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 194: 1661–1671 [DOI] [PubMed] [Google Scholar]
- Raska M., Takahashi K., Czernekova L., Zachova K., Hall S., Moldoveanu Z., et al. (2010) Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem 285: 20860–20869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto-Kim S., Currier J., Cox J., Excler J., Valencia-Micolta A., Thelian D., et al. (2012) Heterologous prime-boost regimens using rAd35 and rMVA vectors elicit stronger cellular immune responses to HIV proteins than homologous regimens. PLoS ONE 7: e45840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd A., Collinson-Streng A., Chatziandreou N., Mullis C., Laeyendecker O., Martens C., et al. (2012) Previously transmitted HIV-1 strains are preferentially selected during subsequent sexual transmissions. J Infect Dis 206: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S., Paris R., Chunsutthiwat S., Premsri N., Namwat C., Bowonwatanuwong C., et al. (2012) Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquired HIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX B/E. J Infect Dis 207: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R., et al. (2009) Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209–2220 [DOI] [PubMed] [Google Scholar]
- Robb M., Rerks-Ngarm S., Nitayaphan S., Pitisuttithum P., Kaewkungwal J., Kunasol P., et al. (2012) Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 12: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. (2013) Non-neutralizing antibodies in prevention of HIV infection. Expert Opin Biol Ther 13: 197–207 [DOI] [PubMed] [Google Scholar]
- Rolland M., Edlefsen P., Larsen B., Tovanabutra S., Sanders-Buell E., Hertz T., et al. (2012) Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490: 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M., Tovanabutra S., deCamp A., Frahm N., Gilbert P., Sanders-Buell E., et al. (2011) Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med 17: 366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N., Graham B., Keefer M., McElrath M., Self S., Weinhold K., et al. (2007) Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: negative results fail to trigger a phase 3 correlates trial. J Acquir Immune Defic Syndr 44: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M., Laeyendecker O., Lee S., Gamiel J., Wawer M., Gray R., et al. (2009) Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis 199: 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S., Liao H., Zhang R., Muldoon M., Watson S., Fischer W. et al. (2010) Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Kuroda M., Santra S., Sasseville V., Simon M., Lifton M., et al. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–860 [DOI] [PubMed] [Google Scholar]
- Schwartz D., Clements M., Gorse G., Belshe R., Izu A., Duliege A., et al. (1993) Induction of HIV-1-neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1 IIIB rgp120 subunit vaccine. Lancet 342: 69–73 [DOI] [PubMed] [Google Scholar]
- Shmelkov E., Nadas A., Swetnam J., Zolla-Pazner S., Cardozo T. (2011) Indirect detection of an epitope-specific response to HIV-1 gp120 immunization in human subjects. PLoS ONE 6: e27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras G., Ferrari G., Shen X., Alam S., Liao H., Pollara J., et al. (2013) Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA 110: 9019-9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Winkel J., Anderson C. (1991) Biology of human immunoglobulin G Fc receptors. J Leukoc Biol 49: 511–524 [DOI] [PubMed] [Google Scholar]
- Walker L., Huber M., Doores K., Falkowska E., Pejchal R., Julien J., et al. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477: 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L., Phogat S., Chan-Hui P., Wagner D., Phung P., Goss J., et al. (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326: 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky S., Wike C., Korber B., Hutto C., Parks W., Rosenblum L., et al. (1992) Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255: 1134–1137 [DOI] [PubMed] [Google Scholar]
- Wu X., Yang Z., Li Y., Hogerkorp C., Schief W., Seaman M., et al. (2010) Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329: 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]