Abstract
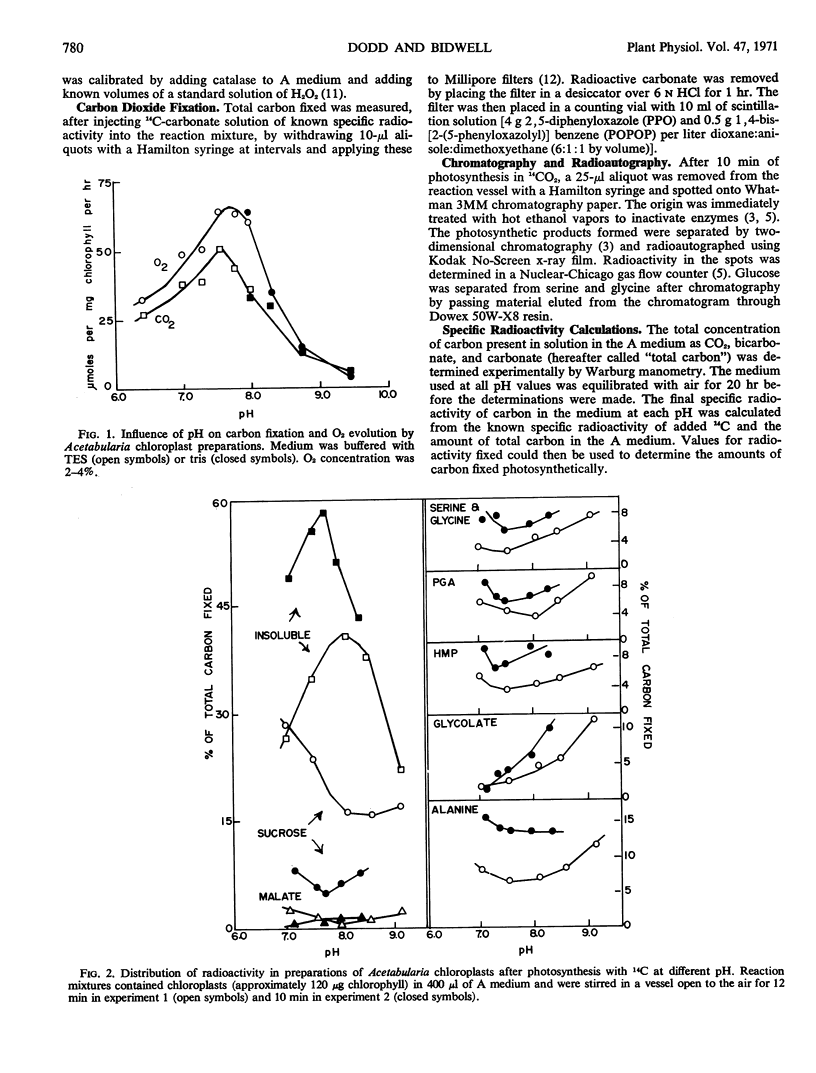
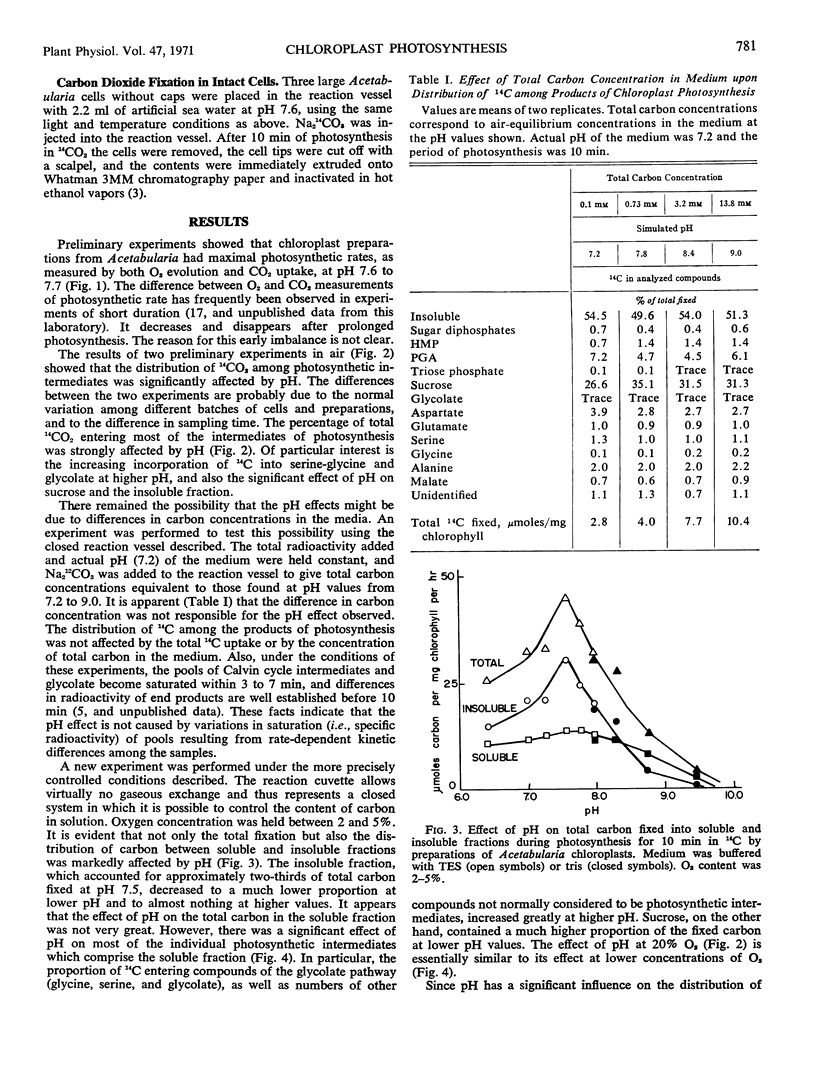
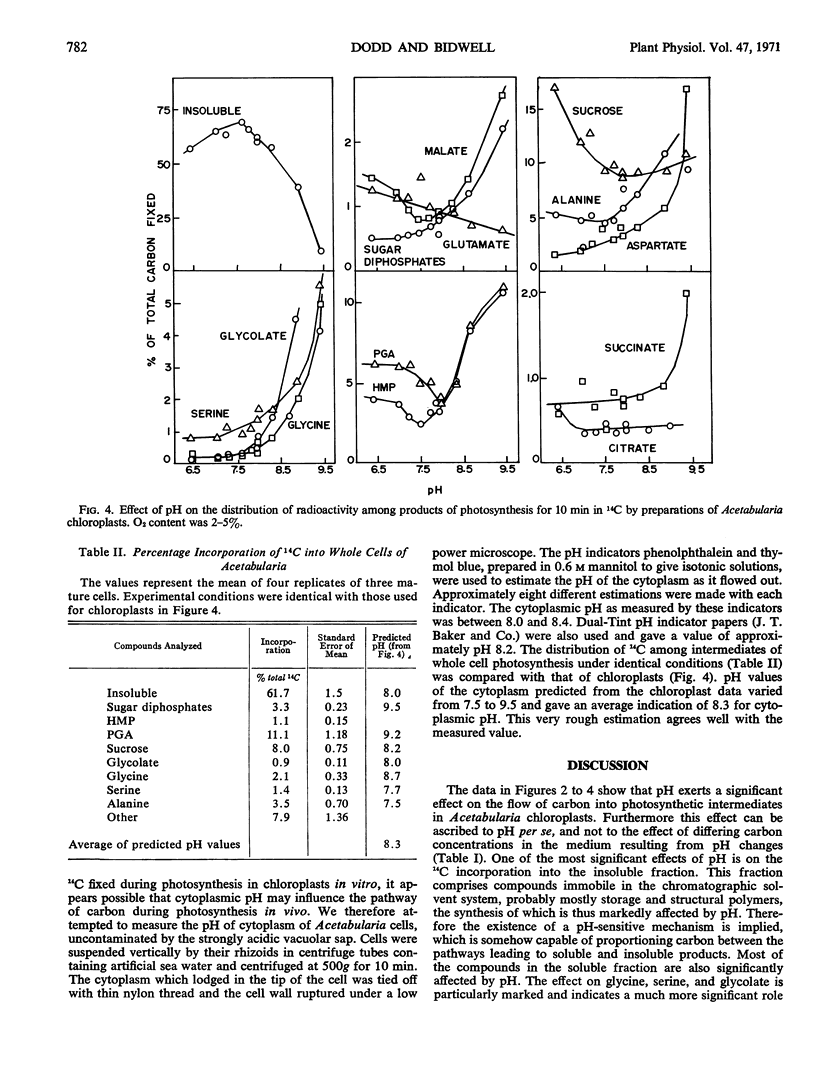
The effect of pH on the pathways of carbon in photosynthesis was examined in chloroplast preparations from Acetabularia mediterranea. The flow of carbon into a number of photosynthetic intermediates, particularly sucrose, glycine, serine, glycolate, and the insoluble fraction, was strongly influenced by pH. At higher pH a much larger portion of the 14C entered intermediates of the glycolate pathway. Although maximal apparent photosynthesis occurred at pH 7.6 to 7.7, cytoplasmic pH was found to be 8.0 to 8.4, using indicators. The pattern of distribution of 14C in intermediates of whole cells was closest to that in chloroplasts at the higher pH range.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B., WHATLEY F. R., ARNON D. I. Photosynthesis by isolated chloroplasts. VI. Rates of conversion of light into chemical energy in photosynthetic phosphorylation. Biochim Biophys Acta. 1958 Jan;27(1):16–23. doi: 10.1016/0006-3002(58)90288-9. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIDWELL R. G. Direct paper chromatography of soluble compounds in small samples of tissue adhering to the paper. Can J Biochem Physiol. 1962 Jun;40:757–761. [PubMed] [Google Scholar]
- Bidwell R. G., Levin W. B., Shephard D. C. Intermediates of photosynthesis in Acetabularia mediterranie chloroplasts. Plant Physiol. 1970 Jan;45(1):70–75. doi: 10.1104/pp.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell R. G., Levin W. B., Shephard D. C. Photosynthesis, Photorespiration and Respiration of Chloroplasts From Acetabularia mediterrania. Plant Physiol. 1969 Jul;44(7):946–954. doi: 10.1104/pp.44.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERTZHAGEN H., KANDLER O. Differences in the pH optimum of the photosynthetic fixation of carbon dioxide in isolated whole and broken chloroplasts. Nature. 1962 Apr 21;194:312–313. doi: 10.1038/194312b0. [DOI] [PubMed] [Google Scholar]
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., CALO N. Studies on carbon dioxide fixation by chloroplasts, the effect of arsenite and other factors. Biochim Biophys Acta. 1960 Nov 4;44:341–347. doi: 10.1016/0006-3002(60)91570-5. [DOI] [PubMed] [Google Scholar]
- Gibbs M., Calo N. Factors Affecting Light Induced Fixation of Carbon Dioxide by Isolated Spinach Chloroplasts. Plant Physiol. 1959 May;34(3):318–323. doi: 10.1104/pp.34.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]


