Abstract
The development of vaccines containing adjuvants has the potential to enhance antibody and cellular immune responses, broaden protective immunity against heterogeneous pathogen strains, enable antigen dose sparing, and facilitate efficacy in immunocompromised populations. Nevertheless, the structural interplay between antigen and adjuvant components is often not taken into account in the published literature. Interactions between antigen and adjuvant formulations should be well characterized to enable optimum vaccine stability and efficacy. This review focuses on the importance of characterizing antigen–adjuvant interactions by summarizing findings involving widely used adjuvant formulation platforms, such as aluminum salts, emulsions, lipid vesicles, and polymer-based particles. Emphasis is placed on the physicochemical basis of antigen–adjuvant associations and the appropriate analytical tools for their characterization, as well as discussing the effects of these interactions on vaccine potency.
Keywords: vaccine adjuvant, vaccine antigen, physicochemical characterization
Introduction
Modern vaccines based on recombinant antigens generally require adjuvant help to generate adequate immune responses. Even live attenuated or inactivated vaccines contain intrinsic adjuvant structures [McKee et al. 2007]. Thus, vaccines can be considered to consist of two principal components: antigen and adjuvant. In general, the mechanism of action of each of these two components is heavily investigated before a vaccine reaches licensing stage. For example, vaccine antigens are carefully screened based on bioinformatic and experimental approaches for their ability to elicit protective immunity [Flower et al. 2010]. Likewise, specific receptors and immune signaling cascades are well known for immunostimulators such as Toll-like receptor (TLR) agonists or C-type lectin receptor (CLR) agonists [Duthie et al. 2011]. While there may be less consensus regarding mechanisms of action of particulate-based adjuvants such as aluminum salts and oil-in-water emulsions, even these adjuvants have been investigated at length to generate data on a range of potential biological mechanisms [Kool et al. 2012; O’Hagan et al. 2012]. However, a thorough analysis of the physicochemical interactions between antigen and adjuvant, and the resulting optimization of those interactions, is too often lacking in the literature. The purpose of this review is to highlight the work that has been reported regarding antigen–adjuvant interactions and generate interest in the need for more investigation in this area in order to optimize vaccine formulations for stability and bioactivity.
Adjuvants are often simplistically classified as immunostimulatory molecules (TLR ligands, CLR ligands, NOD-like receptor (NLR) ligands, saponins, etc.) or delivery systems (aluminum salts, emulsions, lipid vesicles, etc.). In reality, most adjuvants are a combination of these two classes. For instance, aluminum salts and emulsions are not just delivery vehicles since they clearly generate adjuvant activity besides their potential antigen delivery functions. Likewise, immunostimulatory molecules are rarely employed in isolation; in general, they are formulated in some particle-based platform. The best illustration of this concept is perhaps the adjuvant AS04 in the Cervarix® vaccine, approved by the US Food and Drug Administration (FDA) in 2009, which is composed of aluminum oxyhydroxide and a TLR4 ligand (MPL®). Thus, the aluminum salt may serve as an adjuvant itself as well as a delivery vehicle for MPL® and/or the vaccine antigen.
In this review, emphasis is placed on adjuvant formulations rather than unformulated immunostimulatory molecules. While a few vaccines in development contain soluble unformulated immunostimulatory molecules, little information is available regarding interactions of these adjuvants with the antigen. For instance, the most advanced vaccine candidate containing a soluble immunostimulatory molecule is Dynavax’s HEPLISAV, which has completed phase III clinical testing although an FDA committee decided in November 2012 that there was insufficient data to support the safety of the vaccine [FDA, 2012]. While HEPLISAV’s hepatitis B surface antigen forms small particles, the adjuvant itself (a CpG-based TLR9 ligand known as 1018 ISS) is apparently not formulated in any particle-based platform, which may explain why a relatively high dose of 3 mg is necessary, and no information is available regarding interactions between the antigen and adjuvant in HEPLISAV [Heyward, 2012; Sablan et al. 2012].
Most vaccines that contain immunostimulatory molecules employ some type of particle-based formulation for the adjuvant molecule for stabilization, delivery, or dose-sparing purposes. For example, using tetanus toxoid antigen, Diwan and colleagues demonstrated that 10-fold dose sparing of CpG adjuvant is feasible when the adjuvant is formulated in polymeric nanoparticles compared with soluble CpG [Diwan et al. 2004]. Thus, the complete formulation (comprising the immunostimulatory molecule and the particulate platform) becomes the entity of interest when investigating interactions with the antigen. In the following sections, we focus on the interactions of adjuvant formulations with vaccine antigens, beginning with the adjuvant most widely used in vaccines today and for the last century: aluminum salts. Owing to their ubiquity, there are multiple studies delineating the effect of antigen adsorption to aluminum salts, including the affinity of the adsorption interaction and corresponding effects on antigen structure and bioactivity. Emulsions and lipid vesicles will then be addressed, the latter forming one of the most versatile formulation platforms since the antigen can be encapsulated in the vesicles or surface-conjugated. Finally, other formulations falling outside of the above traditional platforms will be discussed.
Aluminum salts
Aluminum salt adjuvants are the most commonly used class of adjuvants and were the first class of adjuvants approved for use in human vaccines [Vogel and Powell, 1995]. The safety and efficacy of these adjuvants are well established; however, their adjuvanticity is not entirely understood. Potential mechanisms of action include serving as a depot for slow antigen release, enhancing the recruitment of and subsequent uptake by immune cells, or direct stimulation of the immune system [Gupta, 1998; Gupta et al. 1995; Hem and Hogenesch, 2007; Noe et al. 2010]. Several detailed reviews on aluminum adjuvants have been written [De Souza Reboucas et al. 2012; Kamerzell et al. 2011; Wilson-Welder et al. 2009]; here we will focus on the physical properties of antigen–adjuvant interactions.
Several types of aluminum salt adjuvants are approved for use, including aluminum oxyhydroxide and aluminum phosphate, of which Alhydrogel® and Adju-Phos® are respective commercial examples [White and Hem, 2000]. At neutral pH, the difference in the physical properties of these two adjuvants is quite significant. Aluminum oxyhydroxide has a point of zero charge (PZC) at pH 11 and aluminum phosphate has a PZC at pH 4–5.5 [al-Shakhshir et al. 1995; Seeber et al. 1991]. Adsorption of antigen by adjuvant is thought to be important for immune response, so when selecting which aluminum salt to use, antigen and adjuvant charge is a critical consideration. Electrostatics are often a dominant force in antigen adsorption by adjuvant [al-Shakhshir et al. 1995]; however, hydrogen bonding, van der Waals forces, hydrophobic interactions, and ligand exchange have also been shown to play a role [al-Shakhshir et al. 1995; Iyer et al. 2004; Peek et al. 2007]. A recent report describes the generation of functionalized aluminum oxide for covalent conjugation of small molecule haptens [Maquieira et al. 2012]. Furthermore, pH, ionic strength, and buffer and excipient selection can have a significant effect on antigen adsorption and adjuvant degradation [al-Shakhshir et al. 1995; Peek et al. 2007; Salnikova et al. 2008].
Antigen adsorption isotherms are easily constructed, since aluminum salts are denser than aqueous solutions and can be removed by centrifugation [Iyer et al. 2004]. The strength of adsorption of antigen by aluminum salts is antigen specific and can have a significant effect on immunopotentiation; modulation of the strength of adsorption by aluminum oxyhydroxide can be achieved by pretreatment with phosphate salts [Hansen et al. 2011]. Antigen adsorption may not always be important for immunogenicity, as has been demonstrated by Romero Mendez and colleagues [Romero Mendez et al. 2007]. In this study, three model vaccines in which protein was not adsorbed by aluminum phosphate produced antibody titers that were similar to those produced by protein adsorbed by aluminum phosphate. Confocal microscopy results suggested that the antigens used in this study were trapped in the void spaces between adjuvant aggregates and that this facilitated uptake of the antigen by dendritic cells. In the case of AS04 with human papilloma virus antigens, the TLR4 agonist (and half the dose of aluminum) could be administered 1 hour, but not 24 hours, before administration of the antigens (with the other half dose of aluminum) and still elicit similar antibody response as contemporaneous administration [Didierlaurent et al. 2009].
Proteins adsorbed by adjuvant are frequently less stable than in solution [Peek et al. 2007] and may be more susceptible to physical and chemical degradation [Estey et al. 2009; Vessely et al. 2009]. Differences in pH at the adjuvant surface can have a significant effect on the rates of antigen degradation [Ljutic et al. 2012], and the pH of the microenvironment around aluminum oxyhydroxide has been shown to be 2 pH units higher than the bulk [Wittayanukulluk et al. 2004]. One must perform a detailed study of the protein on adjuvant and not rely solely on antigen solution studies. When selecting excipients, pH, buffers, and other storage conditions for antigen adsorbed by adjuvant, it is important to be able to evaluate the stability and integrity of the antigen. Peek and colleagues studied the effects of various excipients on the stability of protein in solution and adsorbed by adjuvant [Peek et al. 2007]. They found that many of the same excipients that stabilized a protein in solution also stabilized the protein on adjuvant; however, the adsorbed protein remained less stable than in solution.
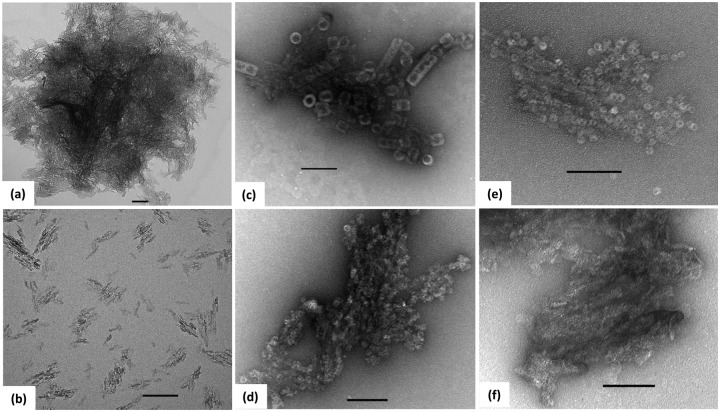
Many of the spectroscopic techniques traditionally used to assess antigens are not suitable or must be modified due to the turbidity of solutions containing aluminum salt. Intrinsic tryptophan fluorescence can be used to assess the tertiary structure of the antigen using a traditional fluorometer and a cuvette designed for front-face fluorescence. Attenuated total reflection Fourier transform infrared (ATR-FTIR) and Raman spectroscopies can be used to examine properties of the secondary structure of the antigen. Differential scanning calorimetry (DSC) can be used to determine the thermal stability of the antigen–adjuvant system, and isothermal titration calorimetry (ITC) can be used to more thoroughly characterize antigen adsorption; however, cleaning the calorimeter after the experiment may be difficult. Ausar and colleagues describes a high-throughput screening (HTS) approach that uses a real-time polymerase chain reaction (RT-PCR) instrument and extrinsic fluorescence dyes such as SYPRO Orange to rapidly screen for stabilizers of protein adsorbed by adjuvant [Ausar et al. 2011]. To assess the chemical stability of the antigen, traditional approaches (high-performance liquid chromatography [HPLC], mass spectrometry, capillary isoelectric focusing) may be used if antigen can be desorbed and isolated from the aluminum salts. Several methods have been used to desorb protein from aluminum including incubation with succinate, urea, phosphate, or various surfactants [Estey et al. 2009; Katz, 1987; Rinella et al. 1998; Vessely et al. 2009]. Aluminum salt adjuvants have been imaged by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) [Burrell et al. 2000; Harris et al. 2012; Lee et al. 2009b]. A recent study describes an ultrasonication treatment of Alhydrogel® to generate finely dispersed crystals that can be negatively stained for visualization of protein on the surface (Figure 1) [Harris et al. 2012].
Figure 1.
TEM images of Alhydrogel untreated (a), or ultrasonicated with a probe sonicator for 10 min (b)—(f). Panels (c)—(f) contain protein adsorbed to Alhydrogel and negatively stained with uranyl acetate. Keyhole limpet hemocyanin (c), respiratory syncytial virus nucleocapsid protein RSV n-RNA (d), anthrax protective antigen PA63 (e), and Escherichia coli outer membrane protein OmpF are shown. Scale bars indicate 100 nm. (Reproduced with permission from Harris et al. [2012]. Copyright © 2012 Elsevier.)
Emulsions
Emulsion-based adjuvant systems have also been widely employed in vaccine development and formulation. Several different classes of emulsions exist, such as oil-in-water (o/w) emulsions, water-in-oil (w/o) emulsions, water-in-oil-in-water (w/o/w) emulsions and protein-stabilized emulsions. O/w emulsions are formulated as oil nanoparticles suspended via surfactant in an oil phase, and have been observed to be more stable in protein-containing formulations than w/o/w emulsions [Barnett et al. 1996]. The w/o emulsions are essentially the inverse of o/w emulsions and are gentle enough on proteins to maintain enzymatic activity of chymotrypsin in the aqueous droplet [Lee and Brody, 2005]. The w/o/w emulsions contain water droplets within larger oil droplets, which are themselves suspended within a bulk aqueous solution. Whereas o/w emulsions are generally preferred for human applications, both w/o and w/o/w emulsions are widely used in veterinary vaccines containing attenuated or inactivated antigens [Aucouturier et al. 2001]. Protein-stabilized emulsions have been extensively characterized and are a class of o/w emulsions, however with proteins as the primary surfactant species. While protein-stabilized emulsions are generally studied for food science applications, the knowledge gained is often relevant for other systems such as vaccines, and for this reason we cite several food science studies below. In each of these systems, hydrophobic and electrostatic forces govern antigen–adjuvant interactions that result in important interprotein interactions and adsorbed protein conformational shifts (Figure 2).
Figure 2.
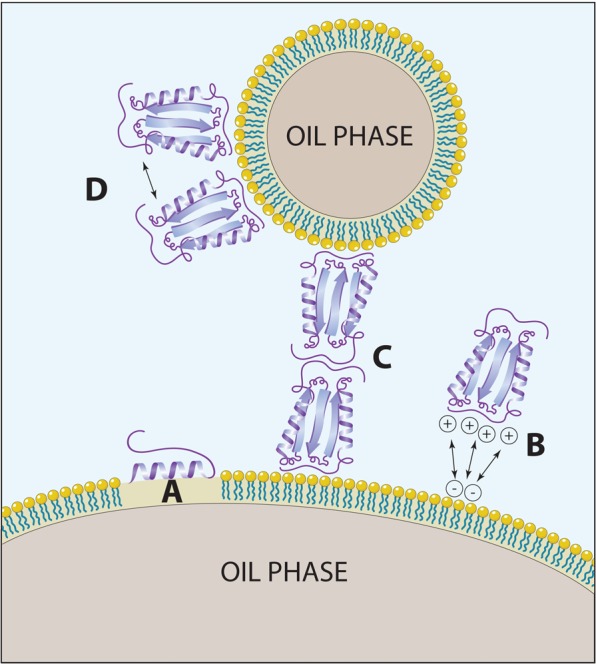
Common protein interactions with emulsion systems. (A) Hydrophobic interactions with the oil phase resulting in α-helical transitions. (B) Electrostatic interactions binding charged surfaces of antigen and emulsion. (C) Protein—protein interactions resulting in flocculation between particles. (D) Protein—protein interactions on the surface of the emulsion, resulting in increased surface viscosity. Image courtesy of Alessandro Baliani © 2013. Adapted from Lucien Barnes’ original artwork.
Within the context of protein-stabilized emulsions, hydrophobicity has been demonstrated to be the primary interaction mechanism [Junghans et al. 2010], in which emulsifying proteins can be easily denatured [Jutila et al. 2000] or displaced by nonionic surfactants such as polysorbates [Courthaudon et al. 1991; Dickinson and Gelin, 1992; Rampon et al. 2003a, 2003b; Stevenson et al. 1997] and sorbitan esters [Cornec et al. 1996], among others [Courthaudon et al. 1991]. This desorption tends to proceed as a gradual replacement of protein with surfactant [Rampon et al. 2003a] at the oil–water interface resulting from a change in protein binding exchange kinetics [Dickinson and Gelin, 1992] in a manner that appears to be a function of protein structure [Cornec et al. 1996; Stevenson et al. 1997] and the timing of surfactant introduction [Courthaudon et al. 1991].
Electrostatic interactions pertain most significantly to protein mixed with membranes generated with ionic surfactants [Chang et al. 2008; Junghans et al. 2010] or ionic proteins [Chen and Dickinson, 1995a, 1995b, 1995c]. These interactions are driven by charge differentials between components, and as such are highly impacted by formulation pH and ionic strength [Chang et al. 2008; Chesko et al. 2005; Tokle and McClements, 2011]. Emulsion particle size is another important factor in protein–emulsion adsorption likely because the net surface area of an emulsion (with constant oil-phase and surfactant concentrations) increases when the particle size decreases, making more surface area available for protein adsorption. Depending on protein concentrations, protein monolayers or multilamellar protein shells can form around emulsions [Chang et al. 2008].
Another significant consideration regarding proteins adsorbed to oils and emulsions is interprotein interactions. The strongest of these interactions is covalent aggregation via disulfide bridges, as has been observed in some o/w emulsions following formulation, perhaps due to oil-phase oxidation products or other low concentration reactive species [Miles et al. 2005]. Strong surface protein interactions between emulsions can result in flocculation events [Rampon et al. 2003a; Zhai et al. 2011]; however, these can commonly be abated by increasing electrostatic repulsion [Zhai et al. 2011], or by adding cosolvents such as glycerol and sorbitol that increase aqueous phase viscosity (slowing particle velocity) and repulsive colloidal interactions between particles, in addition to altering the conformation of adsorbed proteins to avoid exposure of hydrophobic or cysteine residues [Chanasattru et al. 2007]. Further, these interprotein interactions on a single surface can produce dense viscoelastic protein films [Zhai et al. 2011], potentially explaining why high protein concentrations can reduce overall formulation stability [Zhu et al. 2011].
Substantial shifts in protein secondary and tertiary conformation have been observed following adsorption to emulsions and oils. Although some studies have claimed only minor changes in structure using atomic-level techniques such as electron paramagnetic resonance [Berzofsky et al. 1976], advanced spectroscopic methodologies that are sensitive to protein secondary and tertiary structural rearrangements are required to fully understand these interaction systems. FTIR studies have captured changes in secondary structure for proteins adsorbed to emulsions [Jorgensen et al. 2004]. Further, circular dichroism (CD) spectroscopy studies have detected a general decrease in β-sheet structure concurrent with an increase in α-helical content upon adsorption to oil and emulsions, which is lost during desorption [Lee et al. 2009a; Zhai et al. 2011, 2012]. Tertiary structural changes have also been observed via fluorescence spectroscopy such as blue shifts and increases in quantum yield that occur when proteins are adsorbed to oils and emulsions, suggesting that changes in tertiary structure result in shielding of tryptophan residues from solution [Castelain and Genot, 1994; Fox et al. 2012; Husband et al. 2001; Jorgensen et al. 2004]. These structural rearrangements appear to be more resilient to thermal denaturation than nonadsorbed proteins [Zhai et al. 2011, 2012], potentially due to α-helical conformation support imparted by the oil microenvironment. Moreover, the oil phase may have a considerable impact on adsorbed protein structure in comparison to native protein conformation, as oil polarity and chain disorder can vary folding of adsorbed proteins [Herrero et al. 2011; Stevenson et al. 1997; Venien et al. 2000; Zhai et al. 2011, 2012].
Given the multitude of potential interactions and conformational changes between protein antigens and emulsion formulations, it is perhaps surprising that in some cases these interactions, though detectable, do not appear to affect bioactivity [Fox et al. 2012; Ott et al. 1995]. Likewise, significant association between antigen and emulsion does not appear to be critical for adjuvant effects. For example, Ott and colleagues showed that the o/w adjuvant emulsion MF59® does not detectably bind the herpes simplex virus (HSV) antigen gD2 but still generates high-titer antibodies to the antigen [Ott et al. 1995]. Similarly, the HSV antigen gB2 could be made to bind to MF59 to some extent, but this did not improve the elicited immune response at the normal MF59 dose; interestingly, at a lower MF59 dose, the effect of bound gB2 on elicited antibody titers was significant, indicating that a certain density of antigen/emulsion droplet may provide boosted immune responses. A recent report of recombinant HIV protein, gp140, interactions with MF59 found little or no association of the protein with the oil phase as indicated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and only minor changes in protein conformation after extraction from the adjuvant as determined by monoclonal antibody enzyme-linked immunosorbent assays (ELISAs), whereas polymer-based formulations had more significant effects on protein conformation [Lai et al. 2012]. Finally, MF59 could be administered 24 hours before the antigen or 1 hour after the antigen and elicit the same antibody responses as jointly administered adjuvant and antigen. In a similar study, the o/w adjuvant emulsion AS03 could be injected 1 hour before the antigen and induce the same antibody response to an influenza antigen as contemporary administration with the antigen [Morel et al. 2011]. Further, even though a low level of association was detected between antigen and AS03, administration of premixed vaccine compared to administration with two syringes at the same time (one with adjuvant and one with antigen) did not alter the immune response.
Lipid vesicles
Lipid vesicles comprise a class of formulations that generally consist of some sort of lipid bilayer encapsulating an aqueous core. Liposomes, composed of phospholipids and cholesterol, are the most prominent example, with several liposome-based drug delivery products in use as well as GlaxoSmithKline’s liposome-based adjuvant formulation, AS01, in malaria vaccine phase III clinical trials. However, virosomes (liposomes with embedded fusogenic viral proteins) are the more accomplished formulation in the vaccine adjuvant field, with two virosome-based vaccine products licensed in Europe (Inflexal® and Epaxal®). Niosomes are formed with nonionic surfactants instead of phospholipids and have also shown promise as vaccine adjuvant formulations.
The main advantage of lipid-vesicle-based systems is their versatility. A superb review by Watson and colleagues of liposome-based vaccine development, with emphasis on formulation parameters, was recently published [Watson et al. 2012]. Here, we focus only on the antigen association aspect of lipid vesicle adjuvants with emphasis on the most recent reports from the literature. As a general rule, it is thought that some form of association of the antigen with the liposome is desirable, although this may substantially increase the complexity of manufacturing and maintaining stability [Haensler, 2010; Watson et al. 2012]. For example, GlaxoSmithKline’s liposomal system AS01 is apparently a simple mixture with the RTS,S malaria antigen, with no available information regarding extent or mechanism of antigen–adjuvant association. Indeed, Yanasarn and colleagues discovered that simple mixtures of anionic antigens with negatively charged anionic liposomes produced equivalent immune responses to cationic liposomes (even though the cationic liposomes demonstrated a higher level of association with the antigen), although this adjuvant activity depended on the anionic lipids employed [Yanasarn et al. 2011]. In contrast, developers of the CAF01 adjuvant formulation, comprising cationic lipid with the immunostimulatory molecule trehalose dibehenate, have demonstrated that electrostatic association of antigen with adjuvant is necessary for optimal adjuvant activity [Henriksen-Lacey et al. 2010].
Besides electrostatic association, antigens may be covalently bound or chelated to modified lipids or intercalated into the bilayer. For example, virosome adjuvant activity is best when the antigen of interest is associated with the virosome, either through encapsulation (for optimal T-cell responses) or anchored to the bilayer via hydrophobic protein domains or protein lipidation (for optimal antibody responses) [Moser, 2011]. Antigen conjugation to a lipid vesicle surface can be accomplished by covalent or noncovalent attachment, with the conjugation method having a potential effect on the adjuvant activity. For instance, covalent attachment of antigen compared with noncovalent attachment via metal chelation demonstrated improved adjuvant activity; furthermore, antigen attachment via trivalent metal chelation was not better than monovalent linkage despite the higher association affinity of the former [Watson et al. 2011]. New click chemistry methods have shown potential for more straightforward and/or controlled linking of antigen to adjuvant nanoparticles [Mahon et al. 2012], but more studies are needed to evaluate their practical product potential for vaccine applications.
Another important but often overlooked consideration is the antigen density per particle [Vorup-Jensen, 2012; Watson et al. 2012]. Appropriate antigen density on the surface of lipid vesicles and other nanoparticles may result in B-cell receptor cross-linking and enhanced immune responses [Little, 2012]. Moon and colleagues developed a novel multilamellar lipid vesicle delivery system (ICMV) with recombinant malaria antigen chemically linked to the vesicle surface or encapsulated in the vesicle interior (Figure 3), resulting in significantly enhanced antibody titers compared with encapsulated antigen; this lipid vesicle delivery system, in combination with TLR4 agonist, induced a balanced Th1/Th2 type response, broadened antibody specificity while increasing avidity, and caused expansion of helper T cells [Moon et al. 2012]. A well-controlled click chemistry technology was used by Elias and colleagues to show that an intermediate density of targeting proteins on a nanoparticle surface provided better cell binding compared with lower or higher densities [Elias et al. 2012]. Antigen density in virosomes has also been shown to be an important factor for immunogenicity and protection [Homhuan et al. 2004].
Figure 3.
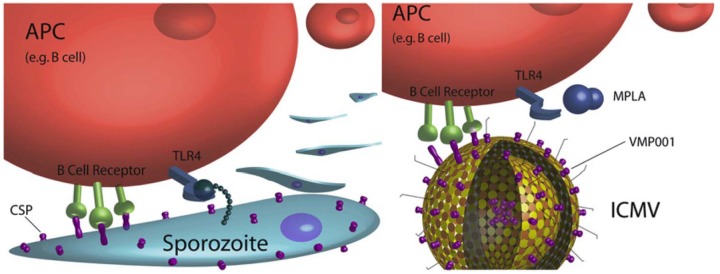
Interbilayer crosslinked multilamellar vesicles (ICMVs) enhance immune responses by presenting surface-linked malaria antigen along with TLR4 agonist to antigen-presenting cells (right), mimicking the malaria sporozoite structure (left). (Reproduced with permission from Little [2012]).
Other adjuvant formulations
IC31 is a binary polyelectrolyte complex consisting of the TLR9-agonist oligodeoxynocleotide mixed with an excess of the antimicrobial peptide KLKL(5)KLK. For negatively charged antigens, adhesion is likely due to coulombic forces. At pH 7.9, any unbound antigen Ag85B-ESAT-6 (pI 4.88) was not detectable by SDS-PAGE and Western blot in the supernatant after ultracentrifugation of the antigen–adjuvant complex for 1 hour at 100,000g, and the Western blot of preformulated antigen to antigen that had been adsorbed and then desorbed from IC31 showed that the antigen was not significantly degraded by adsorption to the adjuvant [Agger et al. 2006]. However, to the best of the authors’ knowledge no characterization of bound antigen structure has been evaluated. Adsorption efficiencies and kinetics depend on the pH of the vaccine formulation, antigen pI, and antigen hydrophobicity. The extent of antigen adsorption and its adsorbed conformation are likely important physicochemical properties of the vaccine formulation. Indeed, association of the antigen with the adjuvant may be necessary for uptake by dendritic cells [Aichinger et al. 2011].
Chitosan nanoparticles, another polyelectrolyte formulation, can be formulated to have either a positive or negative surface charge. The surface charge, buffer type, pH, and antigen, all determine the type and strength of interaction, as well as reactogenicity in vitro and in vivo. A complex of chitosan and dextran sulfate can be formulated to be cationic (excess chitosan) or anionic (excess dextran sulfate). The capsid protein from HIV-1, p24, bound to both positively and negatively charged particles regardless of pH [Drogoz et al. 2008]. Complexes with excess chitosan bound p24 more efficiently than complexes with excess dextran sulfate. Furthermore, acetic acid and ammonium phosphate buffer disrupted adhesion, suggesting that hydrogen bonding is involved in the immobilization process [Drogoz et al. 2008; Schatz et al. 2003]. Adsorption and desorption kinetics depend strongly on the excess electrolyte in the complex. Particles with excess dextran sulfate in a pH 6.2 solution had very rapid sorption kinetics, reaching equilibrium after only 15 hours. Below the isoelectric point of p24, adsorption efficiency was low and reached equilibrium at 35% binding after 24 hours on the same chitosan particles [Drogoz et al. 2008]. Drogoz and colleagues found sorption capacity to be above that predicted by a monolayer model as a function of solid content suggesting absorption into the particle under certain conditions. Thus, the immobilization process is not only directed by the charge of the protein and polyelectrolyte particle, but also by protein/particle ratio and the protein partition coefficient between the solution and the colloid. Another report investigating an analogous protein–polymer interaction concluded that proteins may overcome overall charge repulsion through more specific interactions of polymer with oppositely charge protein patches, which releases counter ions and increases system entropy [Rosenfeldt et al. 2004].
There are a variety of other polymer particles in development as putative vaccine antigen delivery systems. Suitable polymers include poly (ϵ-caprolactone), poly(ethylenimine), poly(γ-glutamic acid), poly(D,L-lactic acid), poly(methyl methacrylate), and poly(uridylic acid), in addition to the chitosan polymer described above [Ferreira et al. 2012]. Polymer particles can be formulated with a variety of immunostimulatory molecules and have been explored in the context of various diseases and both parenteral and mucosal delivery routes [Davis, 2006; Kirby et al. 2008; Murillo et al. 2002; Roman et al. 2008]. As with chitosan and IC31 particles, the charge differential between the antigen and polymer particle is one of the primary drivers of adsorption; as such adsorption is highly impacted by formulation pH and ionic strength [Chesko et al. 2008]. The optimal condition for electrostatic binding is the pH at which surface charge differentials are greatest, although non-Coulombic forces can overcome even charge repulsion [Chesko et al. 2005, 2008]. Thus, adsorption depends on the surface pI and hydrophobicity of the protein, the formulation PZC and surface chemistry, and the pH and ionic strength of the solution [Chesko et al. 2008]. Investigations into polymeric particle development with protein have yielded a variety of methods for modulating important properties such as protein adsorption, size, release rate, and encapsulation efficiency [Blanco and Alonso, 1997; Ho et al. 2008; Jiang and Schwendeman, 2001; Yang et al. 2000]. A recent report by Dey and colleagues employed dynamic light scattering and surface plasmon resonance (SPR) to show that a recombinant HIV glycoprotein, gp140, bound to polyanionic polymer at low pH and slowly disassociated when the pH was raised to 7.4; SDS-PAGE, monoclonal antibody ELISA, and SPR assays confirmed that the protein was stable for several hours at low pH in the presence of the polymer, although some conformational changes were apparent due to reduced antibody binding [Dey et al. 2012]. Interestingly, a complex emulsion–polymer combination adjuvant enhanced antibody responses to gp140 more than either formulation alone [Dey et al. 2012; Lai et al. 2012].
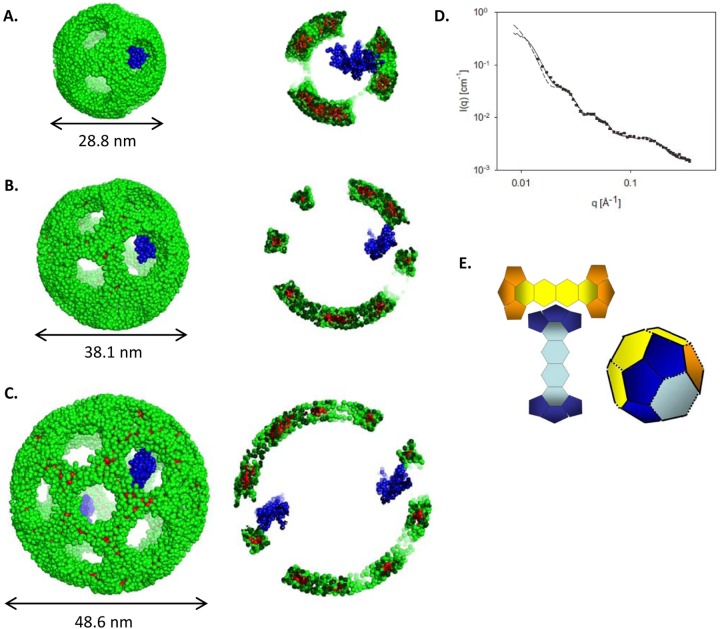
Immune stimulating complexes (ISCOMs) are a class of vaccine adjuvant formulations developed in the mid-1980s [Morein et al. 1984]. These adjuvants consist of mixtures of saponin, cholesterol, and phospholipids in specific stoichiometric ratios. Antigen is either formulated into the ISCOM without chemical modification [Homhuan et al. 2004], or by covalent modification via conjugation to phospholipid or fatty acid, then formulated into the ISCOM [Pedersen et al. 2012; Reid, 1992]. Because ISCOM particles have a negative surface charge [Kersten et al. 1991; Pedersen et al. 2012] as do many soluble proteins, strong adhesion of most soluble proteins is not observed. Attempts to improve adhesion of soluble proteins by treating with extreme pH conditions (pH 2.5) and high lipid concentration increased recovery to 14% [Morein et al. 1990]. Based on the more efficient incorporation of amphipathic antigens such as envelope proteins [Morein et al. 1984], and the need for lipids for incorporation of antigen [Lovgren and Morein, 1988], the major mechanism of association between the ISCOM and antigen is likely hydrophobic in nature. Small-angle X-ray scattering spectroscopy (SAXS) and modeling by Monte Carlo integration suggest that lipid-modified antigen is incorporated into the ISCOM inside of the cage-like structure rather than on the external surface (Figure 4) [Pedersen et al. 2012]. Furthermore, this study showed that ISCOMs likely consist of three particles with different geometry: an icosahedral structure with a mean diameter of 28.8 nm accounting for 10% of the mixture, a tennis ball structure (12 pentagonal and eight hexagonal openings) with a mean diameter of 38.1 nm accounting for 76% of the mixture, and a football (soccer ball) structure with a mean diameter of 48.6 nm accounting for 11% of the mixture. Based on modeling of the SAXS data, the smaller structures contain only one antigen molecule per particle; the larger structure contains two antigen molecules per particle. Positioning of the antigen is likely an important factor in the nature, strength, and duration of the immune response to the vaccine.
Figure 4.
Immune stimulating complex (ISCOM) model structures with antigen positions (blue) from small angle x-ray scattering data (SAXS). (A) Icosahedral structure with a single associated antigen molecule. (B) ‘Tennis ball’ structure with a single associated antigen molecule. (C) ‘Football’ structure with two associated antigen molecules. (D) Experimental SAXS data of ISCOM with loaded antigen. The dashed line is the nonsmeared fit of the data. The solid line is the actual fit of the data with instrumental smearing; modeling by Monte Carlo integration. (E) Schematic of the ‘tennis ball’ model structure showing the orientation of the 12 pentagonal and 8 hexagonal openings. The other structures have only hexagonal openings. (Reproduced with permission from Pedersen et al. [2012]. Copyright © 2012 Elsevier.)
Conclusion
There is much to be learned regarding how antigens and adjuvant formulations interact. In many cases, it appears that antigen–adjuvant association behavior could be further characterized and subsequently optimized for maximum vaccine stability and biological activity. On the other hand, in some cases, direct antigen–adjuvant association may not be necessary or even desirable. For example, adjuvant stockpiling in preparation for an influenza pandemic favors separate vialing of adjuvant and antigen since the dose and strain of the latter is not knowable until the pandemic arrives. However, even in this case, a thorough understanding of antigen–adjuvant interactions is essential to ensure stability after mixing and to prevent unforeseen interaction effects from decreasing vaccine efficacy. Just as regulatory bodies only approve adjuvants in the context of a vaccine product and not by themselves, vaccine developers must also consider vaccines containing adjuvants as a unit with integral interactions and associations, and perform the requisite characterization studies to understand each antigen–adjuvant combination.
Footnotes
Funding: This project has been funded in part with funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (contract number HHSN272200800045C), from the Biomedical Advanced Research and Development Authority, Assistant Secretary for Preparedness and Response, Department of Health and Human Services (contract number HHSO100201000039C), and from the Bill and Melinda Gates Foundation (grant number 42387).
Conflict of interest statement: The authors report no financial conflicts of interest.
Contributor Information
Christopher B. Fox, IDRI, 1124 Columbia Street, Ste 400, Seattle, WA 98104, USA
Ryan M. Kramer, IDRI, Seattle, WA USA
Lucien Barnes V, IDRI, Seattle, WA USA.
Quinton M. Dowling, IDRI, Seattle, WA USA
Thomas S. Vedvick, IDRI, Seattle, WA USA
References
- Agger E., Rosenkrands I., Olsen A., Hatch G., Williams A., Kritsch C., et al. (2006) Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine 24: 5452–5460 [DOI] [PubMed] [Google Scholar]
- Aichinger M., Ginzler M., Weghuber J., Zimmermann L., Riedl K., Schutz G., et al. (2011) Adjuvanting the adjuvant: facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine 29: 426–436 [DOI] [PubMed] [Google Scholar]
- al-Shakhshir R., Regnier F., White J., Hem S. (1995) Contribution of electrostatic and hydrophobic interactions to the adsorption of proteins by aluminium-containing adjuvants. Vaccine 13: 41–44 [DOI] [PubMed] [Google Scholar]
- Aucouturier J., Dupuis L., Ganne V. (2001) Adjuvants designed for veterinary and human vaccines. Vaccine 19: 2666–2672 [DOI] [PubMed] [Google Scholar]
- Ausar S., Chan J., Hoque W., James O., Jayasundara K., Harper K. (2011) Application of extrinsic fluorescence spectroscopy for the high throughput formulation screening of aluminum-adjuvanted vaccines. J Pharm Sci 100(2): 431–440 [DOI] [PubMed] [Google Scholar]
- Barnett P., Pullen L., Williams L., Doel T. (1996) International bank for foot-and-mouth disease vaccine: assessment of Montanide ISA 25 and ISA 206, two commercially available oil adjuvants. Vaccine 14: 1187–1198 [DOI] [PubMed] [Google Scholar]
- Berzofsky J., Schechter A., Kon H. (1976) Does Freund’s adjuvant denature protein antigens? EPR studies of emulsified hemoglobin. J Immunol 115: 270–272 [PubMed] [Google Scholar]
- Blanco M., Alonso M. (1997) Development and characterization of protein-loaded poly(lactide-co-glycolide) nanospheres. Eur J Pharm Biopharm 43: 287–294 [Google Scholar]
- Burrell L., Johnston C., Schulze D., Klein J., White J., Hem S. (2000) Aluminium phosphate adjuvants prepared by precipitation at constant pH. Part II: physicochemical properties. Vaccine 19(2–3): 282–287 [DOI] [PubMed] [Google Scholar]
- Castelain C., Genot C. (1994) Conformational changes of bovine serum albumin upon its adsorption in dodecane-in-water emulsions as revealed by front-face steady-state fluorescence. Biochim Biophys Acta 1199: 59–64 [DOI] [PubMed] [Google Scholar]
- Chanasattru W., Decker E., McClements D. (2007) Inhibition of droplet flocculation in globular-protein stabilized oil-in-water emulsions by polyols. Food Res Int 40: 1161–1169 [Google Scholar]
- Chang C., Knobler C., Gelbart W., Mason T. (2008) Curvature dependence of viral protein structures on encapsidated nanoemulsion droplets. ACS Nano 2: 281–286 [DOI] [PubMed] [Google Scholar]
- Chen J., Dickinson E. (1995a) Protein/surfactant interfacial interactions part 1. Flocculation of emulsions containing mixed protein + surfactants. Coll Surf A: Physicochem Eng Aspects 100: 255–265 [Google Scholar]
- Chen J., Dickinson E. (1995b) Protein/surfactant interfacial interactions part 2. Electrophoretic mobility of mixed protein + surfactant systems. Coll Surf A: Physicochem Eng Aspects 100: 267–277 [Google Scholar]
- Chen J., Dickinson E. (1995c) Protein/surfactant interfacial interactions part 3. Competitive adsorption of protein + surfactant in emulsions. Coll Surf A: Physicochem Eng Aspects 101: 77–85 [Google Scholar]
- Chesko J., Kazzaz J., Ugozzoli M., O’Hagan D., Singh M. (2005) An investigation of the factors controlling the adsorption of protein antigens to anionic PLG microparticles. J Pharm Sci 94: 2510–2519 [DOI] [PubMed] [Google Scholar]
- Chesko J., Kazzaz J., Ugozzoli M., Singh M., O’Hagan D., Madden C., et al. (2008) Characterization of antigens adsorbed to anionic PLG microparticles by XPS and TOF-SIMS. J Pharm Sci 97: 1443–1453 [DOI] [PubMed] [Google Scholar]
- Cornec M., Mackie A., Wilde P., Clark D. (1996) Competitive adsorption of β-lactoglobulin and β-casein with Span 80 at the oil-water interface and the effects on emulsion behavior. Coll Surf A: Physicochem Eng Aspects 114: 237–244 [Google Scholar]
- Courthaudon J., Dickinson E., Dalgleish D. (1991) Competitive adsorption of β-casein and nonionic surfactants in oil-in-water emulsions. J Colloid Interface Sci 145: 390–395 [Google Scholar]
- Davis S. (2006) The use of soluble polymers and polymer microparticles to provide improved vaccine responses after parenteral and mucosal delivery. Vaccine 24S2: S2/7–S2/10 [DOI] [PubMed] [Google Scholar]
- De Souza Reboucas J., Esparza I., Ferrer M., Sanz M., Irache J., Gamazo C. (2012) Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J Biomed Biotechnol 2012: 474605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Burke B., Sun Y., Hartog K., Heeney J., Montefiori D., et al. (2012) Use of a polyanionic carbomer, Carbopol971P, in combination with MF59, improves antibody responses to HIV-1 envelope glycoprotein. Vaccine 30: 2749–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson E., Gelin J. (1992) Influence of emulsifier on competitive adsorption of αs-casein + β-lactoglobulin in oil-in-water emulsions. Coll Surf 63: 329–335 [Google Scholar]
- Didierlaurent A., Morel S., Lockman L., Giannini S., Bisteau M., Carlsen H., et al. (2009) AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 10: 6186–6197 [DOI] [PubMed] [Google Scholar]
- Diwan M., Elamanchili P., Cao M., Samuel J. (2004) Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv 1: 405–412 [DOI] [PubMed] [Google Scholar]
- Drogoz A., Munier S., Verrier B., David L., Domard A., Delair T. (2008) Towards biocompatible vaccine delivery systems: interactions of colloidal PECs based on polysaccharides with HIV-1 p24 antigen. Biomacromol 9: 583–591 [DOI] [PubMed] [Google Scholar]
- Duthie M., Windish H., Fox C., Reed S. (2011) Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev 239: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Poloukhtine A., Popik V., Tsourkas A. (2012) Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estey T., Vessely C., Randolph T., Henderson I., Braun L., Nayar R., et al. (2009) Evaluation of chemical degradation of a trivalent recombinant protein vaccine against botulinum neurotoxin by LysC peptide mapping and MALDI-TOF mass spectrometry. J Pharm Sci 98(9): 2994–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2012) Summary Minutes Vaccines and Related Biological Products Advisory Committee. Food and Drug Administration, Center for Biologics Evaluation and Review, Silver Spring, MD, US [Google Scholar]
- Ferreira S., Gama F., Vilanova M. (2012) Polymeric nanogels as vaccine delivery systems. Nanomedicine, in press. [DOI] [PubMed] [Google Scholar]
- Flower D., Macdonald I., Ramakrishnan K., Davies M., Doytchinova I. (2010) Computer aided selection of candidate vaccine antigens. Immunome Research 6(Suppl. 2): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C., Barnes V L., Evers T., Chesko J., Vedvick T., Coler R., et al. (2012) Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy. Influenza Other Respi Viruses, in press. DOI: 10.1111/irv.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. (1998) Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev 32: 155–172 [DOI] [PubMed] [Google Scholar]
- Gupta R., Rost B., Relyveld E., Siber G. (1995) Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol 6: 229–248 [DOI] [PubMed] [Google Scholar]
- Haensler J. (2010) Liposomal adjuvants: preparation and formulation with antigens. Methods Mol Biol 626: 73–90 [DOI] [PubMed] [Google Scholar]
- Hansen B., Malyala P., Singh M., Sun Y., Srivastava I., Hogenesch H., et al. (2011) Effect of the strength of adsorption of HIV 1 SF162dV2gp140 to aluminum-containing adjuvants on the immune response. J Pharmaceut Sci 100: 3245–3250 DOI: 10.1002/jps.22555 [DOI] [PubMed] [Google Scholar]
- Harris J., Soliakov A., Lewis R., Depoix F., Watkinson A., Lakey J. (2012) Alhydrogel(R) adjuvant, ultrasonic dispersion and protein binding: a TEM and analytical study. Micron 43: 192–200 [DOI] [PubMed] [Google Scholar]
- Hem S., Hogenesch H. (2007) Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines 6: 685–698 [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M., Christensen D., Bramwell V., Lindenstrom T., Agger E., Andersen P., et al. (2010) Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce CMI response. J Control Rel 145: 102–108 [DOI] [PubMed] [Google Scholar]
- Herrero A., Carmona P., Pintado T., Jimenez-Colmenero F., Ruiz-Capillas C. (2011) Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Res Int 44: 360–366 [Google Scholar]
- Heyward W. (2012) Methods and compositions for eliciting an immune response against hepatitis B virus. US Patent US20120263755 A1
- Ho L., Fu Y., Wang G., Chen H., Chang J., Tsai T., et al. (2008) Controlled release carrier of BSA made by W/O/W emulsion method containing PLGA and hydroxyapatite. J Control Rel 128: 142–148 [DOI] [PubMed] [Google Scholar]
- Homhuan A., Prakongpan S., Poomvises P., Maas R., Crommelin D., Kersten G., et al. (2004) Virosome and ISCOM vaccines against Newcastle disease: preparation, characterization and immunogenicity. Eur J Pharm Sci 22: 459–468 [DOI] [PubMed] [Google Scholar]
- Husband F., Garrood M., Mackie A., Burnett G., Wilde P. (2001) Adsorbed protein secondary and tertiary structures by circular dichroism and infrared spectroscopy with refractive index matched emulsions. J Agricul Food Chem 49: 859–866 [DOI] [PubMed] [Google Scholar]
- Iyer S., Robinett R., HogenEsch H., Hem S. (2004) Mechanism of adsorption of hepatitis B surface antigen by aluminum hydroxide adjuvant. Vaccine 22: 1475–1479 [DOI] [PubMed] [Google Scholar]
- Jiang W., Schwendeman S. (2001) Stabilization of a model formalinized protein antigen encapsulated in poly(lactide-co-glycolide)-based microspheres. J Pharm Sci 90: 1558–1569 [DOI] [PubMed] [Google Scholar]
- Jorgensen L., Van De, Weert M., Vermehren C., Bjerregaard S., Frokjaer S. (2004) Probing structural changes of proteins incorporated into water-in-oil emulsions. J Pharm Sci 93: 1847–1859 [DOI] [PubMed] [Google Scholar]
- Junghans A., Champagne C., Cayot P., Loupiac C., Koper I. (2010) Protein-lipid interactions at the air-water interface. Langmuir 26: 12049–12053 [DOI] [PubMed] [Google Scholar]
- Jutila A., Zhu K., Patkar S., Vind J., Svendsen A., Kinnunen P. (2000) Detergent-induced conformational changes of Humicola lanuginose lipase studied by fluorescence spectroscopy. Biophys J 78: 1634–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerzell T., Esfandiary R., Joshi S., Middaugh C., Volkin D. (2011) Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev 63(13): 1118–1159 [DOI] [PubMed] [Google Scholar]
- Katz J. (1987) Desorption of porcine parvovirus from aluminum hydroxide adjuvant with subsequent viral immunoassay or hemagglutination assay. Vet Res Commun 11: 83–92 [DOI] [PubMed] [Google Scholar]
- Kersten G., Spiekstra A., Beuvery E., Crommelin D. (1991) On the structure of immune-stimulating saponin-lipid complexes (ISCOMs). Biochim Biophys Acta 1062: 165–171 [DOI] [PubMed] [Google Scholar]
- Kirby D., Rosenkrands I., Agger E., Andersen P., Coombes A., Perrie Y. (2008) PLGA microspheres for the delivery of a novel subunit TB vaccine. J Drug Target 16: 282–293 [DOI] [PubMed] [Google Scholar]
- Kool M., Fierens K., Lambrecht B. (2012) Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol 61: 927–934 [DOI] [PubMed] [Google Scholar]
- Lai R., Seaman M., Tonks P., Wegmann F., Seilly D., Frost S., et al. (2012) Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund’s adjuvants. PLoS ONE 7: e35083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Brody J. (2005) Single-molecule enzymology of chymotrypsin using water-in-oil emulsion. Biophys J 88: 4303–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lefevre T., Subirade M., Paquin P. (2009a) Effects of ultra-high pressure homogenization on the properties and structure of interfacial protein layer in whey protein-stabilized emulsion. Food Chem 113: 191–195 [Google Scholar]
- Lee S., Jung K., Oh C., Lee Y., Tran T., Kim M. (2009b) Precipitation of fine aluminium hydroxide from Bayer liquors. Hydrometallurgy 98: 156–161 [Google Scholar]
- Little S. (2012) Reorienting our view of particle-based adjuvants for subunit vaccines. Proc Natl Acad Sci U S A 109: 999–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljutic B., Ochs M., Messham B., Ming M., Dookie A., Harper K., et al. (2012) Formulation, stability and immunogenicity of a trivalent pneumococcal protein vaccine formulated with aluminum salt adjuvants. Vaccine 30: 2981–2988 [DOI] [PubMed] [Google Scholar]
- Lovgren K., Morein B. (1988) The requirement of lipids for the formation of immunostimulating complexes (ISCOMs). Biotechnol Appl Biochem 10: 161–172 [PubMed] [Google Scholar]
- Mahon E., Salvati A., Bombelli F., Lynch I., Dawson K. (2012) Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J Control Rel 161: 164–174 [DOI] [PubMed] [Google Scholar]
- Maquieira A., Brun E., Garces-Garcia M., Puchades R. (2012) Aluminum oxide nanoparticles as carriers and adjuvants for eliciting antibodies from non-immunogenic haptens. Anal Chem 84: 9340–9348 [DOI] [PubMed] [Google Scholar]
- McKee A., Munks M., Marrack P. (2007) How do adjuvants work? Important considerations for new generation adjuvants. Immunity 27: 687–690 [DOI] [PubMed] [Google Scholar]
- Miles A., McClellan H., Rausch K., Zhu D., Whitmore M., Singh S., et al. (2005) Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 23: 2530–2539 [DOI] [PubMed] [Google Scholar]
- Moon J., Suh H., Li A., Ockenhouse C., Yadava A., Irvine D. (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A 109: 1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein B., Ekstrom J., Lovgren K. (1990) Increased immunogenicity of a non-amphipathic protein (BSA) after inclusion into ISCOMs. J Immunol Methods 128: 177–181 [DOI] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Hoglund S., Dalsgaard K., Osterhaus A. (1984) ISCOM, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 308: 457–460 [DOI] [PubMed] [Google Scholar]
- Morel S., Diderlaurent A., Bourguignon P., Delhaye S., Baras B., Jacob V., et al. (2011) Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29: 2461–2473 [DOI] [PubMed] [Google Scholar]
- Moser C. (2011) Virosomes. In: Rappuoli R., De Gregorio E. (eds), Novel Immunologic Adjuvants. London: Future Medicine, pp. 43–53 [Google Scholar]
- Murillo M., Gamazo C., Irache J., Goni M. (2002) Polyester microparticles as a vaccine delivery system for brucellosis: influence of the polymer on release, phagocytosis and toxicity. J Drug Target 10: 211–219 [DOI] [PubMed] [Google Scholar]
- Noe S., Green M., HogenEsch H., Hem S. (2010) Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine 28: 3588–3594 [DOI] [PubMed] [Google Scholar]
- O’Hagan D., Ott G., De Gregorio E., Seubert A. (2012) The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine 30: 4341–4348 [DOI] [PubMed] [Google Scholar]
- Ott G., Barchfeld G., Chernoff D., Radhakrishnan R., van Hoogevest P., Van Nest G. (1995) MF59: design and evaluation of a safe and potent adjuvant for human vaccines. In: Powell M., Newman M. (eds), Vaccine Design: The Subunit and Adjuvant Approach. New York: Plenum Press, pp. 277–296 [DOI] [PubMed] [Google Scholar]
- Pedersen J., Oliveira C., Hubschmann H., Arleth L., Manniche S., Kirkby N., et al. (2012) Structure of immune stimulating complex matrices and immune stimulating complexes in suspension determined by small-angle x-ray scattering. Biophys J 102: 2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek L., Martin T., Elk Nation C., Pegram S., Middaugh C. (2007) Effects of stabilizers on the destabilization of proteins upon adsorption to aluminum salt adjuvants. J Pharm Sci 96: 547–557 [DOI] [PubMed] [Google Scholar]
- Rampon V., Genot C., Riaublanc A., Anton M., Axelos M., McClements D. (2003a) Front-face fluorescence spectroscopy study of globular proteins in emulsions: displacement of BSA by a nonionic surfactant. J Agricul Food Chem 51: 2482–2489 [DOI] [PubMed] [Google Scholar]
- Rampon V., Genot C., Riaublanc A., Anton M., Axelos M., McClements D. (2003b) Front-face fluorescence spectroscopy study of globular proteins in emulsions: influence of droplet flocculation. J Agricul Food Chem 51: 2490–2495 [DOI] [PubMed] [Google Scholar]
- Reid G. (1992) Soluble proteins incorporate into ISCOMs after covalent attachment of fatty acid. Vaccine 10: 597–602 [DOI] [PubMed] [Google Scholar]
- Rinella J., Workman R., Hermodson M., White J., Hem S. (1998) Elutability of proteins from aluminum-containing vaccine adjuvants by treatment with surfactants. J Colloid Interface Sci 197: 48–56 [DOI] [PubMed] [Google Scholar]
- Roman B., Irache J., Gomez S., Tsapis N., Gamazo C., Espuelas M. (2008) Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. Eur J Pharm Biopharm 70: 98–108 [DOI] [PubMed] [Google Scholar]
- Romero Mendez I., Shi Y., HogenEsch H., Hem S. (2007) Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine 25: 825–833 [DOI] [PubMed] [Google Scholar]
- Rosenfeldt S., Wittemann A., Ballauff M. (2004) Interaction of proteins with spherical polyelectrolyte brushes in solution as studied by small-angle X-ray scattering. Physical Rev E 70: 061403. [DOI] [PubMed] [Google Scholar]
- Sablan B., Kim D., Barzaga N., Chow W., Cho M., Ahn S., et al. (2012) Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine 30: 2689–2696 [DOI] [PubMed] [Google Scholar]
- Salnikova M., Joshi S., Rytting J., Warny M., Middaugh C. (2008) Preformulation studies of Clostridium difficile toxoids A and B. J Pharmaceut Sci 97: 4194–4207 [DOI] [PubMed] [Google Scholar]
- Schatz C., Viton C., Delair T., Pichot C., Domard A. (2003) Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromol 4: 641–648 [DOI] [PubMed] [Google Scholar]
- Seeber S., White J., Hem S. (1991) Predicting the adsorption of proteins by aluminium-containing adjuvants. Vaccine 9: 201–203 [DOI] [PubMed] [Google Scholar]
- Stevenson E., Horne D., Leaver J. (1997) Displacement of native and thiolated β-casein from oil–water interfaces - effect of heating, ageing and oil phase. Food Hydrocolloids 11: 3–6 [Google Scholar]
- Tokle T., McClements D. (2011) Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: effect of pH, salt, and heating. Food Hydrocolloids 25: 976–982 [Google Scholar]
- Venien A., Levieux D., Dufour E. (2000) Alkanethiol layers for the study of emulsified protein conformation by surface plasmon resonance using monoclonal antibodies. J Colloid Interface Sci 223: 215–222 [DOI] [PubMed] [Google Scholar]
- Vessely C., Estey T., Randolph T., Henderson I., Cooper J., Nayar R., et al. (2009) Stability of a trivalent recombinant protein vaccine formulation against botulinum neurotoxin during storage in aqueous solution. J Pharm Sci 98: 2970–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F., Powell M. (1995) A compendium of vaccine adjuvants and excipients. Pharm Biotechnol 6: 141–228 [DOI] [PubMed] [Google Scholar]
- Vorup-Jensen T. (2012) On the roles of polyvalent binding in immune recognition: Perspectives in the nanoscience of immunology and the immune response to nanomedicines. Adv Drug Del Rev 64: 1759–1781 [DOI] [PubMed] [Google Scholar]
- Watson D., Endsley A., Huang L. (2012) Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 30: 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Platt V., Cao L., Venditto V., Francis C., Szoka J. (2011) Antibody response in mice to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid. Clin Vaccine Immunol 18: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Hem S. (2000) Characterization of aluminium-containing adjuvants. Dev Biol (Basel) 103: 217–228 [PubMed] [Google Scholar]
- Wilson-Welder J., Torres M., Kipper M., Mallapragada S., Wannemuehler M., Narasimhan B. (2009) Vaccine adjuvants: current challenges and future approaches. J Pharm Sci 98: 1278–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittayanukulluk A., Jiang D., Regnier F., Hem S. (2004) Effect of microenvironment pH of aluminum hydroxide adjuvant on the chemical stability of adsorbed antigen. Vaccine 22: 1172–1176 [DOI] [PubMed] [Google Scholar]
- Yanasarn N., Sloat B., Cui Z. (2011) Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol Pharm 8: 1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Chung T., Bai X., Chan W. (2000) Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. Chem Eng Sci 55: 2223–2236 [Google Scholar]
- Zhai J., Hoffmann S., Day L., Lee T., Augustin M., Aguilar M., et al. (2012) Conformational changes of a-lactalbumin adsorbed at oil-water interfaces: interplay between protein structure and emulsion stability. Langmuir 28: 2357–2367 [DOI] [PubMed] [Google Scholar]
- Zhai J., Wooster T., Hoffmann S., Lee T., Augustin M., Aguilar M. (2011) Structural rearrangement of β-lactoglobulin at different oil-water interfaces and its effect on emulsion stability. Langmuir 27: 9227–9236 [DOI] [PubMed] [Google Scholar]
- Zhu D., McClellan H., Dai W., Gebregeorgis E., Kidwell M., Aebig J., et al. (2011) Long term stability of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Montanide ISA 720 and stabilized with glycine. Vaccine 29: 3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]