Abstract
Caveolin (CAV) is an essential component of caveolae, cholesterol-enriched invaginations of the plasma membrane of most mammalian cells. However, CAV is not restricted to plasma membrane caveolae, and pools of CAV are present in myriad intracellular membranes. CAV proteins tightly bind cholesterol and contribute to regulation of cholesterol fluxes and distributions within cells. In this context, we recently demonstrated that CAV1 regulates the poorly-understood process controlling mitochondria cholesterol levels. Cholesterol accumulates in mitochondrial membranes in the absence of CAV1, promoting the organelle’s dysfunction with important metabolic consequences for cells and animals. In this Interchange Article we suggest a working hypothesis that addresses the role of CAV1 within the homeostatic network that regulates the influx/efflux of mitochondrial cholesterol.
Keywords: Caveolin, caveolae, cholesterol, mitochondria, glutathione, oxidative stress
Caveolin regulates intracellular cholesterol fluxes
Caveolae are distinctive invaginations of the plasma membrane of most mammalian cells (1). Organized as highly condensed domains, they have significant accumulations of both glycosphingolipids and cholesterol. Caveolin (CAV) is an essential component of caveolae: a clear phenotype of CAV1 and CAV3 deficient mice (CAV−/−) is the complete loss of caveolae (2–5). Conversely, CAV expression in cells results in assembly of caveolae at the cell surface (6, 7). Lipids are also indispensable caveolar components; cholesterol depletion causes flattening of caveolae (8). Further, there is an important interplay between lipids and proteins: cholesterol addition accelerates CAV transport to the plasma membrane (9), whereas this transport is inhibited by depletion of glycosphingolipids (10).
While critical for caveolae, CAV proteins likely have additional functions as they are not entirely restricted to caveolae in the plasma membrane. Intracellular CAV is present in myriad locations, including in the Golgi complex (11, 12), the endoplasmic reticulum (ER)/Golgi network (13), cis-Golgi cisternae (14), in TGN-derived vesicles of epithelial cells (15, 16), exocytic and endocytic carriers (17, 18), endosomes (19–22), cytosolic complexes with chaperones (23), cytosolic lipid droplets (24–27), secretory vesicles (28), mitochondria (28) and peroxisomes (29). Whether these non-caveolar pools of CAV are connected under physiological conditions by specific trafficking pathways and the biological significance of the protein in each location also remains unclear (30).
Functionally, CAV1 binds cholesterol with high affinity (31), and CAV’s ability to move between these compartments might contribute to regulation of cholesterol fluxes and distributions within cells (1, 9, 25). In support of this general statement, an ectopically expressed CAV dominant negative mutant (CAVDGV) promotes a complex intracellular lipid imbalance. Expression of CAVDGV increases the level of free cholesterol in late endosomes but depletes cholesterol in the Golgi complex and the plasma membrane. Interestingly, although CAVDGV irreversibly accumulates in the membranes of the ER and on lipid droplets (32), deregulation of cholesterol fluxes modify signaling pathways at the cell surface, such as the H-Ras mediated activation of Raf-1. The inhibitory effect of CAVDGV on signaling is completely reversed by replenishing the cell membrane with cholesterol and reproduced by depletion of membrane cholesterol (33). Therefore, by modulation of intracellular cholesterol fluxes CAV might facilitate multiple cellular processes potentially occurring in virtually all the organelles of cells.
In this context, we recently demonstrated that CAV1 participates in regulation of mitochondria cholesterol levels (34). In the absence of CAV1, cholesterol accumulates in mitochondrial membranes. This accumulation has a high biological cost. Combination of in vivo and in vitro analysis revealed that cholesterol accumulation promotes the organelle’s dysfunction at 4 connected levels: i) it reduces the fluidity of the mitochondrial membrane ii) it reduces the efficiency of the respiratory chain, iii) it increases generation of reactive oxygen species (ROS), and iv) it impairs the uptake of glutathione (mGSH), a key mitochondrial anti-oxidant. Clearly pinpointing causality of cholesterol levels in the organelle malfunction, cholesterol depletion of CAV1−/− mitochondria to the wt levels restored mitochondrial function. Conversely, cholesterol loading of wt type mitochondria to CAV1−/− levels reproduced mitochondrial failure. Importantly, re-expression by retroviral infection of CAV1 in CAV1−/− cells rescued mitochondrial functionality, demonstrating direct causality of CAV1 in mitochondrial dysfunction but also temporality in the accumulation of cholesterol in mitochondria. Thus, cells can potentially modulate mitochondrial cholesterol levels by regulation of CAV1 expression. Indeed, CAV1 expression is up-regulated at the transcriptional level by cholesterol (35).
At the cellular level, the combination of these 4 factors has 3 decisive consequences: i) it causes reduced cellular proliferation when the availability of glucose is reduced, ii) it causes mitochondrial failure that promotes apoptosis in a situation when the function of the organelle is required, and iii) it results in an amplified susceptibility to apoptotic inducers such as cytokines and mitochondrial toxins. Indeed, CAV1−/− cells undergo a ROS-mediated apoptosis during nutrient limitation (induced by 2-deoxyglucose, Figure 1), during the shift from aerobic glycolysis to mitochondrial oxidative phosphorylation (OXPHOS, promoted by dichloroacetate), or when cells are challenged with pro-apoptotic factors (such as TNF alpha or the agonistic anti-Fas antibody Jo2). Supplementation with anti-oxidants highly reduces the apoptosis that CAV1−/− cells show in the above situations, clearly pinpointing ROS as the apoptogenic triggers. At the animal level, such a mitochondrial dysfunction also predisposes CAV1−/− animals to mitochondrial related diseases such as steatohepatitis or the progressive neurodegeneration occurring during Huntington’s and Alzehimer’s diseases (see also (36)). Further, during liver regeneration after partial hepatectomy - in which glucose levels in serum rapidly fall- CAV1−/− mice showed reduced cellular proliferation and low survival (37). Supplementation of CAV1−/− mice with glucose restores liver regeneration and mice survival by re-activation of hepatocyte proliferation.
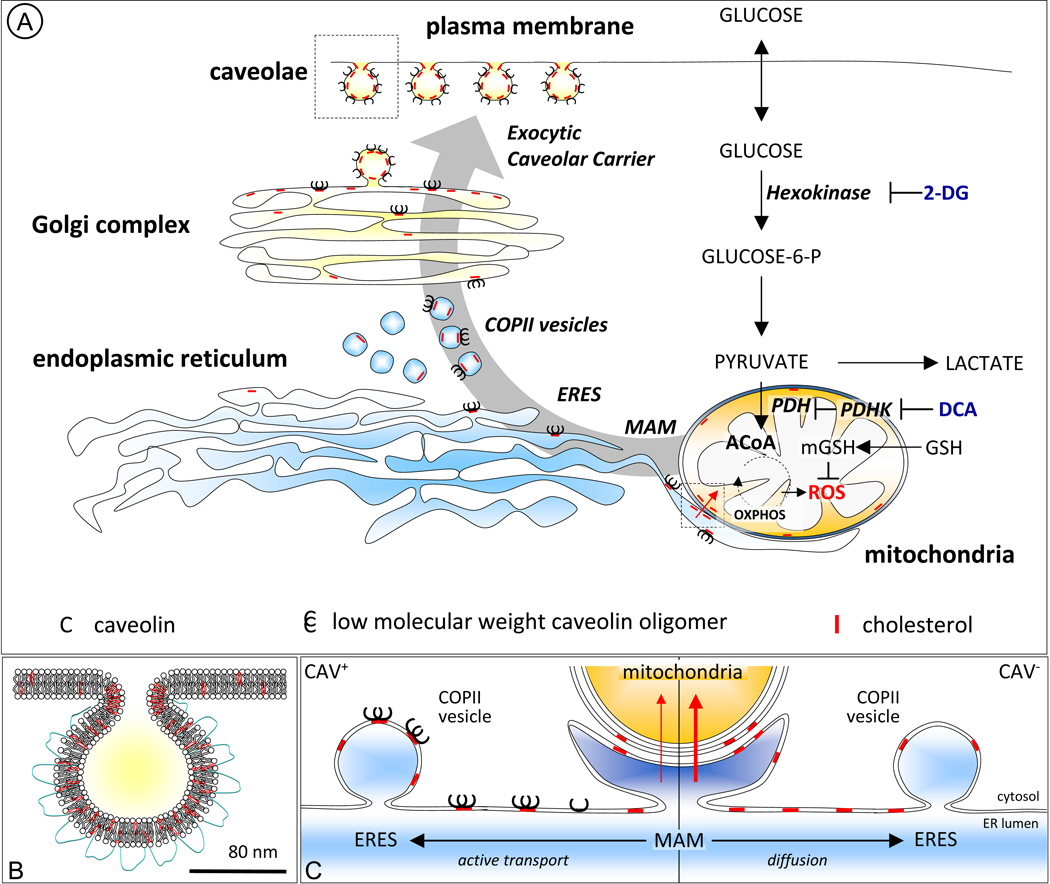
Figure 1. Caveolin regulates the influx of mitochondrial cholesterol: metabolic implications.
(A) CAV (black C) is synthesized in the ER and rapidly goes through a first stage of oligomerization into low molecular weight oligomers in a process stabilized by cholesterol (red slashes) (44). Immediately after synthesis CAV is recruited to ER exit sites (ERES) (48) and the coat protein II (COPII) machinery mediates its transport to the Golgi complex. Exit from the Golgi complex is associated with formation of high molecular weight oligomers and incorporation into cholesterol-enriched lipid-raft structures. Finally, the mature caveola-like exocytic carrier is incorporated into the plasma membrane (1, 17) (detail in B). The physical association between the ER and mitochondria, known as MAM, participates of the influx of cholesterol into the organelle (42). CAV1 is a MAM resident protein (43) and thus it is in a position to modulate cholesterol fluxes from/to MAM. We postulate that newly-synthesized CAV binds cholesterol in the ER and the CAV-cholesterol complexes are rapidly cleared into ERES and transported to the Golgi complex. This immediate forward transport of CAV (active transport) reduces overall levels of newly synthesized cholesterol in the membranes of the ER and thus cholesterol levels in MAM and ultimately the influx of cholesterol into the mitochondria. In contrast, in the absence of CAV (right panel situation in C) cholesterol accumulates in the bulk of the ER, including MAM, and thus increases its transport into mitochondria. Glucose is internalized by cells and rapidly phosphorylated by the key rate-limiting enzyme hexokinase (61) this process can be inhibited with 2-deoxyglucose (2-DG) which is a glucose analogue that acts as a competitive inhibitor of glucose phosphorylation. Dichloroacetate (DCA) is an inhibitor of pyruvate dehydrogenase kinase (PDK) (62). Inhibition of PDK results in the activation of pyruvate dehydrogenase (PDH), a gate-keeping enzyme that shifts glucose metabolism from lactate production to mitochondrial oxidative phosphorylation (OXPHOS). Mitochondrial OXPHOS represents a major source of ROS, especially in a low energy context (63), and ROS are effective apoptogenic molecules (64). Mitochondrial glutathione (mGSH) is a key antioxidant imported from the cytosol to modulate the levels of ROS, the oxidative state of the cell, and ultimately apoptosis (41).
How does caveolin regulate mitochondrial cholesterol?
The evidence that caveolae are organelles enriched in cholesterol with respect to the rest of the plasma membrane was already provided in pioneering studies (38). In contrast, mitochondria are cholesterol-poor organelles and very little is known about the homeostatic network that regulates the influx/efflux of mitochondrial cholesterol (39). Elucidation of these pathways has become essential since mitochondrial cholesterol plays a key role not only in organelle’s function but also in disease progression (40). It may acquire epidemic dimensions, for instance, because cholesterol accumulates in hepatic mitochondria of obese ob/ob mice (41). Excellent reviews on the mechanisms that regulate cholesterol trafficking in cells and mitochondria can be found in the literature (39). Thus, here we will specifically centre our analysis on the emerging role of CAV1 in the regulation of mitochondrial cholesterol. In this Interchange Article we will propose a working hypothesis - based on the new experimental data, combined with previous published results- to address a possible mechanism for how cholesterol accumulates in the mitochondria of CAV1 deficient cells.
In principle, CAV1 could directly modulate cholesterol in the mitochondria by trafficking through the organelle. Although this has been proposed (28), it does not appear likely here. In our experimental conditions we could not detect CAV1 in mitochondria purified from wt livers (34). However, it is increasingly accepted that cholesterol partially reaches mitochondria through specialized domains of the ER called mitochondrial associated membranes (MAM) (42). Interestingly, CAV1 was recently described as a MAM resident protein (43) and thus is in principle in an excellent position to regulate cholesterol fluxes from and into MAM (Figure 1). In support of this possibility, we detected CAV1 in a crude fraction containing mitochondria and associated endoplasmic reticulum (34). Further, CAV1 is established to bind cholesterol in the ER (44), and transports this newly synthesized cholesterol from the ER to the plasma membrane (23, 45). Accordingly, cholesterol efflux to the cell surface is more rapid in cells expressing CAV1 (45) and CAV1 expression enhances cholesterol efflux in hepatic cells (46). Similarly, cholesterol addition rapidly accelerates the exocytic trafficking of newly-synthesized CAV1 from the ER to the plasma membrane (9). Taken together, we believe that this data supports the hypothesis that CAV1 promotes cholesterol efflux out of the ER, reducing availability of cholesterol in MAM, and thus ultimately the entry of cholesterol into mitochondria (Figure 1).
At the molecular level, CAV is an integral membrane protein, synthesized in the ER in a signal recognition particle (SRP)-dependent manner (47). Then, the newly synthesized protein rapidly goes through a first stage of oligomerization into low molecular weight oligomers in a process stabilized by binding with cholesterol (44, 47, 48). Immediately after synthesis CAV is recruited to ER exit sites (ERES) and the coat protein II (COPII) machinery mediates its transport to the Golgi complex (48). This immediate forward transport of CAV into the exocytic pathway will directly reduce the levels of newly synthesized cholesterol in the membranes of the ER, and ultimately the availability of mitochondrial cholesterol via MAM (Figure 1). Next, CAV transport through the Golgi complex is relatively slower and Golgi pools of newly synthesized CAV are observed in many cell types (9). Exit of CAV from the Golgi complex is associated with formation of high molecular weight oligomers (48) and masking of specific CAV epitopes by incorporation into cholesterol-enriched lipid-raft structures (9). This most probably occurs in late Golgi compartments which are especially enriched in glycosphingolipids and in cholesterol (39). Finally, the mature caveola-exocytic carrier is incorporated into the plasma membrane (1, 17). At the cell surface auxiliary proteins such as PTRF/cavins stabilize the organelle (19, 48, 49) and - in contrast to the intracellular pools of the protein- CAV becomes relatively immobile (18). Because cholesterol homeostasis is so important, there are clearly multiple regulatory mechanisms. In BHK cells, the exocytic pathway through the Golgi complex contributes only partially to the transport of newly synthesized cholesterol to the cell surface, and it was estimated as controlling approximately 20% of the total (50). Alternatively, it was proposed that a soluble form of CAV associated with chaperone complexes also cooperates in the transport of newly synthesized cholesterol from the ER through the cytoplasm to caveolae (23). Although questions remain about the relative importance of this cytosolic pool, CAV alone cannot control all cholesterol transport and in many cases - especially in optimal culture conditions- a deficit on this pathway will only have a mild impact in cells.
The hypothesis that CAV1 promotes cholesterol efflux out of the ER and thus ultimately reduces the entry of cholesterol into mitochondria predicted that steroid biosynthesis should be affected by the absence of CAV1. In steroidogenic cells, after synthesis at the ER, cholesterol is transported into mitochondria to become pregnenolone, the precursor of steroids, by the P450 side chain cleavage enzyme (CYP11A1). Then, pregnenolone leaves the mitochondrion to undergo enzymatic transformation into final steroid products. Mitochondrial cholesterol availability is the rate-determining step in steroid biosynthesis (51), so pregnenolone levels indicate the rate of mitochondrial cholesterol influx. In support to our hypothesis, we demonstrated that reduction of CAV1 protein levels in steroidogenic F2-CHO proportionally increased pregnenolone biosynthesis. We confirmed that the same mechanism occurs in animals: concentration of serum steroids (pregnenolone, corticosterone and testosterone) were found to be significantly higher in CAV1−/− mice (34).
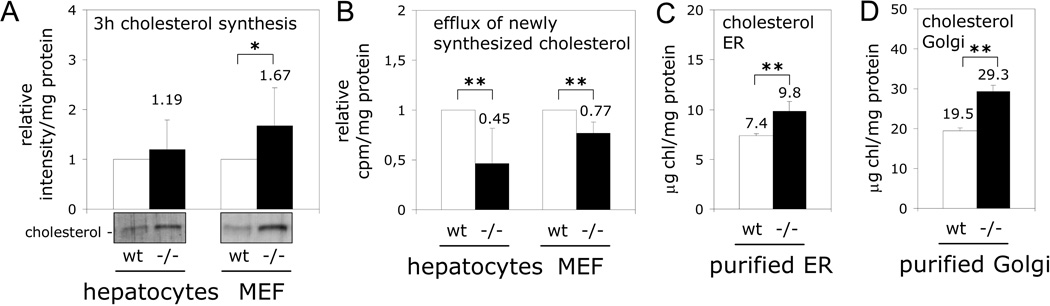
Moreover, the model proposed in Figure 1 makes 4 additional predictions that we have experimentally tested in this Interchange Article (Figure 2). First, in CAV1 deficient cells, there should be an intracellular accumulation of newly-synthesized cholesterol caused by the reduced transport of the new cholesterol into the plasma membrane. To test this, cells were incubated with the radio-labeled cholesterol precursor 14[C]-acetate, and intracellular accumulation of newly-synthesized cholesterol determined after just 3 hours by thin layer chromatography. As shown in Figure 2A and 2B, CAV1 deficient cells accumulate radio-labeled cholesterol and early arrival of this cholesterol to the cell surface, using a non-specific acceptor such as cyclodextrin, is reduced in CAV1 deficient hepatocytes and mouse embryonic fibroblasts (MEFs). Third, as a result of the reduced efflux, cholesterol levels throughout the exocytic pathway followed by CAV1 (48) should be higher in CAV1−/− cells. Indeed, as predicted by others (52), here we have measured by HPLC a significant enrichment in free cholesterol levels in ER purified from the liver of CAV1 deficient mice (Figure 2C, 32.79±10.33%). Finally, our last prediction is that cholesterol levels in the Golgi complex should be also higher in CAV deficient cells because CAV exit from the Golgi complex is associated with insertion into lipid-raft domains (1, 17). After acquisition of raft properties a single CAV protein seems to be able to organize an estimated of approximately 140 molecules of cholesterol (53). Indeed, as shown in Figure 2D, there is a significant enrichment (53.86±5.01%) in the levels of free cholesterol present in a Golgi fraction purified from CAV1−/− mice liver. Therefore, these results support the working model proposed in Figure 1 and strongly implicate CAV in the crucial lipid transport occurring throughout the exocytic pathway.
Figure 2. Inefficient exocytic transport of newly-synthesized cholesterol in CAV1−/− cells.
(A) Primary hepatocytes and MEFs were incubated for 3 hours with 14[C]-acetate and its incorporation into free cholesterol analyzed by TLC (example in bottom). Band intensity was calculated by densitometry and is shown as relative to the intensity of the band corresponding to wt cells in each experiment. (B) Efflux of radio-labeled cholesterol to cyclodextrin was measured during the last hour. The results are shown as relative to the efflux calculated in wt cells in each experiment. (C and D) Free cholesterol content of the ER and Golgi complex isolated from wt (white bars) and CAV1−/− mice liver (black bars) and measured by HPLC. Statistical significances were determined in at least 5 independent experiments using the Student’s t test, *P<0.05, **P<0.01.
Concluding remarks
In conclusion, the cholesterol binding capacity of CAV1, in combination with complex intracellular trafficking, converts CAV1 into an excellent candidate to function as a sensitive lipid organizing molecule not only at the cell surface but also on intracellular membranes. In support of this general idea, we have particularly demonstrated that in the absence of CAV1, cholesterol accumulates in mitochondrial membranes and the exocytic pathway. With the available data we favor a model in which the increased influx of cholesterol into mitochondria is a consequence of inefficient cholesterol efflux out of the ER. Cholesterol accumulation in the ER could account for the cholesterol increase in mitochondria, since the magnitudes of the increases are similar (32.79±10.33% for ER (Figure 2C) and 39.16±6.38% for mitochondria (34)).
This hypothesis of CAV as a cholesterol transporter proposes an alternative view of caveolae. It has been suggested that mammalian cells use caveolae and lipid droplets to ensure a supply of lipid-raft components to the cell when needed (1) and indeed, regulatory circuits that coordinate their functions are followed by CAV (9, 32, 54, 55). However, since cholesterol levels in different sub-cellular membranes can change the organelles function (as we saw for mitochondria), it is likely important to have ‘storehouse’ locations where it is possible to rapidly sequester as well as release extra cholesterol until needed, but where such changes will not impair cellular function. We hypothesize that caveolae may serve this role. If so, it would explain the dynamic nature of caveolae, specially in response to changes in cholesterol levels, and the fact that the precise contribution of caveolae and CAV to cell biology and animal physiology has remained difficult to elucidate, in spite of many investigations searching for the fundamental function of caveolae (1).
As might be expected from subtle impairment of cholesterol homeostasis, the CAV1 null animal lacks a clear fundamental phenotype. Nonetheless, there are a variety of mild phenotypes that link the loss of CAV function with unrelated diseases (3, 56, 57), which are likely explained by metabolic failure and oxidative stress (58). By discovery that CAV1 loss alters mitochondrial function, we provide a single underlying mechanism. In a physiological context, cells continuously switch between aerobic glycolysis and OXPHOS, and thus a lack of CAV function would progressively result in sustained oxidative stress and apoptosis, contributing to disease pathogenesis.
Methods
Animals, cells, and primary hepatocytes were cultured, treated or isolated exactly as previously described (34). Cholesterol in subcellular fractions was measured by HPLC as described (34, 41). Purified ER and Golgi were obtained from mice liver as described elsewhere (59). Statistical analysis of differences were determined using the Student's t test, *P<0.05, **P<0.01. All data shown in the graphs are the mean ± SD.
Cholesterol synthesis
Intracellular accumulation of newly synthesized cholesterol was determined by thin layer chromatography (TLC) by using 14[C]-acetate as the precursor. Cells plated in 6-well culture plates the day before and finally labeled by incubation with 1µCi/ml of 14[C]-acetate for 3 hours at 37°C. After washing with PBS, cells were trypsinized, centrifuged and resuspended in 100µl of PBS. An aliquot of the homogenate was taken for protein determination and lipids were extracted according to Bligh and Dyer (60) and separated by TLC by using petroleum ether/diethyl ether/acetic acid (60:40:1) as the solvent. Plates were exposed during 7 days for autoradiography. The band corresponding to cholesterol was identified by co-migration with a cholesterol standard. Band intensity was calculated by densitometry and is shown as relative to the intensity of the band corresponding to wt cells in each independent experiment.
Cholesterol efflux
Cells were plated in 6-well culture plates, growth overnight and labeled by incubation with 1µCi/ml of 14[C]-acetate for 2 hours at 37°C. After labeling, cells were rinsed three times with DMEM and incubated for 1 hour at 37°C with serum-free DMEM or DMEM containing 10mM β-cyclodextrin. 10µl aliquots were taken out for the liquid scintillation counter and cells were harvested to determine protein concentrations. Cholesterol incorporated on the plasma membrane was calculated as the cpm obtained in the presence of β-cyclodextrin after subtracting the cpm obtained in free DMEM. Results are shown as relative to the efflux calculated for wt cells in each independent experiment.
Acknowledgments
AP is supported by grants (BFU2008-00345, CSD2009-00016 and Marató de TV3 PI041315), MM (PI10/02114), SPG (GM64624/NIH), and JCFC (SAF2009-11417 and HI2007-0244/MCI, P50-AA-11999/NIAAA/NIH and Marató de TV3 PI041315). We would like to thank Dr Carlos Enrich, Dr Francesc Tebar, Dr Anna Colell, Dr Elina Ikonen and the members of AP’s and JCFC’s labs for scientific discussion and Dr. Amèrica Giménez and Josep Mª Marimon from the Animal Facility (UB), and Alba Fajardo, Anna Colell, Adam Kassan, Albert Herms and Susana Nuñez for technical assistance.
References
- 1.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 2.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 3.Le Lay S, Kurzchalia TV. Getting rid of caveolins: Phenotypes of caveolin-deficient animals. Biochim Biophys Acta. 2005 doi: 10.1016/j.bbamcr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, Shirani J, Razani B, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160(6):2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277(10):8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 6.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995;92(19):8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J Cell Biol. 1998;140(3):617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 9.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16(4):2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng ZJ, Singh RD, Sharma DK, Holicky EL, Hanada K, Marks DL, Pagano RE. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell. 2006;17(7):3197–3210. doi: 10.1091/mbc.E05-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gkantiragas I, Brugger B, Stuven E, Kaloyanova D, Li XY, Lohr K, Lottspeich F, Wieland FT, Helms JB. Sphingomyelin-enriched microdomains at the Golgi complex. Mol Biol Cell. 2001;12(6):1819–1833. doi: 10.1091/mbc.12.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11(4):424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 13.Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127(5):1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luetterforst R, Stang E, Zorzi N, Carozzi A, Way M, Parton RG. Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule. J Cell Biol. 1999;145(7):1443–1459. doi: 10.1083/jcb.145.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol. 1998;140(4):795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170(5):769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118(6):767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132(1):113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pol A, Calvo M, Lu A, Enrich C. The "early-sorting" endocytic compartment of rat hepatocytes is involved in the intracellular pathway of caveolin-1 (VIP-21) Hepatology. 1999;29(6):1848–1857. doi: 10.1002/hep.510290602. [DOI] [PubMed] [Google Scholar]
- 21.Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell. 2000;11(8):2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pol A, Calvo M, Enrich C. Isolated endosomes from quiescent rat liver contain the signal transduction machinery. Differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 1998;441(1):34–38. doi: 10.1016/s0014-5793(98)01517-8. [DOI] [PubMed] [Google Scholar]
- 23.Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273(11):6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- 24.Ostermeyer AG, Paci JM, Zeng Y, Lublin DM, Munro S, Brown DA. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol. 2001;152(5):1071–1078. doi: 10.1083/jcb.152.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152(5):1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Caveolin-2 is targeted to lipid droplets, a new "membrane domain" in the cell. J Cell Biol. 2001;152(5):1079–1085. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A. Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 2006;7(9):1254–1269. doi: 10.1111/j.1600-0854.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 28.Li WP, Liu P, Pilcher BK, Anderson RG. Cell-specific targeting of caveolin-1 to caveolae, secretory vesicles, cytoplasm or mitochondria. J Cell Sci. 2001;114(Pt 7):1397–1408. doi: 10.1242/jcs.114.7.1397. [DOI] [PubMed] [Google Scholar]
- 29.Woudenberg J, Rembacz KP, van den Heuvel FA, Woudenberg-Vrenken TE, Buist-Homan M, Geuken M, Hoekstra M, Deelman LE, Enrich C, Henning RH, Moshage H, Faber KN. Caveolin-1 is enriched in the peroxisomal membrane of rat hepatocytes. Hepatology. 51(5):1744–1753. doi: 10.1002/hep.23460. [DOI] [PubMed] [Google Scholar]
- 30.Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17(2):51–57. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92(22):10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingelmo-Torres M, Gonzalez-Moreno E, Kassan A, Hanzal-Bayer M, Tebar F, Herms A, Grewal T, Hancock JF, Enrich C, Bosch M, Gross SP, Parton RG, Pol A. Hydrophobic and basic domains target proteins to lipid droplets. Traffic. 2009;10(12):1785–1801. doi: 10.1111/j.1600-0854.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF, Parton RG. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1(2):98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 34.Bosch M, Mari M, Herms A, Fernandez A, Fajardo A, Kassan A, Giralt A, Colell A, Balgoma D, Barbero E, Gonzalez-Moreno E, Matias N, Tebar F, Balsinde J, Camps M, et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr Biol. 21(8):681–686. doi: 10.1016/j.cub.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bist A, Fielding PE, Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci U S A. 1997;94(20):10693–10698. doi: 10.1073/pnas.94.20.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, Bonds JA, Schilling JM, Miyanohara A, Headrick J, Ali SS, Roth DM, Patel PM, Patel HH. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 5(12):e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, Enrich C, Parton RG, Pol A. Caveolin-1 is essential for liver regeneration. Science. 2006;313(5793):1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 38.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 39.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Ruiz C, Mari M, Colell A, Morales A, Caballero F, Montero J, Terrones O, Basanez G, Fernandez-Checa JC. Mitochondrial cholesterol in health and disease. Histol Histopathol. 2009;24(1):117–132. doi: 10.14670/HH-24.117. [DOI] [PubMed] [Google Scholar]
- 41.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4(3):185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19(2):81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36(3):500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monier S, Dietzen DJ, Hastings WR, Lublin DM, Kurzchalia TV. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 1996;388(2–3):143–149. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- 45.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271(46):29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 46.Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem. 2004;279(14):14140–14146. doi: 10.1074/jbc.M311061200. [DOI] [PubMed] [Google Scholar]
- 47.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6(7):911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic. 11(3):361–382. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8(4):310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci U S A. 2000;97(15):8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110(7):881–890. doi: 10.1172/JCI16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291(2):H677–H686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- 53.Ortegren U, Karlsson M, Blazic N, Blomqvist M, Nystrom FH, Gustavsson J, Fredman P, Stralfors P. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur J Biochem. 2004;271(10):2028–2036. doi: 10.1111/j.1432-1033.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 54.Le Lay S, Hajduch E, Lindsay MR, Le Liepvre X, Thiele C, Ferre P, Parton RG, Kurzchalia T, Simons K, Dugail I. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic. 2006;7(5):549–561. doi: 10.1111/j.1600-0854.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 55.Pol A, Martin S, Fernandez MA, Ferguson C, Carozzi A, Luetterforst R, Enrich C, Parton RG. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol Biol Cell. 2004;15(1):99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim CA, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, Semple RK, O'Rahilly S, Dugail I, Capeau J, Lathrop M, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 58.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Croze EM, Morre DJ. Isolation of plasma membrane, golgi apparatus, and endoplasmic reticulum fractions from single homogenates of mouse liver. J Cell Physiol. 1984;119(1):46–57. doi: 10.1002/jcp.1041190109. [DOI] [PubMed] [Google Scholar]
- 60.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 61.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism. 1962;11:1098–1112. [PubMed] [Google Scholar]
- 62.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11(5):388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 64.Avery SV. Molecular targets of oxidative stress. Biochem J. 434(2):201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]